Ibα-XYL1 Interfered Expression Decreases Starch Granule Size and Increases Soluble Sugar Content to Improve Steamed Sweetpotato Storage Root Taste
Abstract
1. Introduction
2. Results
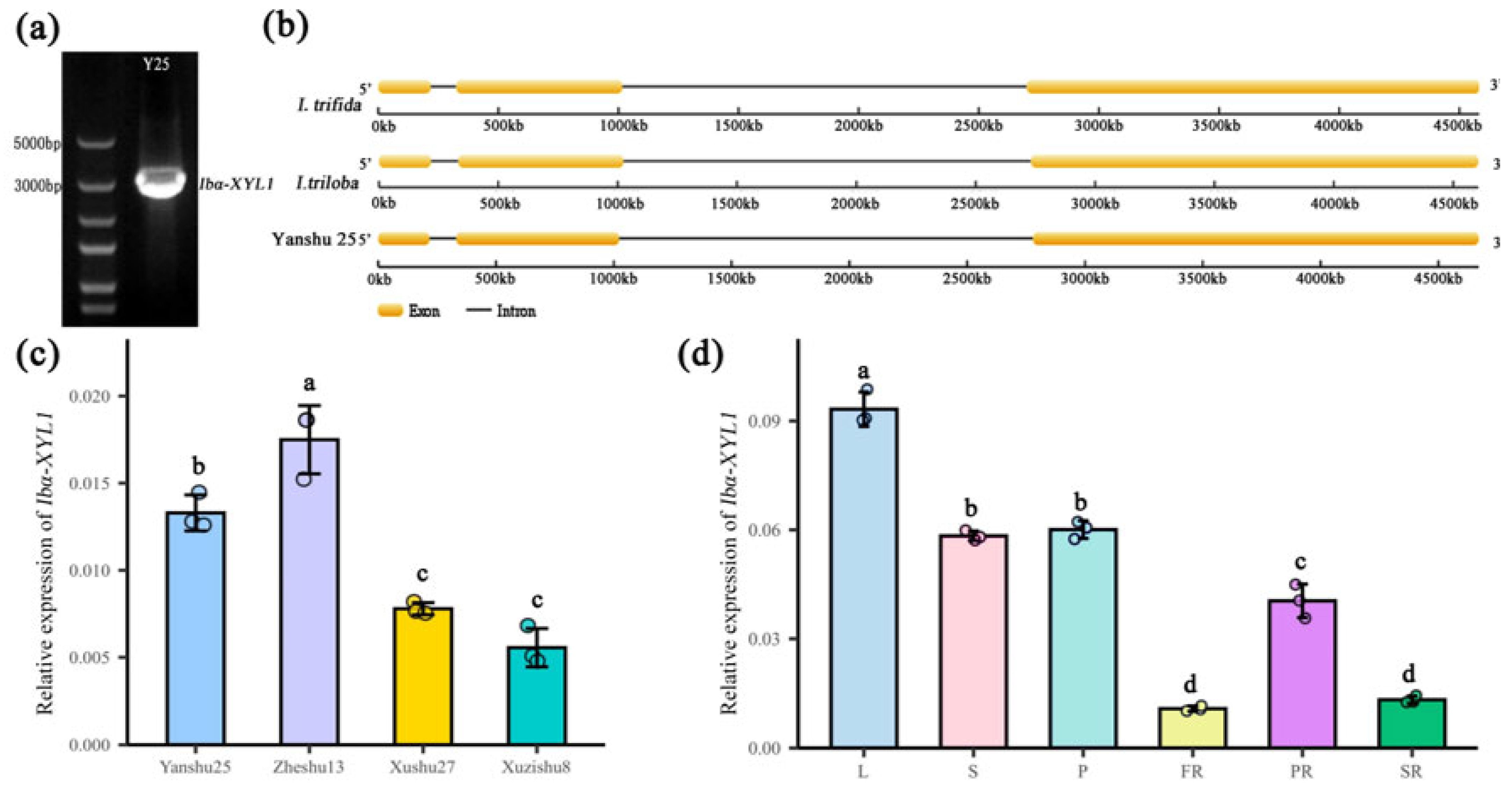
2.1. Cloning, Sequence and Expression Pattern Analysis of Ibα-XYL1
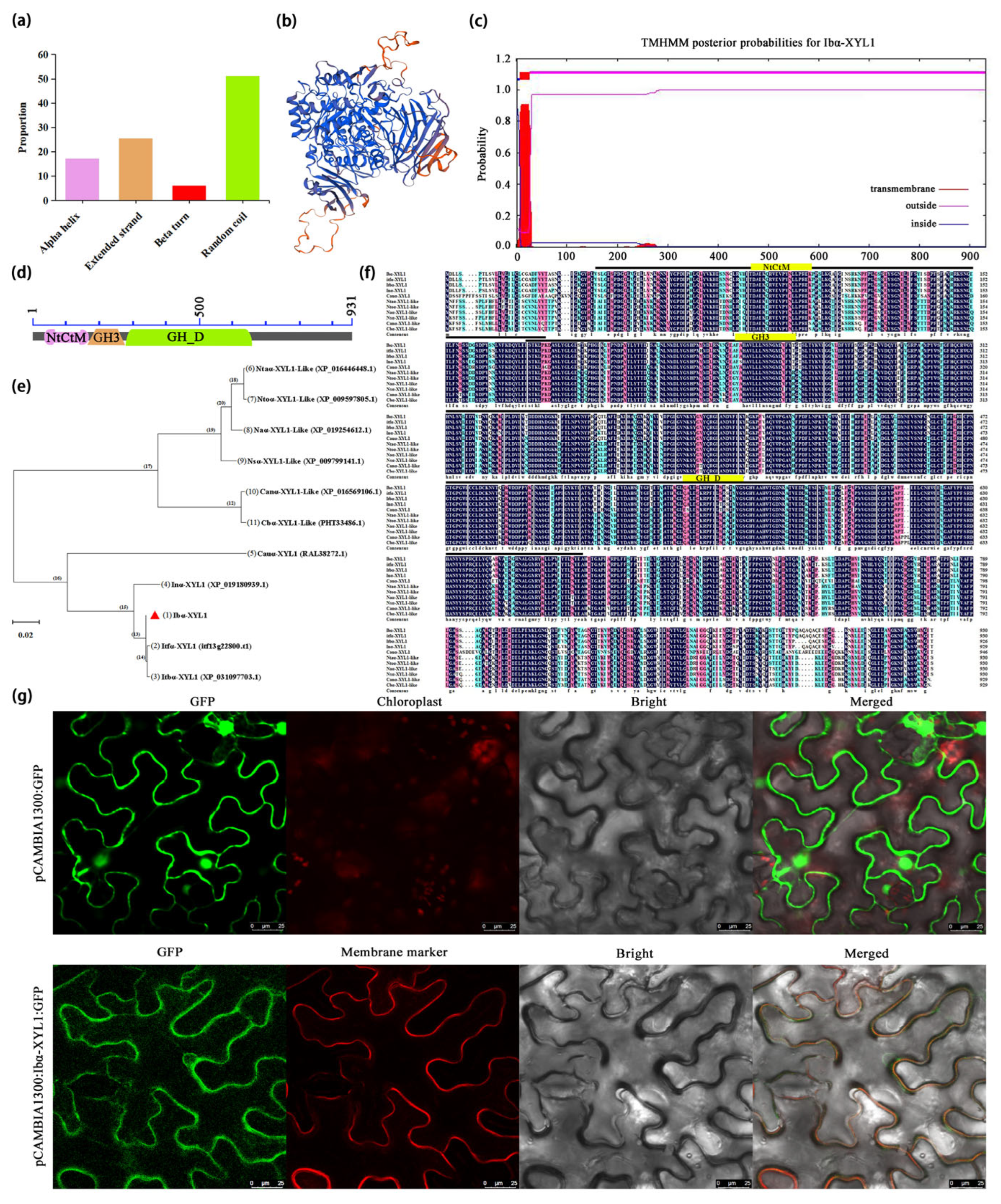
2.2. Ibα-XYL1 Protein Structure, Properties Analysis, and Subcellular Localization
2.3. Acquisition, Phenotypes, Yield Traits, and Anthocyanin Content Analysis of Transgenic SPSR
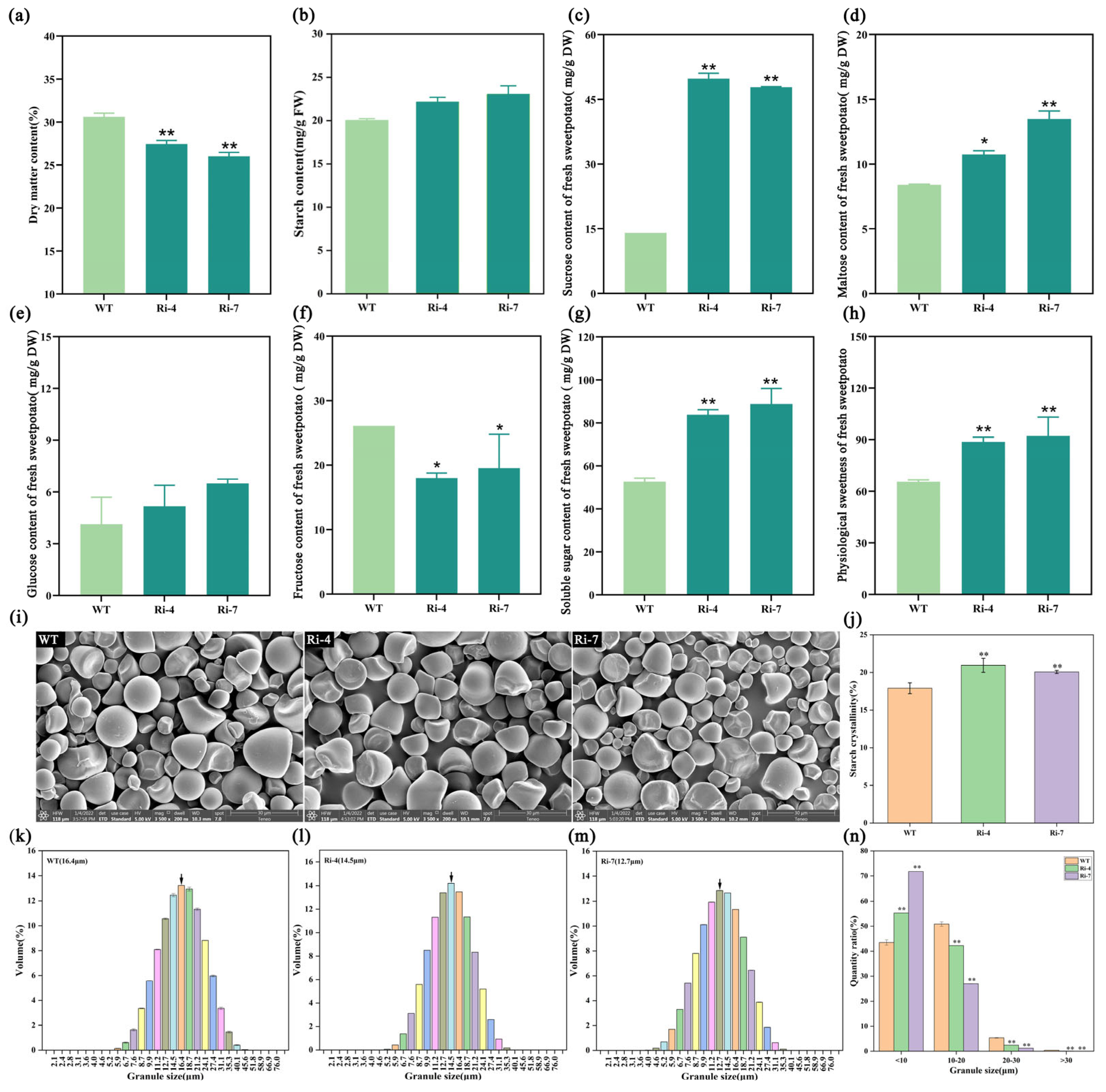
2.4. Interference with the Expression of Ibα-XYL1 Decreases Starch Granule Size and Increases Soluble Sugar Content of Fresh Transgenic SPSR
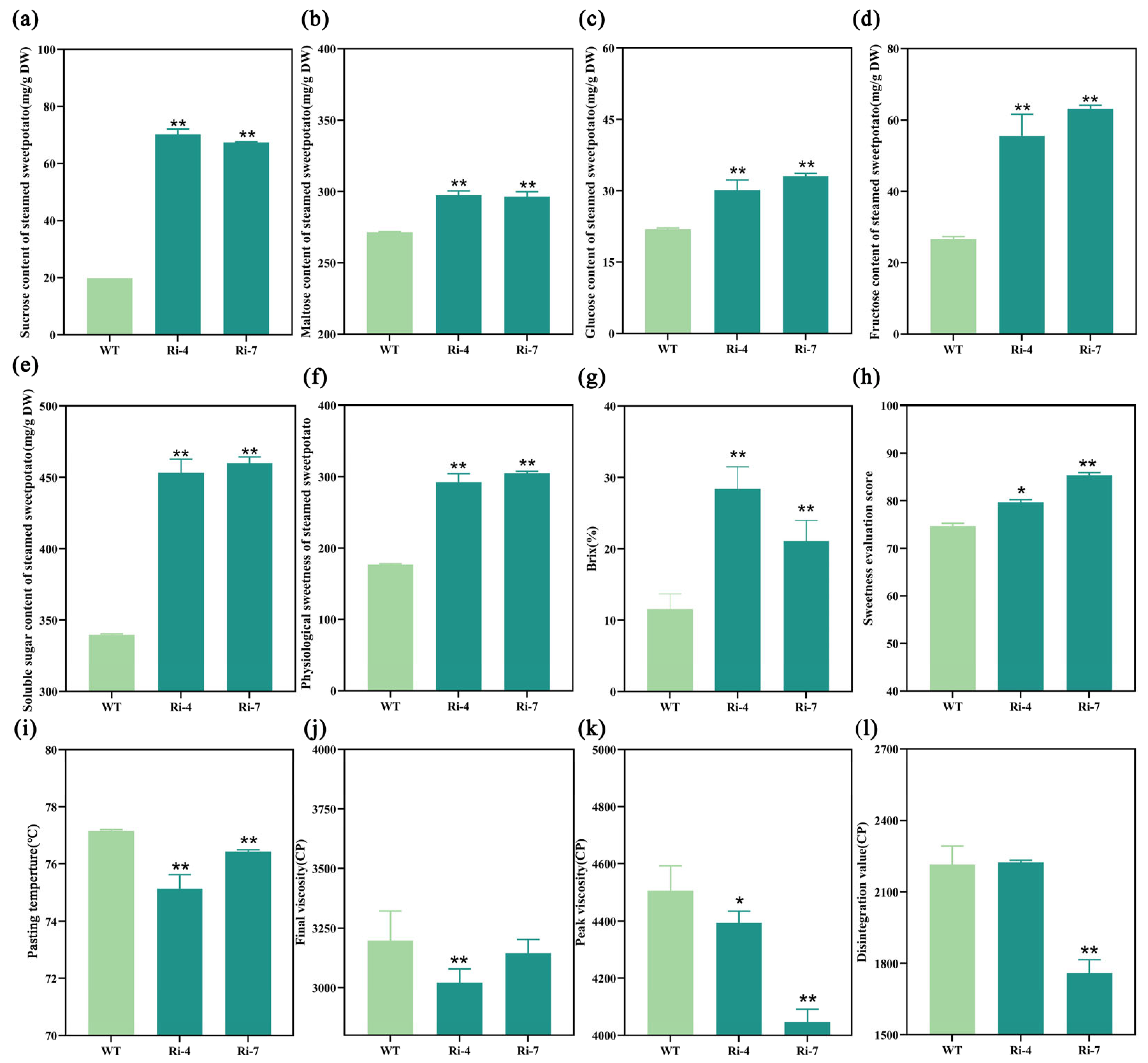
2.5. Interference with the Expression of Ibα-XYL1 Improved Soluble Sugar Content, Sweetness, and Taste of Steamed Transgenic SPSR
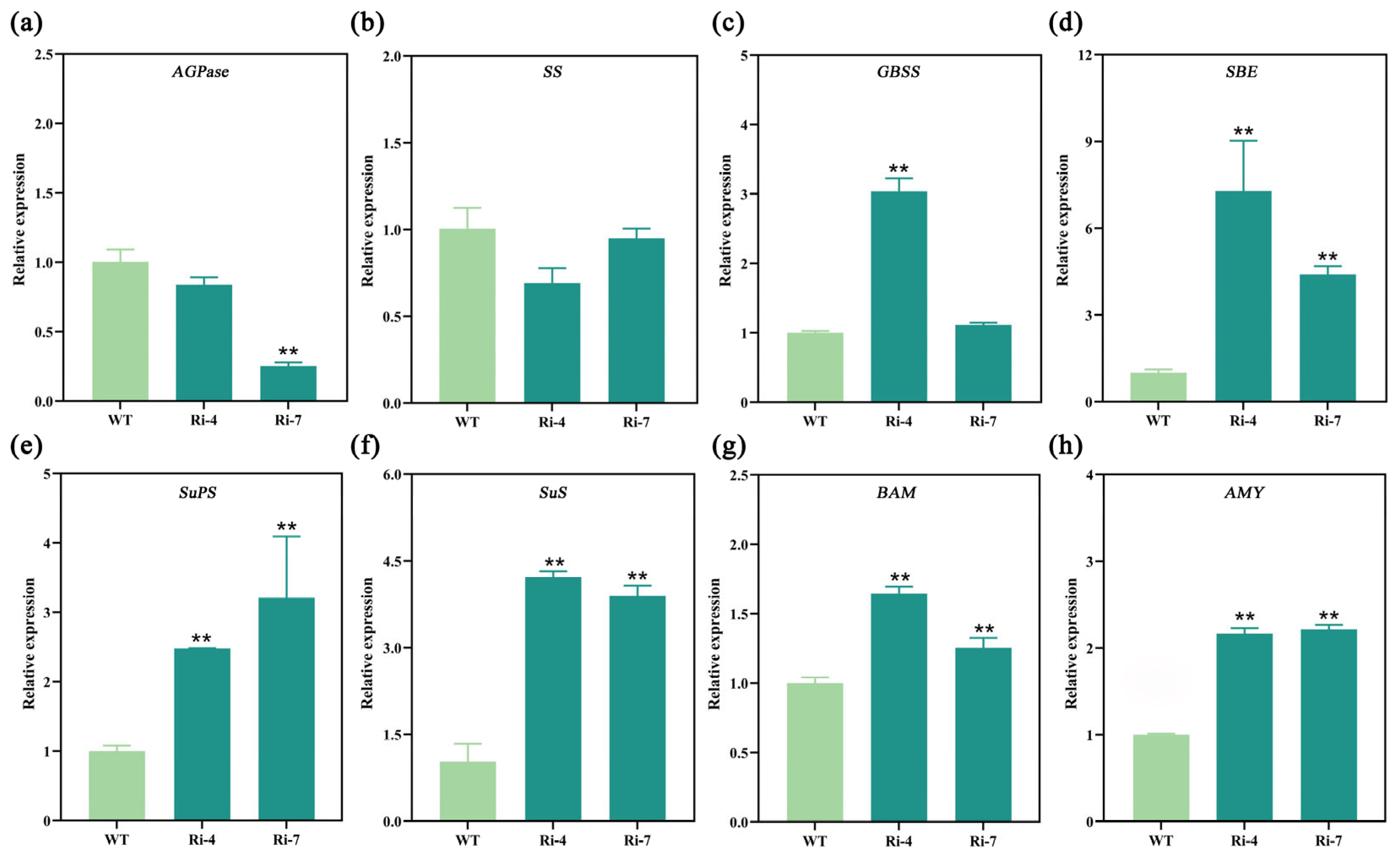
2.6. Interference with the Expression of Ibα-XYL1 Affects the Expression of Genes Related to Starch and Sucrose Metabolic Pathways
3. Discussion
3.1. Interference with the Expression of Ibα-XYL1 Did Not Affect Yield Traits and Anthocyanin Content in SPSR
3.2. Interference with the Expression of Ibα-XYL1 Decreases Starch Granule Size and Change Starch Gelatinization Properties
3.3. Interference with the Expression of Ibα-XYL1 Increases the Soluble Sugar Content of Fresh and Steamed SPSR, and Improves the Sweetness and Taste of Steamed SPSR
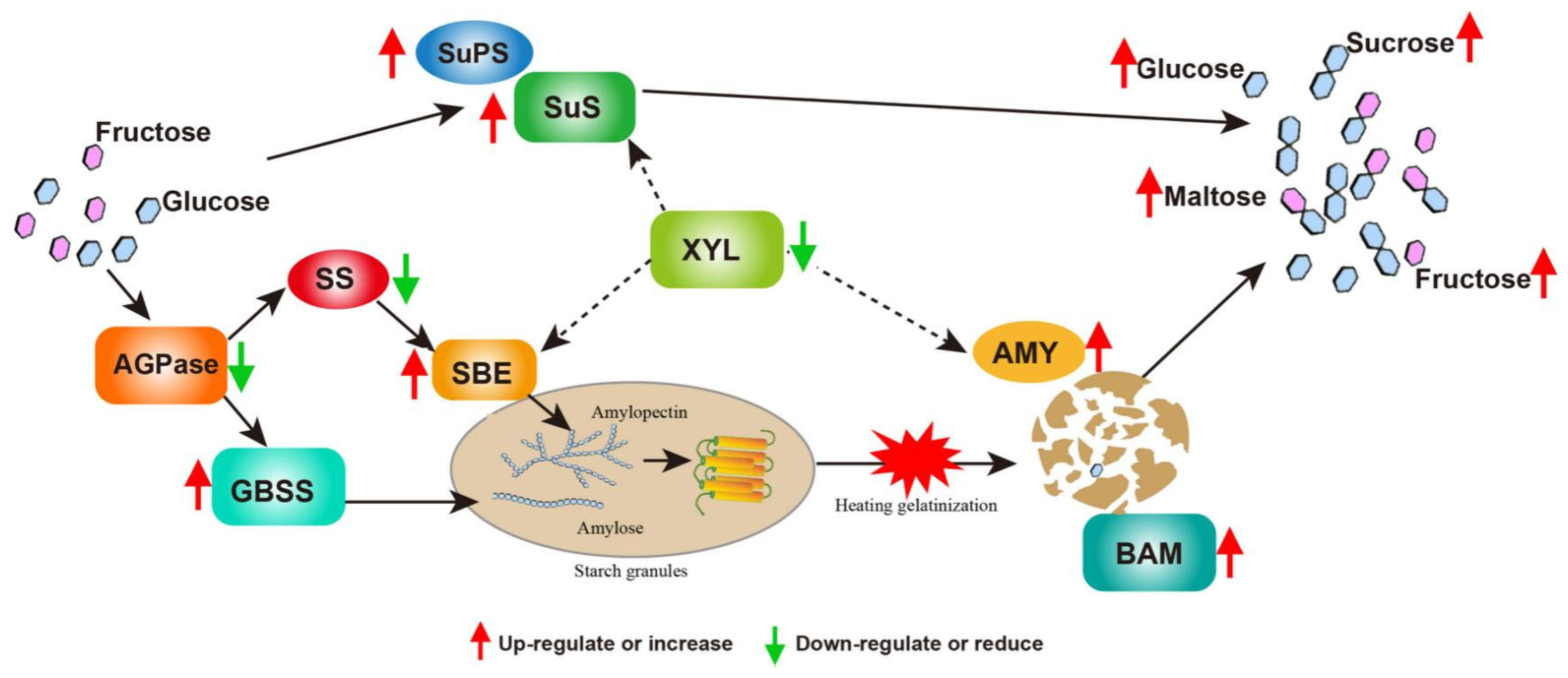
3.4. Molecular Model Predictions of Interference with the Expression of Ibα-XYL1 Regulation for Improving SPSR Taste
4. Materials and Methods
4.1. Plant Materials
4.2. Ibα-XYL1 Cloning and Expression Pattern Analysis
4.3. Ibα-XYL1 Protein Sequence, Structure, Properties Analysis, and Subcellular Localization
4.4. Transgenic Sweetpotato Plants Production and Phenotypic Analysis
4.5. Transgenic Sweetpotato Physicochemical Properties Analysis
4.6. Gene Expression Related to Starch and Sucrose Regulatory Networks Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, C.; Han, J.; Chen, D.; Zong, S.; Liu, J.; Kan, J.; Qian, C.; Jin, C. Recent advances on the biological activities of purple sweetpotato anthocyanins. Food Biosci. 2023, 53, 102670. [Google Scholar]
- Kitahara, K.; Nakamura, Y.; Otani, M.; Hamada, T.; Nakayachi, O.; Takahata, Y. Carbohydrate components in sweetpotato storage roots: Their diversities and genetic improvement. Breed. Sci. 2017, 67, 62–72. [Google Scholar] [CrossRef]
- Shen, S.F.; Xiang, C.; Wu, L.H.; Li, B.; Luo, Z.G. Analysis on the characteristics of soluble sugar components in sweetpotato storage root and its relationship with taste. Sci. Agric. Sin. 2021, 54, 34–45. [Google Scholar]
- Wang, H.; Feng, Y.; Guo, K.; Shi, L.; Xu, X.; Wei, C. Structural, thermal, pasting and digestion properties of starches from developing root tubers of sweet potato. Foods 2024, 13, 1103. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ohara-Takada, A.; Kuranouchi, T.; Masuda, R.; Katayama, K. Mechanism for maltose generation by heating in the storage roots of sweet potato cultivar ‘quick sweet’ containing starch with a low pasting temperature. J. Jpn. Soc. Food Sci. Technol. 2014, 61, 62–69. [Google Scholar] [CrossRef]
- Ren, Z.; Zhao, H.; He, S.Z.; Zhai, H.; Zhao, N.; Liu, Q.C. Overexpression of IbSnRK1 enhances nitrogen uptake and carbon assimilation in transgenic sweetpotato. J. Integr. Agric. 2018, 17, 296–305. [Google Scholar] [CrossRef]
- Morrison, T.A.; Pressey, R.; Kays, S.J. Changes in α- and β-amylase during storage of sweetpotato lines with varying starch hydrolysis potential. J. Am. Soc. Hortic. Sci. 1993, 118, 236–242. [Google Scholar] [CrossRef]
- Amankwaah, V.A.; Williamson, S.; Olukolu, B.A.; Truong, V.D.; Carey, E.; Ssali, R.; Yencho, G.C. Interrelations of α- and β-amylase activity with starch, sugars, and culinary and nutritional quality attributes in sweetpotato storage roots. J. Sci. Food Agric. 2024, 104, 4662–4670. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kuranouchi, T.; Ohara-Takada, A.; Katayama, K. The effects of β-amylase activity and starch pasting temperature on maltose generation in steamed storage roots of sweet potato. J. Jpn. Soc. Food Sci. Technol. 2014, 61, 577–585. [Google Scholar] [CrossRef]
- Wang, L.T.; Wang, A.Y.; Hsieh, C.W.; Chen, C.Y.; Sung, H.Y. Vacuolar invertases in sweet potato: Molecular cloning, characterization, and analysis of gene expression. J. Agric. Food Chem. 2005, 53, 3672. [Google Scholar] [CrossRef]
- Su, Z.X.; Gao, R.F.; Tang, W.; Yan, H.; Hou, M.; Wang, X.; Zhang, Y.G.; Ma, D.F.; Li, Q. Temporal and spatial expression of IbFBA5 gene and its relationship with carbohydrate accumulation in sweetpotato (Ipomoea batatas). Plant Physiol. J. 2017, 53, 857–864. [Google Scholar]
- Moracci, M.; Ponzano, B.C.; Trincone, A.; Fusco, S.; De Rosa, M.; Van der Oost, J.; Sensen, C.W.; Charlebois, R.L.; Rossi, M. Identification and molecular characterization of the first α-xylosidase from an archaeon. J. Biol. Chem. 2000, 275, 22082–22089. [Google Scholar] [CrossRef] [PubMed]
- Larsbrink, J.; Izumi, A.; Ibatullin, F.M.; Nakhai, A.; Gilbert, H.J.; Davies, G.J.; Brumer, H. Structural and enzymatic characterization of a glycoside hydrolase family 31 α-xylosidase from Cellvibrio japonicus involved in xyloglucan saccharification. Biochem. J. 2011, 436, 567–580. [Google Scholar] [CrossRef]
- Scott-Craig, J.S.; Borrusch, M.S.; Banerjee, G.; Harvey, C.M.; Walton, J.D. Biochemical and molecular characterization of secreted α-xylosidase from Aspergillus niger. J. Biol. Chem. 2011, 286, 42848–42854. [Google Scholar] [CrossRef]
- Nakai, H.; Tanizawa, S.; Ito, T.; Kamiya, K.; Kim, Y.M.; Yamamoto, T.; Matsubara, K.; Sakai, M.; Sato, H.; Imbe, T.; et al. Function-unknown glycoside hydrolase family 31 proteins, mrnas of which were expressed in rice ripening and germinating stages, are α-glucosidase and α-xylosidase. J. Biochem. 2007, 142, 491–500. [Google Scholar] [CrossRef]
- Shigeyama, T.; Watanabe, A.; Tokuchi, K.; Toh, S.; Sakurai, N.; Shibuya, N.; Kawakami, N. A-xylosidase plays essential roles in xyloglucan remodelling, maintenance of cell wall integrity, and seed germination in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 5615–5629. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Kameyama, A.; Yaoi, K. Identification and characterization of α-xylosidase involved in xyloglucan degradation in aspergillus oryzae. Appl. Microbiol. Biotechnol. 2020, 104, 201–210. [Google Scholar] [CrossRef]
- Sampedro, J.; Sieiro, C.; Revilla, G.; Gonzalez-Villa, T.; Zarra, I. Cloning and expression pattern of a gene encoding an α-xylosidase active against xyloglucan oligosaccharides from Arabidopsis. Plant Physiol. 2001, 126, 910–920. [Google Scholar] [CrossRef]
- Li, C.; Kou, M.; Arisha, M.H.; Tang, W.; Ma, M.; Yan, H.; Wang, X.X.; Wang, X.; Zhang, Y.G.; Liu, Y.J.; et al. Transcriptomic and metabolic profiling of high-temperature treated storage roots reveals the mechanism of saccharification in sweetpotato (Ipomoea batatas (L.) Lam.). Int. J. Mol. Sci. 2021, 22, 6641. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, H.; Jin, Y.L.; Zhu, J.C.; Ma, D.F. Analysis and perspectives of sweetpotato industry contributing to national food security in China. Jiangsu J. Agric. Sci. 2022, 38, 1484–1491. [Google Scholar]
- Imparl-Radosevich, J.M.; Gameon, J.R.; Mckean, A.; Wetterberg, D.; Keeling, P.L.; Guan, H. Understanding catalytic properties and functions of maize starch synthase isozymes. J. Appl. Glyosci. 2003, 50, 177–182. [Google Scholar] [CrossRef]
- Li, N.; Zhang, S.; Zhao, Y.; Li, B.; Zhang, J. Over-expression of agpase genes enhances seed weight and starch content in transgenic maize. Planta 2011, 233, 241–250. [Google Scholar] [CrossRef]
- Hou, F.Y.; Qin, F.; Li, Z.; Li, A.X.; Dong, S.X.; Wang, Q.M.; Zhang, L.M. Cloning and expression analysis of enzyme genes related to starch synthesis in sweetpotato. Mol. Plant Breed. 2019, 17, 7663–7668. [Google Scholar]
- Wang, S.; Yeh, K.; Tsai, C. Regulation of starch granule-bound starch synthase I gene expression by circadian clock and sucrose in the source tissue of sweet potato. Plant Sci. 2011, 161, 635–644. [Google Scholar] [CrossRef]
- Kuriki, T.; Stewart, D.C.; Preiss, J. Construction of chimeric enzymes out of maize endosperm branching enzymes I and II. J. Biol. Chem. 1997, 272, 28999–29004. [Google Scholar] [CrossRef]
- Guo, Z.; Jia, X.; Zhao, B.; Zeng, S.; Xiao, J.; Zheng, B. C-type starches and their derivatives: Structure and function. Ann. N. Y. Acad. Sci. 2017, 1398, 47–61. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Yu, M.; Goff, H.D.; Chen, M.; Zhong, F. Analysis of kinetic parameters and mechanisms of nanocrystalline cellulose inhibition of α-amylase and alpha-glucosidase in simulated digestion of starch. Food Funct. 2020, 11, 4719–4731. [Google Scholar] [CrossRef]
- Hu, X.; Kuang, S.; Zhang, A.; Zhang, W.; Chen, M.; Yin, X.; Chen, K. Characterization of starch degradation related genes in postharvest kiwifruit. Int. J. Mol. Sci. 2016, 17, 2112. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.; Ji, C.Y.; Park, S.; Jeong, J.C.; Lee, H.; Kwak, S. Stable internal reference genes for the normalization of real-time PCR in different sweetpotato cultivars subjected to abiotic stress conditions. PLoS ONE 2012, 7, 51502. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wei, Z.H.; Shang, M.Q.; Jiang, Z.C.; Zhai, H.; Xing, S.H.; Wang, Z.; He, S.Z.; Gao, S.P.; Zhao, N.; Zhang, H.; et al. Natural allelic variation of basic helix-loop-helix transcription factor 25 regulates carotenoid biosynthesis in sweet potato. Plant Biotechnol. J. 2025, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Li, C.; Song, W.H.; Shen, Y.; Tang, W.; Zhang, Y.G.; Wang, X.; Yan, H.; Gao, R.F.; Ahmad, M.Q.; et al. Identification and functional characterization of a flavonol synthase gene from sweet potato [Ipomoea batatas (L.) Lam.]. Front. Plant Sci. 2023, 14, 1181173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.C.; Zhai, H.; Wang, Y.; Zhang, D.P. Efficient plant regeneration from embryogenic suspension cultures of sweetpotato. Vitr. Cell. Dev. Biol.-Plant 2001, 37, 564–567. [Google Scholar] [CrossRef]
- Li, C.; Kou, M.; Song, W.H.; Arisha, M.H.; Gao, R.F.; Tang, W.; Yan, H.; Wang, X.; Zhang, Y.G.; Li, Q. Comparative analysis of saccharification characteristics of different type sweetpotato cultivars. Foods 2023, 12, 3785. [Google Scholar] [CrossRef]
- Xu, X.; Wu, S.; Chen, K.; Zhang, H.; Zhou, S.; Lv, Z.; Chen, Y.; Cui, P.; Cui, Z.; Lu, G.Q. Comprehensive evaluation of raw eating quality in 81 sweet potato (Ipomoea batatas (L.) Lam) varieties. Foods 2023, 12, 261. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Kou, M.; You, C.; Ma, M.; Song, W.; Tang, W.; Yan, H.; Gao, R.; Wang, X.; Zhang, Y.; et al. Ibα-XYL1 Interfered Expression Decreases Starch Granule Size and Increases Soluble Sugar Content to Improve Steamed Sweetpotato Storage Root Taste. Int. J. Mol. Sci. 2025, 26, 5015. https://doi.org/10.3390/ijms26115015
Li C, Kou M, You C, Ma M, Song W, Tang W, Yan H, Gao R, Wang X, Zhang Y, et al. Ibα-XYL1 Interfered Expression Decreases Starch Granule Size and Increases Soluble Sugar Content to Improve Steamed Sweetpotato Storage Root Taste. International Journal of Molecular Sciences. 2025; 26(11):5015. https://doi.org/10.3390/ijms26115015
Chicago/Turabian StyleLi, Chen, Meng Kou, Chang You, Meng Ma, Weihan Song, Wei Tang, Hui Yan, Runfei Gao, Xin Wang, Yungang Zhang, and et al. 2025. "Ibα-XYL1 Interfered Expression Decreases Starch Granule Size and Increases Soluble Sugar Content to Improve Steamed Sweetpotato Storage Root Taste" International Journal of Molecular Sciences 26, no. 11: 5015. https://doi.org/10.3390/ijms26115015
APA StyleLi, C., Kou, M., You, C., Ma, M., Song, W., Tang, W., Yan, H., Gao, R., Wang, X., Zhang, Y., & Li, Q. (2025). Ibα-XYL1 Interfered Expression Decreases Starch Granule Size and Increases Soluble Sugar Content to Improve Steamed Sweetpotato Storage Root Taste. International Journal of Molecular Sciences, 26(11), 5015. https://doi.org/10.3390/ijms26115015




