Immune Responses of Mango Callus Infected by Agrobacterium tumefaciens Inhibited Transformation
Abstract
1. Introduction
2. Results
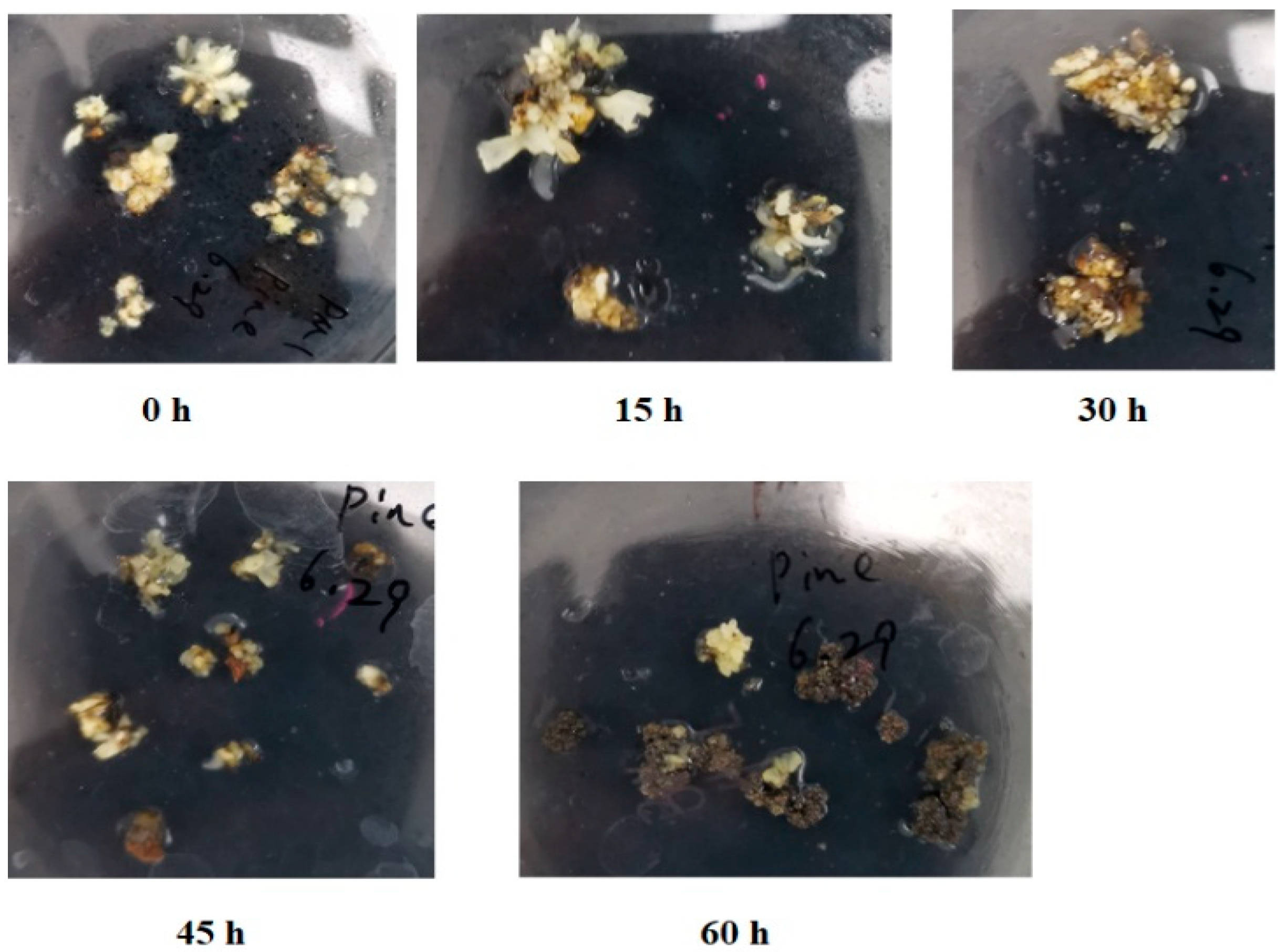
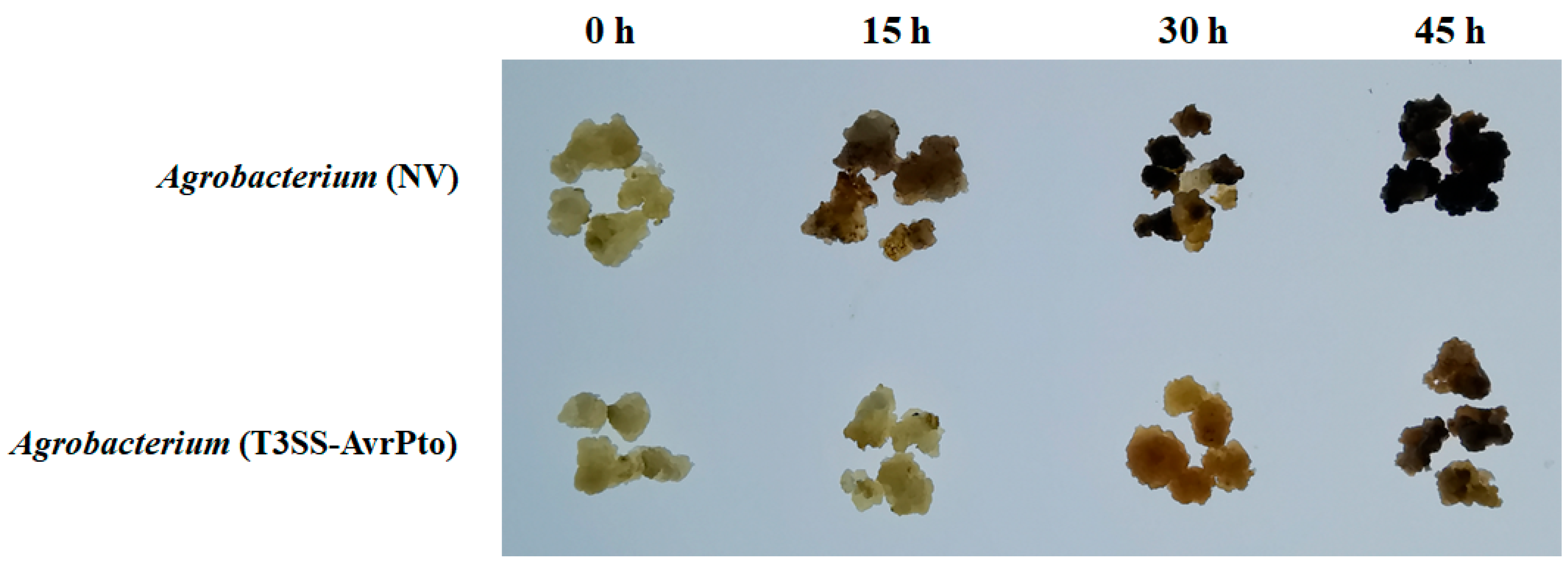
2.1. Browning and Necrosis of Mango Callus After Agrobacterium Infection
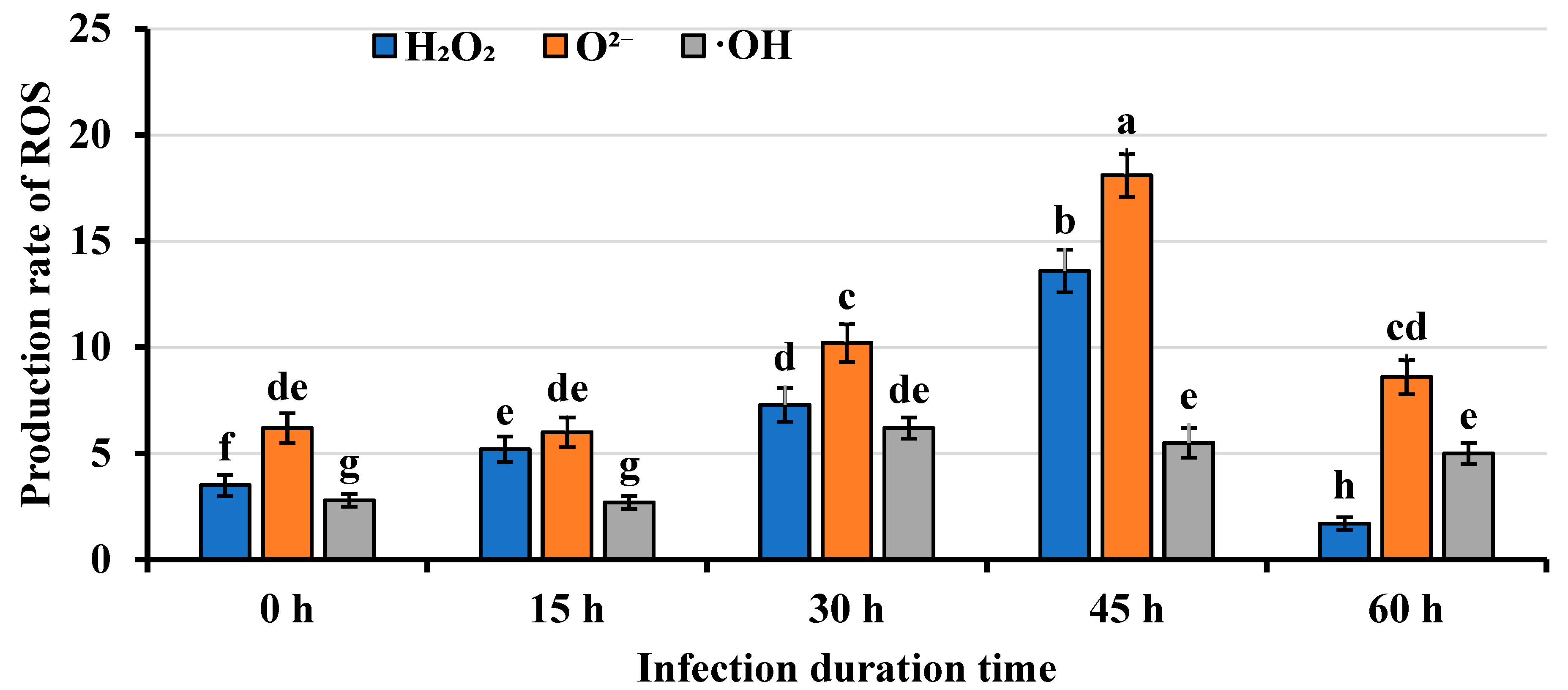
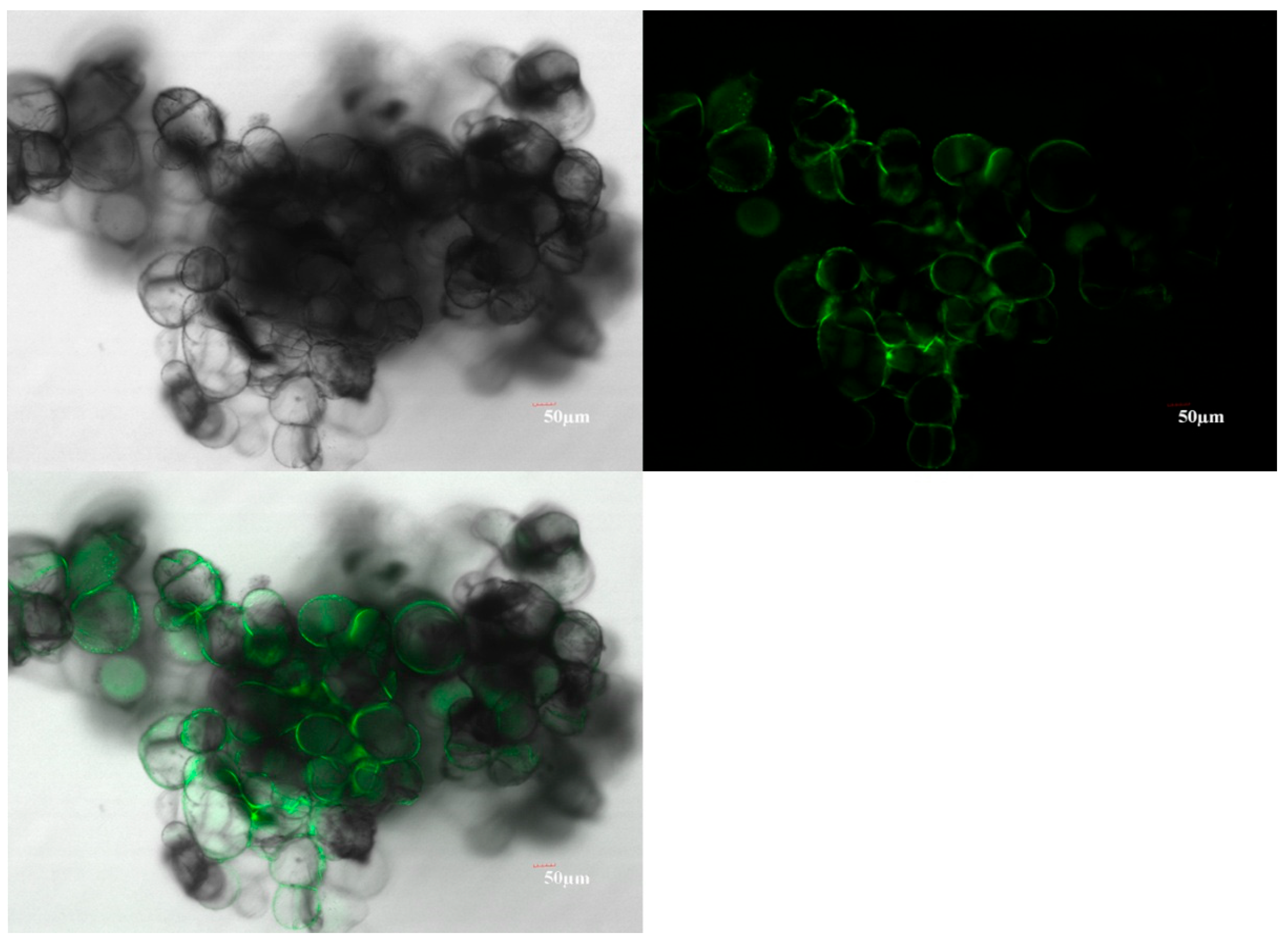
2.2. Mango Callus Infected by Agrobacterium Contained More Reactive Oxygen Species (ROS) than Control
2.3. Identification of Key Metabolites Associated with Browning in Mango Calluses Infected by Agrobacterium
2.4. The Expression Levels of ROS-Related Genes and Gallic Acid Synthesis Genes Were Up-Regulated in Callus Infected by Agrobacterium
2.5. Validation of Immune-Related Gene Expression Patterns in Agrobacterium-Infected Mango Callus
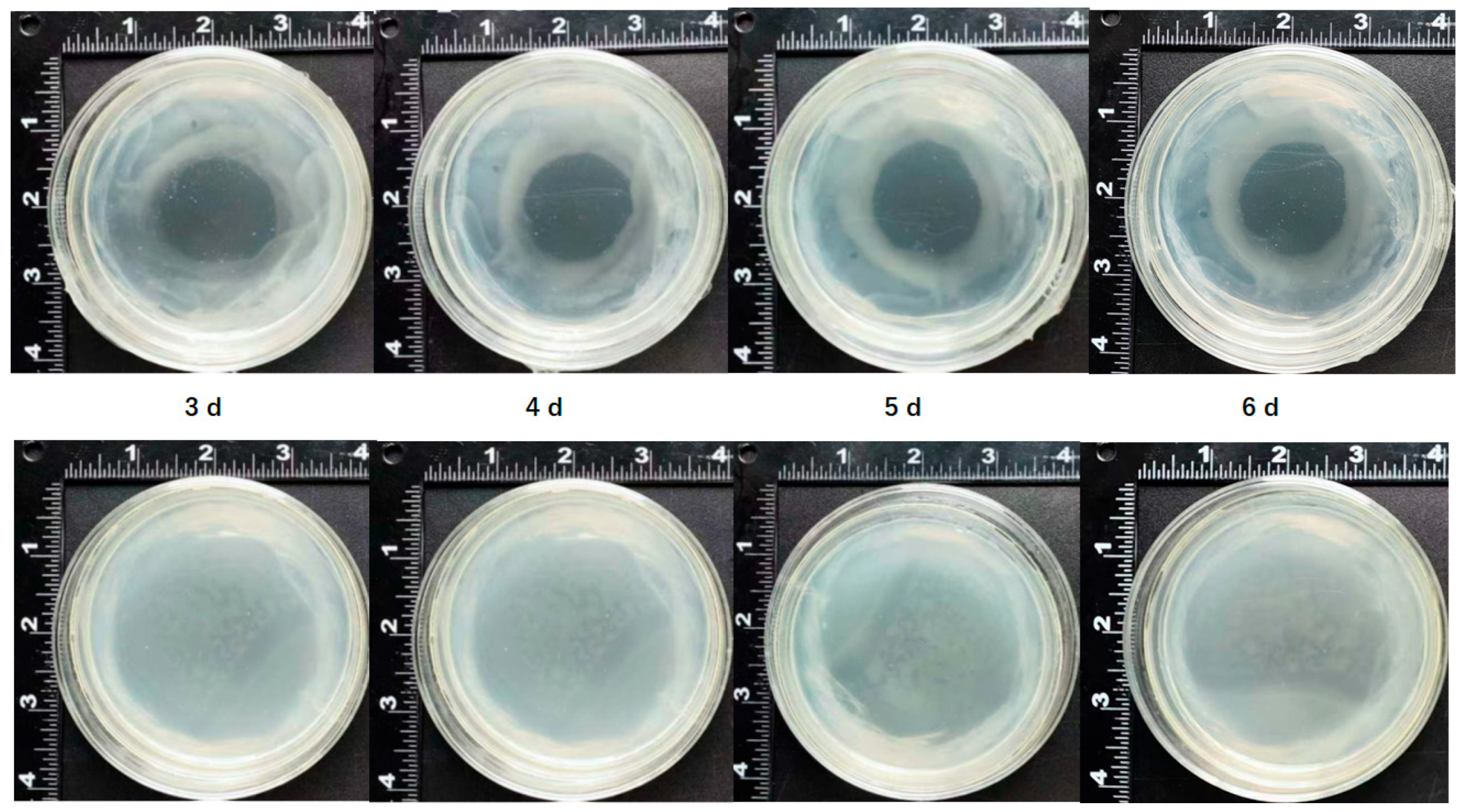
2.6. The Inhibitory Effect of Gallic Acid on Agrobacterium Growth
2.7. Suppression of Immune Responses in Mango Callus by Agrobacterium (T3SS-AvrPto)
2.8. Mango Callus Infected by Agrobacterium (T3SS-AvrPto) Produced Less ROS than Those Infected by Agrobacterium (NV)
2.9. Suppression of Immune Responses in Mango Callus Infected by Agrobacterium (T3SS-AvrPto) Compared to Agrobacterium (NV)
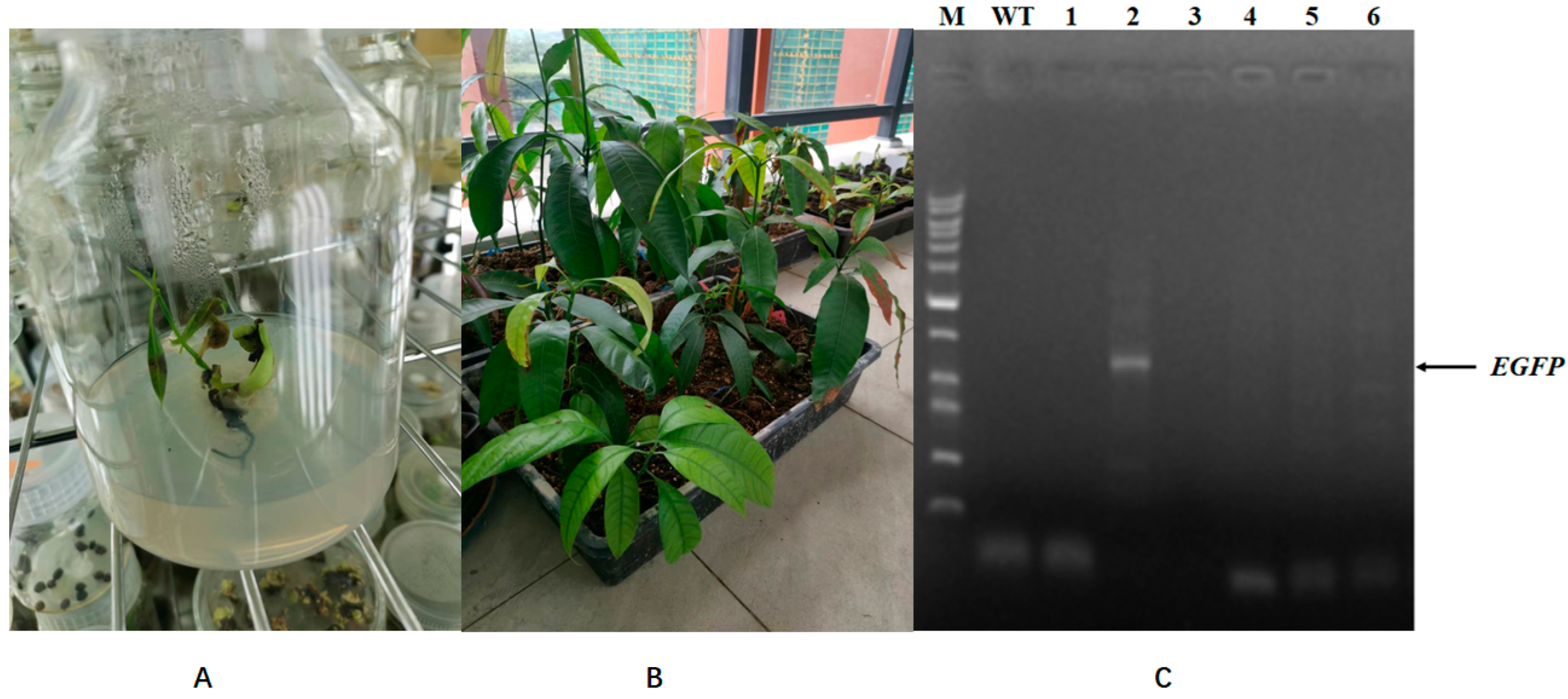
2.10. Agrobacterium Harboring Type III Secretion Gene Cluster (T3SS) and Effector Gene AvrPto Had Higher Infection Efficiency than Control Agrobacterium
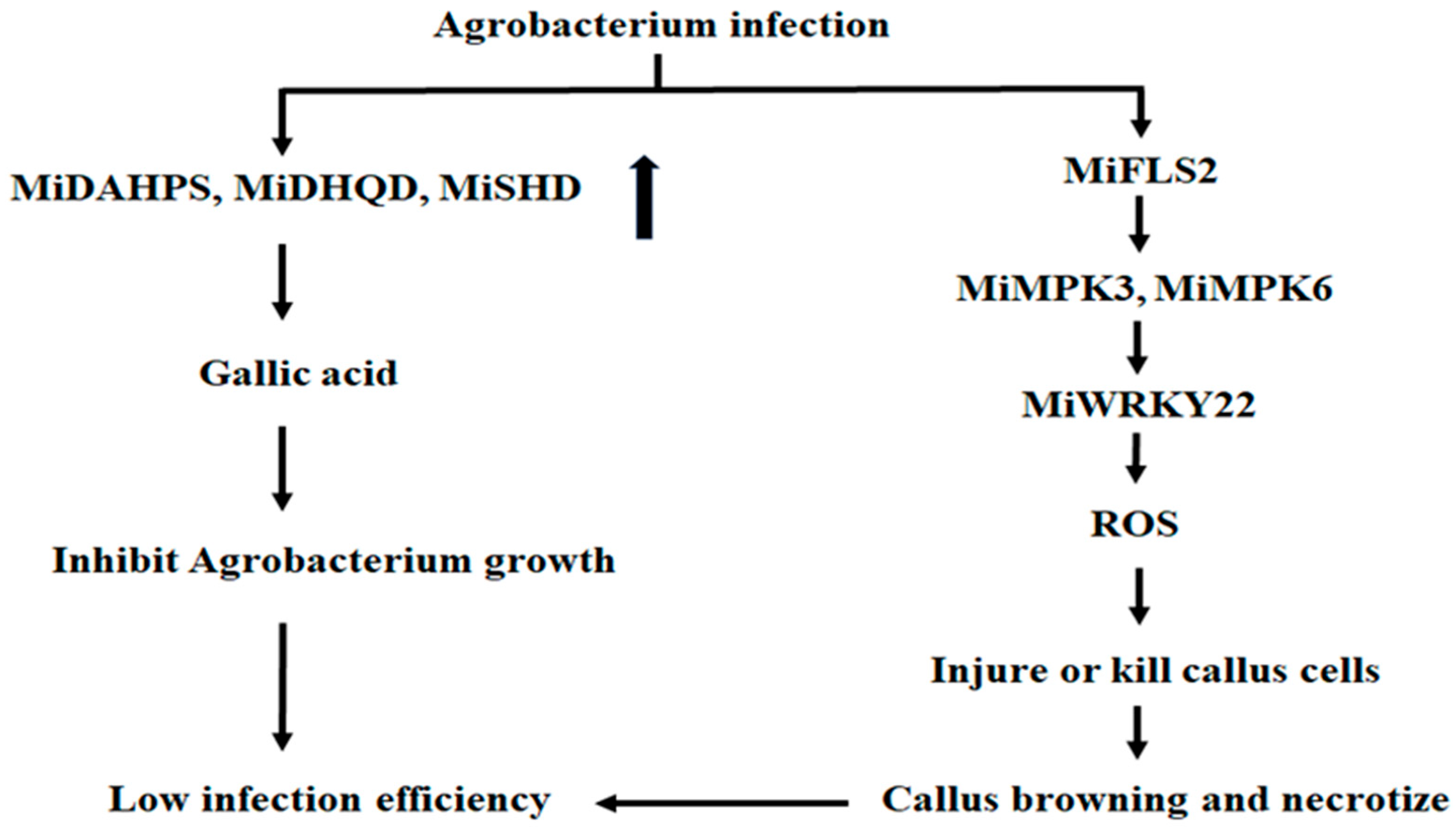
3. Discussion
4. Materials and Methods
4.1. Agrobacterium Strain
4.2. Construction of Agrobacterium Strains
4.3. Mango Embryogenic Callus Induction
4.4. Mango Callus Transformation
4.5. Metabolome and Transcriptome Experiments
4.6. QRT-PCR
4.7. Confocal Observation
4.8. Determination of ROS Contents in Mitochondria Isolated from Mango Callus
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dofuor, A.K.; Quartey, N.K.; Osabutey, A.F.; Antwi-Agyakwa, A.K.; Asante, K.; Boateng, B.O.; Ablormeti, F.K.; Lutuf, H.; Osei-Owusu, J.; Osei, J.H.N.; et al. Mango anthracnose disease: The current situation and direction for future research. Front. Microbiol. 2023, 14, 1168203. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.B.; Saxena, S.P.; Patel, K.M.; Patel, A.T. Estimation of crop loss caused by important insect-pests of mango. Int. J. Biol. Sci. 2022, 4, 194–197. [Google Scholar] [CrossRef]
- Bally, I.S.E.; Dillon, N.L. Mango (Mangifera indica L.) Breeding. In Advances in Plant Breeding Strategies: Fruits; Springer Nature: Berlin, Germany, 2018; pp. 811–896. ISBN 303023415X. [Google Scholar]
- Litz, R.E.; Hormaza, J.I. Mangifera indica mango. In Biotechnology of Fruit and Nut Crops, 2nd ed.; Litz, R.E., Pliego-Alfaro, F., Hormaza, J.I., Eds.; CAB International: Wallingford, UK, 2020; pp. 27–43. ISBN 9781780648279. [Google Scholar]
- Petri, C.; Litz, R.E.; Singh, S.K.; Hormaza, J.I. In vitro culture and genetic transformation in mango. In The Mango Genome, a Compendium of Plant Genomes; Kole, C., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; pp. 131–151. ISBN 2199-4781. [Google Scholar]
- Pandey, K.; Karthik, K.; Singh, S.K.; Vinod; Sreevathsa, R.; Srivastav, M. Amenability of an Agrobacterium tumefaciens-mediated shoot apical meristem-targeted in planta transformation strategy in Mango (Mangifera indica L.). GM Crops Food 2022, 13, 342–354. [Google Scholar] [CrossRef]
- Adjei, M.O.; Zhao, H.; Tao, X.; Yang, L.; Deng, S.; Li, X.; Mao, X.; Li, S.; Huang, J.; Luo, R.; et al. Using a protoplast transformation system to enable functional studies in Mangifera indica L. Int. J. Mol. Sci. 2023, 24, 11984. [Google Scholar] [CrossRef]
- Chen, X.; He, S.; Wang, Z.; Zhai, Y.; Guo, W.; Li, X. Production of transgenic periclinal chimeras in pumpkin—A tool for revealing cell fates of L1 meristem. Plant Biol. 2023, 26, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Aida, R.; Sasaki, K.; Yoshioka, S.; Noda, N. Distribution of cell layers in floral organs of chrysanthemum analyzed with periclinal chimeras carrying a transgene encoding fluorescent protein. Plant Cell Rep. 2020, 39, 609–619. [Google Scholar] [CrossRef]
- Li, M.; Wang, D.; Long, X.; Hao, Z.; Lu, Y.; Zhou, Y.; Peng, Y.; Cheng, T.; Shi, J.; Chen, J. Agrobacterium-Mediated genetic transformation of embryogenic callus in a Liriodendron hybrid (L. Chinense × L. Tulipifera). Front. Plant Sci. 2022, 13, 802128. [Google Scholar] [CrossRef]
- Nakajima, I.; Endo, M.; Haji, T.; Moriguchi, T.; Yamamoto, T. Embryogenic callus induction and Agrobacterium-mediated genetic transformation of ‘Shine Muscat’ grape. Plant Biotechnol. 2020, 37, 185–194. [Google Scholar] [CrossRef]
- Chen, Z.; Debernardi, J.M.; Dubcovsky, J.; Gallavotti, A. Recent advances in crop transformation technologies. Nat. Plants 2022, 8, 1343–1351. [Google Scholar] [CrossRef]
- Zhang, D.; Ren, L.; Chen, G.; Zhang, J.; Reed, B.M.; Shen, X. ROS-induced oxidative stress and apoptosis-like event directly affect the cryopreserved embryogenic callus cell viability in Agapanthus praecox. Plant Cell Rep. 2015, 34, 1499–1513. [Google Scholar] [CrossRef]
- Christie, P.J. Type IV secretion: The Agrobacterium VirB/D4 and related conjugation systems. Biochim. Biophys. Acta 2004, 1694, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Raman, V.; Rojas, C.M.; Vasudevan, B.; Dunning, K.; Kolape, J.; Oh, S.; Yun, J.; Yang, L.; Li, G.; Pant, B.D.; et al. Agrobacterium expressing a type III secretion system delivers Pseudomonas effectors into plant cells to enhance transformation. Nat. Commun. 2022, 13, 2581. [Google Scholar] [CrossRef] [PubMed]
- Zavaliev, R.; Mohan, R.; Chen, T.; Dong, X. Formation of NPR1 condensates promotes cell survival during plant immune response. Cell 2020, 182, 1093–1108. [Google Scholar] [CrossRef]
- Peng, T.; Tao, X.; Xia, Z.; Hu, S.; Xue, J.; Zhu, Q.; Pan, X.; Zhang, Q.; Li, S. Pathogen hijacks programmed cell death signaling by arginine ADPR-deacylization of caspases. Mol. Cell 2022, 82, 1806–1820. [Google Scholar] [CrossRef]
- Cornelis, G.R. The type III secretion injectisome. Nat. Rev. Microbiol. 2006, 4, 811–825. [Google Scholar] [CrossRef]
- Yang, F.; Li, G.; Felix, G.; Albert, M.; Guo, M. Engineered Agrobacterium improves transformation by mitigating plant immunity detection. New Phytol. 2022, 237, 2493–2504. [Google Scholar] [CrossRef]
- Büttner, D. Behind the lines–actions of bacterial type III effector proteins in plant cells. FEMS Microbiol. Rev. 2016, 40, 894–937. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, G.; Zhou, J. Receptor kinases in plant-pathogen interactions: More than pattern recognition. Plant Cell 2017, 29, 618–637. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, L.; Perl, A.; Chen, S.; Ma, H. Proteomic changes in grape embryogenic callus in response to Agrobacterium tumefaciens-mediated transformation. Plant Sci. 2011, 181, 485–495. [Google Scholar] [CrossRef]
- Perl, A.; Lotan, O.; Abu-Abied, M.; Holland, D. Establishment of an Agrobacterium-mediated transformation system for grape (Vitis vinifera L.): The role of antioxidants during grape-Agrobacterium interactions. Nat. Biotechnol. 1996, 14, 624–628. [Google Scholar] [CrossRef]
- Hansen, G. Evidence of Agrobacterium-induced apoptosis in maize cells. Mol. Plant Microbe Interact. 2000, 13, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Parrot, D.L.; Anderson, A.J.; Carman, J.G. Agrobacterium induces plant cell death in wheat (Triticum aestivum L.). Physiol. Mol. Plant Pathol. 2002, 60, 59–69. [Google Scholar] [CrossRef]
- Cai, J.; Chen, T.; Wang, Y.; Qin, G.; Tian, S. SlREM1 triggers cell death by activating an oxidative burst and other regulators. Plant Physiol. 2020, 183, 717–732. [Google Scholar] [CrossRef]
- Sun, L.L.; Shao, Y.N.; You, M.X.; Li, C.H. ROS-mediated BNIP3-dependent mitophagy promotes coelomocyte survival in Apostichopus japonicus in response to Vibrio splendidus infection. Zool. Res. 2022, 43, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Sun, J.; Zhang, T.; Tang, Y.; Liu, J.; Gao, C.; Zhai, Y.; Guo, Y.; Feng, L.; Zhang, X.; et al. Comparative transcriptome analyses of different Rheum officinale tissues reveal differentially expressed genes associated with anthraquinone, catechin, and gallic acid biosynthesis. Genes 2022, 13, 1592. [Google Scholar] [CrossRef]
- Zhu, Y.; Kun, K.; Zhang, X.; Ma, H.; Yang, L.; Wei, L.; Yang, L.; Jiang, M. Antibacterial effect of gallic acid in UV C light treatment against Escherichia coli O157:H7 and the underlying mechanism. Food Bioprocess Technol. 2024, 17, 2216–2231. [Google Scholar] [CrossRef]
- Delplace, F.; Huard-Chauveau, C.; Berthome, R.; Roby, D. Network organization of the plant immune system: From pathogen perception to robust defense induction. Plant J. 2022, 109, 447–470. [Google Scholar] [CrossRef] [PubMed]
- Ngou, B.P.M.; Ding, P.; Jones, J.D.G. Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell 2022, 34, 1447–1478. [Google Scholar] [CrossRef]
- Liu, T.; Cao, L.; Cheng, Y.; Ji, J.; Wei, Y.; Wang, C.; Duan, K. MKK4/5-MPK3/6 cascade regulates Agrobacterium-mediated transformation by modulating plant immunity in Arabidopsis. Front. Plant Sci. 2021, 12, 731690. [Google Scholar] [CrossRef]
- Ding, L.; Li, Y.; Wu, Y.; Li, T.; Geng, R.; Cao, J.; Zhang, W.; Tan, X. Plant disease resistance-related signaling pathways: Recent progress and future prospects. Int. J. Mol. Sci. 2022, 23, 16200. [Google Scholar] [CrossRef]
- Zhou, J.M.; Zhang, Y. Plant immunity: Danger perception and signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Mishra, A.K.; Chakrabarty, D. Agrobacterium-mediated gene transfer: Recent advancements and layered immunity in plants. Planta 2022, 256, 37. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.A.; Liu, Z.; Binns, A.N. Agrobacterium Protocols; Wang, K., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 43–54. ISBN 9781588298430. [Google Scholar]
- Hall, A.; Donohue, T.; Peters, J. Complete sequences of conjugal helper plasmids pRK2013 and pEVS104. MicroPubl. Biol. 2023, 10, 17912. [Google Scholar]
- Figurski, D.H.; Helinski, D.R. Replication of an origin-containing derivative of plasmid RK2. dependent on a plasmid function provided in trans. Proc. Nati. Acad. Sci. USA 1979, 76, 1648–1652. [Google Scholar] [CrossRef]
- Xu, W.; Wu, H.; Luo, C.; Li, L.; Liang, Q.; Ma, X.; Yao, Q.; Gao, Y.; Wang, S. Callus proliferation, early somatic embryogenesis and cytological observation of mango ‘Jinhuang’. Mol. Plant Breed. 2019, 17, 3001–3008. (In Chinese) [Google Scholar]
- Iqbal, A.; Qiang, D.; Xiangru, W.; Huiping, G.; Hengheng, Z.; Xiling, Z.; Meizhen, S. Integrative physiological, transcriptome and metabolome analysis reveals the involvement of carbon and flavonoid biosynthesis in low phosphorus tolerance in cotton. Plant Physiol. Biochem. 2023, 196, 302–317. [Google Scholar] [CrossRef]
- Lee, M.Y.; Song, Y.S.; Ryu, K.H. Development of infectious transcripts from full-length and GFP-tagged cDNA clones of Pepper mottle virus and stable systemic expression of GFP in tobacco and pepper. Virus Res. 2011, 155, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Vanlerberghe, G.C.; McIntosh, L. Lower growth temperature increases alternative pathway capacity and alternative oxidase protein in tobacco. Plant Physiol. 1992, 100, 115–119. [Google Scholar] [CrossRef]
- Wang, A.G.; Luo, G.H. Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol. Commun. 1990, 6, 55–57. [Google Scholar]
- Simontacchi, M.; Puntarulo, S. Oxygen radical generation by isolated microsomes from soybean seedling. Plant Physiol. 1992, 100, 1263–1268. [Google Scholar] [CrossRef]
- Nash, T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem. J. 1953, 55, 416–421. [Google Scholar] [CrossRef] [PubMed]
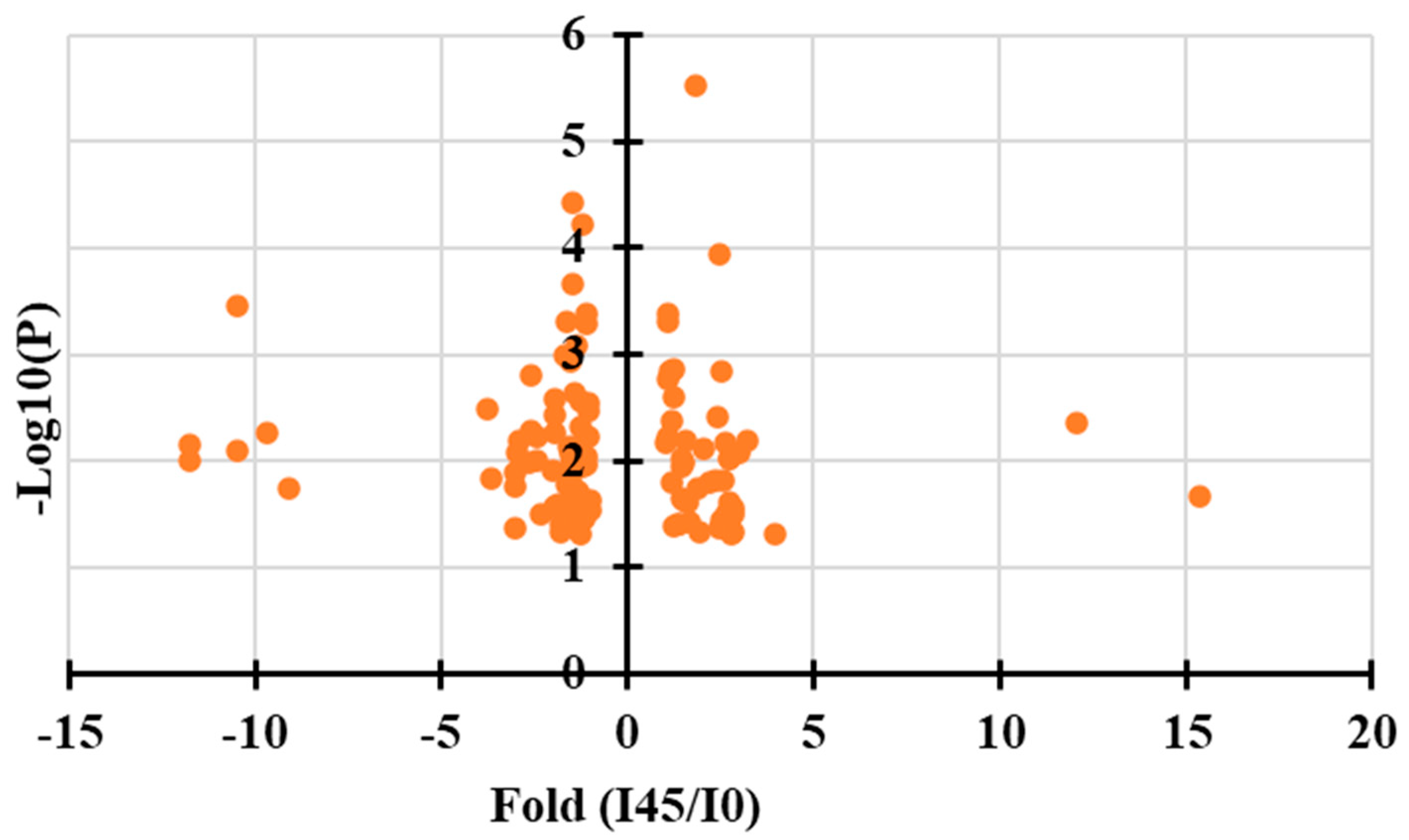
| Index | Compounds | Fold (I45/I0) | VIP Value |
|---|---|---|---|
| Gallic acid | Organic acid | 15.2 | 1.96 |
| Phosphoenolpyruvic acid | Organic acid | 3.9 | 1.22 |
| D-erythrose-4-phosphate | Phosphorylated derivatives of erythritol | 4.1 | 1.36 |
| Ubiquinone | Liposoluble quinone compound | 11.5 | 1.25 |
| Dihydrotricetin | Flavonoids | 3.5 | 1.26 |
| Dihydrokaempferol | Flavonoid | 3.8 | 1.19 |
| Cyanidin | Antioxidant | 3.7 | 1.28 |
| Query | Symbol | Fold (I45/I0) | Gene Annotation | Subject |
|---|---|---|---|---|
| LOC123211694 | MiDAHPS1 | 5.2 | Mangifera indica 3-oxoacyl-[acyl-carrier-protein] synthase | XM_044646917.1 |
| LOC123210632 | MiDHQD1 | 3 | Mangifera indica bifunctional 3-dehydrogenate dehydratase/shikimate dehydrogenase | XM_044644036.1 |
| LOC123236405 | MiDHQD2 | 3.7 | Mangifera indica bifunctional 3-dehydrogenate dehydratase/shikimate dehydrogenase | XM_044644035.1 |
| LOC123207068 | MiSHD1 | 2.4 | Mangifera indica bifunctional 3-dehydrogenate dehydratase/shikimate dehydrogenase | XM_044644037.1 |
| LOC123215035 | MiSHD2 | 2.2 | Mangifera indica bifunctional 3-dehydrogenate dehydratase/shikimate dehydrogenase | XM_044644039.1 |
| LOC123225839 | MiCAT1 | 2.9 | Mangifera indica catalase isozyme 1 | XM_044655447.1 |
| LOC123228490 | MiCAT2 | 2.6 | Mangifera indica catalase isozyme 1 | XM_044655447.1 |
| LOC123217301 | MiGOX1 | 1.7 | Mangifera indica peroxisomal (S)-2-hydroxy-acid oxidase-like | XM_044639087.1 |
| LOC123227499 | MiGOX2 | 1.9 | Mangifera indica peroxisomal (S)-2-hydroxy-acid oxidase-like | XM_044618393.1 |
| LOC123204838 | MiFOX1 | 3.4 | Mangifera indica FAD-linked sulfhydryl oxidase ERV1-like | XM_044652170.1 |
| LOC123227490 | MiFOX2 | 3.8 | Mangifera indica FAD-linked sulfhydryl oxidase ERV1-like | XM_044625447.1 |
| LOC123206396 | MiPOD1 | 2.8 | Mangifera indica peroxidase A2-like | XM_044652117.1 |
| LOC123208403 | MiPOD2 | 2.5 | Mangifera indica lignin-forming anionic peroxidase-like | XM_044627935.1 |
| LOC123217403 | MiNADO1 | 3.2 | Mangifera indica respiratory burst oxidase homolog protein D-like | XM_044606776.1 |
| LOC123228596 | MiNADO2 | 3.6 | Mangifera indica respiratory burst oxidase homolog protein A-like | XM_044630849.1 |
| LOC123217494 | MiFLS2-1 | 2.1 | Mangifera indica LRR receptor-like serine/threonine-protein kinase FLS2 | XM_044604788.1 |
| LOC123227485 | MiMPK3-1 | 1.7 | Mangifera indica mitogen-activated protein kinase kinase 3-like | XM_044610886.1 |
| LOC123210475 | MiMPK6-1 | 2.6 | Mangifera indica mitogen-activated protein kinase kinase 6 | XM_044607731.1 |
| LOC123210574 | MiWRKY22-1 | 1.5 | Mangifera indica WRKY transcription factor 22-like | XM_044646893.1 |
| LOC123226579 | MiNHL10-1 | 3.8 | Mangifera indica NDR1-like protein | XM_044626069.1 |
| Gene | 0 h | 15 h | 30 h | 45 h | 60 h |
|---|---|---|---|---|---|
| MiDAHPS1 | 1.0 ± 0.1 | 1.5 ± 0.1 | 1.9 ± 0.1 | 5.0 ± 0.3 | 4.0 ± 0.2 |
| MiDHQD1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.1 | 1.9 ± 0.2 | 1.6 ± 0.2 |
| MiSHD1 | 1.5 ± 0.1 | 1.7 ± 0.1 | 1.9 ± 0.2 | 3.6 ± 0.2 | 2.8 ± 0.2 |
| MiCAT1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.2 ± 0.1 | 1.9 ± 0.2 | 1.5 ± 0.2 |
| MiGOX1 | 2.3 ± 0.2 | 2.5 ± 0.2 | 2.8 ± 0.2 | 3.9 ± 0.3 | 3.2 ± 0.3 |
| MiFOX1 | 1.6 ± 0.1 | 1.7 ± 0.1 | 2.1 ± 0.2 | 5.6 ± 0.3 | 5.0 ± 0.2 |
| MiPOD1 | 3.1 ± 0.2 | 3.2 ± 0.2 | 3.5 ± 0.2 | 7.3 ± 0.5 | 6.5 ± 0.4 |
| MiNADO1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.8 ± 0.1 | 3.6 ± 0.2 | 3.0 ± 0.2 |
| MiFLS2-1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.1 ± 0.1 | 1.5 ± 0.1 | 1.2 ± 0.1 |
| MiPK3-1 | 5.2 ± 0.3 | 5.5 ± 0.3 | 5.8 ± 0.3 | 8.5 ± 0.5 | 7.1 ± 0.4 |
| MiPK6-1 | 3.3 ± 0.2 | 3.5 ± 0.2 | 3.6 ± 0.3 | 7.2 ± 0.5 | 5.7 ± 0.3 |
| MiWRKY22-1 | 1.6 ± 0.1 | 1.9 ± 0.2 | 2.1 ± 0.2 | 3.6 ± 0.2 | 2.8 ± 0.2 |
| MiNHL10-1 | 2.6 ± 0.2 | 2.6 ± 0.2 | 2.8 ± 0.2 | 8.7 ± 0.5 | 7.2 ± 0.4 |
| Callus | Infection Time (h) | H2O2 (μmol/mg Protein) | O2− (nmol/(min mg Protein)) | •OH (nmol/(min mg Protein)) |
|---|---|---|---|---|
| NV | 0 h | 3.4 ± 0.5 | 6.3 ± 0.6 | 3.0 ± 0.2 |
| NV | 15 h | 5.0 ± 0.6 | 5.8 ± 0.5 | 2.6 ± 0.2 |
| NV | 30 h | 7.1 ± 0.8 | 10.0 ± 1.0 | 6.1 ± 0.5 |
| NV | 45 h | 13.3 ± 1.3 | 17.9 ± 1.5 | 5.3 ± 0.4 |
| NV | 60 h | 1.5 ± 0.3 | 8.3 ± 0.7 | 5.2 ± 0.4 |
| T3SS-AvrPto | 0 h | 3.6 ± 0.4 | 6.1 ± 0.5 | 2.9 ± 0.2 |
| T3SS-AvrPto | 15 h | 3.9 ± 0.5 | 6.3 ± 0.6 | 2.7 ± 0.2 |
| T3SS-AvrPto | 30 h | 4.5 ± 0.5 | 6.6 ± 0.5 | 3.0 ± 0.2 |
| T3SS-AvrPto | 45 h | 4.7 ± 0.5 | 6.9 ± 0.6 | 3.5 ± 0.3 |
| T3SS-AvrPto | 60 h | 4.4 ± 0.6 | 6.5 ± 0.6 | 3.3 ± 0.3 |
| Gene | 0 h (NV) | 15 h (NV) | 30 h (NV) | 45 h (NV) | 60 h (NV) | 0 h (T) | 15 h (T) | 30 h (T) | 45 h (T) | 60 h (T) |
|---|---|---|---|---|---|---|---|---|---|---|
| MiDAHPS1 | 1.0 ± 0.1 | 1.5 ± 0.1 | 1.9 ± 0.1 | 5.1 ± 0.3 | 4.0 ± 0.2 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.1 | 2.0 ± 0.2 | 1.5 ± 0.1 |
| MiDHQD1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.1 | 1.9 ± 0.2 | 1.6 ± 0.2 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 1.1 ± 0.2 | 0.8 ± 0.1 |
| MiSHD1 | 1.5 ± 0.1 | 1.7 ± 0.1 | 1.9 ± 0.1 | 3.6 ± 0.2 | 2.8 ± 0.2 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.6 ± 0.1 | 2.1 ± 0.2 | 1.8 ± 0.1 |
| MiCAT1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.2 ± 0.1 | 1.9 ± 0.2 | 1.5 ± 0.2 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.3 ± 0.1 | 1.0 ± 0.1 |
| MiGOX1 | 2.3 ± 0.1 | 2.5 ± 0.1 | 2.8 ± 0.1 | 3.9 ± 0.2 | 3.2 ± 0.2 | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.1 | 2.6 ± 0.1 | 2.4 ± 0.1 |
| MiFOX1 | 1.6 ± 0.1 | 1.7 ± 0.1 | 2.1 ± 0.1 | 5.6 ± 0.3 | 5.0 ± 0.2 | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.8 ± 0.1 | 2.7 ± 0.2 | 2.3 ± 0.1 |
| MiPOD1 | 3.1 ± 0.1 | 3.2 ± 0.1 | 3.5 ± 0.1 | 7.3 ± 0.3 | 6.5 ± 0.2 | 3.0 ± 0.1 | 3.1 ± 0.1 | 3.3 ± 0.1 | 4.6 ± 0.2 | 3.8 ± 0.1 |
| MiNADO1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.8 ± 0.1 | 3.6 ± 0.2 | 2.8 ± 0.2 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.1 | 2.1 ± 0.2 | 1.8 ± 0.1 |
| MiFLS2-1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.1 ± 0.1 | 1.5 ± 0.1 | 1.2 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 |
| MiPK3-1 | 5.2 ± 0.2 | 5.5 ± 0.2 | 5.8 ± 0.2 | 8.5 ± 0.3 | 7.1 ± 0.3 | 5.1 ± 0.2 | 5.2 ± 0.2 | 5.4 ± 0.2 | 6.6 ± 0.2 | 6.1 ± 0.2 |
| MiPK6-1 | 3.3 ± 0.2 | 3.5 ± 0.2 | 3.6 ± 0.3 | 7.2 ± 0.5 | 5.7 ± 0.3 | 3.2 ± 0.2 | 3.3 ± 0.2 | 3.4 ± 0.2 | 4.9 ± 0.2 | 4.5 ± 0.2 |
| MiWRKY22-1 | 1.6 ± 0.1 | 1.9 ± 0.1 | 2.1 ± 0.1 | 3.6 ± 0.2 | 2.8 ± 0.2 | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.9 ± 0.1 | 2.5 ± 0.1 | 2.2 ± 0.1 |
| MiNHL10-1 | 2.6 ± 0.2 | 2.6 ± 0.2 | 2.8 ± 0.2 | 8.5 ± 0.5 | 7.2 ± 0.5 | 2.6 ± 0.2 | 2.6 ± 0.2 | 2.7 ± 0.2 | 3.6 ± 0.3 | 3.4 ± 0.3 |
| Medium | Formulation |
|---|---|
| M1 | B5 Mac elements, MS microelements, 4% sucrose, 10% coconut water, 500 mgL−1 glutamine, 0.7% agar, 0.2% activated charcoal, 3.0 mgL−1 2,4-D, 0.5 mgL−1 KT, 0.5 mgL−1 ZT, pH = 5.8 |
| M2 | B5 Mac elements, MS microelements, 4% sucrose, 10% coconut water, 500 mgL−1 glutamine, 0.7% agar, 0.2% activated charcoal, 1.0 mgL−1 2,4-D, 0.5 mgL−1 KT, 0.5 mgL−1 GA3, pH = 5.8 |
| M3 | B5 Mac elements, MS microelements, 4% sucrose, 10% coconut water, 500 mgL−1 glutamine, 0.7% agar, 0.2% activated charcoal, 1.0 mgL−1 2,4-D, 0.5 mgL−1 KT, 0.5 mgL−1 GA3, 20 μgmL−1 chloromycetin, 400 mgL−1 timentin, with a pH of 5.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, H.; Jewel, Z.A.; Faruk, O.; He, L.; Wei, Q.; Zhan, R.; Chang, S. Immune Responses of Mango Callus Infected by Agrobacterium tumefaciens Inhibited Transformation. Int. J. Mol. Sci. 2025, 26, 5006. https://doi.org/10.3390/ijms26115006
Shu H, Jewel ZA, Faruk O, He L, Wei Q, Zhan R, Chang S. Immune Responses of Mango Callus Infected by Agrobacterium tumefaciens Inhibited Transformation. International Journal of Molecular Sciences. 2025; 26(11):5006. https://doi.org/10.3390/ijms26115006
Chicago/Turabian StyleShu, Haiyan, Zilhas Ahmed Jewel, Omor Faruk, Luqiong He, Qing Wei, Rulin Zhan, and Shenghe Chang. 2025. "Immune Responses of Mango Callus Infected by Agrobacterium tumefaciens Inhibited Transformation" International Journal of Molecular Sciences 26, no. 11: 5006. https://doi.org/10.3390/ijms26115006
APA StyleShu, H., Jewel, Z. A., Faruk, O., He, L., Wei, Q., Zhan, R., & Chang, S. (2025). Immune Responses of Mango Callus Infected by Agrobacterium tumefaciens Inhibited Transformation. International Journal of Molecular Sciences, 26(11), 5006. https://doi.org/10.3390/ijms26115006






