Exploring the Healing Powers of Histatins: From Oral Health to Therapeutics
Abstract
1. Introduction
2. Gene Expression and Transcriptional Regulation
3. Post-Translational Modifications
4. Structure and Functional Domains of Histatins
5. Domain-Specific Functions
5.1. Antifungal Properties
5.2. Antibacterial Properties
5.3. Enamel Fortification
5.4. Immunomodulation
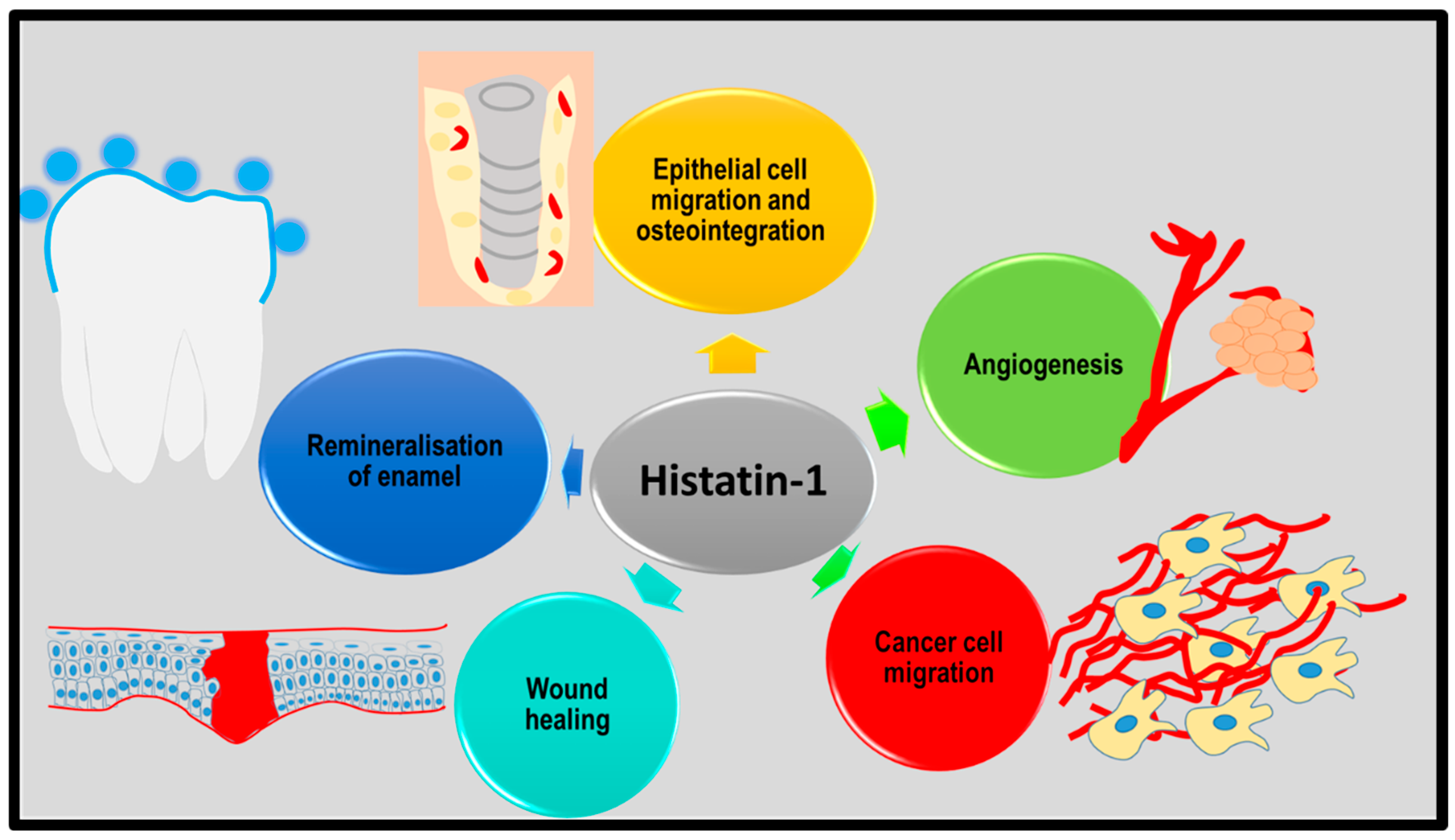
5.5. Wound Healing
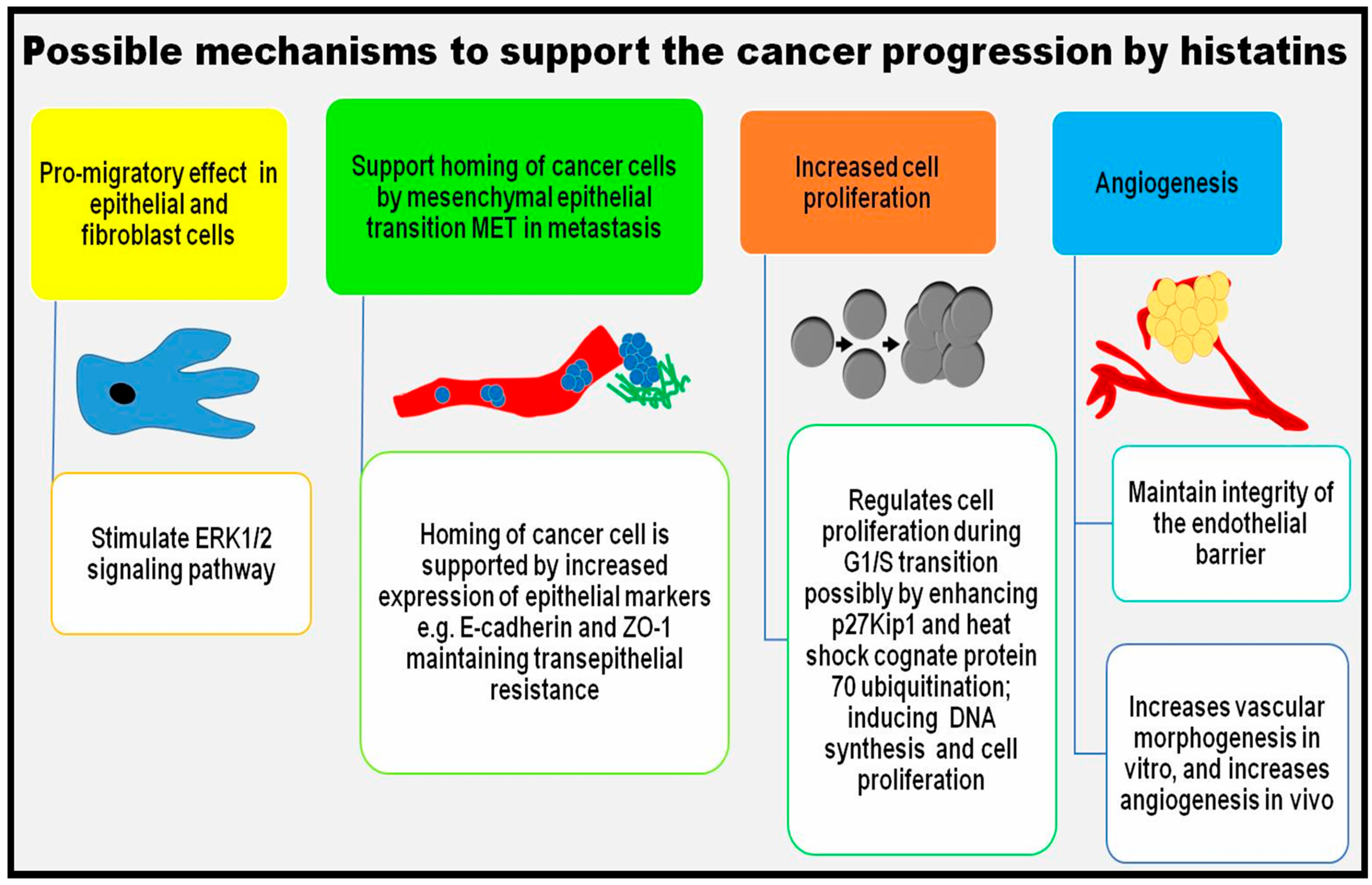
5.6. Possible Role in Cancer
6. Biomedical Applications of Histatins
6.1. Histatins as Biomarkers
6.2. Histatins as Therapeutic Peptides
6.2.1. Antimicrobial Therapy

6.2.2. Caries Prevention Therapy
6.2.3. Tissue Bioengineering
6.2.4. Anticancer Therapy
7. Overcoming Limitations in Therapeutic Applications of Histatins
7.1. Combined Histatin Preparations for Enhanced Functionality
7.2. Overcoming Proteolytic Instability
7.3. Achieving Gradual and Constant Release of Histatins
7.4. Improving Histatin Delivery in Therapeutics
7.5. Modifying Peptide Length to Ensure Best Possible Drug Efficiency
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roblegg, E.; Coughran, A.; Sirjani, D. Saliva: An all-rounder of our body. Eur. J. Pharm. Biopharm. 2019, 142, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Edgar, W.M. Saliva: Its secretion, composition and functions. Br. Dent. J. 1992, 172, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Kashyap, N.; Avinash, A.; Chevvuri, R.; Sagar, M.K.; Shrikant, K. The composition, function and role of saliva in maintaining oral health: A review. Int. J. Contemp. Dent. Med. Rev. 2017, 2017, 011217. [Google Scholar]
- Castagnola, M.; Scarano, E.; Passali, G.C.; Messana, I.; Cabras, T.; Iavarone, F.; Di Cintio, G.; Fiorita, A.; De Corso, E.; Paludetti, G. Salivary biomarkers and proteomics: Future diagnostic and clinical utilities. Acta Otorhinolaryngol. Ital. 2017, 37, 94–101. [Google Scholar] [CrossRef]
- Teo, C.B.; Tan, B.K.J.; Collins, D.C. Editorial: Non-invasive Technology Advances in Oncology. Front. Digit. Health 2021, 3, 676216. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, M.; Zhu, J.; Zhang, H.; Duan, Z.; Wang, S.; Liao, Z.; Liu, W. Developments in diagnostic applications of saliva in human organ diseases. Med. Nov. Technol. Devices 2022, 13, 100115. [Google Scholar] [CrossRef]
- Khurshid, Z.; Najeeb, S.; Mali, M.; Moin, S.F.; Raza, S.Q.; Zohaib, S.; Sefat, F.; Zafar, M.S. Histatin peptides: Pharmacological functions and their applications in dentistry. Saudi Pharm. J. 2017, 25, 25–31. [Google Scholar] [CrossRef]
- Campese, M.; Sun, X.; Bosch, J.A.; Oppenheim, F.G.; Helmerhorst, E.J. Concentration and fate of histatins and acidic proline-rich proteins in the oral environment. Arch. Oral Biol. 2009, 54, 345–353. [Google Scholar] [CrossRef]
- Xu, T.; Levitz, S.M.; Diamond, R.D.; Oppenheim, F.G. Anticandidal activity of major human salivary histatins. Infect. Immun. 1991, 59, 2549–2554. [Google Scholar] [CrossRef]
- Oppenheim, F.G.; Xu, T.; McMillian, F.M.; Levitz, S.M.; Diamond, R.D.; Offner, G.D.; Troxler, R.F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 1988, 263, 7472–7477. [Google Scholar] [CrossRef]
- Torres, P.; Castro, M.; Reyes, M.; Torres, V.A. Histatins, wound healing, and cell migration. Oral Dis. 2018, 24, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Melino, S.; Gallo, M.; Trotta, E.; Mondello, F.; Paci, M.; Petruzzelli, R. Metal-binding and nuclease activity of an antimicrobial peptide analogue of the salivary histatin 5. Biochemistry 2006, 45, 15373–15383. [Google Scholar] [CrossRef]
- Troxler, R.F.; Offner, G.D.; Xu, T.; Vanderspek, J.C.; Oppenheim, F.G. Structural relationship between human salivary histatins. J. Dent. Res. 1990, 69, 2–6. [Google Scholar] [CrossRef]
- Imamura, Y.; Fujigaki, Y.; Oomori, Y.; Ouryouji, K.; Yanagisawa, S.; Miyazawa, H.; Wang, P.L. Transcriptional regulation of the salivary histatin gene: Finding of a strong positive regulatory element and its binding protein. J. Biochem. 2009, 145, 279–288. [Google Scholar] [CrossRef]
- Xu, L.; Lal, K.; Pollock, J.J. Histatins 2 and 4 are autoproteolytic degradation products of human parotid saliva. Oral Microbiol. Immunol. 1992, 7, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Salih, E.; Oppenheim, F.G.; Helmerhorst, E.J. Kinetics of histatin proteolysis in whole saliva and the effect on bioactive domains with metal-binding, antifungal, and wound-healing properties. FASEB J. 2009, 23, 2691–2701. [Google Scholar]
- Messana, I.; Cabras, T.; Pisano, E.; Sanna, M.T.; Olianas, A.; Manconi, B.; Pellegrini, M.; Paludetti, G.; Scarano, E.; Fiorita, A.; et al. Trafficking and postsecretory events responsible for the formation of secreted human salivary peptides: A proteomics approach. Mol. Cell. Proteom. 2008, 7, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, L.M.; Azen, E.A. Histatins, a family of salivary histidine-rich proteins, are encoded by at least two loci (HIS1 and HIS2). Biochem. Biophys. Res. Commun. 1989, 160, 495–502. [Google Scholar] [CrossRef]
- Castagnola, M.; Inzitari, R.; Rossetti, D.V.; Olmi, C.; Cabras, T.; Piras, V.; Nicolussi, P.; Sanna, M.T.; Pellegrini, M.; Giardina, B.; et al. A cascade of 24 histatins (histatin 3 fragments) in human saliva. Suggestions for a pre-secretory sequential cleavage pathway. J. Biol. Chem. 2004, 279, 41436–41443. [Google Scholar] [CrossRef]
- Cabras, T.; Fanali, C.; Monteiro, J.A.; Amado, F.; Inzitari, R.; Desiderio, C.; Scarano, E.; Giardina, B.; Castagnola, M.; Messana, I. Tyrosine polysulfation of human salivary histatin 1. A post-translational modification specific of the submandibular gland. J. Proteome Res. 2007, 6, 2472–2480. [Google Scholar] [CrossRef]
- Brewer, D.; Hunter, H.; Lajoie, G. NMR studies of the antimicrobial salivary peptides histatin 3 and histatin 5 in aqueous and nonaqueous solutions. Biochem. Cell Biol. 1998, 76, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.; Raj, P.A.; Bobek, L.A. Candidacidal activity of recombinant human salivary histatin-5 and variants. Infect. Immun. 1996, 64, 5000–5007. [Google Scholar] [CrossRef] [PubMed]
- Melino, S.; Santone, C.; Di Nardo, P.; Sarkar, B. Histatins: Salivary peptides with copper(II)- and zinc(II)-binding motifs: Perspectives for biomedical applications. FEBS J. 2014, 281, 657–672. [Google Scholar] [CrossRef]
- Norris, H.L.; Kumar, R.; Ong, C.Y.; Xu, D.; Edgerton, M. Zinc Binding by Histatin 5 Promotes Fungicidal Membrane Disruption in C. albicans and C. glabrata. J. Fungi 2020, 6, 124. [Google Scholar] [CrossRef]
- Son, K.N.; Lee, H.; Shah, D.; Kalmodia, S.; Miller, R.C.; Ali, M.; Balasubramaniam, A.; Cologna, S.M.; Kong, H.; Shukla, D.; et al. Histatin-1 is an endogenous ligand of the sigma-2 receptor. FEBS J. 2021, 288, 6815–6827. [Google Scholar] [CrossRef]
- Raj, P.A.; Edgerton, M.; Levine, M.J. Salivary histatin 5: Dependence of sequence, chain length, and helical conformation for candidacidal activity. J. Biol. Chem. 1990, 265, 3898–3905. [Google Scholar] [CrossRef]
- Raj, P.A.; Marcus, E.; Sukumaran, D.K. Structure of human salivary histatin 5 in aqueous and nonaqueous solutions. Biopolymers 1998, 45, 51–67. [Google Scholar] [CrossRef]
- Melino, S.; Rufini, S.; Sette, M.; Morero, R.; Grottesi, A.; Paci, M.; Petruzzelli, R. Zn(2+) ions selectively induce antimicrobial salivary peptide histatin-5 to fuse negatively charged vesicles. Identification and characterization of a zinc-binding motif present in the functional domain. Biochemistry 1999, 38, 9626–9633. [Google Scholar] [CrossRef]
- Grogan, J.; McKnight, C.J.; Troxler, R.F.; Oppenheim, F.G. Zinc and copper bind to unique sites of histatin 5. FEBS Lett. 2001, 491, 76–80. [Google Scholar] [CrossRef]
- Huang, X.; Atwood, C.S.; Moir, R.D.; Hartshorn, M.A.; Vonsattel, J.P.; Tanzi, R.E.; Bush, A.I. Zinc-induced Alzheimer’s Abeta1-40 aggregation is mediated by conformational factors. J. Biol. Chem. 1997, 272, 26464–26470. [Google Scholar] [CrossRef]
- Porciatti, E.; Milenkovic, M.; Gaggelli, E.; Valensin, G.; Kozlowski, H.; Kamysz, W.; Valensin, D. Structural characterization and antimicrobial activity of the Zn(II) complex with P113 (demegen), a derivative of histatin 5. Inorg. Chem. 2010, 49, 8690–8698. [Google Scholar] [CrossRef] [PubMed]
- Tay, W.M.; Hanafy, A.I.; Angerhofer, A.; Ming, L.J. A plausible role of salivary copper in antimicrobial activity of histatin-5--metal binding and oxidative activity of its copper complex. Bioorg. Med. Chem. Lett. 2009, 19, 6709–6712. [Google Scholar] [CrossRef] [PubMed]
- Gornowicz, A.; Tokajuk, G.; Bielawska, A.; Maciorkowska, E.; Jablonski, R.; Wojcicka, A.; Bielawski, K. The assessment of sIgA, histatin-5, and lactoperoxidase levels in saliva of adolescents with dental caries. Med. Sci. Monit. 2014, 20, 1095–1100. [Google Scholar] [PubMed]
- Li, X.S.; Reddy, M.S.; Baev, D.; Edgerton, M. Candida albicans Ssa1/2p is the cell envelope binding protein for human salivary histatin 5. J. Biol. Chem. 2003, 278, 28553–28561. [Google Scholar] [CrossRef]
- Vylkova, S.; Sun, J.N.; Edgerton, M. The role of released ATP in killing Candida albicans and other extracellular microbial pathogens by cationic peptides. Purinergic Signal. 2007, 3, 91–97. [Google Scholar] [CrossRef]
- Raj, P.A.; Soni, S.D.; Levine, M.J. Membrane-induced helical conformation of an active candidacidal fragment of salivary histatins. J. Biol. Chem. 1994, 269, 9610–9619. [Google Scholar] [CrossRef]
- Situ, H.; Balasubramanian, S.V.; Bobek, L.A. Role of alpha-helical conformation of histatin-5 in candidacidal activity examined by proline variants. Biochim. Biophys. Acta 2000, 1475, 377–382. [Google Scholar] [CrossRef]
- Kavanagh, K.; Dowd, S. Histatins: Antimicrobial peptides with therapeutic potential. J. Pharm. Pharmacol. 2004, 56, 285–289. [Google Scholar] [CrossRef]
- Helmerhorst, E.J.; Breeuwer, P.; van’t Hof, W.; Walgreen-Weterings, E.; Oomen, L.C.; Veerman, E.C.; Amerongen, A.V.; Abee, T. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J. Biol. Chem. 1999, 274, 7286–7291. [Google Scholar] [CrossRef]
- Komatsu, T.; Salih, E.; Helmerhorst, E.J.; Offner, G.D.; Oppenheim, F.G. Influence of histatin 5 on Candida albicans mitochondrial protein expression assessed by quantitative mass spectrometry. J. Proteome Res. 2011, 10, 646–655. [Google Scholar] [CrossRef]
- Gusman, H.; Travis, J.; Helmerhorst, E.J.; Potempa, J.; Troxler, R.F.; Oppenheim, F.G. Salivary histatin 5 is an inhibitor of both host and bacterial enzymes implicated in periodontal disease. Infect. Immun. 2001, 69, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Gyurko, C.; Lendenmann, U.; Troxler, R.F.; Oppenheim, F.G. Candida albicans mutants deficient in respiration are resistant to the small cationic salivary antimicrobial peptide histatin 5. Antimicrob. Agents Chemother. 2000, 44, 348–354. [Google Scholar] [CrossRef]
- Veerman, E.C.; Nazmi, K.; Van’t Hof, W.; Bolscher, J.G.; Den Hertog, A.L.; Nieuw Amerongen, A.V. Reactive oxygen species play no role in the candidacidal activity of the salivary antimicrobial peptide histatin 5. Biochem. J. 2004, 381 Pt 2, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.N.; Li, W.; Jang, W.S.; Nayyar, N.; Sutton, M.D.; Edgerton, M. Uptake of the antifungal cationic peptide Histatin 5 by Candida albicans Ssa2p requires binding to non-conventional sites within the ATPase domain. Mol. Microbiol. 2008, 70, 1246–1260. [Google Scholar] [CrossRef]
- Kumar, R.; Chadha, S.; Saraswat, D.; Bajwa, J.S.; Li, R.A.; Conti, H.R.; Edgerton, M. Histatin 5 uptake by Candida albicans utilizes polyamine transporters Dur3 and Dur31 proteins. J. Biol. Chem. 2011, 286, 43748–43758. [Google Scholar] [CrossRef] [PubMed]
- Mochon, A.B.; Liu, H. The antimicrobial peptide histatin-5 causes a spatially restricted disruption on the Candida albicans surface, allowing rapid entry of the peptide into the cytoplasm. PLoS Pathog. 2008, 4, e1000190. [Google Scholar] [CrossRef]
- Jang, W.S.; Bajwa, J.S.; Sun, J.N.; Edgerton, M. Salivary histatin 5 internalization by translocation, but not endocytosis, is required for fungicidal activity in Candida albicans. Mol. Microbiol. 2010, 77, 354–370. [Google Scholar] [CrossRef]
- Tsai, H.; Bobek, L.A. Studies of the mechanism of human salivary histatin-5 candidacidal activity with histatin-5 variants and azole-sensitive and -resistant Candida species. Antimicrob. Agents Chemother. 1997, 41, 2224–2228. [Google Scholar] [CrossRef]
- Rothstein, D.M.; Spacciapoli, P.; Tran, L.T.; Xu, T.; Roberts, F.D.; Dalla Serra, M.; Buxton, D.K.; Oppenheim, F.G.; Friden, P. Anticandida activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrob. Agents Chemother. 2001, 45, 1367–1373. [Google Scholar] [CrossRef]
- Jang, W.S.; Li, X.S.; Sun, J.N.; Edgerton, M. The P-113 fragment of histatin 5 requires a specific peptide sequence for intracellular translocation in Candida albicans, which is independent of cell wall binding. Antimicrob. Agents Chemother. 2008, 52, 497–504. [Google Scholar] [CrossRef]
- Stewart, L.J.; Hong, Y.; Holmes, I.R.; Firth, S.J.; Ahmed, Y.; Quinn, J.; Santos, Y.; Cobb, S.L.; Jakubovics, N.S.; Djoko, K.Y. Salivary Antimicrobial Peptide Histatin-5 Does Not Display Zn(II)-Dependent or -Independent Activity against Streptococci. ACS Infect. Dis. 2023, 9, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Puri, S.; McCall, A.; Norris, H.L.; Russo, T.; Edgerton, M. Human Salivary Protein Histatin 5 Has Potent Bactericidal Activity against ESKAPE Pathogens. Front. Cell. Infect. Microbiol. 2017, 7, 41. [Google Scholar] [CrossRef]
- MacKay, B.J.; Denepitiya, L.; Iacono, V.J.; Krost, S.B.; Pollock, J.J. Growth-inhibitory and bactericidal effects of human parotid salivary histidine-rich polypeptides on Streptococcus mutans. Infect. Immun. 1984, 44, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Xu, T.; Helmerhorst, E.J.; Ori, G.; Troxler, R.F.; Lally, E.T.; Oppenheim, F.G. Inhibitory effect of synthetic histatin 5 on leukotoxin from Actinobacillus actinomycetemcomitans. Oral Microbiol. Immunol. 2002, 17, 143–149. [Google Scholar] [CrossRef]
- Murakami, Y.; Nagata, H.; Amano, A.; Takagaki, M.; Shizukuishi, S.; Tsunemitsu, A.; Aimoto, S. Inhibitory effects of human salivary histatins and lysozyme on coaggregation between Porphyromonas gingivalis and Streptococcus mitis. Infect. Immun. 1991, 59, 3284–3286. [Google Scholar] [CrossRef]
- Murakami, Y.; Shizukuishi, S.; Tsunemitsu, A.; Nakashima, K.; Kato, Y.; Aimoto, S. Binding of a histidine-rich peptide to Porphyromonas gingivalis. FEMS Microbiol. Lett. 1991, 82, 253–256. [Google Scholar] [CrossRef][Green Version]
- Skog, A.E.; Corucci, G.; Tully, M.D.; Fragneto, G.; Gerelli, Y.; Skepo, M. Interaction of a Histidine-Rich Antimicrobial Saliva Peptide with Model Cell Membranes: The Role of Histidines. Langmuir 2023, 39, 7694–7706. [Google Scholar] [CrossRef]
- Shimotoyodome, A.; Kobayashi, H.; Tokimitsu, I.; Matsukubo, T.; Takaesu, Y. Statherin and histatin 1 reduce parotid saliva-promoted Streptococcus mutans strain MT8148 adhesion to hydroxyapatite surfaces. Caries Res. 2006, 40, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Borgwardt, D.S.; Martin, A.D.; Van Hemert, J.R.; Yang, J.; Fischer, C.L.; Recker, E.N.; Nair, P.R.; Vidva, R.; Chandrashekaraiah, S.; Progulske-Fox, A.; et al. Histatin 5 binds to Porphyromonas gingivalis hemagglutinin B (HagB) and alters HagB-induced chemokine responses. Sci. Rep. 2014, 4, 3904. [Google Scholar] [CrossRef]
- Li, R.; Hou, M.; Yu, L.; Luo, W.; Kong, J.; Yu, R.; Liu, R.; Li, Q.; Tan, L.; Pan, C.; et al. Anti-biofilm effect of salivary histatin 5 on Porphyromonas gingivalis. Appl. Microbiol. Biotechnol. 2023, 107, 5179–5189. [Google Scholar] [CrossRef]
- Kreth, J.; Merritt, J.; Pfeifer, C.S.; Khajotia, S.; Ferracane, J.L. Interaction between the Oral Microbiome and Dental Composite Biomaterials: Where We Are and Where We Should Go. J. Dent. Res. 2020, 99, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Enax, J.; Ganss, B.; Amaechi, B.T.; Schulze Zur Wiesche, E.; Meyer, F. The composition of the dental pellicle: An updated literature review. Front. Oral Health 2023, 4, 1260442. [Google Scholar] [CrossRef]
- Hardestam, J.; Petterson, L.; Ahlm, C.; Evander, M.; Lundkvist, A.; Klingstrom, J. Antiviral effect of human saliva against hantavirus. J. Med. Virol. 2008, 80, 2122–2126. [Google Scholar] [CrossRef]
- Zolin, G.V.S.; Fonseca, F.H.D.; Zambom, C.R.; Garrido, S.S. Histatin 5 Metallopeptides and Their Potential against Candida albicans Pathogenicity and Drug Resistance. Biomolecules 2021, 11, 1209. [Google Scholar] [CrossRef]
- Fabian, T.K.; Hermann, P.; Beck, A.; Fejerdy, P.; Fabian, G. Salivary defense proteins: Their network and role in innate and acquired oral immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.E.; Goldberg, H.A.; Tabbara, N.; Mendes, F.M.; Siqueira, W.L. Histatin 1 resists proteolytic degradation when adsorbed to hydroxyapatite. J. Dent. Res. 2011, 90, 268–272. [Google Scholar] [CrossRef]
- Oppenheim, F.G.; Salih, E.; Siqueira, W.L.; Zhang, W.; Helmerhorst, E.J. Salivary proteome and its genetic polymorphisms. Ann. N. Y. Acad. Sci. 2007, 1098, 22–50. [Google Scholar] [CrossRef]
- Yin, A.; Margolis, H.C.; Yao, Y.; Grogan, J.; Oppenheim, F.G. Multi-component adsorption model for pellicle formation: The influence of salivary proteins and non-salivary phospho proteins on the binding of histatin 5 onto hydroxyapatite. Arch. Oral Biol. 2006, 51, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J.; Zuo, Y.; Xu, T.; Choi, J.R.; Troxler, R.F.; Oppenheim, F.G. Functional comparison of native and recombinant human salivary histatin 1. J. Dent. Res. 1995, 74, 1837–1844. [Google Scholar] [CrossRef]
- Siqueira, W.L.; Margolis, H.C.; Helmerhorst, E.J.; Mendes, F.M.; Oppenheim, F.G. Evidence of intact histatins in the in vivo acquired enamel pellicle. J. Dent. Res. 2010, 89, 626–630. [Google Scholar] [CrossRef]
- Hay, D.I. The interaction of human parotid salivary proteins with hydroxyapatite. Arch. Oral Biol. 1973, 18, 1517–1529. [Google Scholar] [CrossRef]
- Kawasaki, K.; Weiss, K.M. Mineralized tissue and vertebrate evolution: The secretory calcium-binding phosphoprotein gene cluster. Proc. Natl. Acad. Sci. USA 2003, 100, 4060–4065. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, W.L.; Oppenheim, F.G. Small molecular weight proteins/peptides present in the in vivo formed human acquired enamel pellicle. Arch. Oral Biol. 2009, 54, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.N.; Custodio, W.; Hatibovic-Kofman, S.; Lee, Y.H.; Xiao, Y.; Siqueira, W.L. Proteome and peptidome of human acquired enamel pellicle on deciduous teeth. Int. J. Mol. Sci. 2013, 14, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Pussinen, P.J.; Kopra, E.; Pietiainen, M.; Lehto, M.; Zaric, S.; Paju, S.; Salminen, A. Periodontitis and cardiometabolic disorders: The role of lipopolysaccharide and endotoxemia. Periodontol. 2000 2022, 89, 19–40. [Google Scholar] [CrossRef]
- Tsukahara, F.; Maru, Y. Identification of novel nuclear export and nuclear localization-related signals in human heat shock cognate protein 70. J. Biol. Chem. 2004, 279, 8867–8872. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Wang, P.L. Salivary histatin 3 inhibits heat shock cognate protein 70-mediated inflammatory cytokine production through toll-like receptors in human gingival fibroblasts. J. Inflamm. 2014, 11, 4. [Google Scholar] [CrossRef]
- Lee, S.M.; Son, K.N.; Shah, D.; Ali, M.; Balasubramaniam, A.; Shukla, D.; Aakalu, V.K. Histatin-1 Attenuates LPS-Induced Inflammatory Signaling in RAW264.7 Macrophages. Int. J. Mol. Sci. 2021, 22, 7856. [Google Scholar] [CrossRef]
- Di Natale, C.; De Benedictis, I.; De Benedictis, A.; Marasco, D. Metal-Peptide Complexes as Promising Antibiotics to Fight Emerging Drug Resistance: New Perspectives in Tuberculosis. Antibiotics 2020, 9, 337. [Google Scholar] [CrossRef]
- Paquette, D.W.; Waters, G.S.; Stefanidou, V.L.; Lawrence, H.P.; Friden, P.M.; O’Connor, S.M.; Sperati, J.D.; Oppenheim, F.G.; Hutchens, L.H.; Williams, R.C. Inhibition of experimental gingivitis in beagle dogs with topical salivary histatins. J. Clin. Periodontol. 1997, 24, 216–222. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Neves, C.; Buskermolen, J.; Roffel, S.; Waaijman, T.; Thon, M.; Veerman, E.; Gibbs, S. Human saliva stimulates skin and oral wound healing in vitro. J. Tissue Eng. Regen. Med. 2019, 13, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Waasdorp, M.; Krom, B.P.; Bikker, F.J.; van Zuijlen, P.P.M.; Niessen, F.B.; Gibbs, S. The Bigger Picture: Why Oral Mucosa Heals Better Than Skin. Biomolecules 2021, 11, 1165. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, I.A.; Nazmi, K.; Bolscher, J.G.; Veerman, E.C.; Stap, J. Histatin-1, a histidine-rich peptide in human saliva, promotes cell-substrate and cell-cell adhesion. FASEB J. 2015, 29, 3124–3132. [Google Scholar] [CrossRef]
- van Dijk, I.A.; Ferrando, M.L.; van der Wijk, A.E.; Hoebe, R.A.; Nazmi, K.; de Jonge, W.J.; Krawczyk, P.M.; Bolscher, J.G.M.; Veerman, E.C.I.; Stap, J. Human salivary peptide histatin-1 stimulates epithelial and endothelial cell adhesion and barrier function. FASEB J. 2017, 31, 3922–3933. [Google Scholar] [CrossRef]
- Torres, P.; Diaz, J.; Arce, M.; Silva, P.; Mendoza, P.; Lois, P.; Molina-Berrios, A.; Owen, G.I.; Palma, V.; Torres, V.A. The salivary peptide histatin-1 promotes endothelial cell adhesion, migration, and angiogenesis. FASEB J. 2017, 31, 4946–4958. [Google Scholar] [CrossRef]
- Imatani, T.; Kato, T.; Minaguchi, K.; Okuda, K. Histatin 5 inhibits inflammatory cytokine induction from human gingival fibroblasts by Porphyromonas gingivalis. Oral Microbiol. Immunol. 2000, 15, 378–382. [Google Scholar] [CrossRef]
- Dillingh, M.R.; van Poelgeest, E.P.; Malone, K.E.; Kemper, E.M.; Stroes, E.S.G.; Moerland, M.; Burggraaf, J. Characterization of inflammation and immune cell modulation induced by low-dose LPS administration to healthy volunteers. J. Inflamm. 2014, 11, 28. [Google Scholar] [CrossRef]
- Oudhoff, M.J.; Bolscher, J.G.; Nazmi, K.; Kalay, H.; van ‘t Hof, W.; Amerongen, A.V.; Veerman, E.C. Histatins are the major wound-closure stimulating factors in human saliva as identified in a cell culture assay. FASEB J. 2008, 22, 3805–3812. [Google Scholar] [CrossRef]
- Chen, J.D.; Kim, J.P.; Zhang, K.; Sarret, Y.; Wynn, K.C.; Kramer, R.H.; Woodley, D.T. Epidermal growth factor (EGF) promotes human keratinocyte locomotion on collagen by increasing the alpha 2 integrin subunit. Exp. Cell Res. 1993, 209, 216–223. [Google Scholar] [CrossRef]
- Oudhoff, M.J.; Kroeze, K.L.; Nazmi, K.; van den Keijbus, P.A.; van’t Hof, W.; Fernandez-Borja, M.; Hordijk, P.L.; Gibbs, S.; Bolscher, J.G.; Veerman, E.C. Structure-activity analysis of histatin, a potent wound healing peptide from human saliva: Cyclization of histatin potentiates molar activity 1000-fold. FASEB J. 2009, 23, 3928–3935. [Google Scholar] [CrossRef]
- Oudhoff, M.J.; van den Keijbus, P.A.; Kroeze, K.L.; Nazmi, K.; Gibbs, S.; Bolscher, J.G.; Veerman, E.C. Histatins enhance wound closure with oral and non-oral cells. J. Dent. Res. 2009, 88, 846–850. [Google Scholar] [CrossRef]
- Sun, W.; Ma, D.; Bolscher, J.G.M.; Nazmi, K.; Veerman, E.C.I.; Bikker, F.J.; Sun, P.; Lin, H.; Wu, G. Human Salivary Histatin-1 Promotes Osteogenic Cell Spreading on Both Bio-Inert Substrates and Titanium SLA Surfaces. Front. Bioeng. Biotechnol. 2020, 8, 584410. [Google Scholar] [CrossRef]
- Boink, M.A.; van den Broek, L.J.; Roffel, S.; Nazmi, K.; Bolscher, J.G.; Gefen, A.; Veerman, E.C.; Gibbs, S. Different wound healing properties of dermis, adipose, and gingiva mesenchymal stromal cells. Wound Repair Regen. 2016, 24, 100–109. [Google Scholar] [CrossRef]
- Cheng, L.; Lei, X.; Yang, Z.; Kong, Y.; Xu, P.; Peng, S.; Wang, J.; Chen, C.; Dong, Y.; Hu, X.; et al. Histatin 1 enhanced the speed and quality of wound healing through regulating the behaviour of fibroblast. Cell Prolif. 2021, 54, e13087. [Google Scholar] [CrossRef]
- Shi, Z.; Yao, C.; Shui, Y.; Li, S.; Yan, H. Research progress on the mechanism of angiogenesis in wound repair and regeneration. Front. Physiol. 2023, 14, 1284981. [Google Scholar] [CrossRef]
- Mateluna, C.; Torres, P.; Rodriguez-Pena, M.; Silva, P.; Matthies, D.J.; Criollo, A.; Bikker, F.J.; Bolscher, J.G.M.; Wilson, C.A.M.; Zapata-Torres, G.; et al. Identification of VEGFR2 as the Histatin-1 receptor in endothelial cells. Biochem. Pharmacol. 2022, 201, 115079. [Google Scholar] [CrossRef]
- Sandri, C.; Caccavari, F.; Valdembri, D.; Camillo, C.; Veltel, S.; Santambrogio, M.; Lanzetti, L.; Bussolino, F.; Ivaska, J.; Serini, G. The R-Ras/RIN2/Rab5 complex controls endothelial cell adhesion and morphogenesis via active integrin endocytosis and Rac signaling. Cell Res. 2012, 22, 1479–1501. [Google Scholar] [CrossRef]
- Lin, Z.; Li, R.; Liu, Y.; Zhao, Y.; Ao, N.; Wang, J.; Li, L.; Wu, G. Histatin1-modified thiolated chitosan hydrogels enhance wound healing by accelerating cell adhesion, migration and angiogenesis. Carbohydr. Polym. 2020, 230, 115710. [Google Scholar] [CrossRef]
- Lei, X.; Cheng, L.; Lin, H.; Pang, M.; Yao, Z.; Chen, C.; Forouzanfar, T.; Bikker, F.J.; Wu, G.; Cheng, B. Human Salivary Histatin-1 Is More Efficacious in Promoting Acute Skin Wound Healing Than Acellular Dermal Matrix Paste. Front. Bioeng. Biotechnol. 2020, 8, 999. [Google Scholar] [CrossRef]
- Ma, D.; Sun, W.; Fu, C.; Nazmi, K.; Veerman, E.C.I.; Jaspers, R.T.; Bolscher, J.G.M.; Bikker, F.J.; Wu, G. GPCR/endocytosis/ERK signaling/S2R is involved in the regulation of the internalization, mitochondria-targeting and -activating properties of human salivary histatin 1. Int. J. Oral Sci. 2022, 14, 42. [Google Scholar] [CrossRef]
- Shah, D.; Son, K.N.; Kalmodia, S.; Lee, B.S.; Ali, M.; Balasubramaniam, A.; Shukla, D.; Aakalu, V.K. Wound Healing Properties of Histatin-5 and Identification of a Functional Domain Required for Histatin-5-Induced Cell Migration. Mol. Ther. Methods Clin. Dev. 2020, 17, 709–716. [Google Scholar] [CrossRef]
- Lei, X.; Yang, Y.; Zheng, J.; Liang, L.; Cheng, L.; Dong, Y.; Qiu, B.; Bikker, F.J.; Forouzanfar, T.; Cheng, B.; et al. The cyclization of human salivary Histatin 1 via click chemistry for skin wound healing. Eur. J. Pharm. Sci. 2025, 204, 106978. [Google Scholar] [CrossRef]
- Imamura, Y.; Fujigaki, Y.; Oomori, Y.; Usui, S.; Wang, P.L. Cooperation of salivary protein histatin 3 with heat shock cognate protein 70 relative to the G1/S transition in human gingival fibroblasts. J. Biol. Chem. 2009, 284, 14316–14325. [Google Scholar] [CrossRef]
- Jiang, W.P.; Wang, Z.; Xu, L.X.; Peng, X.; Chen, F. Diagnostic model of saliva peptide finger print analysis of oral squamous cell carcinoma patients using weak cation exchange magnetic beads. Biosci. Rep. 2015, 35, e00211. [Google Scholar] [CrossRef]
- Fang, X.N.; Yin, M.; Li, H.; Liang, C.; Xu, C.; Yang, G.W.; Zhang, H.X. Comprehensive analysis of competitive endogenous RNAs network associated with head and neck squamous cell carcinoma. Sci. Rep. 2018, 8, 10544. [Google Scholar] [CrossRef]
- O’Donnell, R.K.; Kupferman, M.; Wei, S.J.; Singhal, S.; Weber, R.; O’Malley, B.; Cheng, Y.; Putt, M.; Feldman, M.; Ziober, B.; et al. Gene expression signature predicts lymphatic metastasis in squamous cell carcinoma of the oral cavity. Oncogene 2005, 24, 1244–1251. [Google Scholar] [CrossRef]
- Toruner, G.A.; Ulger, C.; Alkan, M.; Galante, A.T.; Rinaggio, J.; Wilk, R.; Tian, B.; Soteropoulos, P.; Hameed, M.R.; Schwalb, M.N.; et al. Association between gene expression profile and tumor invasion in oral squamous cell carcinoma. Cancer Genet. Cytogenet. 2004, 154, 27–35. [Google Scholar] [CrossRef]
- Wongpanuwich, W.; Yodsanga, S.; Chaisuparat, R.; Amornphimoltham, P. Association Between PD-L1 and Histatin1, 3 Expression in Advanced Head and Neck Squamous Cell Carcinoma. Anticancer Res. 2022, 42, 2689–2699. [Google Scholar] [CrossRef]
- Cabras, T.; Pisano, E.; Mastinu, A.; Denotti, G.; Pusceddu, P.P.; Inzitari, R.; Fanali, C.; Nemolato, S.; Castagnola, M.; Messana, I. Alterations of the salivary secretory peptidome profile in children affected by type 1 diabetes. Mol. Cell. Proteom. 2010, 9, 2099–2108. [Google Scholar] [CrossRef]
- de Gutierrez, G.M.; Marin, L.M.; Xiao, Y.; Escalante-Herrera, A.; Santos, M.; Siqueira, W.L. Detection of periodontal disease activity based on histatin degradation in individuals with cerebral palsy. Heliyon 2022, 8, e10134. [Google Scholar] [PubMed]
- Lal, K.; Pollock, J.J.; Santarpia, R.P., 3rd; Heller, H.M.; Kaufman, H.W.; Fuhrer, J.; Steigbigel, R.T. Pilot study comparing the salivary cationic protein concentrations in healthy adults and AIDS patients: Correlation with antifungal activity. J. Acquir. Immune Defic. Syndr. 1992, 5, 904–914. [Google Scholar] [PubMed]
- Mandel, I.D.; Barr, C.E.; Turgeon, L. Longitudinal study of parotid saliva in HIV-1 infection. J. Oral Pathol. Med. 1992, 21, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.P.; Buzalaf, M.A.R.; Silva, N.C.; Ventura, T.M.O.; Toniolo, J.; Rodrigues, J.A. Proteomic profile of the acquired enamel pellicle of children with early childhood caries and caries-free children. Eur. J. Oral Sci. 2023, 131, e12944. [Google Scholar] [CrossRef]
- Elbawendi, M.I.; Badreldin, H.M.; Ismail, A.A.A.; Khalil, M.A. Uses of histatin 5 as biomarkers for caries risk assessment. Al-Azhar J. Dent. Sci. 2024, 27, 571–578. [Google Scholar] [CrossRef]
- Contini, C.; Olianas, A.; Serrao, S.; Deriu, C.; Iavarone, F.; Boroumand, M.; Bizzarro, A.; Lauria, A.; Faa, G.; Castagnola, M.; et al. Top-Down Proteomics of Human Saliva Highlights Anti-inflammatory, Antioxidant, and Antimicrobial Defense Responses in Alzheimer Disease. Front. Neurosci. 2021, 15, 668852. [Google Scholar]
- Arakido, Y.; Yamamori, T. Effect of Mental stress on human Salivary proteins concerning bitter taste. Ohu Univ. Repos. 2007, 34, 137–144. [Google Scholar]
- Calderón-Santiago, M.; Luque de Castro, M.D. The dual trend in histatins research. TrAC Trends Anal. Chem. 2009, 28, 1011–1018. [Google Scholar] [CrossRef]
- Chaiben, C.L.; Batista, T.B.D.; Penteado, C.A.S.; Barbosa, M.C.M.; Ventura, T.M.O.; Dionizio, A.; Rosa, E.A.R.; Buzalaf, M.A.R.; Azevedo-Alanis, L.R. Salivary proteome analysis of crack cocaine dependents. Arch. Oral Biol. 2021, 121, 104952. [Google Scholar]
- Foratori-Junior, G.A.; Ventura, T.M.O.; Grizzo, L.T.; Carpenter, G.H.; Buzalaf, M.A.R.; Sales-Peres, S.H.C. Label-Free Quantitative Proteomic Analysis Reveals Inflammatory Pattern Associated with Obesity and Periodontitis in Pregnant Women. Metabolites 2022, 12, 1091. [Google Scholar] [CrossRef]
- Torres, P.; Hernandez, N.; Mateluna, C.; Silva, P.; Reyes, M.; Solano, L.; Venegas, S.; Criollo, A.; Nazmi, K.; Bikker, F.J.; et al. Histatin-1 is a novel osteogenic factor that promotes bone cell adhesion, migration, and differentiation. J. Tissue Eng. Regen. Med. 2021, 15, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Kalmodia, S.; Son, K.N.; Cao, D.; Lee, B.S.; Surenkhuu, B.; Shah, D.; Ali, M.; Balasubramaniam, A.; Jain, S.; Aakalu, V.K. Presence of Histatin-1 in Human Tears and Association with Aqueous Deficient Dry Eye Diagnosis: A Preliminary Study. Sci. Rep. 2019, 9, 10304. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, K.; Rai, V.K. Recent advances in the antifungal drug delivery to oral mucosa. Pharmaspire 2021, 13, 157–167. [Google Scholar]
- Welling, M.M.; Brouwer, C.P.; van ‘t Hof, W.; Veerman, E.C.; Amerongen, A.V. Histatin-derived monomeric and dimeric synthetic peptides show strong bactericidal activity towards multidrug-resistant Staphylococcus aureus in vivo. Antimicrob. Agents Chemother. 2007, 51, 3416–3419. [Google Scholar] [CrossRef]
- Tsai, H.; Bobek, L.A. Human salivary histatins: Promising anti-fungal therapeutic agents. Crit. Rev. Oral Biol. Med. 1998, 9, 480–497. [Google Scholar] [CrossRef]
- Edgerton, M.; Koshlukova, S.E.; Lo, T.E.; Chrzan, B.G.; Straubinger, R.M.; Raj, P.A. Candidacidal activity of salivary histatins. Identification of a histatin 5-binding protein on Candida albicans. J. Biol. Chem. 1998, 273, 20438–20447. [Google Scholar] [CrossRef]
- Zheng, Y.; Yuan, W.; Liu, H.; Huang, S.; Bian, L.; Guo, R. Injectable supramolecular gelatin hydrogel loading of resveratrol and histatin-1 for burn wound therapy. Biomater. Sci. 2020, 8, 4810–4820. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, X.; Zhao, X.; Chen, B.; Li, X.; Li, Y.; Chen, Y.; Chen, C.; Lu, H.; Liu, J. Acellular dermal matrix decorated with collagen-affinity peptide accelerate diabetic wound healing through sustained releasing Histatin-1 mediated promotion of angiogenesis. Int. J. Pharm. 2022, 624, 122017. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, C.; Zhao, M.; Liu, N.; Chen, Z.; Liu, J.; Li, G.; Deng, Y.; Sai, X.; Huang, H.; et al. Histatin-1 loaded multifunctional, adhesive and conductive biomolecular hydrogel to treat diabetic wound. Int. J. Biol. Macromol. 2022, 209 Pt A, 1020–1031. [Google Scholar] [CrossRef]
- Qiao, L.; Liang, Y.; Chen, J.; Huang, Y.; Alsareii, S.A.; Alamri, A.M.; Harraz, F.A.; Guo, B. Antibacterial conductive self-healing hydrogel wound dressing with dual dynamic bonds promotes infected wound healing. Bioact. Mater. 2023, 30, 129–141. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shi, A.; Ma, D.; Bolscher, J.G.M.; Nazmi, K.; Veerman, E.C.I.; Bikker, F.J.; Lin, H.; Wu, G. All-trans retinoic acid and human salivary histatin-1 promote the spreading and osteogenic activities of pre-osteoblasts in vitro. FEBS Open Bio 2020, 10, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Shi, A.; Shen, C.; Liu, Y.; Wu, G.; Feng, J. Human salivary histatin-1 (Hst1) promotes bone morphogenetic protein 2 (BMP2)-induced osteogenesis and angiogenesis. FEBS Open Bio 2020, 10, 1503–1515. [Google Scholar] [CrossRef]
- Torres, P.; Flores, V.; Flores, T.; Silva, P.; Gonzalez, L.; Cordova, L.A.; Reyes, M.; Torres, V.A. The salivary peptide histatin-1 enhances bone repair in vivo. Biochem. Biophys. Res. Commun. 2023, 676, 207–212. [Google Scholar] [CrossRef]
- Wu, A.; Pathak, J.L.; Li, X.; Cao, W.; Zhong, W.; Zhu, M.; Wu, Q.; Chen, W.; Han, Q.; Jiang, S.; et al. Human Salivary Histatin-1 Attenuates Osteoarthritis through Promoting M1/M2 Macrophage Transition. Pharmaceutics 2023, 15, 1272. [Google Scholar] [CrossRef]
- Castro, M.; Torres, P.; Solano, L.; Cordova, L.A.; Torres, V.A. Histatin-1 counteracts the cytotoxic and antimigratory effects of zoledronic acid in endothelial and osteoblast-like cells. J. Periodontol. 2019, 90, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, M.; Kato, T.; Matsuzaka, K.; Hayakawa, T.; Shiba, K. Prevention of biofilm formation on titanium surfaces modified with conjugated molecules comprised of antimicrobial and titanium-binding peptides. Biofouling 2010, 26, 103–110. [Google Scholar] [CrossRef]
- Jenwanichkul, P.; Amornphimoltham, P. In Vitro Anticancer Activity of Histatin-1 Combination with Cisplatin in Head and Neck Cancer Cell Lines. Exp. Oncol. 2024, 46, 101–109. [Google Scholar] [CrossRef]
- Anjomshoa, M.; Amirheidari, B. Nuclease-like metalloscissors: Biomimetic candidates for cancer and bacterial and viral infections therapy. Coord. Chem. Rev. 2022, 458, 214417. [Google Scholar] [CrossRef]
- Ikonomova, S.P.; Moghaddam-Taaheri, P.; Wang, Y.; Doolin, M.T.; Stroka, K.M.; Hube, B.; Karlsson, A.J. Effects of histatin 5 modifications on antifungal activity and kinetics of proteolysis. Protein Sci. 2020, 29, 480–493. [Google Scholar] [CrossRef]
- Lu, J.; Xu, H.; Xia, J.; Ma, J.; Xu, J.; Li, Y.; Feng, J. D- and Unnatural Amino Acid Substituted Antimicrobial Peptides With Improved Proteolytic Resistance and Their Proteolytic Degradation Characteristics. Front. Microbiol. 2020, 11, 563030. [Google Scholar] [CrossRef] [PubMed]
- Moffa, E.B.; Machado, M.A.; Mussi, M.C.; Xiao, Y.; Garrido, S.S.; Giampaolo, E.T.; Siqueira, W.L. In Vitro Identification of Histatin 5 Salivary Complexes. PLoS ONE 2015, 10, e0142517. [Google Scholar] [CrossRef] [PubMed]
- Martin-Serrano, A.; Gomez, R.; Ortega, P.; de la Mata, F.J. Nanosystems as Vehicles for the Delivery of Antimicrobial Peptides (AMPs). Pharmaceutics 2019, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Voltan, A.R.; Quindos, G.; Alarcon, K.P.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.; Chorilli, M. Fungal diseases: Could nanostructured drug delivery systems be a novel paradigm for therapy? Int. J. Nanomed. 2016, 11, 3715–3730. [Google Scholar] [CrossRef]
- Zambom, C.R.; da Fonseca, F.H.; Crusca, E., Jr.; da Silva, P.B.; Pavan, F.R.; Chorilli, M.; Garrido, S.S. A Novel Antifungal System With Potential for Prolonged Delivery of Histatin 5 to Limit Growth of Candida albicans. Front. Microbiol. 2019, 10, 1667. [Google Scholar] [CrossRef]
- Mickels, N.; McManus, C.; Massaro, J.; Friden, P.; Braman, V.; Agostino, R.D.; Oppenheim, F.; Warbington, M.; Dibart, S.; Dyke, T.V. Clinical and microbial evaluation of a histatin-containing mouthrinse in humans with experimental gingivitis. J. Clin. Periodontol. 2001, 28, 404–410. [Google Scholar] [CrossRef]
- Lu, G.; Ju, X.; Zhu, M.; Ou, J.; Xu, D.; Li, K.; Jiang, W.; Wan, C.; Tian, Y.; Niu, Z. Histatin 5-Inspired Short-Chain Peptides Selectively Combating Pathogenic Fungi with Multifaceted Mechanisms. Adv. Healthc. Mater. 2024, 13, e2303755. [Google Scholar] [CrossRef]
- Zuo, Y.; Xu, T.; Troxler, R.F.; Li, J.; Driscoll, J.; Oppenheim, F.G. Recombinant histatins: Functional domain duplication enhances candidacidal activity. Gene 1995, 161, 87–91. [Google Scholar] [CrossRef]
- Marin, L.M.; Xiao, Y.; Cury, J.A.; Siqueira, W.L. Engineered Salivary Peptides Reduce Enamel Demineralization Provoked by Cariogenic S. mutans Biofilm. Microorganisms 2022, 10, 742. [Google Scholar] [CrossRef]
- Schnaider, L.; Rosenberg, A.; Kreiser, T.; Kolusheva, S.; Gazit, E.; Berman, J. Peptide Self-Assembly Is Linked to Antibacterial, but Not Antifungal, Activity of Histatin 5 Derivatives. mSphere 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Ikonomova, S.P.; Moghaddam-Taaheri, P.; Jabra-Rizk, M.A.; Wang, Y.; Karlsson, A.J. Engineering improved variants of the antifungal peptide histatin 5 with reduced susceptibility to Candida albicans secreted aspartic proteases and enhanced antimicrobial potency. FEBS J. 2018, 285, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Chaudhary, M.; Khanna, G.; Rishi, P.; Kaur, I.P. Envisaging Antifungal Potential of Histatin 5: A Physiological Salivary Peptide. J. Fungi 2021, 7, 1070. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, G.; Vilk, G.; Welch, I. Methods and Compositions Comprising Cyclic Analogues of Histatin 5 for Treating Wounds. US 2014/0065119 A1, 10 November 2011. [Google Scholar]
- Babu, U.M.; VanDine, R.W.; Sambursky, R.P. Histatins as Therapeutic Agents for Ocular Surface Disease. WO 2016/060916 A1, 15 October 2014. [Google Scholar]
- Periathamby, A.R.; Dentino, A.R. Modified Dental Prosthesis. US 7781531 B2, 10 August 2017. [Google Scholar]
- Cheng, D.; Oppenheim, F.; Helmerhorst, E. Antifungal Formulation and Method of Preparation. WO 20090/05798 A2, 8 January 2009. [Google Scholar]
- Jernberg, G.R. Selectively Targeted Antimicrobials for the Treatment of Periodontal Disease. US 2010/0202983 A1, 9 February 2009. [Google Scholar]
- Groot, F.; Sanders, R.W.; ter Brake, O.; Nazmi, K.; Veerman, E.C.; Bolscher, J.G.; Berkhout, B. Histatin 5-derived peptide with improved fungicidal properties enhances human immunodeficiency virus type 1 replication by promoting viral entry. J. Virol. 2006, 80, 9236–9243. [Google Scholar] [CrossRef]
- Situ, H.; Tsai, H.; Bobek, L.A. Construction and characterization of human salivary histatin-5 multimers. J. Dent. Res. 1999, 78, 690–698. [Google Scholar] [CrossRef] [PubMed]
| Histatin Type | Disease Name | References |
|---|---|---|
| Histatin-1 | Type I diabetes | [110] |
| Histatin-1 | Periodontal disease | [111] |
| Histatin-1 | AIDS | [112,113] |
| Histatin-5 | Caries | [114,115] |
| Histatin-3 or Histatin-5 | Alzheimer’s disease | [116] |
| Histatin-5 | Stress | [117] |
| Histatin-2 | Addictions including cocaine | [118,119] |
| Histatin-3 | Obesity in pregnancy with periodontitis | [120] |
| Histatin-1 | Bone diseases | [121] |
| Histatin-1 | Aqueous-deficient dry eye disease (ADDE) | [122] |
| Histatin-3 | Oral squamous cell carcinoma | [109] |
| Name | Modification/ Engineering | Purpose/ Applications | References |
|---|---|---|---|
| Repeat-histatin-3 Repeat-histatin-3-repeat | Functional domain was repeated in tandem | 5 times increased candidacidal activity | [148] |
| DR9-RR14 | Hybrid of histatin-3 with statherin | Inhibit enamel demineralisation | [149] |
| Three histatin-5 proline variants 1:H21P 2:H19P/H21P 3:E16P/H19P/H21P | One or more residues were replaced with proline (potent α-helix breaker) | α-helix may not be important for candidacidal activity of histatin-5 | [37] |
| ATCUN-C16 (modified histatin-5) | Contains two metal-binding centres, ATCUN motif (Cu-binding) and a Zn-binding motif | Assumes a more stable conformation and possesses nuclease activity, making it a suitable candidate for anticancer treatment and a biotechnological tool | [12] |
| Dhvar2 and modified dhvar2 (L7F) (modified histatin-5) | L7F (KRLFKEFLFSLRKY), required to facilitate peptide self-assembly into ordered nanostructures | Antimicrobial peptides with the ability to self-assemble into ordered amyloid-like nanostructures, facilitating their antibacterial activity and stable antifungal activities | [150] |
| P-113 Histatin-5 (C-terminal modification) | 12-amino-acid sequence amidated on C terminus, reducing propensity to make an α-helix | Two-fold increase in fungicidal activity after amidation. LD50 = 2.3 ± 0.65 µg/mL | [49] |
| Histatin-5 (K17R) | Lysine at position 17 substituted for arginine in histatin-5 | Confers increased resistance to proteolysis by Saps | [151] |
| Histatin-5 (K17L) | Lysine at position 17 substituted for leucine in histatin-5 | Enhanced antifungal activity | [151] |
| Histatin-5 (K11R) | Lysine at position 11 substituted for arginine | Enhanced antifungal activity | [151] |
| W-histatin-5 | Tryptophan (W) added in histatin-5 sequence | Prolonged fungicidal activity | [145] |
| Patents of histatin-5 and derivatives | |||
| Cyclic analogues of histatin-5 U.S. Patent. 2011 November 10 (US 2014/0065119A1) | The invention focuses on the use of cyclic analogues of histatin-5 for the treatment of wounds. Cyclable amino acids can be incorporated to induce cyclisation in histatin-5 and its derivatives. | Cyclisation improves stability and cellular uptake of histatin-5. Therapeutically effective doses range from 0.01 mg to 100 mg per kg of body weight. A suitable absorbent hydrogel can be developed for topical application. Histatin-5, along with other therapeutic agents, can be used for wound healing. | [152,153] |
| WO 2016/060916 A1 | The invention focuses on the utilisation of combined histatin-5 and histatin-1 as therapeutic agents for ocular surface diseases such as dry eyes. | Histatin-5, being a modulator of inflammatory cytokines, can be incorporated in anti-inflammatory formulations along with other therapeutics. The preferred weight-to-weight ratios of histatin-5 to cHistatin-1 were 1:1, 6:1, 1:10, and 1:15. Histatin-5 and histatin-1 were combined in ranges from 1 μg to 10 mg/mL. Both histatins were mixed with 0.1% to 1% glycerin to form sterile eye drops. Histatin-5, along with rapamycin, can be administered to treat dry eyes in patients suffering from autoimmune diseases such as Sjogren’s syndrome. | [152,154] |
| US 7781531 B2 | Dentures conventionally made from poly (methyl methacrylate) lead to denture-induced stomatitis in the user due to adhesion of C. albicans. | This invention focuses on the incorporation of histatin-5 with phosphate-containing co-polymers in dentures. Phosphate anions facilitate the adhesion of cationic histatin molecule overdentures to limit the induced complications. Adsorption of histatin-5 increases with an increase in the negative charge on the polymer. | [152,155] |
| WO 2009/005798 A2 | The invention is a histatin-5 derivative-based mouth rinse formulation with improved antifungal activity. | Amidation at the carboxyl terminus of the histatin-5 derivative resulted in a two-fold increase in antimicrobial activity. | [152,156] |
| US 2010/0202983 A1 | The invention describes the utilisation of carrier agents for the delivery of histatins and their derivatives for the treatment of periodontal disease. | Carrier agents and histatins are covalently coupled to form a complex. The formed complex ensures sustained release of histatins with better penetration and retention. | [152,157] |
| Name | Modification | Application | Reference |
|---|---|---|---|
| M21 (modified histatin-5) | K13T | Reduced fungicidal activity | [22] |
| M71 (modified histatin-5) | K13E | Reduced fungicidal activity | [22] |
| Dhvar2 (modified histatin-5) | Increased HIV-1 replication by promoting the envelope-mediated cell entry process | Modification of antimicrobial peptides in order to improve their activity against a pathogen may have unpredictable and unwanted side effects on other pathogens | [158] |
| LL37 and melittin (modified histatin-5) | Enhanced antifungal activity with increased growth of Lactobacillus species | Unwanted side effects on other commensals | [147] |
| Histatin-5— Histatin-5; Histatin-5—C16, C16—C16) | More potent histatin-5 molecules may be achieved by duplication of the functional domain of histatin-5 (C16, residues 9–24 of histatin-5) | Decreased candidacidal activity | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, S.; You, Y.; Waseem, A. Exploring the Healing Powers of Histatins: From Oral Health to Therapeutics. Int. J. Mol. Sci. 2025, 26, 5019. https://doi.org/10.3390/ijms26115019
Usman S, You Y, Waseem A. Exploring the Healing Powers of Histatins: From Oral Health to Therapeutics. International Journal of Molecular Sciences. 2025; 26(11):5019. https://doi.org/10.3390/ijms26115019
Chicago/Turabian StyleUsman, Saima, Yvonne You, and Ahmad Waseem. 2025. "Exploring the Healing Powers of Histatins: From Oral Health to Therapeutics" International Journal of Molecular Sciences 26, no. 11: 5019. https://doi.org/10.3390/ijms26115019
APA StyleUsman, S., You, Y., & Waseem, A. (2025). Exploring the Healing Powers of Histatins: From Oral Health to Therapeutics. International Journal of Molecular Sciences, 26(11), 5019. https://doi.org/10.3390/ijms26115019







