Blurred by a “Puff of Smoke”—A Case-Based Review on the Challenging Recognition of Coexisting CNS Demyelinating Disease and Moyamoya Angiopathy
Abstract
1. Introduction
2. Case Presentation
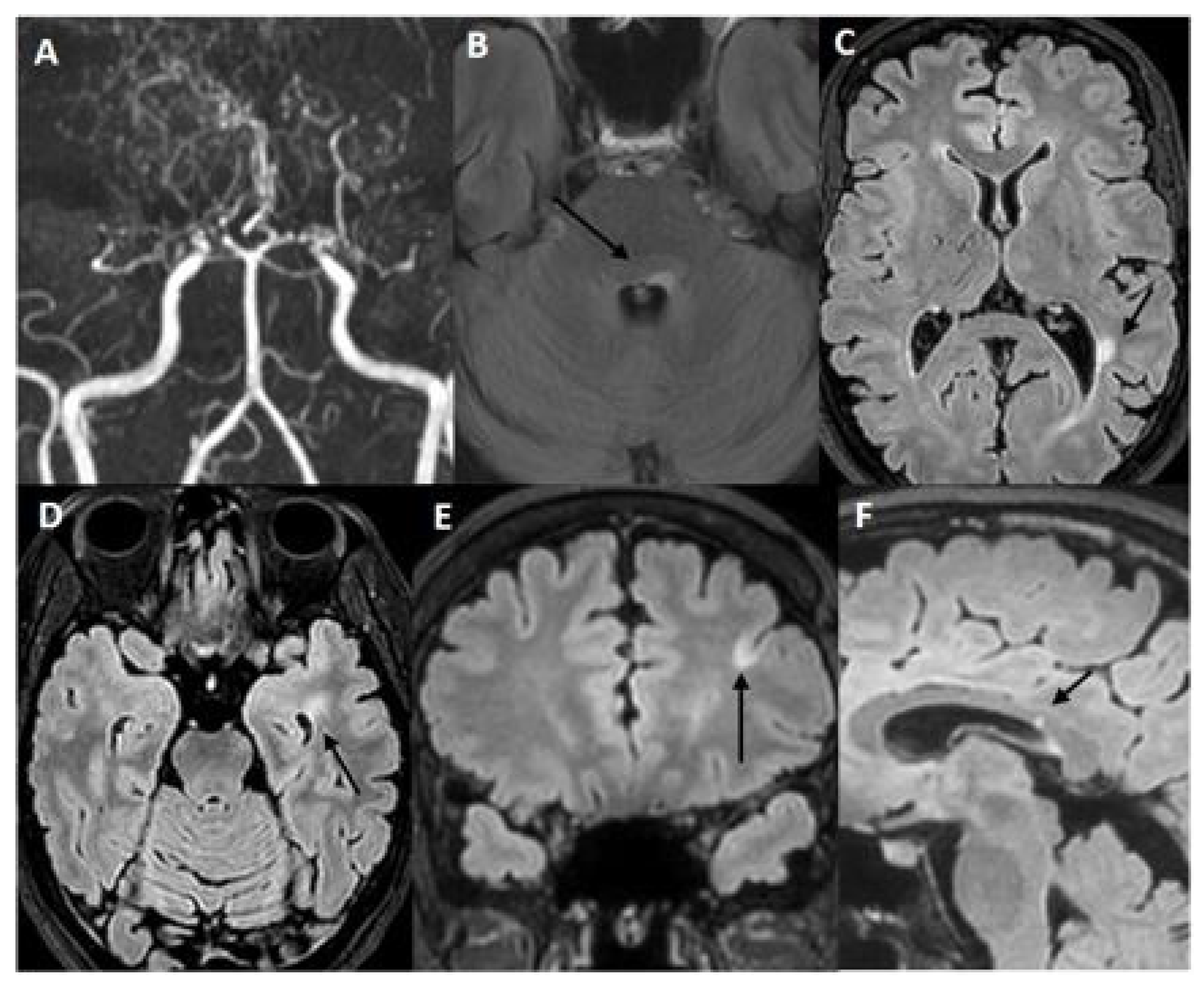
2.1. Case 1: A 45-Year-Old Woman Was Referred to Our Cerebrovascular Department for MMA
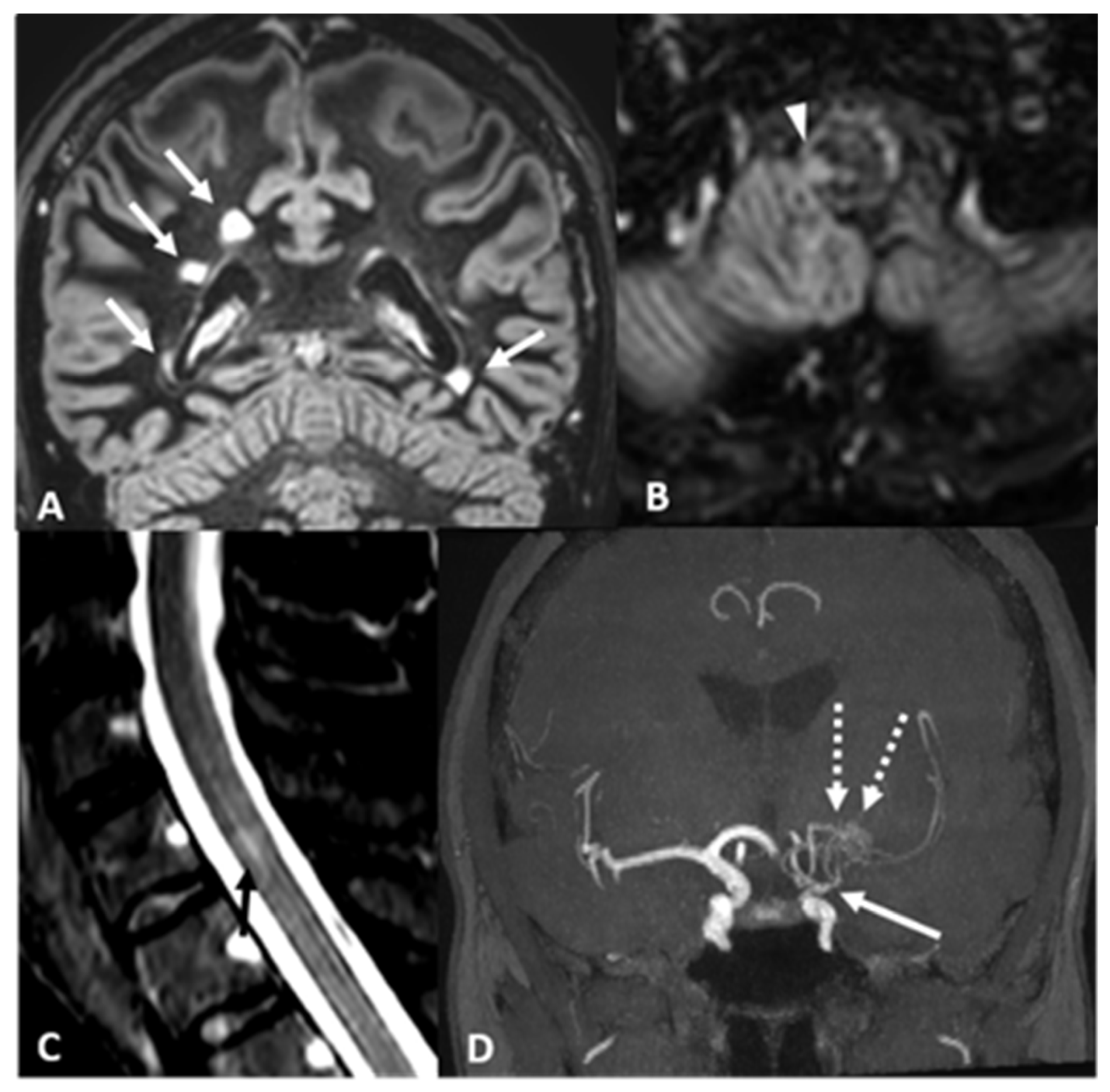
2.2. Case 2: A 43 Years-Old Woman Was Evaluated in Our Institute Because of the Neuroradiological Finding of Unilateral MMA
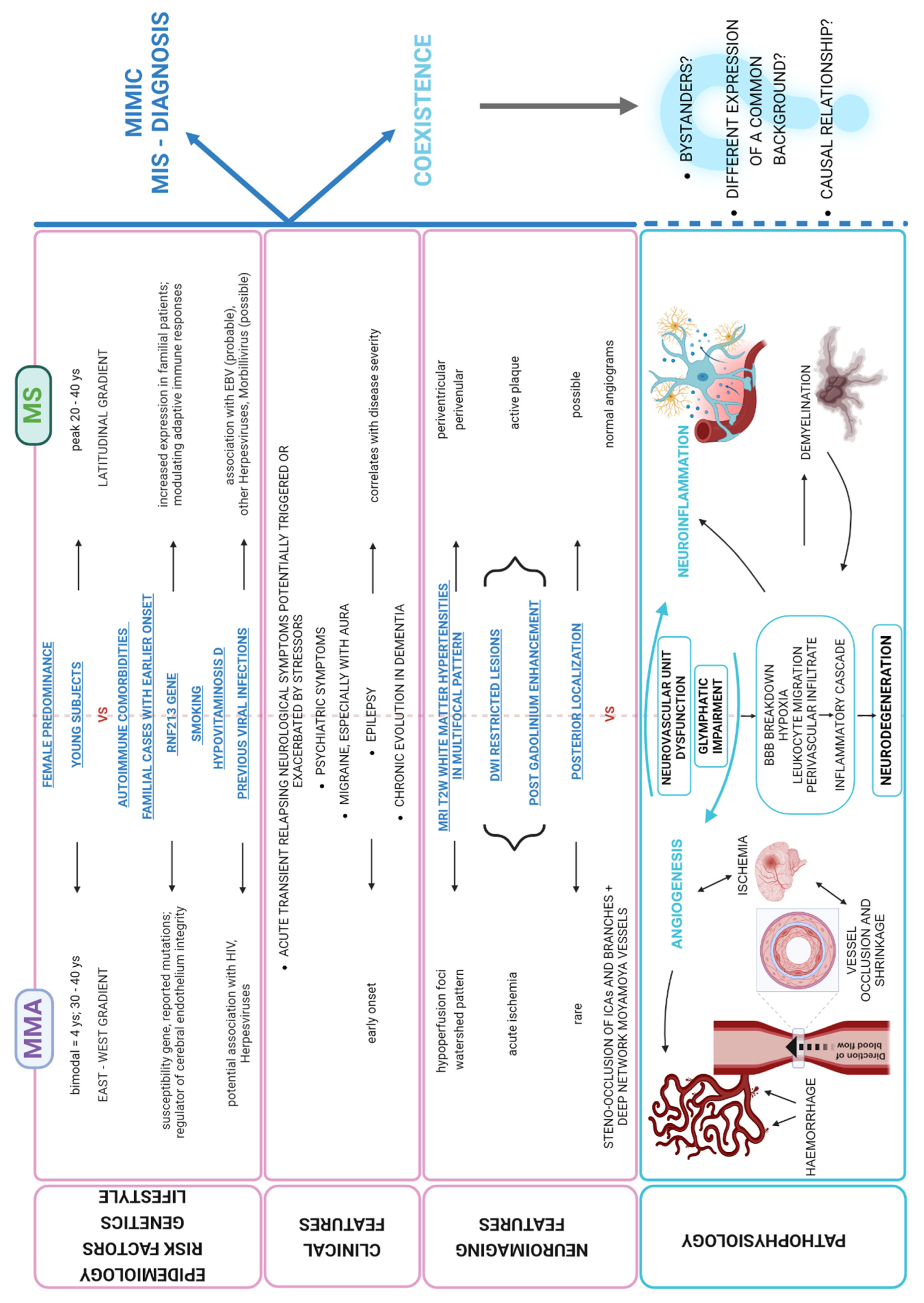
3. Discussion
- -
- On one hand, it is indeed imperative to diagnose and promptly treat MMA at the early stages with antiplatelets and revascularization surgery, before irreversible stroke occurs.On the other hand, the definite diagnosis of MMA should not automatically exclude MS as a possible concomitant disease, especially considering the higher incidence of MS [6] and the role that autoimmune mechanisms potentially play in both these conditions. Thus, it may be reasonable to recommend CSF analysis and spine MRI in the diagnostic work-up of MMA, especially in the presence of atypical features, e.g., when MRI lesions are not exclusively located in the typical borderzone territories (‘watershed lesions’) and/or when their morphology may suggest a different etiology.
| Case | Country/ Ethnicity | Age/Sex | Clinical Presentation | T2-w MRI White Matter Hyperintensities Location | Gadolinium Enhancement | Spine MRI | CSF OB | VEP | Other Tests | Angiogram Findings | Diagnosis | Any Clinical Stroke? | Treatment | FU | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| For MMA | For NMO | ||||||||||||||
| [66] | Japan/ n.s. | 52/F | Blindness, sensory-motor impairment lower limbs, urinary retention | Not specified (Hemorrhage in right thalamus) Longitudinal thoracic spinal cord | Brain: − Spine: + | + | − | + | ANA (+ 1:80) Anti SS-A/B Ab (+) AQP4 Ab (+) ANCA (−) Anticardiolipin (−) | Bilateral ACA and MCA occlusion, bilateral MM network | MMA + NMO + Systemic sclerosis | Hemorrhage | n.s. (hemorrhagic) | High dose i.v. corticosteroid pulse | 4 month |
| [67] | Hong Kong/East Asian | 62/F | Gait impairment to tetraparesis and sensory impairment | Brain: − Longitudinal cervico-thoracic (C2-T3) lesions | Spine: n.s. | + | n.s. | + | Anti SS-A Ab (+) AQP4 Ab (+) ANA (−) | Bilateral ICAD and MCA occlusion, bilateral MM network | MMA + NMO | n.s. | n.s. | High dose i.v. corticosteroid pulse, followed by oral tapering and azathioprine, followed by cyclophosphamide | 8 month |
| [26] | China/ n.s. | 43/F | Left sensory impairment | Brain: centrum semiovale + DWI restriction in right thalamus, hemosiderin deposition in left basal ganglia Thoracic spinal cord | Brain: n.s. Spine: n.s. | + | n.s. | + | AQP4 Ab (+) | Bilateral ACA and MCA, right PCA and right ICAD stenosis, bilateral MM network | MMA + NMO | Hemorrhagic and ischemic | (hemorrhagic) Antiplatelet | I.v. and oral corticosteroids, azathioprine | 6 month |
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukui, M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya’ disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin. Neurol. Neurosurg. 1997, 99 (Suppl. S2), S238–S240. [Google Scholar] [CrossRef] [PubMed]
- ‘t Hart, B.A.; Brok, H.P.; Amor, S.; Bontrop, R.E. The major histocompatibility complex influences the ethiopathogenesis of MS-like disease in primates at multiple levels. Hum. Immunol. 2001, 62, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.P.; Lublin, F.D.; McFarland, H.F.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef]
- Yoon, H.K.; Shin, H.J.; Chang, Y.W. “Ivy sign” in childhood moyamoya disease: Depiction on FLAIR and contrast-enhanced T1-weighted MR images. Radiology 2002, 223, 384–389. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef]
- Dorfman, L.J.; Fischbein, N.J.; Woodard, J.I.; Choudhri, O.; Bell-Stephens, T.E.; Steinberg, G.K. Moyamoya disease can masquerade as multiple sclerosis. Neurologist 2012, 18, 398–403. [Google Scholar] [CrossRef]
- Zaheer, F.; Berger, J.R. Moyamoya: Another multiple sclerosis mimic. Mult. Scler. Relat. Disord. 2012, 1, 100–103. [Google Scholar] [CrossRef]
- Si, X.; Li, L.; Fang, Y.; Yan, Y.; Pu, J. A Patient With Multiple Sclerosis and Coexisting Moyamoya Disease: Why and How. Front. Neurol. 2020, 11, 516587. [Google Scholar] [CrossRef]
- Koul, P.; Patel, A.; Chaudhry, F.; Steinklein, J.; Harel, A. A patient with concurrent multiple sclerosis and moyamoya disease. Mult. Scler. Relat. Disord. 2021, 54, 103151. [Google Scholar] [CrossRef]
- Shamrani, F.A.; Al-Khamis, F.; Al Ghanimi, I.; Khuda, I.; Al Jaafari, D.; Alabdali, M. Moyamoya Disease in Patient with Multiple Sclerosis. Bahrain Med. Bull. 2015, 37. Available online: https://www.bahrainmedicalbulletin.com/september_2015/MoyaMoya.pdf (accessed on 18 May 2025). [CrossRef]
- Bower, R.S.; Mallory, G.W.; Nwojo, M.; Kudva, Y.C.; Flemming, K.D.; Meyer, F.B. Moyamoya disease in a primarily white, midwestern US population: Increased prevalence of autoimmune disease. Stroke 2013, 44, 1997–1999. [Google Scholar] [CrossRef] [PubMed]
- Graf, J.; Schwitalla, J.C.; Albrecht, P.; Veltkamp, R.; Berlit, P.; Hartung, H.P.; Aktas, O.; Kraemer, M. Misdiagnoses and delay of diagnoses in Moyamoya angiopathy-a large Caucasian case series. J. Neurol. 2019, 266, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Canavero, I.; Vetrano, I.G.; Zedde, M.; Pascarella, R.; Gatti, L.; Acerbi, F.; Nava, S.; Ferroli, P.; Parati, E.A.; Bersano, A. Clinical Management of Moyamoya Patients. J. Clin. Med. 2021, 10, 3628. [Google Scholar] [CrossRef]
- Frohman, T.C.; Davis, S.L.; Beh, S.; Greenberg, B.M.; Remington, G.; Frohman, E.M. Uhthoff’s phenomena in MS—Clinical features and pathophysiology. Nat. Rev. Neurol. 2013, 9, 535–540. [Google Scholar] [CrossRef]
- Trivedi, J.K. Cognitive deficits in psychiatric disorders: Current status. Indian J. Psychiatry 2006, 48, 10–20. [Google Scholar] [CrossRef]
- Kraemer, M.; Berlit, P. Primary central nervous system vasculitis and moyamoya disease: Similarities and differences. J. Neurol. 2010, 257, 816–819. [Google Scholar] [CrossRef]
- Haller, S.; Kövari, E.; Herrmann, F.R.; Cuvinciuc, V.; Tomm, A.M.; Zulian, G.B.; Lovblad, K.O.; Giannakopoulos, P.; Bouras, C. Do brain T2/FLAIR white matter hyperintensities correspond to myelin loss in normal aging? A radiologic-neuropathologic correlation study. Acta Neuropathol. Commun. 2013, 1, 14. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Li, H.; Xiao, L.; Zhou, Z.; Chen, K.; Gui, L.; Hou, X.; Fan, R.; Chen, K.; et al. Clinical, Radiological and Pathological Characteristics Between Cerebral Small Vessel Disease and Multiple Sclerosis: A Review. Front. Neurol. 2022, 13, 841521. [Google Scholar] [CrossRef]
- Love, S. Demyelinating diseases. J. Clin. Pathol. 2006, 59, 1151–1159. [Google Scholar] [CrossRef]
- Bersano, A.; Khan, N.; Fuentes, B.; Acerbi, F.; Canavero, I.; Tournier-Lasserve, E.; Vajcoczy, P.; Zedde, M.L.; Hussain, S.; Lémeret, S.; et al. European Stroke Organisation (ESO) Guidelines on Moyamoya angiopathy Endorsed by Vascular European Reference Network (VASCERN). Eur. Stroke J. 2023, 8, 55–84. [Google Scholar] [CrossRef]
- Funaki, T.; Takahashi, J.C.; Yoshida, K.; Takagi, Y.; Fushimi, Y.; Kikuchi, T.; Mineharu, Y.; Okada, T.; Morimoto, T.; Miyamoto, S. Periventricular anastomosis in moyamoya disease: Detecting fragile collateral vessels with MR angiography. J. Neurosurg. 2016, 124, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- McNicholas, N.; Hutchinson, M.; McGuigan, C.; Chataway, J. 2017 McDonald diagnostic criteria: A review of the evidence. Mult. Scler. Relat. Disord. 2018, 24, 48–54. [Google Scholar] [CrossRef]
- Preziosa, P.; Martinelli, V.; Ferrè, L.; Guaschino, C.; Simionato, F.; Moiola, L.; Comi, G.; Filippi, M. Moyamoya disease mimicking the first attack of multiple sclerosis. J. Neurol. 2017, 264, 1005–1007. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, W.; Guo, X.; Shi, Z.; Sun, Z.; Gao, L.; Jin, F.; Wang, J.; Chen, W.; Yang, Y. CT perfusion assessment of Moyamoya syndrome before and after direct revascularization (superficial temporal artery to middle cerebral artery bypass). Eur. Radiol. 2016, 26, 254–261. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zheng, Y.; Cai, M.T.; Wu, L.; Zhang, B.R. Moyamoya disease presenting as thalamic infarction in a patient with neuromyelitis optica spectrum disorder. CNS Neurosci. Ther. 2019, 25, 412–414. [Google Scholar] [CrossRef]
- Liu, W.; Morito, D.; Takashima, S.; Mineharu, Y.; Kobayashi, H.; Hitomi, T.; Hashikata, H.; Matsuura, N.; Yamazaki, S.; Toyoda, A.; et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS ONE 2011, 6, e22542. [Google Scholar] [CrossRef]
- Kamada, F.; Aoki, Y.; Narisawa, A.; Abe, Y.; Komatsuzaki, S.; Kikuchi, A.; Kanno, J.; Niihori, T.; Ono, M.; Ishii, N.; et al. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J. Hum. Genet. 2011, 56, 34–40. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, X.; Sheng, J.; Fu, Y.; Nie, D.; You, X.; Chen, Y.; Yang, X.; Ling, Q.; Zhang, H.; et al. RNF213 promotes Treg cell differentiation by facilitating K63-linked ubiquitination and nuclear translocation of FOXO1. Nat. Commun. 2024, 15, 5961. [Google Scholar] [CrossRef]
- Vilariño-Güell, C.; Zimprich, A.; Martinelli-Boneschi, F.; Herculano, B.; Wang, Z.; Matesanz, F.; Urcelay, E.; Vandenbroeck, K.; Leyva, L.; Gris, D.; et al. Exome sequencing in multiple sclerosis families identifies 12 candidate genes and nominates biological pathways for the genesis of disease. PLoS Genet. 2019, 15, e1008180, Erratum in PLoS Genet. 2020, 16, e1008737. [Google Scholar] [CrossRef]
- Manini, A.; Pantoni, L. Genetic Causes of Cerebral Small Vessel Diseases: A Practical Guide for Neurologists. Neurology 2023, 100, 766–783. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Chauhan, G.; Violleau, M.H.; Vojinovic, D.; Jian, X.; Bis, J.C.; Li, S.; Saba, Y.; Grenier-Boley, B.; Yang, Q.; et al. Association of variants in HTRA1 and NOTCH3 with MRI-defined extremes of cerebral small vessel disease in older subjects. Brain 2019, 142, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Hosoki, S.; Yamaguchi, E.; Ishiyama, H.; Abe, S.; Yoshimoto, T.; Tanaka, T.; Hattori, Y.; Liao, Y.C.; Lee, Y.C.; et al. Blended Phenotype of NOTCH3 and RNF213 Variants with Accelerated Large and Small Artery Crosstalk: A Case Report and Literature Review. Neurol. Genet. 2024, 10, e200176. [Google Scholar] [CrossRef]
- Bersano, A.; Guey, S.; Bedini, G.; Nava, S.; Hervé, D.; Vajkoczy, P.; Tatlisumak, T.; Sareela, M.; van der Zwan, A.; Klijn, C.J.; et al. European Moyamoya Disease Initiative. Research Progresses in Understanding the Pathophysiology of Moyamoya Disease. Cerebrovasc. Dis. 2016, 41, 105–118. [Google Scholar] [CrossRef]
- Weng, L.; Cao, X.; Han, L.; Zhao, H.; Qiu, S.; Yan, Y.; Wang, X.; Chen, X.; Zheng, W.; Xu, X.; et al. Association of increased Treg and Th17 with pathogenesis of moyamoya disease. Sci. Rep. 2017, 7, 3071. [Google Scholar] [CrossRef]
- Wanifuchi, H.; Kagawa, M.; Takeshita, M.; Izawa, M.; Maruyama, S.; Kitamura, K. Autoimmune antibody in moyamoya disease. No Shinkei Geka 1986, 14, 31–55. (In Japanese) [Google Scholar]
- Jeong, H.C.; Kim, Y.J.; Yoon, W.; Joo, S.P.; Lee, S.S.; Park, Y.W. Moyamoya syndrome associated with systemic lupus erythematosus. Lupus 2008, 17, 679–682. [Google Scholar] [CrossRef]
- Malik, S.; Russman, A.N.; Katramados, A.M.; Silver, B.; Mitsias, P.D. Moyamoya syndrome associated with Graves’ disease: A case report and review of the literature. J. Stroke Cerebrovasc. Dis. 2011, 20, 528–536. [Google Scholar] [CrossRef]
- Paciaroni, M.; Micheli, S.; Caso, V.; Venti, M.; Alberti, A.; Milia, P.; Balucani, C.; Biagini, S.; Agnelli, G. Angiographic findings of moyamoya vessels in a patient with rheumatoid arthritis. Cerebrovasc. Dis. 2005, 20, 415–416. [Google Scholar] [CrossRef]
- Lin, Y.H.; Huang, H.; Hwang, W.Z. Moyamoya disease with Sjogren disease and autoimmune thyroiditis presenting with left intracranial hemorrhage after messenger RNA-1273 vaccination: A case report. Medicine 2022, 101, e28756. [Google Scholar] [CrossRef]
- Annunziata, P.; De Santi, L.; Di Rezze, S.; Millefiorini, E.; Capello, E.; Mancardi, G.; De Riz, M.; Scarpini, E.; Vecchio, R.; Patti, F. Clinical features of Sjogren’s syndrome in patients with multiple sclerosis. Acta Neurol. Scand. 2011, 124, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Leal Rato, M.; Santos, M.; de Sá, J.; Ferreira, J. Comorbid autoimmune disorders in people with multiple sclerosis: A retrospective cohort study. J. Neuroimmunol. 2023, 385, 578226. [Google Scholar] [CrossRef] [PubMed]
- OMIM. Available online: https://www.omim.org/entry/618999 (accessed on 27 February 2025).
- Benveniste, E.N.; Liu, Y.; McFarland, B.C.; Qin, H. Involvement of the janus kinase/signal transducer and activator of transcription signaling pathway in multiple sclerosis and the animal model of experimental autoimmune encephalomyelitis. J. Interferon Cytokine Res. 2014, 34, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Chen, C.; Shi, G.; Zhou, Y.; Wei, Y.; Wu, L.; Sun, L.; Zhang, T. JAK inhibition ameliorated experimental autoimmune encephalomyelitis by blocking GM-CSF-driven inflammatory signature of monocytes. Acta Pharm. Sin. B. 2023, 13, 4185–4201. [Google Scholar] [CrossRef]
- Demartini ZJr Teixeira, B.C.; Koppe, G.L.; Gatto, L.A.M.; Roman, A.; Munhoz, R.P. Moyamoya disease and syndrome: A review. Radiol. Bras. 2022, 55, 31–37. [Google Scholar] [CrossRef]
- Phi, J.H.; Wang, K.C.; Lee, J.Y.; Kim, S.K. Moyamoya Syndrome: A Window of Moyamoya Disease. J. Korean Neurosurg. Soc. 2015, 57, 408–414. [Google Scholar] [CrossRef]
- Houkin, K.; Ito, M.; Sugiyama, T.; Shichinohe, H.; Nakayama, N.; Kazumata, K.; Kuroda, S. Review of past research and current concepts on the etiology of moyamoya disease. Neurol. Med. Chir. 2012, 52, 267–277. [Google Scholar] [CrossRef]
- Imani, S.Z.H.; Hojati, Z.; Khalilian, S.; Dehghanian, F.; Kheirollahi, M.; Khorrami, M.; Shaygannejad, V.; Mirmosayyeb, O. Expression and clinical significance of IL7R, NFATc2, and RNF213 in familial and sporadic multiple sclerosis. Sci. Rep. 2021, 11, 19260. [Google Scholar] [CrossRef]
- Cashion, J.M.; Young, K.M.; Sutherland, B.A. How does neurovascular unit dysfunction contribute to multiple sclerosis? Neurobiol. Dis. 2023, 178, 106028. [Google Scholar] [CrossRef]
- Yang, R.; Dunn, J.F. Reduced cortical microvascular oxygenation in multiple sclerosis: A blinded, case-controlled study using a novel quantitative near-infrared spectroscopy method. Sci. Rep. 2015, 5, 16477. [Google Scholar] [CrossRef]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernán, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef]
- Degelman, M.L.; Herman, K.M. Smoking and multiple sclerosis: A systematic review and meta-analysis using the Bradford Hill criteria for causation. Mult. Scler. Relat. Disord. 2017, 17, 207–216. [Google Scholar] [CrossRef]
- Hedström, A.K.; Olsson, T.; Alfredsson, L. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult. Scler. 2012, 18, 1334–1336. [Google Scholar] [CrossRef]
- Munger, K.L.; Bentzen, J.; Laursen, B.; Stenager, E.; Koch-Henriksen, N.; Sørensen, T.I.; Baker, J.L. Childhood body mass index and multiple sclerosis risk: A long-term cohort study. Mult. Scler. 2013, 19, 1323–1329. [Google Scholar] [CrossRef]
- Pepper, R.E.; Pitman, K.A.; Cullen, C.L.; Young, K.M. How Do Cells of the Oligodendrocyte Lineage Affect Neuronal Circuits to Influence Motor Function, Memory and Mood? Front. Cell. Neurosci. 2018, 12, 399. [Google Scholar] [CrossRef]
- Holley, J.E.; Newcombe, J.; Whatmore, J.L.; Gutowski, N.J. Increased blood vessel density and endothelial cell proliferation in multiple sclerosis cerebral white matter. Neurosci. Lett. 2010, 470, 65–70. [Google Scholar] [CrossRef]
- Pantoni, L.; Garcia, J.H.; Gutierrez, J.A. Cerebral white matter is highly vulnerable to ischemia. Stroke 1996, 27, 1641–1646, discussion 1647. [Google Scholar] [CrossRef]
- Rinholm, J.E.; Hamilton, N.B.; Kessaris, N.; Richardson, W.D.; Bergersen, L.H.; Attwell, D. Regulation of oligodendrocyte development and myelination by glucose and lactate. J. Neurosci. 2011, 31, 538–548. [Google Scholar] [CrossRef]
- Cunnea, P.; McMahon, J.; O’Connell, E.; Mashayekhi, K.; Fitzgerald, U.; McQuaid, S. Gene expression analysis of the microvascular compartment in multiple sclerosis using laser microdissected blood vessels. Acta Neuropathol. 2010, 119, 601–615. [Google Scholar] [CrossRef]
- Taccone, F.S.; Su, F.; De Deyne, C.; Abdellhai, A.; Pierrakos, C.; He, X.; Donadello, K.; Dewitte, O.; Vincent, J.L.; De Backer, D. Sepsis is associated with altered cerebral microcirculation and tissue hypoxia in experimental peritonitis. Crit. Care Med. 2014, 42, e114–e122. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tanaka, M.; Kondo, S.; Okamoto, K.; Hirai, S. Clinical significance of reduced cerebral metabolism in multiple sclerosis: A combined PET and MRI study. Ann. Nucl. Med. 1998, 12, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Du, S.Q.; Wang, X.R.; Xiao, L.Y.; Tu, J.F.; Zhu, W.; He, T.; Liu, C.Z. Molecular Mechanisms of Vascular Dementia: What Can Be Learned from Animal Models of Chronic Cerebral Hypoperfusion? Mol. Neurobiol. 2017, 54, 3670–3682. [Google Scholar] [CrossRef]
- Bruscolini, A.; Sacchetti, M.; La Cava, M.; Gharbiya, M.; Ralli, M.; Lambiase, A.; De Virgilio, A.; Greco, A. Diagnosis and management of neuromyelitis optica spectrum disorders—An update. Autoimmun. Rev. 2018, 17, 195–200. [Google Scholar] [CrossRef]
- Asai, Y.; Nakayasu, H.; Fusayasu, E.; Nakashima, K. Moyamoya disease presenting as thalamic hemorrhage in a patient with neuromyelitis optica and Sjögren’s syndrome. J. Stroke Cerebrovasc. Dis. 2012, 21, 619.e7–619.e9. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.H.; Ip, V.H.; Au, L.; Siu, D.; Leung, T.; Xiong, L.; Wong, K.S. Moyamoya disease in a patient with neuromyelitis optica. Oxf. Med. Case Rep. 2014, 2014, 13–15. [Google Scholar] [CrossRef][Green Version]
| Gene (Transcript) | Nucleotide Change | Aminoacid Change | ACMG Class * | Disease (OMIM) |
|---|---|---|---|---|
| RNF213 (NM_001256071.3) | c.12092T>C | p.Ile4031Thr | III (VUS) | Moyamoya (*607151) |
| NOTCH3 (NM_000435.3) | c.2738C>T | p.Pro913Leu | III (VUS) | Cerebral arteriopathy (*600276) |
| COL4A1 (NM_0018450.6) | c.1588C>T | p.Pro530Ser | III (VUS) | Microangiopathy and leukoencephalopathy, pontine, autosomal dominant (*618564) |
| JAK1 (NM_002227.4) | c.1513G>A | p.Gly505Ser | III (VUS) | Autoinflammation, Immune dysregulation (*618999) |
| Case | Country/Ethnicity | Age/ Sex | Clinical Presentation | T2-w MRI White Matter Hyperintensities Location | Gadolinium Enhancement | Spine MRI | CSF OB | VEP | Other Tests | Angiogram Findings | Diagnosis | Any Clinical Stroke? | Treatment | FU | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| For MMA | For MS | ||||||||||||||
| [6] (case 3) | US/ n.s. | 44/F | Intermittent left hemiparesis | Multiple subcortical lesions (n.s.) | n.s. | − | + | − | n.s. | Left ICA occlusion, severe right ICAD stenosis, bilateral MM network | MMA + MS | Ischemic stroke | Bilateral bypass surgery, Aspirin | b-interferon, glatiramer acetate, high dose i.v. corticosteroids | 6 month |
| [6] (case 9) | US/ n.s. | 31/F | Limb numbness and weakness, memory impairment | Multiple subcortical lesions (n.s.) | n.s. | n.s. | n.s. | n.s. | n.s. | Right MCA occlusion, R ACA stenosis | MMA + MS | n.s. | Right bypass surgery | n.s. | 18 month |
| [7] (case 2) | US/ n.s. | 44/M | Right hemiparesis; impaired vision | Periventricular | Brain: − | n.p. | − | + | ANA (+)—ENA (−) | Left ACA, MCA and PCA stenosis | MMA mimicking/ associated with MS (discussion not conclusive) | TIA | Aspirin + Clopidogrel | b-interferon (stopped after MMA evidence) | n.s. |
| [8] | China/ n.s. | 42/M | Right hemiparesis, speech impairment | Cervical spinal cord; Frontal, parietal, temporal lobe | Brain: + Spine: + | + | + | + | Anti-b2-glycoprotein I IgA (++) | Bilateral ICAD and right MCA occlusion; Left ACA, MCA, PCA stenosis | MMA + MS | Ischemic stroke (during steroid administration) | Aspirin + Clopidogrel; bilateral bypass surgery | High dose i.v. methylprednisolone | 3 month |
| [9] | US/ Caucasian | 57/F | Episodic gait dysfunction; feet paresthesias; myelopathic signs | Cervical and thoracic spinal cord; bilateral frontal lobes; right pons; right cerebellum | Brain: spotty + Spine: − | + | + | NA | ANA (+ 1:80) Ab anti AQP4 and anti MOG (−) Ab anti cardiolipin (−) | Bilateral MCA and ACA occlusion, MM network | MMA + MS | No | n.s. | n.s. | n.s. |
| [10] | Saudi Arabia/ n.s. | 16/F | Left hemiparesis | Periventricular lesion | n.s. | n.s. | + | n.s. | n.s. | Bilateral steno-occlusion ICAs-MCAs, R-ACA and R-PCA stenosis, lentriculostriate network | MMA + MS | Ischemic stroke (2) | Aspirin; bilateral by-pass surgery | Interferon beta and steroids | n.s. |
| Our Case 1 | Italy/ Caucasian | 45/F | Blurred vision and diplopia | Bilateral brainstem, left frontal and temporal lobe, right temporal lobe, cerebellar hemispheres, left periventricular region | Brain: | − | + | − | AntiAQP4 (−) Anti-ds DNA (+ 1:20) ANA-ENA-ANCA-Anti cardiolipin and beta2GP (−) | Bilateral MCAs and ACAs narrow, thick network of collateral vessels | MMA + MS | TIA | By-pass surgery, Aspirin | Corticosteroids Teriflunomide | 4 years |
| Our Case 2 | Italy/ Caucasian | 43/F | Left hemi-anesthesia (transient, recurring) | Bilateral supratentorial periventricular with corpus callosum involvement, left cerebellar hemisphere; bulbospinal tract; cervical spine | Brain: − Spine: − | + | + | − | AntiAQP4 (−) Anti-ds DNA-ANA-ENA-ANCA-Anti cardiolipin and beta2GP(−) G20210A Factor II and MTHFRC1677T (heterozigosis) Total body PET (−) | Left MCA and distal ICA steno-occlusion, MM network | MMA + MS | no | Aspirin | Dimethylfumarate Glatiramer acetate Teriflunomide | 8 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canavero, I.; Rifino, N.; Antozzi, C.; Caldiera, V.; Colombo, E.; Carrozzini, T.; Ganci, G.; Ferroli, P.; Acerbi, F.; Storti, B.; et al. Blurred by a “Puff of Smoke”—A Case-Based Review on the Challenging Recognition of Coexisting CNS Demyelinating Disease and Moyamoya Angiopathy. Int. J. Mol. Sci. 2025, 26, 5030. https://doi.org/10.3390/ijms26115030
Canavero I, Rifino N, Antozzi C, Caldiera V, Colombo E, Carrozzini T, Ganci G, Ferroli P, Acerbi F, Storti B, et al. Blurred by a “Puff of Smoke”—A Case-Based Review on the Challenging Recognition of Coexisting CNS Demyelinating Disease and Moyamoya Angiopathy. International Journal of Molecular Sciences. 2025; 26(11):5030. https://doi.org/10.3390/ijms26115030
Chicago/Turabian StyleCanavero, Isabella, Nicola Rifino, Carlo Antozzi, Valentina Caldiera, Elena Colombo, Tatiana Carrozzini, Giuseppe Ganci, Paolo Ferroli, Francesco Acerbi, Benedetta Storti, and et al. 2025. "Blurred by a “Puff of Smoke”—A Case-Based Review on the Challenging Recognition of Coexisting CNS Demyelinating Disease and Moyamoya Angiopathy" International Journal of Molecular Sciences 26, no. 11: 5030. https://doi.org/10.3390/ijms26115030
APA StyleCanavero, I., Rifino, N., Antozzi, C., Caldiera, V., Colombo, E., Carrozzini, T., Ganci, G., Ferroli, P., Acerbi, F., Storti, B., Boncoraglio, G. B., Potenza, A., Pollaci, G., Gorla, G., Ciceri, E., De Marco, P., Gatti, L., & Bersano, A. (2025). Blurred by a “Puff of Smoke”—A Case-Based Review on the Challenging Recognition of Coexisting CNS Demyelinating Disease and Moyamoya Angiopathy. International Journal of Molecular Sciences, 26(11), 5030. https://doi.org/10.3390/ijms26115030








