GDF5 rs143384 Polymorphism Associated with Developmental Dysplasia of the Hip in Brazilian Patients: A Case-Control Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
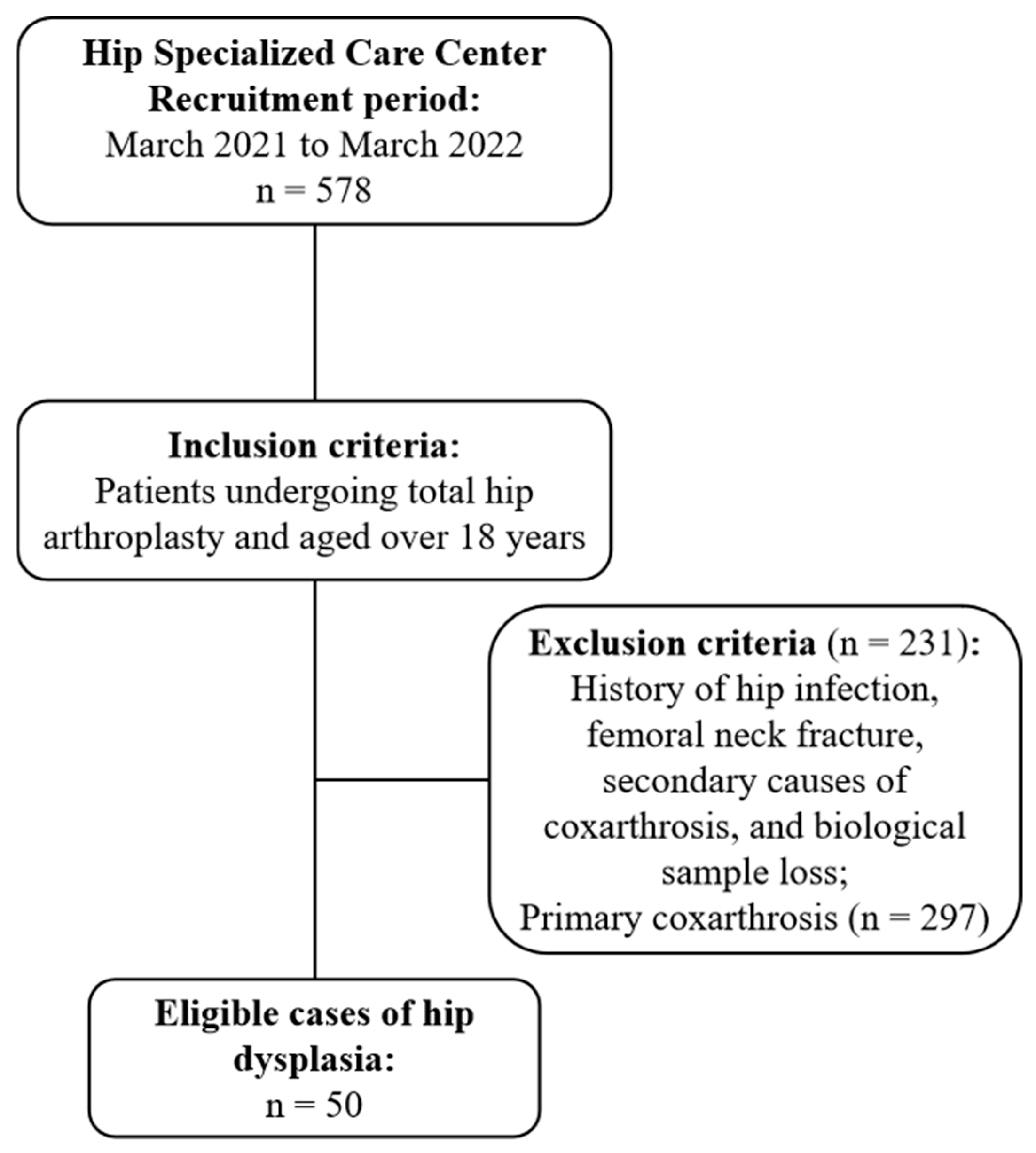
4.1. Study Population
4.2. Clinical Evaluation
4.3. Sample Collection and Polymorphisms Genotyping Analysis
4.4. Data Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gala, L.; Clohisy, J.C.; Beaulé, P.E. Hip Dysplasia in the Young Adult. J. Bone Joint Surg. Am. 2016, 98, 63–73. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.P.; Marshall, B.J.; Davison, J.; Clohisy, J.C.; Willey, M.C. Prevalence of Radiographic Hip Dysplasia in the General Adult Population: A Systematic Review. Iowa Orthop. J. 2024, 44, 145–149. [Google Scholar] [PubMed]
- Harsanyi, S.; Zamborsky, R.; Krajciova, L.; Kokavec, M.; Danisovic, L. Developmental Dysplasia of the Hip: A Review of Etiopathogenesis, Risk Factors, and Genetic Aspects. Medicina 2020, 56, 153. [Google Scholar] [CrossRef] [PubMed]
- Guarniero, R. Dysplasia of Hip Development: Update. Rev. Bras. Ortop. 2010, 45, 116–121. [Google Scholar] [CrossRef]
- Motta, G.G.B.; Chiovatto, A.R.S.; Chiovatto, E.D.; Duarte, M.L.; Rodrigues, N.V.M.; Iared, W. Prevalence of Developmental Dysplasia of the Hip in a Maternity Hospital in São Paulo, Brazil. Rev. Bras. Ortop. 2021, 56, 664–670. [Google Scholar] [CrossRef]
- Delgado-Arellanes, I.; Temperly, M.K.; Martin, E.M.; Davison, J.C.; Kochuyt, A.; Westermann, R.W.; Willey, M.C. Hip Dysplasia Diagnosed After Skeletal Maturity: Factors Associated With Progression to Osteoarthritis. Iowa Orthop. J. 2024, 44, 49–60. [Google Scholar]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Semciw, A.I.; Mechlenburg, I.; Tønning, L.C.; Stewart, C.J.; Kemp, J.L. Pain, Function and Quality of Life Are Impaired in Adults Undergoing Periacetabular Osteotomy (PAO) for Hip Dysplasia: A Systematic Review and Meta-Analysis. Hip Int. 2024, 34, 96–114. [Google Scholar] [CrossRef]
- Lim, J.; Choi, A.; Kim, B. The Effects of Resistance Training on Pain, Strength, and Function in Osteoarthritis: Systematic Review and Meta-Analysis. J. Pers. Med. 2024, 14, 1130. [Google Scholar] [CrossRef]
- Gambling, T.S.; Long, A. Psycho-social impact of developmental dysplasia of the hip and of differential access to early diagnosis and treatment: A narrative study of young adults. SAGE Open Med. 2019, 7, 2050312119836010. [Google Scholar] [CrossRef]
- Hatzikotoulas, K.; Roposch, A.; DDH Case Control Consortium; Shah, K.M.; Clark, M.J.; Bratherton, S.; Limbani, V.; Steinberg, J.; Zengini, E.; Warsame, K.; et al. Genome-wide Association Study of Developmental Dysplasia of the Hip Identifies an Association with GDF5. Commun. Biol. 2018, 1, 56. [Google Scholar] [CrossRef] [PubMed]
- Kenanidis, E.; Gkekas, N.K.; Karasmani, A.; Anagnostis, P.; Christofilopoulos, P.; Tsiridis, E. Genetic Predisposition to Developmental Dysplasia of the Hip. J. Arthroplasty 2020, 35, 291–300.e1. [Google Scholar] [CrossRef]
- Jacobsen, K.K.; Laborie, L.B.; Kristiansen, H.; Schäfer, A.; Gundersen, T.; Zayats, T.; Rosendahl, K. Genetics of Hip Dysplasia—A Systematic Literature Review. BMC Musculoskelet. Disord. 2024, 25, 762. [Google Scholar] [CrossRef] [PubMed]
- Kiapour, A.M.; Cao, J.; Young, M.; Capellini, T.D. The Role of Gdf5 Regulatory Regions in Development of Hip Morphology. PLoS ONE 2018, 13, e0202785. [Google Scholar] [CrossRef]
- Dodd, A.W.; Syddall, C.M.; Loughlin, J. A Rare Variant in the Osteoarthritis-Associated Locus GDF5 Is Functional and Reveals a Site That Can Be Manipulated to Modulate GDF5 Expression. Eur. J. Hum. Genet. 2013, 21, 517–521. [Google Scholar] [CrossRef]
- Reynard, L.N.; Bui, C.; Canty-Laird, E.G.; Young, D.A.; Loughlin, J. Expression of the Osteoarthritis-Associated Gene GDF5 Is Modulated Epigenetically by DNA Methylation. Hum. Mol. Genet. 2011, 20, 3358–3369. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, G.; Jin, W.; Li, Y.; Zhang, Y. Positive Selection on the Osteoarthritis-Risk and Decreased-Height Associated Variants at the GDF5 Gene in East Asians. PLoS ONE 2012, 7, e42553. [Google Scholar] [CrossRef]
- Capellini, T.D.; Chen, H.; Cao, J.; Doxey, A.C.; Kiapour, A.M.; Schoor, M.; Kingsley, D.M. Ancient Selection for Derived Alleles at a GDF5 Enhancer Influencing Human Growth and Osteoarthritis Risk. Nat. Genet. 2017, 49, 1202–1210. [Google Scholar] [CrossRef]
- Meng, W.; Adams, M.J.; Palmer, C.N.A.; 23andMe Research Team; Shi, J.; Auton, A.; Ryan, K.A.; Jordan, J.M.; Mitchell, B.D.; Jackson, R.D.; et al. Genome-wide Association Study of Knee Pain Identifies Associations with GDF5 and COL27A1 in UK Biobank. Commun. Biol. 2019, 2, 321. [Google Scholar] [CrossRef]
- Styrkarsdottir, U.; Stefansson, O.A.; Gunnarsdottir, K.; Thorleifsson, G.; Lund, S.H.; Stefansdottir, L.; Juliusson, K.; Agustsdottir, A.B.; Zink, F.; Halldorsson, G.H.; et al. GWAS of Bone Size Yields Twelve Loci That Also Affect Height, BMD, Osteoarthritis or Fractures. Nat. Commun. 2019, 10, 2054. [Google Scholar] [CrossRef]
- Yan, S.; Nie, H.; Bu, G.; Yuan, W.; Wang, S. The Effect of Common Variants in GDF5 Gene on the Susceptibility to Chronic Postsurgical Pain. J. Orthop. Surg. Res. 2021, 16, 420. [Google Scholar] [CrossRef]
- Takahata, Y.; Hagino, H.; Kimura, A.; Urushizaki, M.; Yamamoto, S.; Wakamori, K.; Murakami, T.; Hata, K.; Nishimura, R. Regulatory Mechanisms of Prg4 and Gdf5 Expression in Articular Cartilage and Functions in Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 4672. [Google Scholar] [CrossRef] [PubMed]
- Rouault, K.; Scotet, V.; Autret, S.; Gaucher, F.; Dubrana, F.; Tanguy, D.; El Rassi, C.Y.; Fenoll, B.; Férec, C. Evidence of Association Between GDF5 Polymorphisms and Congenital Dislocation of the Hip in a Caucasian Population. Osteoarthr. Cartil. 2010, 18, 1144–1149. [Google Scholar] [CrossRef]
- Al-Essa, R.S.; Aljahdali, F.H.; Alkhilaiwi, R.M.; Philip, W.; Jawadi, A.H.; Khoshal, K.I. Diagnosis and Treatment of Developmental Dysplasia of the Hip: A Current Practice of Paediatric Orthopaedic Surgeons. J. Orthop. Surg. 2017, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nandhagopal, T.; Tiwari, V.; De Cicco, F.L. Developmental Dysplasia of the Hip. [Updated 2024 May 4]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563157/ (accessed on 15 February 2025).
- Hall, M.; van der Esch, M.; Hinman, R.S.; Peat, G.; de Zwart, A.; Quicke, J.G.; Runhaar, J.; Knoop, J.; van der Leeden, M.; de Rooij, M.; et al. How Does Hip Osteoarthritis Differ from Knee Osteoarthritis? Osteoarthr. Cartil. 2022, 30, 32–41. [Google Scholar] [CrossRef]
- Kondo, K.; Jingushi, S.; Ohfuji, S.; Sofue, M.; Itoman, M.; Matsumoto, T.; Hamada, Y.; Shindo, H.; Takatori, Y.; Yamada, H.; et al. Factors Associated with Functional Limitations in the Daily Living Activities of Japanese Hip Osteoarthritis Patients. Int. J. Rheum. Dis. 2017, 20, 1372–1382. [Google Scholar] [CrossRef]
- Lindberg, A.W.; Bompadre, V.; Satchell, E.K.; Larson, A.C.R.; White, K.K. Patient Factors Associated with Delayed Diagnosis of Developmental Dysplasia of the Hip. J. Child Orthop. 2017, 11, 223–228. [Google Scholar] [CrossRef]
- Committee on Quality Improvement; Subcommittee on Developmental Dysplasia of the Hip; American Academy of Pediatrics. Clinical practice guideline: Early detection of developmental dysplasia of the hip. Pediatrics 2000, 105 Pt 1, 896–905. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Mabuchi, A.; Shi, D.; Kubo, T.; Takatori, Y.; Saito, S.; Fujioka, M.; Sudo, A.; Uchida, A.; Yamamoto, S.; et al. A Functional Polymorphism in the 5′ UTR of GDF5 Is Associated with Susceptibility to Osteoarthritis. Nat. Genet. 2007, 39, 529–533. [Google Scholar] [CrossRef]
- Chueire, A.G.; Rejali, W.A.; Santos, A.F. Protrusão Acetabular. Acta Ortop. Bras. 2002, 10, 52–57. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, W.; Shao, Z. Association between GDF5 rs143383 Genetic Polymorphism and Musculoskeletal Degenerative Diseases Susceptibility: A Meta-Analysis. BMC Med. Genet. 2018, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.G.S.; Vasconcelos, B.M.C.; Pujoni, H.P.; Nogueira, M.C.; Oliveira, V.M.; Chaoubah, A. Epidemiology and costs of surgical treatment of developmental dysplasia of hip in the Brazilian Public Health System in a decade. Einstein 2021, 19, eGS5625. [Google Scholar] [CrossRef] [PubMed]
- Takano, B.Y.; Peixoto, M.E.d.A.; Júnior, T.d.R.; Rocha, A.L.d.O.; Volpatto, L.; Duarte, M.C.B.; Valente, M.d.S.; Rosa, L.G.S.; Wieth, D.M.; Peixoto, M.d.A.; et al. Developmental dysplasia of the hip: Etiopathogenesis, pathology and advances in diagnosis. Braz. J. Implantol. Health Sci. 2024, 6, 5250–5261. [Google Scholar] [CrossRef]
- Araujo Junior, A.E.P.; de Azevedo, G.B.L.; Moliterno, L.A.M.; Tavares, R.H.; Cardoso, J.V.; de Souza, G.R.; Guimarães, J.A.M.; Defino, H.L.A.; Perini, J.A. Association of Polymorphism in Leptin Receptor Gene with Susceptibility of Adolescent Idiopathic Scoliosis. Eur. Spine J. 2024, 33, 646–654. [Google Scholar] [CrossRef]
- Lopes, L.R.; Guimarães, J.A.M.; Amaral, M.V.G.; Pereira, C.G.; Wainchtock, V.S.; Goes, R.A.; Miranda, V.A.R.; Perini, J.A. Genetic Polymorphisms in COL1A2 Gene and the Risk of Tendinopathy: Case-Control Study. Rev. Bras. Ortop. 2023, 58, 478–486. [Google Scholar] [CrossRef]
- de Azevedo, G.B.L.; Perini, J.A.; Araújo Junior, A.E.P.; Moliterno, L.A.M.; Andrande, R.M.; Guimarães, J.A.M.; Defino, H.L.A. Association of FBN1 Polymorphism with Susceptibility of Adolescent Idiopathic Scoliosis: A Case-Control Study. BMC Musculoskelet. Disord. 2022, 23, 430. [Google Scholar] [CrossRef] [PubMed]
- Perini, J.A.; Lopes, L.R.; Guimarães, J.A.M.; Goes, R.A.; Pereira, L.F.A.; Pereira, C.G.; Mandarino, M.; Villardi, A.M.; de Sousa, E.B.; Cossich, V.R.A. Influence of Type I Collagen Polymorphisms and Risk of Anterior Cruciate Ligament Rupture in Athletes: A Case-Control Study. BMC Musculoskelet. Disord. 2022, 23, 154. [Google Scholar] [CrossRef]
- Lopes, L.R.; de Miranda, V.A.R.; Guimarães, J.A.M.; de Araujo Souza, G.G.; Wainchtock, V.S.; Grangeiro Neto, J.A.; de Araújo Goes, R.; Perini, J.A. Association of TNF-α -308G>A Polymorphism with Susceptibility to Tendinopathy in Athletes: A Case-Control Study. BMC Sports Sci. Med. Rehabil. 2021, 13, 51. [Google Scholar] [CrossRef]
- Pena, S.D.; Di Pietro, G.; Fuchshuber-Moraes, M.; Genro, J.P.; Hutz, M.H.; Kehdy, F.S.; Kohlrausch, F.; Magno, L.A.; Montenegro, R.C.; Moraes, M.O.; et al. The genomic ancestry of individuals from different geographical regions of Brazil is more uniform than expected. PLoS ONE 2011, 6, e17063. [Google Scholar] [CrossRef]
- World Health Organization. Physical Status: The Use of and Interpretation of Anthropometry, Report of a WHO Expert Committee; WHO: Geneva, Switzerland, 1995. [Google Scholar]
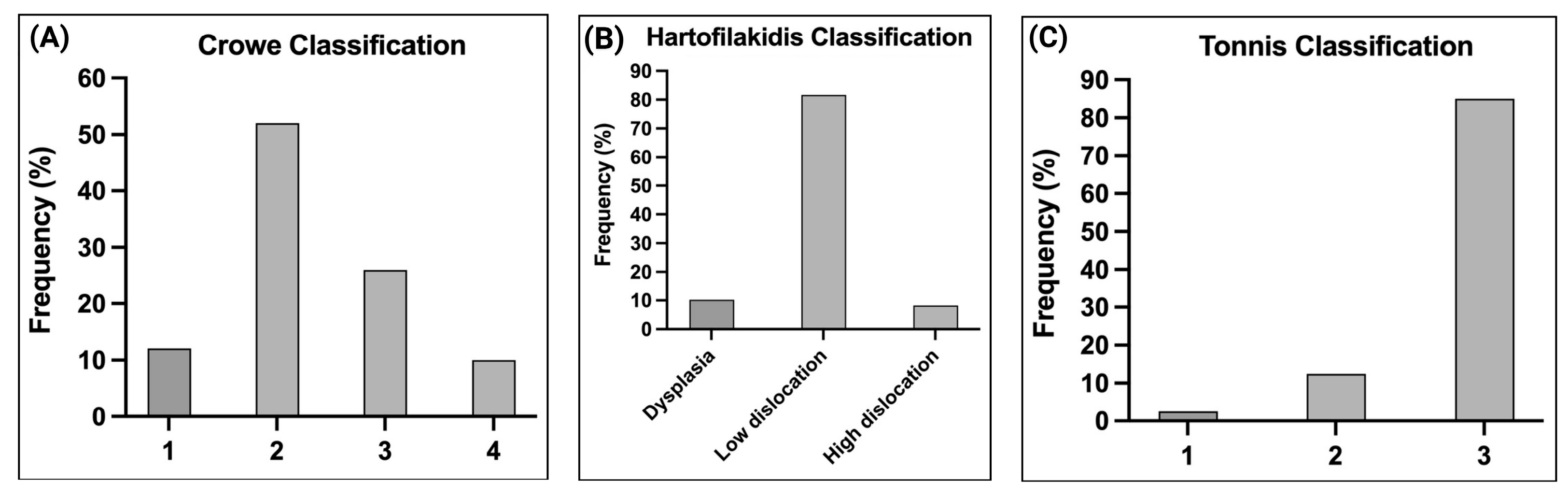
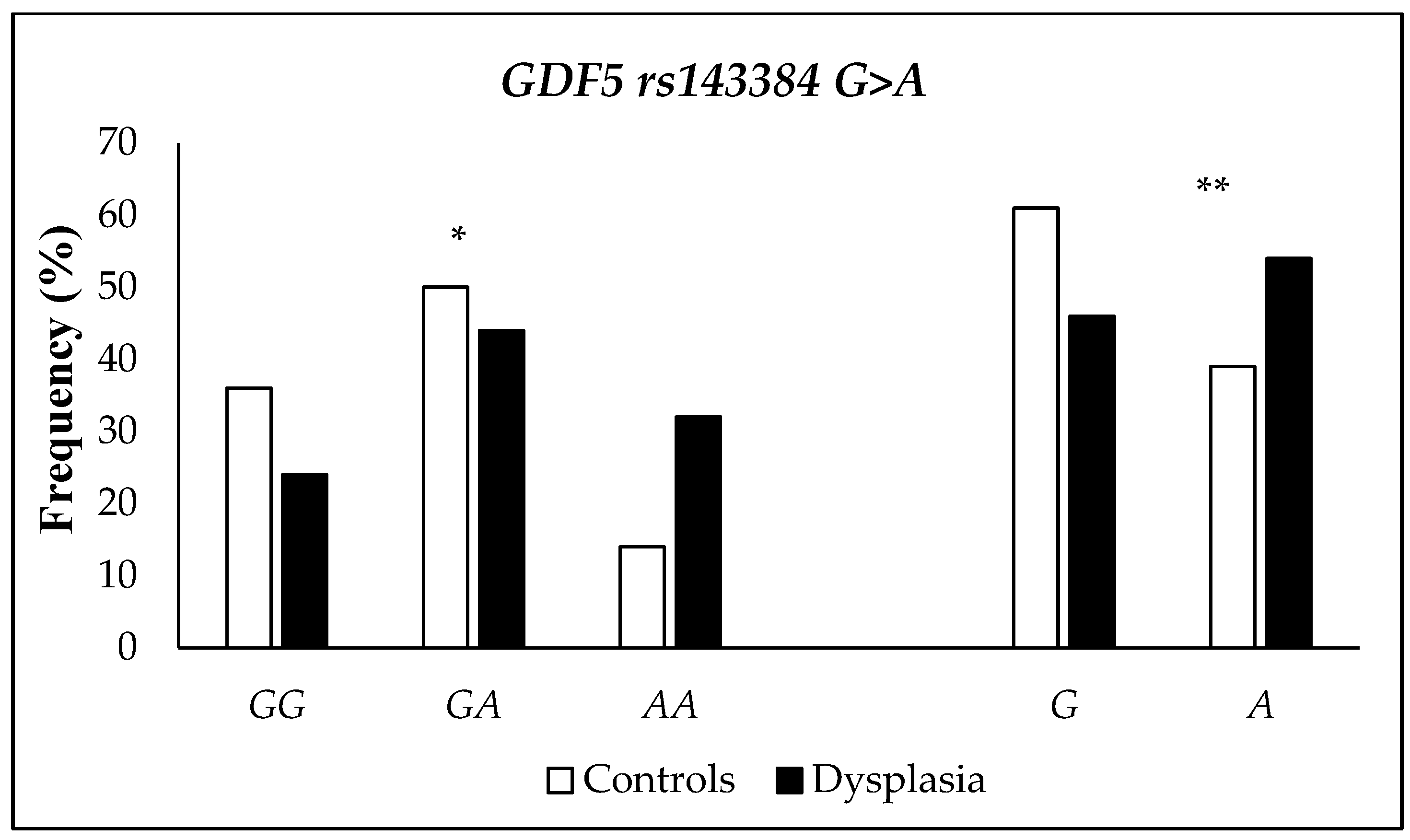
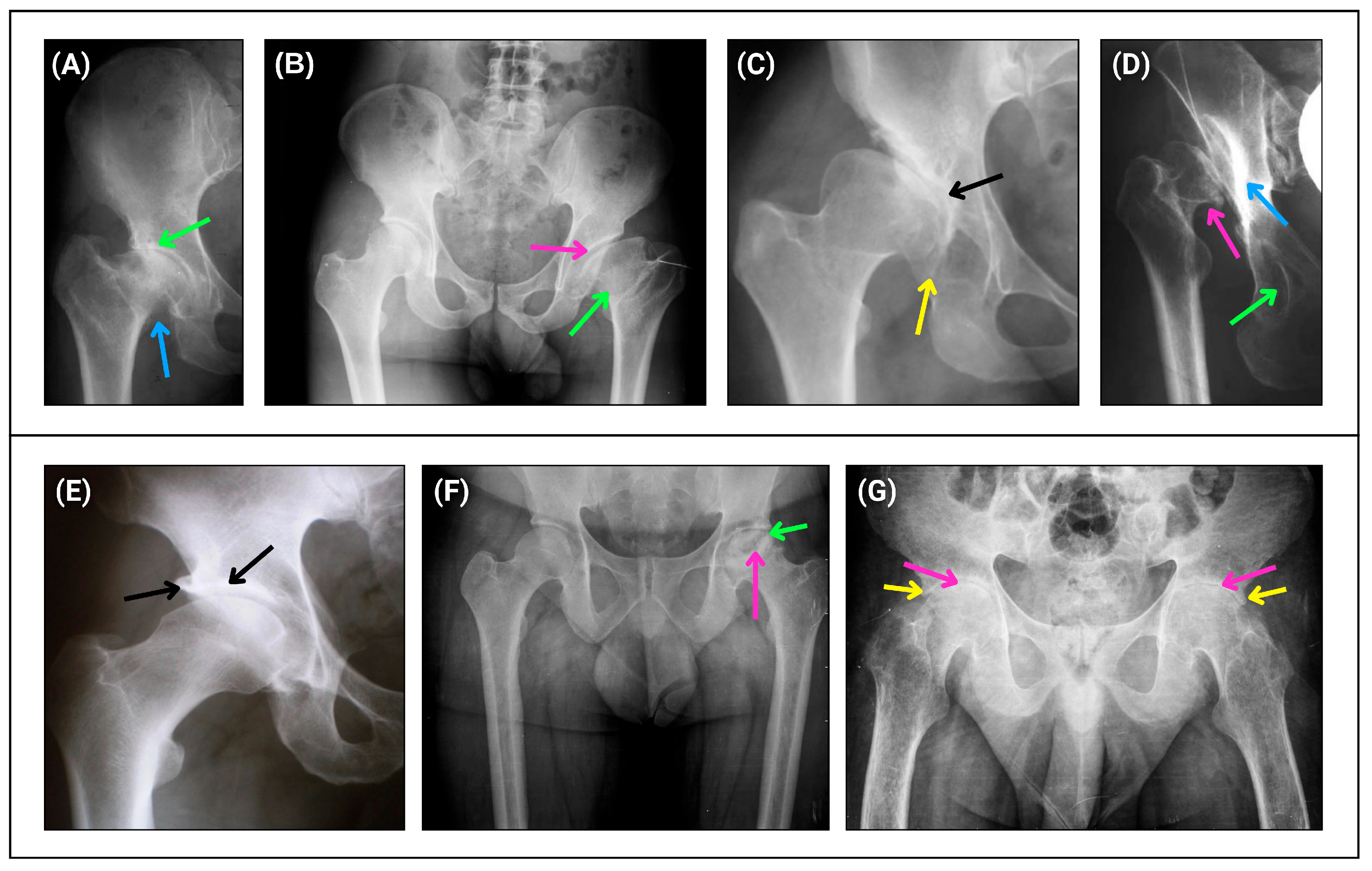
| Characteristics | n (%) | Characteristics | n (%) | Characteristics | n (%) |
|---|---|---|---|---|---|
| Lameness | Support | Climbing and descending stairs | |||
| None | 2 (4) | None | 22 (44) | Normally | 3 (6) |
| Mild | 6 (12) | One crutch | 1 (2) | With support on the handrail | 29 (58) |
| Moderate | 24 (48) | Cane almost always | 20 (40) | With great effort | 16 (32) |
| Severe | 18 (36) | Cane for long walks | 5 (10) | Cannot | 2 (4) |
| Missing | 2 | ||||
| Pain | Sit | Distance able to walk | |||
| None | 2 (4) | For one hour | 13 (26) | Home walker | 11 (22) |
| Mild | 5 (10) | For up to one hour | 7 (14) | Up to three blocks | 16 (32) |
| Moderate without limit | 13 (26) | For up to half an hour | 23 (46) | Three blocks | 9 (18) |
| Moderate with limit | 13 (26) | Cannot sit without pain | 7 (14) | Up to six blocks | 6 (12) |
| Intense | 15 (30) | Six blocks | 4 (8) | ||
| Disabling | 2 (4) | Unlimited | 4 (8) | ||
| Putting on socks | Deformity | Public transport | |||
| No difficulties | 3 (6) | No | 36 (73.5) | No | 6 (12.2) |
| With difficulties | 31 (62) | Yes | 13 (26.5) | Yes | 43 (87.8) |
| Cannot | 16 (32) | Missing | 1 | Missing | 1 |
| Disease side | Disease evolution | ||||
| Right | 10 (20) | <20 years | 19 (38) | ||
| Left | 16 (32) | 21–30 years | 24 (48) | ||
| Bilateral | 24 (48) | >40 years | 7 (14) | ||
| Cohorts | N a | rs143384 A Allele | p-Value b | Reference |
|---|---|---|---|---|
| Brazilian | 778 | 0.419 | Reference | Present study |
| American | 13,356 | 0.666 | <0.001 | gnomAD c |
| African | 1396 | 0.011 | <0.001 | gnomAD c |
| African American | 74,830 | 0.104 | <0.001 | gnomAD c |
| Amerindigenous | 4525 | 0.873 | <0.001 | gnomAD c |
| European | 9233 | 0.555 | <0.001 | gnomAD c |
| East Asian | 44,794 | 0.741 | <0.001 | gnomAD c |
| South Asian | 90,402 | 0.426 | 0.69 | gnomAD c |
| Characteristic | Controls n (%) | DDH n (%) | p-Value |
|---|---|---|---|
| Age (years) | |||
| ≤30 | 56 (37.3) | 7 (14) | <0.001 |
| 31–40 | 44 (29.3) | 10 (20) | |
| 41–50 | 32 (21.3) | 13 (26) | |
| 51–60 | 18 (12.0) | 13 (26) | |
| ≥61 | 0 (0) | 7 (14) | |
| BMI (kg/m2) | |||
| ≤18.5 | 1 (0.7) | 2 (4) | 0.19 |
| 18.6–24.9 | 47 (31.3) | 10 (20) | |
| 25–29.9 | 54 (36) | 17 (34) | |
| 30–39.9 | 48 (32) | 21 (42) | |
| Sex | |||
| Men | 77 (51.3) | 14 (28) | 0.004 |
| Women | 73 (48.7) | 36 (72) | |
| Skin color a | |||
| White | 52 (34.7) | 28 (56) | 0.008 |
| Non-white | 98 (65.3) | 22 (44) |
| Polymorphism rs143384 G>A | Controls (n = 150) | DDH (n = 50) | p-Value | OR (95% CI) | p-Value | ORa (95% CI) |
|---|---|---|---|---|---|---|
| Genotypes | n (%) | |||||
| GG | 54 (36) | 12 (24) | 1 a | 1 a | ||
| GA | 75 (50) | 22 (44) | 0.49 | 1.32 (0.60–2.90) | 0.81 | 0.90 (0.38–2.12) |
| AA | 21 (14) | 16 (32) | 0.006 | 3.43 (1.39–8.45) | 0.25 | 1.39 (0.79–2.44) |
| GG + GA | 129 (86) | 34 (68) | 0.005 | 1 a | 0.08 | 1 a |
| AA | 21 (14) | 16 (32) | 2.89 (1.36–6.13) | 1.47 (0.96–2.26) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perini, J.A.; Cunha, R.W.C.; Cury Fernandes, M.B.; Peixoto, L.P.; Guimarães, J.A.M.; Cavalcanti, A.d.S.; Cardoso, J.V. GDF5 rs143384 Polymorphism Associated with Developmental Dysplasia of the Hip in Brazilian Patients: A Case-Control Study. Int. J. Mol. Sci. 2025, 26, 5012. https://doi.org/10.3390/ijms26115012
Perini JA, Cunha RWC, Cury Fernandes MB, Peixoto LP, Guimarães JAM, Cavalcanti AdS, Cardoso JV. GDF5 rs143384 Polymorphism Associated with Developmental Dysplasia of the Hip in Brazilian Patients: A Case-Control Study. International Journal of Molecular Sciences. 2025; 26(11):5012. https://doi.org/10.3390/ijms26115012
Chicago/Turabian StylePerini, Jamila Alessandra, Raphael Wallace Campos Cunha, Marco Bernardo Cury Fernandes, Lourenço Pinto Peixoto, João Antônio Matheus Guimarães, Amanda dos Santos Cavalcanti, and Jéssica Vilarinho Cardoso. 2025. "GDF5 rs143384 Polymorphism Associated with Developmental Dysplasia of the Hip in Brazilian Patients: A Case-Control Study" International Journal of Molecular Sciences 26, no. 11: 5012. https://doi.org/10.3390/ijms26115012
APA StylePerini, J. A., Cunha, R. W. C., Cury Fernandes, M. B., Peixoto, L. P., Guimarães, J. A. M., Cavalcanti, A. d. S., & Cardoso, J. V. (2025). GDF5 rs143384 Polymorphism Associated with Developmental Dysplasia of the Hip in Brazilian Patients: A Case-Control Study. International Journal of Molecular Sciences, 26(11), 5012. https://doi.org/10.3390/ijms26115012







