Dihydrocapsaicin Enhances Tumor Necrosis Factor-α-Induced Apoptosis and G1 Cell Cycle Arrest in Human Cervical Cancer Cells Through TAK1-Mediated NF-κB and EGFR Pathways
Abstract
1. Introduction
2. Results
2.1. Treatment of Cancer Cells with TNF-α and Dihydrocapsaicin Decreased Cell Viability
2.2. Effect of Dihydrocapsaicin on TNF-α-Mediated G1 Cell Cycle Arrest at 12 h
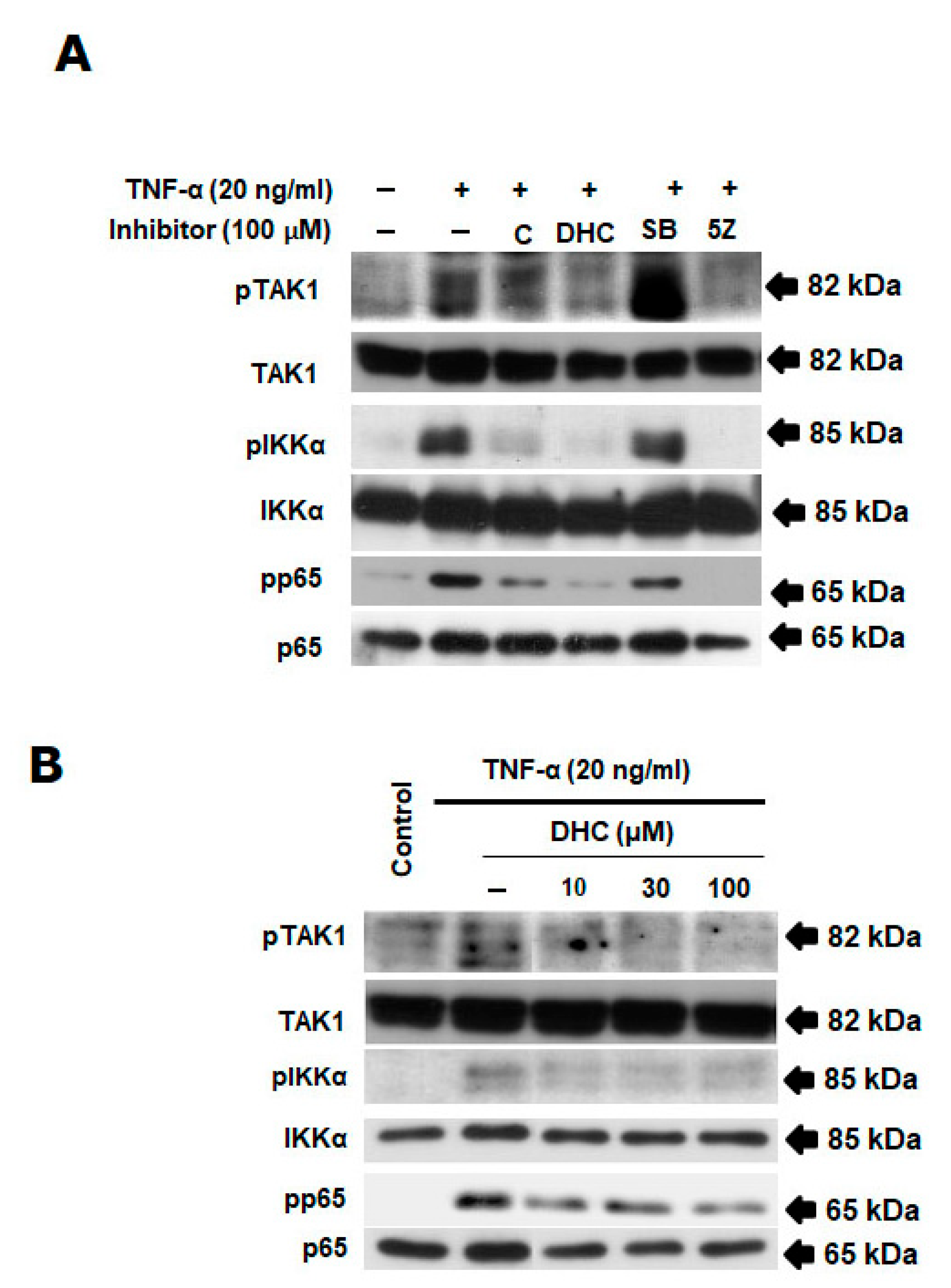
2.3. Effect of Dihydrocapsaicin and TNF-α on TAK1/NF-κB Signaling Pathway
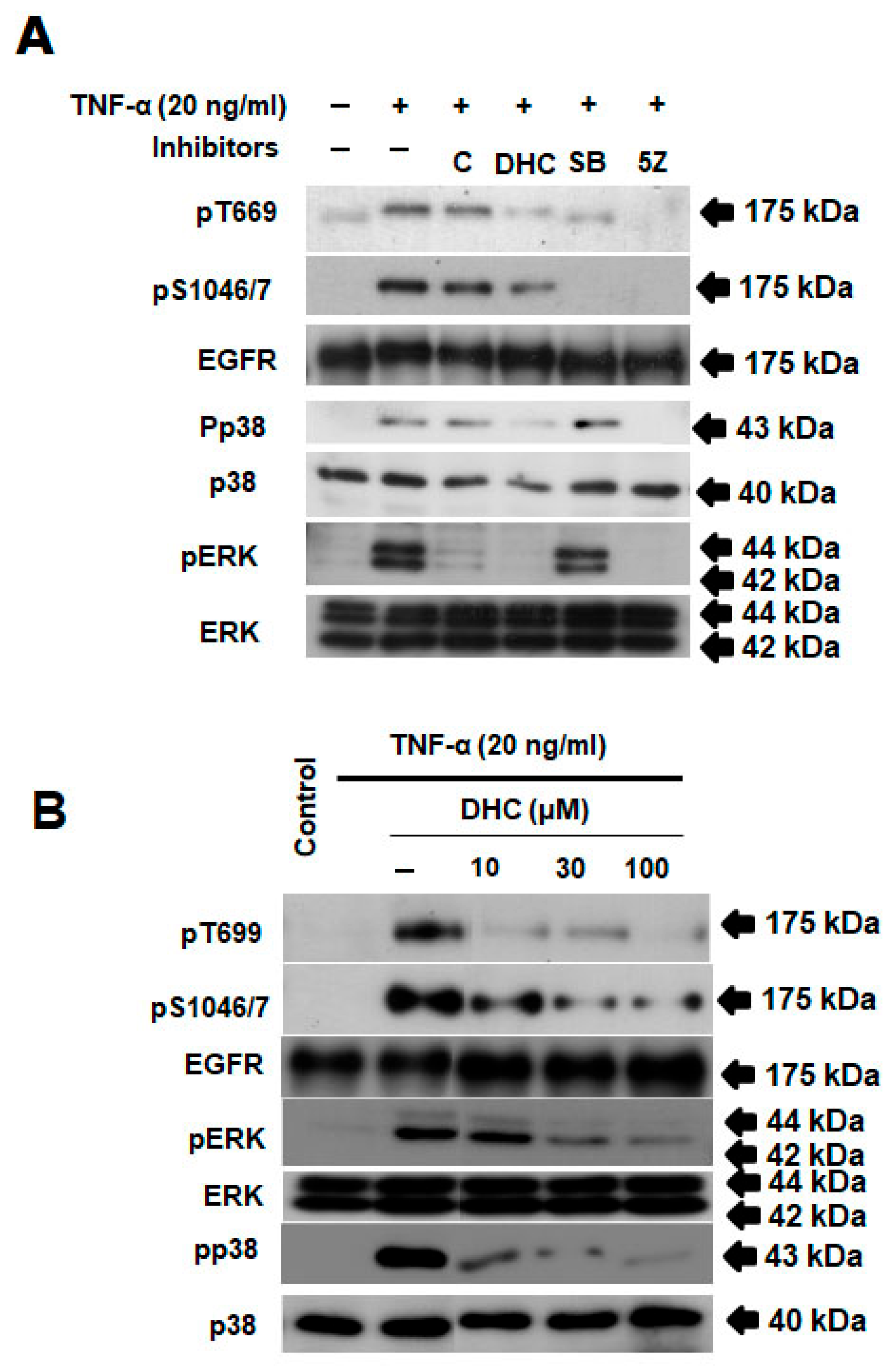
2.4. Effect of Dihydrocapsaicin and TNF-α-on EGFR/p38/Erk Signaling Pathway
2.5. Effect of Dihydrocapsaicin and TNF-α-on Akt/JNK Signaling Pathway
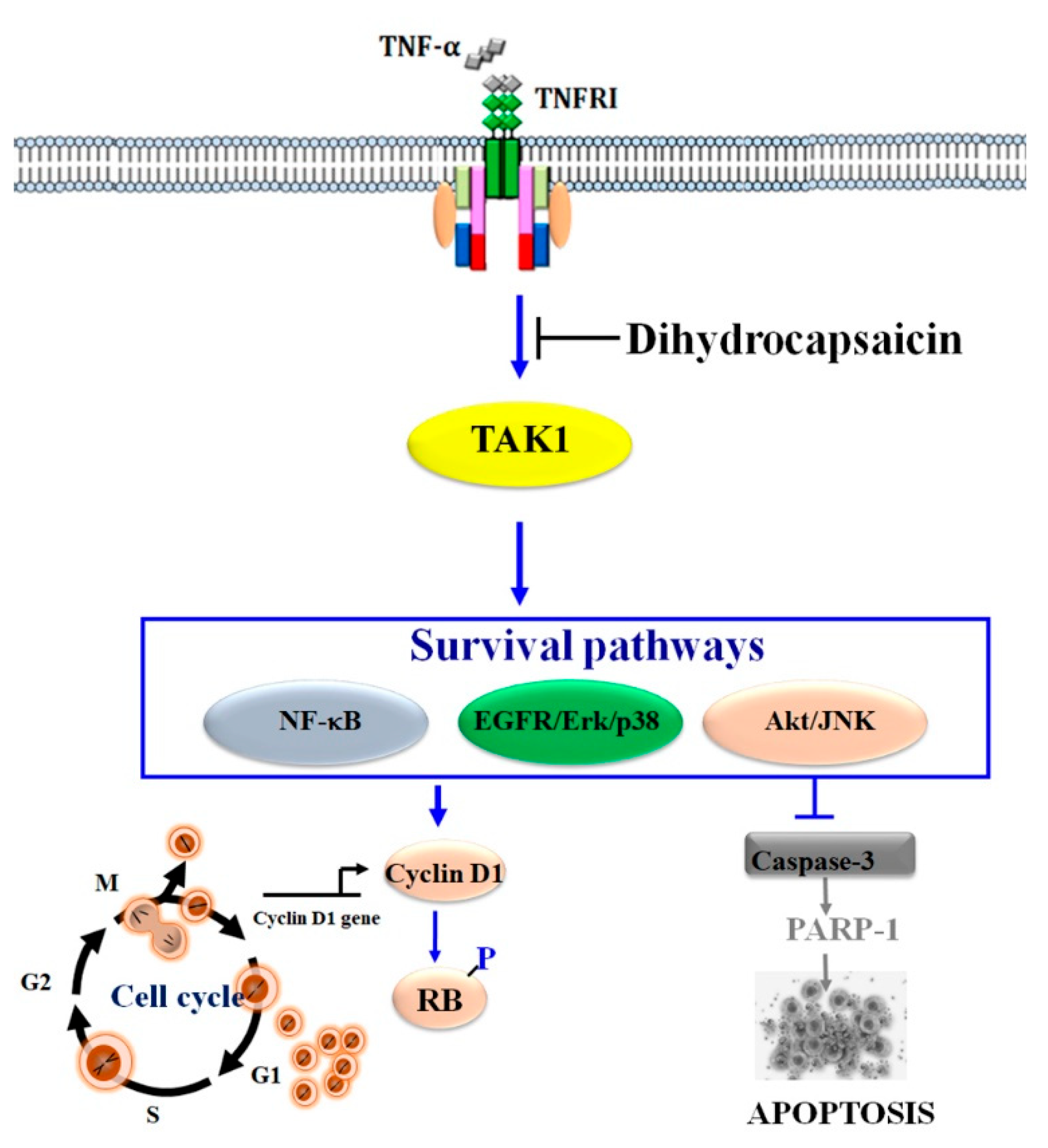
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Proliferation Assay
4.2. Cell Cycle Analysis
4.3. Preparation of Cell Extracts
4.4. Immunoblotting
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbero, G.F.; Ruiz, A.G.; Liazid, A.; Palma, M.; Vera, J.C.; Barroso, C.G. Evolution of total and individual capsaicinoids content in peppers during ripening of Capsicum annuum L. Food Chem. 2014, 153, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Sanatombi, K.; Sharma, G.J. Capsaicin content and pungency of different Capsicum spp. cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2008, 6, 89–90. [Google Scholar]
- Arora, R.; Gill, N.S.; Chauhan, G.; Rana, A.C. An overview about versatile molecule capsaicin. Int. J. Pharm. Sci. Drug Res. 2011, 3, 280–286. [Google Scholar] [CrossRef]
- Srinivasan, K. Biological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kim, C.S.; Han, I.S.; Kawada, T.; Yu, R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007, 581, 4389–4396. [Google Scholar] [CrossRef]
- Clark, R.; Lee, S.H. Anticancer properties of capsaicin against human cancer. Anticancer Res. 2016, 36, 837–843. [Google Scholar]
- Mori, A.; Lehmann, S.; O’Kelly, J.; Kumagai, T.; Desmond, J.C.; Pervan, M.; Koeffler, H.P. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006, 66, 3222–3229. [Google Scholar] [CrossRef]
- Macho, A.; Calzado, M.A.; Muñoz-Blanco, J.; Gómez-Díaz, C.; Gajate, C.; Mollinedo, F.; Navas, P.; Muñoz, E. Selective induction of apoptosis by capsaicin in transformed cells: The role of reactive oxygen species and calcium. Cell Death Differ. 1999, 6, 155–165. [Google Scholar] [CrossRef]
- Luo, X.J.; Peng, J.; Li, Y.J. Recent advances in the study on capsaicinoids and capsinoids. Eur. J. Pharmacol. 2011, 650, 1–7. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, Y.S.; Lim, S.C.; Hou, Y.F.; Chang, I.Y.; You, H.J. Dihydrocapsaicin (DHC), a saturated structural analog of capsaicin, induces autophagy in human cancer cells in a catalase-regulated manner. Autophagy 2008, 4, 1009–10192. [Google Scholar] [CrossRef]
- Chapa-Oliver, A.M.; Mejía-Teniente, L. Capsaicin: From plants to a cancer-suppressing agent. Molecules 2016, 21, 931. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xiang, G.H.; Tang, T.; Tang, Y.; Zhao, L.Y.; Liu, D.; Zhang, Y.R.; Tang, J.T.; Zhou, S.; Wu, D.H. Capsaicin and dihydrocapsaicin induce apoptosis in human glioma cells via ROS and Ca2+ mediated mitochondrial pathway. Mol. Med. Rep. 2016, 14, 4198–4208. [Google Scholar] [CrossRef]
- Shi, S.; Li, C.; Zhang, Y.; Deng, C.; Liu, W.; Du, J.; Li, Q.; Ji, Y.; Guo, L.; Liu, L.; et al. Dihydrocapsaicin Inhibits Cell Proliferation and Metastasis in Melanoma via Down-regulating β-Catenin Pathway. Front. Oncol. 2021, 23, 11. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 1 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef]
- Musgrove, E.A.; Caldon, C.E.; Barraclough, J.; Stone, A.; Sutherland, R.L. Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer 2011, 11, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. Cancer cell cycles. Science 1996, 274, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.A. The retinoblastoma protein and cell cycle control. Cell 1995, 81, 323–330. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-κB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef]
- Sakurai, H.; Suzuki, S.; Kawasaki, N.; Nakano, H.; Okazaki, T.; Chino, A.; Doi, T.; Saiki, I. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-κB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J. Biol. Chem. 2003, 278, 36916–36923. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Shirakabe, K.; Shibuya, H.; Irie, K.; Oishi, I.; Ueno, N.; Nishida, E.; Matsumoto, K. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science 1995, 270, 2008–2011. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Deng, L.; Hong, M.; Akkaraju, G.R.; Inoue, J.; Chen, Z.J. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 2001, 412, 346–351. [Google Scholar] [CrossRef]
- Pomerantz, J.L.; Baltimore, D. NF-κB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J. 1999, 18, 6694–6704. [Google Scholar] [CrossRef]
- Nishimura, M.; Shin, M.S.; Singhirunnusorn, P.; Suzuki, S.; Kawanishi, M.; Koizumi, K.; Saiki, I.; Sakurai, H. TAK1-mediated serine/threonine phosphorylation of epidermal growth factor receptor via p38/extracellular signal-regulated kinase: NF-jB-independent survival pathways in tumor necrosis factor alpha signaling. Mol. Cell Biol. 2009, 29, 5529–5539. [Google Scholar] [CrossRef]
- Shin, M.S.; Shinghirunnusorn, P.; Sugishima, Y.; Nishimura, M.; Suzuki, S.; Koizumi, K.; Saiki, I.; Sakurai, H. Cross interference with TNF-a-induced TAK1 activation via EGFR-mediated p38 phosphorylation of TAK1-binding protein 1. Biochim. Biophys. Acta 2009, 793, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. Integrating cell-signalling pathways with NF-jB and IKK function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef]
- Ninomiya-Tsuji, J.; Kishimoto, K.; Hiyama, A.; Inoue, J.; Cao, Z.; Matsumoto, K. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 1999, 398, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Sanjo, H.; Takeda, K.; Ninomiya-Tsuji, J.; Yamamoto, M.; Kawai, T.; Matsumoto, K.; Takeuchi, O.; Akira, S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 2005, 6, 1087–1095. [Google Scholar] [CrossRef]
- Sakurai, H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol. Sci. 2012, 33, 522–530. [Google Scholar] [CrossRef]
- Winograd-Katz, S.E.; Levitzki, A. Cisplatin induces PKB/Akt activation and p38 (MAPK) phosphorylation of the epidermal growth factor receptor. Oncogene 2006, 25, 7381–7390. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, W.; Wang, H.; Chen, J.; Zhao, Y. EGFR-mediated phosphorylation at Thr669 regulates EGFR internalization through interaction with p38 MAPK in response to osmotic stress. Sci. Rep. 2017, 7, 43415. [Google Scholar]
- D’Amours, D.; Sallmann, F.R.; Dixit, V.M.; Poirier, G.G. Gain-of-function of poly (ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: Implications for apoptosis. J. Cell Sci. 2001, 114, 3771–3778. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonyarat, C.; Sakurai, H.; Hayakawa, Y.; Chaiwiwatrakul, S.; Kaewamatawong, R.; Supapaan, T.S.; Duangjit, S.; Sethabouppha, B.; Waiwut, P. Dihydrocapsaicin Enhances Tumor Necrosis Factor-α-Induced Apoptosis and G1 Cell Cycle Arrest in Human Cervical Cancer Cells Through TAK1-Mediated NF-κB and EGFR Pathways. Int. J. Mol. Sci. 2025, 26, 5011. https://doi.org/10.3390/ijms26115011
Boonyarat C, Sakurai H, Hayakawa Y, Chaiwiwatrakul S, Kaewamatawong R, Supapaan TS, Duangjit S, Sethabouppha B, Waiwut P. Dihydrocapsaicin Enhances Tumor Necrosis Factor-α-Induced Apoptosis and G1 Cell Cycle Arrest in Human Cervical Cancer Cells Through TAK1-Mediated NF-κB and EGFR Pathways. International Journal of Molecular Sciences. 2025; 26(11):5011. https://doi.org/10.3390/ijms26115011
Chicago/Turabian StyleBoonyarat, Chantana, Hiroaki Sakurai, Yoshihiro Hayakawa, Suchada Chaiwiwatrakul, Rawiwun Kaewamatawong, Teeraporn Sadira Supapaan, Sureewan Duangjit, Benjabhorn Sethabouppha, and Pornthip Waiwut. 2025. "Dihydrocapsaicin Enhances Tumor Necrosis Factor-α-Induced Apoptosis and G1 Cell Cycle Arrest in Human Cervical Cancer Cells Through TAK1-Mediated NF-κB and EGFR Pathways" International Journal of Molecular Sciences 26, no. 11: 5011. https://doi.org/10.3390/ijms26115011
APA StyleBoonyarat, C., Sakurai, H., Hayakawa, Y., Chaiwiwatrakul, S., Kaewamatawong, R., Supapaan, T. S., Duangjit, S., Sethabouppha, B., & Waiwut, P. (2025). Dihydrocapsaicin Enhances Tumor Necrosis Factor-α-Induced Apoptosis and G1 Cell Cycle Arrest in Human Cervical Cancer Cells Through TAK1-Mediated NF-κB and EGFR Pathways. International Journal of Molecular Sciences, 26(11), 5011. https://doi.org/10.3390/ijms26115011





