Retrospective Observational Study of CSF-Derived HIV-1 Tat and Vpr Amino Acid Sequences in a South African Pediatric Cohort with HIV Subtype C
Abstract
1. Introduction
2. Results
2.1. Study Characteristics
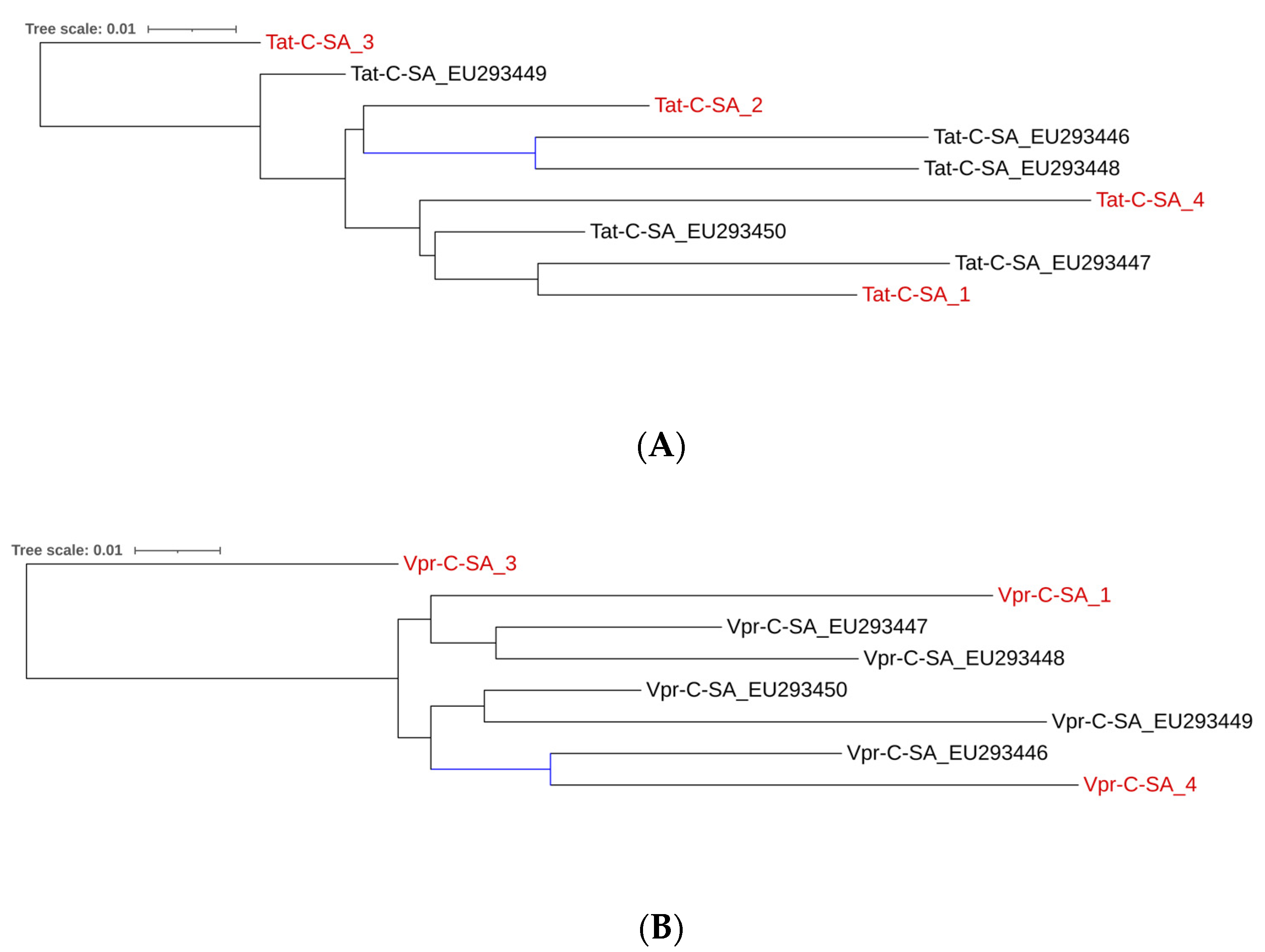
2.2. Subtype C Tat and Vpr Sequences in Study Participants
2.3. Comparison Between South African Subtype C CSF-Derived and PBMC-Derived Tat and Vpr Sequences
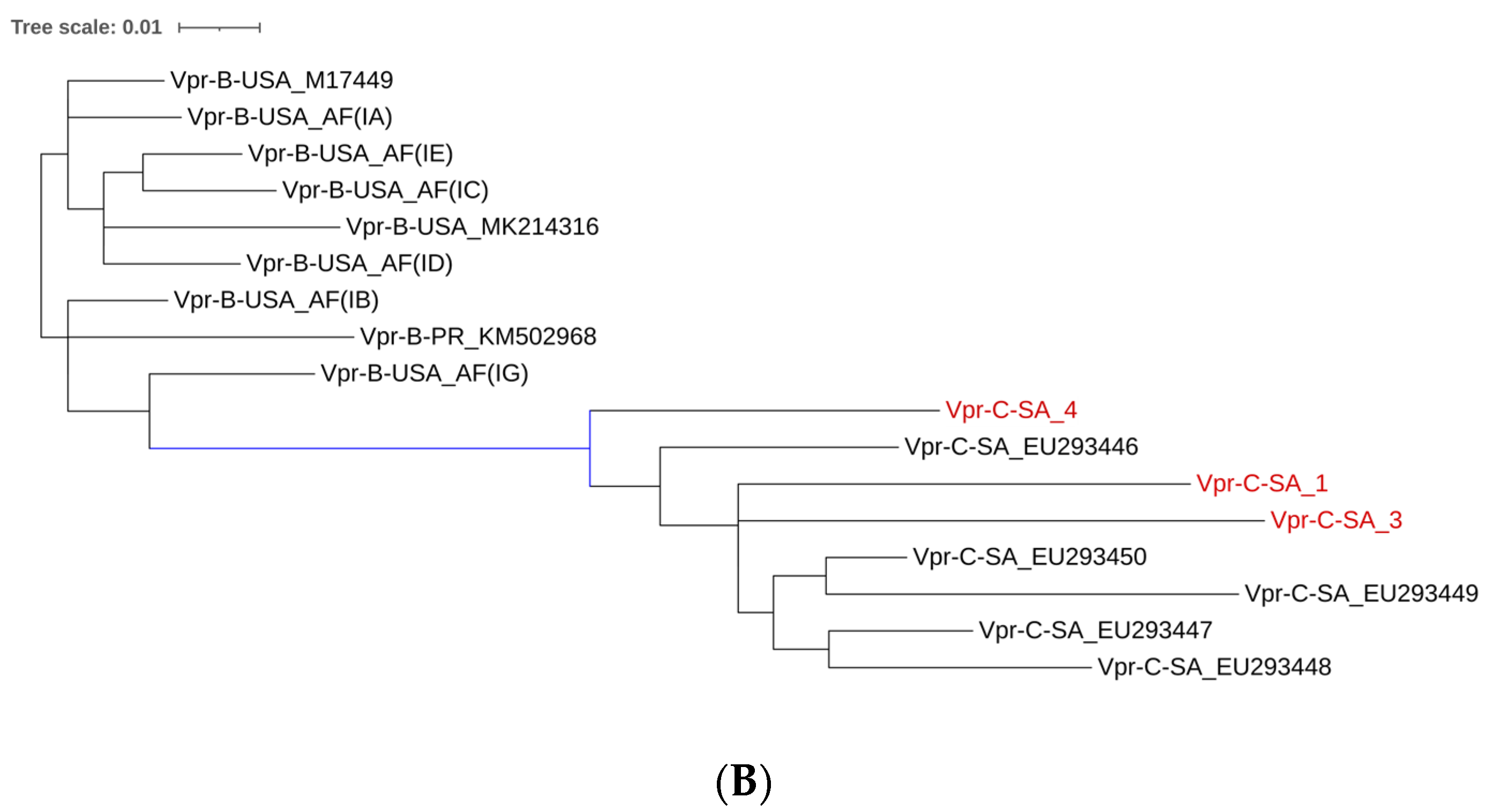
2.4. Comparison Between CSF-Derived Subtype C Sequences and PBMC-Derived Tat and Vpr Sequences Across Various Geographical Regions
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Sample Characteristics
4.2. Laboratory Assessment of CSF
4.3. Bioinformatics Analysis
4.4. Database Sequences
4.5. Phylogenetic Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABI | Applied biosystems |
| BBB | Blood-brain barrier |
| cART | Combination antiretroviral therapy |
| CCL2 | Chemokine ligand 2 |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| HIV | Human immunodeficiency virus |
| MWCO | Molecular weight cut off |
| PBMC | Peripheral blood mononuclear cells |
| Tat | Transactivator of transcription |
| TB | Tuberculosis |
| TYMP | Thymidine phosphorylase |
| Vpr | Viral protein R |
References
- Nyamweya, S.; Hegedus, A.; Jaye, A.; Rowland-Jones, S.; Flanagan, K.L.; Macallan, D.C. Comparing HIV-1 and HIV-2 infection: Lessons for viral immunopathogenesis. Rev. Med. Virol. 2013, 23, 221–240. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. The Path That Ends AIDS: UNAIDS Global AIDS Update 2023; UNAIDS: Geneva, Switzerland, 2023. [Google Scholar]
- Winston, A.; Antinori, A.; Cinque, P.; Fox, H.S.; Gisslen, M.; Henrich, T.J.; Letendre, S.; Persaud, D.; Price, R.W.; Spudich, S. Defining cerebrospinal fluid HIV RNA escape: Editorial review AIDS. Aids 2019, 33, S107–S111. [Google Scholar] [CrossRef] [PubMed]
- Thirion, A.; Loots, D.T.; Williams, M.E.; Solomons, R.; Mason, S. An exploratory investigation of the CSF metabolic profile of HIV in a South African paediatric cohort using GCxGC-TOF/MS. Metabolomics 2024, 20, 33. [Google Scholar] [CrossRef]
- Thirion, A.; Loots, D.T.; Williams, M.E.; Solomons, R.; Mason, S. 1H-NMR metabolomics investigation of CSF from children with HIV reveals altered neuroenergetics due to persistent immune activation. Front. Neurosci. 2024, 18, 1270041. [Google Scholar] [CrossRef]
- González-Scarano, F.; Martín-García, J. The neuropathogenesis of AIDS. Nat. Rev. Immunol. 2005, 5, 69–81. [Google Scholar] [CrossRef]
- Santerre, M.; Wang, Y.; Arjona, S.; Allen, C.; Sawaya, B.E. Differential Contribution of HIV-1 Subtypes B and C to Neurological Disorders: Mechanisms and Possible Treatments. AIDS Rev. 2019, 21, 76–83. [Google Scholar] [CrossRef]
- Sawaya, B.E.; Khalili, K.; Gordon, J.; Taube, R.; Amini, S. Cooperative interaction between HIV-1 regulatory proteins Tat and Vpr modulates transcription of the viral genome. J. Biol. Chem. 2000, 275, 35209–35214. [Google Scholar] [CrossRef]
- Tzitzivacos, D.B.; Tiemessen, C.; Stevens, W.; Papathanasopoulos, M. Viral genetic determinants of nonprogressive HIV type 1 subtype C infection in antiretroviral drug-naive children. AIDS Res. Hum. Retroviruses 2009, 25, 1141–1148. [Google Scholar] [CrossRef]
- Li, W.; Huang, Y.; Reid, R.; Steiner, J.; Malpica-Llanos, T.; Darden, T.A.; Shankar, S.K.; Mahadevan, A.; Satishchandra, P.; Nath, A. NMDA receptor activation by HIV-Tat protein is clade dependent. J. Neurosci. 2008, 28, 12190–12198. [Google Scholar] [CrossRef]
- Spector, C.; Mele, A.R.; Wigdahl, B.; Nonnemacher, M.R. Genetic variation and function of the HIV-1 Tat protein. Med. Microbiol. Immunol. 2019, 208, 131–169. [Google Scholar] [CrossRef]
- Ruiz, A.P.; Ajasin, D.O.; Ramasamy, S.; DesMarais, V.; Eugenin, E.A.; Prasad, V.R. A Naturally Occurring Polymorphism in the HIV-1 Tat Basic Domain Inhibits Uptake by Bystander Cells and Leads to Re16duced Neuroinflammation. Sci. Rep. 2019, 9, 3308. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.E.; Zulu, S.S.; Stein, D.J.; Joska, J.A.; Naudé, P.J.W. Signatures of HIV-1 subtype B and C Tat proteins and their effects in the neuropathogenesis of HIV-associated neurocognitive impairments. Neurobiol. Dis. 2020, 136, 104701. [Google Scholar] [CrossRef]
- Williams, M.E. 1334. Amino Acid Sequence Diversity of HIV-1 Viral Proteins Tat and Vpr in South African People Living with HIV: Implications for Neurocognitive Function. Open Forum Infect. Dis. 2023, 10 (Suppl. S2), ofad500-1171. [Google Scholar] [CrossRef]
- Ruhanya, V.; Jacobs, G.B.; Paul, R.H.; Joska, J.A.; Seedat, S.; Nyandoro, G.; Glashoff, R.H.; Engelbrecht, S. HIV-1 Subtype C Vpr Amino Acid Residue 45Y and Specific Conserved Fragments Are Associated with Neurocognitive Impairment and Markers of Viral Load. AIDS Res. Hum. Retroviruses 2023, 39, 166–175. [Google Scholar] [CrossRef]
- Asia, L.K.; Van Vuren, E.J.; Lindeque, Z.; Williams, M.E. A pilot investigation of the association between HIV-1 Vpr amino acid sequence diversity and the tryptophan-kynurenine pathway as a potential mechanism for neurocognitive impairment. Virol. J. 2024, 21, 47. [Google Scholar] [CrossRef]
- Asia, L.K.; Van Vuren, E.J.; Kruger, I.M.; Williams, M.E. A Pilot Investigation of the Association Between Vpr Amino Acid Substitutions and Peripheral Immune Marker Levels in People with Human Immunodeficiency Virus: Implications for Neurocognitive Impairment. Open Forum Infect. Dis. 2024, 11, ofae111. [Google Scholar] [CrossRef]
- Williams, M.E.; Asia, L.K.; Lindeque, Z.; Jansen van Vuren, E. The association between HIV-1 Tat and Vif amino acid sequence variation, inflammation and Trp-Kyn metabolism: An exploratory investigation. BMC Infect. Dis. 2024, 24, 943. [Google Scholar] [CrossRef]
- James, T.; Nonnemacher, M.R.; Wigdahl, B.; Krebs, F.C. Defining the roles for Vpr in HIV-1-associated neuropathogenesis. J. Neurovirol. 2016, 22, 403–415. [Google Scholar] [CrossRef]
- Jacquot, G.; Le Rouzic, E.; David, A.; Mazzolini, J.; Bouchet, J.; Bouaziz, S.; Niedergang, F.; Pancino, G.; Benichou, S. Localization of HIV-1 Vpr to the nuclear envelope: Impact on Vpr functions and virus replication in macrophages. Retrovirology 2007, 4, 84. [Google Scholar] [CrossRef]
- McMullen, K.; Bateman, K.; Stanley, A.; Combrinck, M.; Engelbrecht, S.; Bryer, A. Viral protein R polymorphisms in the pathogenesis of HIV-associated acute ischaemic stroke: A case-control study. J. Neurovirol. 2021, 27, 137–144. [Google Scholar] [CrossRef]
- Lum, J.J.; Cohen, O.J.; Nie, Z.; Weaver, J.G.; Gomez, T.S.; Yao, X.J.; Lynch, D.; Pilon, A.A.; Hawley, N.; Kim, J.E.; et al. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J. Clin. Investig. 2003, 111, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Hightower, G.K.; Wong, J.K.; Heaton, R.; Woods, S.; Grant, I.; Marcotte, T.D.; Ellis, R.J.; Letendre, S.L.; Collier, A.C.; et al. Genetic features of cerebrospinal fluid-derived subtype B HIV-1 tat. J. Neurovirol. 2012, 18, 81–90. [Google Scholar] [CrossRef]
- Na, H.; Acharjee, S.; Jones, G.; Vivithanaporn, P.; Noorbakhsh, F.; McFarlane, N.; Maingat, F.; Ballanyi, K.; Pardo, C.A.; Cohen, E.A.; et al. Interactions between human immunodeficiency virus (HIV)-1 Vpr expression and innate immunity influence neurovirulence. Retrovirology 2011, 8, 44. [Google Scholar] [CrossRef]
- Tong, C.Y.W.; Costelloe, S.; Hubb, J.; Mullen, J.; O’Shea, S.; Marta, M.; Kulasegaram, R.; Rackstraw, S. Deep Sequencing of HIV-1 in Cerebrospinal Fluid. Clin. Infect. Dis. 2015, 61, 1022–1025. [Google Scholar] [CrossRef]
- Pillai, S.K.; Pond, S.L.K.; Liu, Y.; Good, B.M.; Strain, M.C.; Ellis, R.J.; Letendre, S.; Smith, D.M.; Günthard, H.F.; Grant, I.; et al. Genetic attributes of cerebrospinal fluid-derived HIV-1 env. Brain 2006, 129, 1872–1883. [Google Scholar] [CrossRef]
- Vazquez-Guillen, J.M.; Palacios-Saucedo, G.C.; Rivera-Morales, L.G.; Garcia-Campos, J.; Ortiz-Lopez, R.; Noguera-Julian, M.; Paredes, R.; Vielma-Ramirez, H.J.; Ramirez, T.J.; Chavez-Garcia, M.; et al. Mutations Related to Antiretroviral Resistance Identified by Ultra-Deep Sequencing in HIV-1 Infected Children Under Structured Interruptions of HAART. PLoS ONE 2016, 11, e0147591. [Google Scholar] [CrossRef]
- Williams, M.E. HIV-1 Vif protein sequence variations in South African people living with HIV and their influence on Vif-APOBEC3G interaction. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 325–338. [Google Scholar] [CrossRef]
- Ronsard, L.; Raja, R.; Panwar, V.; Saini, S.; Mohankumar, K.; Sridharan, S.; Padmapriya, R.; Chaudhuri, S.; Ramachandran, V.G.; Banerjea, A.C. Genetic and functional characterization of HIV-1 Vif on APOBEC3G degradation: First report of emergence of B/C recombinants from North India. Sci. Rep. 2015, 5, 15438. [Google Scholar] [CrossRef]
- Sturdevant, C.B.; Dow, A.; Jabara, C.B.; Joseph, S.B.; Schnell, G.; Takamune, N.; Mallewa, M.; Heyderman, R.S.; Van Rie, A.; Swanstrom, R. Central nervous system compartmentalization of HIV-1 subtype C variants early and late in infection in young children. PLoS Pathog. 2012, 8, e1003094. [Google Scholar] [CrossRef]
- Dara, J.; Dow, A.; Cromwell, E.; Sturdevant, C.B.; Mallewa, M.; Swanstrom, R.; Van Rie, A.; Prasad, V.R. Multivariable analysis to determine if HIV-1 Tat dicysteine motif is associated with neurodevelopmental delay in HIV-infected children in Malawi. Behav. Brain Funct. 2015, 11, 38. [Google Scholar] [CrossRef]
- Wei, F.; Wang, X.; Liu, L.; Gao, R.; Shi, Y.; Zhang, Y.; Qiao, L.; Chen, D. Characterization of HIV type 1 env gene in cerebrospinal fluid and blood of infected Chinese patients. AIDS Res. Hum. Retroviruses 2011, 27, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.V.P.; Pham, H.V.; Dinh, T.T.; Nguyen, T.H.; Vu, Q.T.H.; Vu, N.T.P.; Le, P.T.B.; Nguyen, L.V.; Le, H.T.; Vu, P.T.; et al. Characterization of envelope sequence of HIV virus in children infected with HIV in Vietnam. SAGE Open Med. 2020, 8, 2050312120937198. [Google Scholar] [CrossRef]
- Mishra, M.; Vetrivel, S.; Siddappa, N.B.; Ranga, U.; Seth, P. Clade-specific differences in neurotoxicity of human immunodeficiency virus-1 B and C Tat of human neurons: Significance of dicysteine C30C31 motif. Ann. Neurol. 2008, 63, 366–376. [Google Scholar] [CrossRef]
- Paul, R.H.; Joska, J.A.; Woods, C.; Seedat, S.; Engelbrecht, S.; Hoare, J.; Heaps, J.; Valcour, V.; Ances, B.; Baker, L.M.; et al. Impact of the HIV Tat C30C31S dicysteine substitution on neuropsychological function in patients with clade C disease. J. Neurovirol. 2014, 20, 627–635. [Google Scholar] [CrossRef]
- Williams, M.E.; Ruhanya, V.; Paul, R.H.; Ipser, J.C.; Stein, D.J.; Joska, J.A.; Naudé, P.J.W. An investigation of the HIV Tat C31S and R57S mutation on peripheral immune marker levels in South African participants: A pilot study. J. Med. Virol. 2022, 94, 2936–2938. [Google Scholar] [CrossRef]
- Cowley, D.; Gray, L.R.; Wesselingh, S.L.; Gorry, P.R.; Churchill, M.J. Genetic and functional heterogeneity of CNS-derived tat alleles from patients with HIV-associated dementia. J. Neurovirol. 2011, 17, 70–81. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Li, J.; Deng, R. Differences in drug resistance of HIV-1 genotypes in CSF and plasma and analysis of related factors. Virulence 2023, 14, 2171632. [Google Scholar] [CrossRef]
- Yezid, H.; Konate, K.; Debaisieux, S.; Bonhoure, A.; Beaumelle, B. Mechanism for HIV-1 Tat insertion into the endosome membrane. J. Biol. Chem. 2009, 284, 22736–22746. [Google Scholar] [CrossRef]
- Campbell, G.R.; Watkins, J.D.; Singh, K.K.; Loret, E.P.; Spector, S.A. Human immunodeficiency virus type 1 subtype C Tat fails to induce intracellular calcium flux and induces reduced tumor necrosis factor production from monocytes. J. Virol. 2007, 81, 5919–5928. [Google Scholar] [CrossRef]
- Struck, D.; Lawyer, G.; Ternes, A.M.; Schmit, J.C.; Bercoff, D.P. COMET: Adaptive context-based modeling for ultrafast HIV-1 subtype identification. Nucleic Acids Res. 2014, 42, e144. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thirion, A.; Mason, S.; Loots, D.T.; Solomons, R.; Williams, M.E. Retrospective Observational Study of CSF-Derived HIV-1 Tat and Vpr Amino Acid Sequences in a South African Pediatric Cohort with HIV Subtype C. Int. J. Mol. Sci. 2025, 26, 5008. https://doi.org/10.3390/ijms26115008
Thirion A, Mason S, Loots DT, Solomons R, Williams ME. Retrospective Observational Study of CSF-Derived HIV-1 Tat and Vpr Amino Acid Sequences in a South African Pediatric Cohort with HIV Subtype C. International Journal of Molecular Sciences. 2025; 26(11):5008. https://doi.org/10.3390/ijms26115008
Chicago/Turabian StyleThirion, Anicia, Shayne Mason, Du Toit Loots, Regan Solomons, and Monray Edward Williams. 2025. "Retrospective Observational Study of CSF-Derived HIV-1 Tat and Vpr Amino Acid Sequences in a South African Pediatric Cohort with HIV Subtype C" International Journal of Molecular Sciences 26, no. 11: 5008. https://doi.org/10.3390/ijms26115008
APA StyleThirion, A., Mason, S., Loots, D. T., Solomons, R., & Williams, M. E. (2025). Retrospective Observational Study of CSF-Derived HIV-1 Tat and Vpr Amino Acid Sequences in a South African Pediatric Cohort with HIV Subtype C. International Journal of Molecular Sciences, 26(11), 5008. https://doi.org/10.3390/ijms26115008






