Vitamin D in the Prevention and Treatment of Inflammatory Skin Diseases
Abstract
1. Introduction
1.1. Vitamin D Synthesis and Functions
1.2. Vitamin D and Epidermal Proliferation and Differentiation
2. Vitamin D and Psoriasis
The Association Between Vitamin D and Psoriasis
3. Vitamin D and Atopic Dermatitis
3.1. The Role of Vitamin D in the Pathophysiology of Atopic Dermatitis
3.2. The Association Between Serum Levels of Vitamin D and Atopic Dermatitis
4. Vitamin D and Acne
4.1. The Role of Vitamin D in Acne Pathogenesis
4.2. The Effects of Vitamin D Supplementation on Acne
5. Vitamin D and Hidradenitis Suppurativa
The Role of Vitamin D in Hidradenitis Suppurativa
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1,25(OH)2D | 1,25-dihydroxyvitamin D |
| 25(OH)D | 25-hydroxyvitamin D |
| 7-DHC | 7-dehydrocholesterol |
| AD | Atopic dermatitis |
| AMPs | Antimicrobial peptides |
| BMD | Bone mineral density |
| BMI | Body mass index |
| BPO | Benzoyl peroxide |
| CaR | Calcium receptor |
| CRP | C-reactive protein |
| CYP27A1 | 25-hydroxylase |
| CYP27B1 | 1α-hydroxylase |
| DLQI | The Dermatology Life Quality Index |
| EASI | Eczema area and severity index |
| ESR | Erythrocyte sedimentation rate |
| GFR | Glomerular filtration rate |
| HiSQOL | Hidradenitis Suppurativa Quality of Life |
| Hs | Hidradenitis suppurativa |
| HS-PGA | Hidradenitis Suppurativa Physician’s Global Assessment scale |
| IHS4 | International Hidradenitis Suppurativa Severity Scoring System |
| MED | Mediator complex |
| MMP | Matrix metalloproteinase |
| N | Number of patients |
| NA | Not applicable |
| PAPASH | Pyogenic arthritis + pyoderma gangrenosum + acne + hidradenitis suppurativa |
| PASH | Pyoderma gangrenosum + acne + hidradenitis suppurativa |
| PASI | Psoriasis Area and Severity Index |
| PGA | Physician’s Global Assessment scale |
| PI3K | Phosphatidyl inositol 3 kinase |
| PIP5K1α | Phosphatidyl inositol 4-phosphate 5-kinase 1α |
| PLC-γ1 | Phospholipase C-γ1 |
| RCT | Randomized controlled trial |
| SCORAD | Severity Scoring of Atopic Dermatitis |
| SRC | Steroid Receptor Coactivator |
| SREBP-1 | Sterol regulatory binding protein-1 |
| SS | Sartorius score (SS) |
| TBS | Trabecular bone score |
| TEWL | Trans epidermal water loss |
| TNF-α | Tumor necrosis factor-α |
| VDR | Vitamin D receptor |
References
- Holick, M.F.; Chen, T.C.; Lu, Z.; Sauter, E. Vitamin D and Skin Physiology: A D-Lightful Story. J. Bone Miner. Res. 2007, 22, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick Michael, F. Sunlight and Vitamin D A Global Perspective for Health. Dermato-Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Gummert, J.F. Nonclassical Vitamin D Actions. Nutrients 2010, 2, 408–425. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and the Skin: Physiology and Pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Ambagaspitiya, S.S.; Appuhamillage, G.A.; Wimalawansa, S.J. Impact of Vitamin D on Skin Aging, and Age-Related Dermatological Conditions. Front. Biosci. (Landmark Ed.) 2025, 30, 25463. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D Sources, Metabolism, and Deficiency: Available Compounds and Guidelines for Its Treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. The Role of the Parent Compound Vitamin D with Respect to Metabolism and Function: Why Clinical Dose Intervals Can Affect Clinical Outcomes. J. Clin. Endocrinol. Metab. 2013, 98, 4619–4628. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Bikle, D.D.; Pillai, S. Vitamin D, Calcium, and Epidermal Differentiation. Endocr. Rev. 1993, 14, 3–19. [Google Scholar]
- Taracha-Wisniewska, A.; Parks, E.G.C.; Miller, M.; Lipinska-Zubrycka, L.; Dworkin, S.; Wilanowski, T. Vitamin D Receptor Regulates the Expression of the Grainyhead-Like 1 Gene. Int. J. Mol. Sci. 2024, 25, 7913. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Christakos, S. New Aspects of Vitamin D Metabolism and Action-Addressing the Skin as Source and Target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, B.; Relhan, V.; Goel, K.; Kochhar, A.M.; Garg, V.K. Vitamin D and Skin Diseases: A Review. Indian. J. Dermatol. Venereol. Leprol. 2015, 81, 344–355. [Google Scholar] [CrossRef]
- Menon, G.K.; Lee, S.E.; Lee, S.H. An Overview of Epidermal Lamellar Bodies: Novel Roles in Biological Adaptations and Secondary Barriers. J. Dermatol. Sci. 2018, 92, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Elias, P.M. Role of Lipids in the Formation and Maintenance of the Cutaneous Permeability Barrier. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 280–294. [Google Scholar] [CrossRef]
- Gniadecki, R. Stimulation versus Inhibition of Keratinocyte Growth by 1,25-Dihydroxyvitamin D3: Dependence on Cell Culture Conditions. J. Investig. Dermatol. 1996, 106, 510–516. [Google Scholar] [CrossRef]
- Reichrath, J. Vitamin D and the Skin: An Ancient Friend, Revisited. Exp. Dermatol. 2007, 16, 618–625. [Google Scholar] [CrossRef]
- Hawker, N.P.; Pennypacker, S.D.; Chang, S.M.; Bikle, D.D. Regulation of Human Epidermal Keratinocyte Differentiation by the Vitamin D Receptor and Its Coactivators DRIP205, SRC2, and SRC3. J. Investig. Dermatol. 2007, 127, 874–880. [Google Scholar] [CrossRef]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal Expression of 25-Hydroxyvitamin d(3)-1 Alpha-Hydroxylase. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. North. Am. 2017, 46, 815–843. [Google Scholar] [CrossRef]
- Oda, Y.; Sihlbom, C.; Chalkley, R.J.; Huang, L.; Rachez, C.; Chang, C.P.B.; Burlingame, A.L.; Freedman, L.P.; Bikle, D.D. Two Distinct Coactivators, DRIP/Mediator and SRC/P160, Are Differentially Involved in Vitamin D Receptor Transactivation during Keratinocyte Differentiation. Mol. Endocrinol. 2003, 17, 2329–2339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oda, Y.; Uchida, Y.; Moradian, S.; Crumrine, D.; Elias, P.M.; Bikle, D.D. Vitamin D Receptor and Coactivators SRC2 and 3 Regulate Epidermis-Specific Sphingolipid Production and Permeability Barrier Formation. J. Investig. Dermatol. 2009, 129, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Schauber, J.; Dorschner, R.A.; Coda, A.B.; Büchau, A.S.; Liu, P.T.; Kiken, D.; Helfrich, Y.R.; Kang, S.; Elalieh, H.Z.; Steinmeyer, A.; et al. Injury Enhances TLR2 Function and Antimicrobial Peptide Expression through a Vitamin D-Dependent Mechanism. J. Clin. Investig. 2007, 117, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Schauber, J.; Dorschner, R.A.; Yamasaki, K.; Brouha, B.; Gallo, R.L. Control of the Innate Epithelial Antimicrobial Response Is Cell-Type Specific and Dependent on Relevant Microenvironmental Stimuli. Immunology 2006, 118, 509–519. [Google Scholar] [CrossRef]
- Celli, A.; Sanchez, S.; Behne, M.; Hazlett, T.; Gratton, E.; Mauro, T. The Epidermal Ca2+ Gradient: Measurement Using the Phasor Representation of Fluorescent Lifetime Imaging. Biophys. J. 2010, 98, 911–921. [Google Scholar] [CrossRef]
- Tu, C.L.; Chang, W.; Bikle, D.D. The Extracellular Calcium-Sensing Receptor Is Required for Calcium-Induced Differentiation in Human Keratinocytes. J. Biol. Chem. 2001, 276, 41079–41085. [Google Scholar] [CrossRef]
- Tu, C.L.; Chang, W.; Xie, Z.; Bikle, D.D. Inactivation of the Calcium Sensing Receptor Inhibits E-Cadherin-Mediated Cell-Cell Adhesion and Calcium-Induced Differentiation in Human Epidermal Keratinocytes. J. Biol. Chem. 2007, 283, 3519–3528. [Google Scholar] [CrossRef]
- Xie, Z.; Singleton, P.A.; Bourguignon, L.Y.W.; Bikle, D.D. Calcium-Induced Human Keratinocyte Differentiation Requires Src- and Fyn-Mediated Phosphatidylinositol 3-Kinase-Dependent Activation of Phospholipase C-Γ1. Mol. Biol. Cell 2005, 16, 3236–3246. [Google Scholar] [CrossRef]
- Yuspa, S.H.; Kilkenny, A.E.; Steinert, P.M.; Roop, D.R. Expression of Murine Epidermal Differentiation Markers Is Tightly Regulated by Restricted Extracellular Calcium Concentrations In Vitro. J. Cell Biol. 1989, 109, 1207–1217. [Google Scholar] [CrossRef]
- Ratnam, A.V.; Bikle, D.D.; Cho, J.-K. 1,25 Dihydroxyvitamin D3 Enhances the Calcium Response of Keratinocytes. J. Cell Physiol. 1999, 178, 188–196. [Google Scholar] [CrossRef]
- Tu, C.L.; Crumrine, D.; Man, M.Q.; Chang, W.; Elalieh, H.; You, M.; Elias, P.M.; Bikle, D.D. Ablation of the Calcium-Sensing Receptor in Keratinocytes Impairs Epidermal Differentiation and Barrier Function. J. Investig. Dermatol. 2012, 132, 2350–2359. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Bikle, D.D. Phospholipase C-Γ1 Is Required for Calcium-Induced Keratinocyte Differentiation. J. Biol. Chem. 1999, 274, 20421–20424. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.C.; Shafaee, S.; Lee, D.; Bikle, D.D. Requirement of an AP-1 Site in the Calcium Response Region of the Involucrin Promoter. J. Biol. Chem. 2000, 275, 24080–24088. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G.; Bragazzi, N.L.; Karimkhani Aksut, C.; Wu, D.; Alicandro, G.; McGonagle, D.; Guo, C.; Dellavalle, R.; Grada, A.; Wong, P.; et al. The Global, Regional, and National Burden of Psoriasis: Results and Insights From the Global Burden of Disease 2019 Study. Front. Med. 2021, 8, 743180. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA J. Am. Med. Assoc. 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Brożyna, A.A.; Slominski, R.M.; Nedoszytko, B.; Zmijewski, M.A.; Slominski, A.T. Vitamin D Signaling in Psoriasis: Pathogenesis and Therapy. Int. J. Mol. Sci. 2022, 23, 8575. [Google Scholar] [CrossRef]
- Benhadou, F.; Mintoff, D.; Del Marmol, V. Psoriasis: Keratinocytes or Immune Cells—Which Is the Trigger? Dermatology 2019, 235, 91–100. [Google Scholar] [CrossRef]
- Priyadarssini, M.; Divya Priya, D.; Indhumathi, S.; Rajappa, M.; Chandrashekar, L.; Thappa, D.M. Immunophenotyping of T Cells in the Peripheral Circulation in Psoriasis. Br. J. Biomed. Sci. 2016, 73, 174–179. [Google Scholar] [CrossRef]
- Yamamoto, E.; Jørgensen, T.N. Immunological Effects of Vitamin D and Their Relations to Autoimmunity. J. Autoimmun. 2019, 100, 7–16. [Google Scholar] [CrossRef]
- Wei, R.; Christakos, S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients 2015, 7, 8251–8260. [Google Scholar] [CrossRef]
- Xie, Z.; Komuves, L.; Yu, Q.C.; Elalieh, H.; Ng, D.C.; Leary, C.; Chang, S.; Crumrine, D.; Bikle, D.D.; Yoshizawa, T.; et al. Lack of the Vitamin D Receptor Is Associated with Reduced Epidermal Differentiation and Hair Follicle Growth. J. Investig. Dermatol. 2002, 118, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, J.; Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of Terminal Differentiation of Cultured Mouse Epidermal Cells by 1alpha,25-Dihydroxyvitamin D3. Endocrinology 1983, 3, 1950–1957. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.N. Diagnosis and Treatment of Pediatric Psoriasis: Current and Future. Am. J. Clin. Dermatol. 2013, 14, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Kokelj, F.; Lavaroni, G.; Guadagnini, A. UVB versus UVB plus Calcipotriol (MC 903) Therapy for Psoriasis Vulgaris. Acta Derm. Venereol. 1995, 75, 386–387. [Google Scholar] [CrossRef]
- Gollnick, H.; Altmeyer, P.; Kaufmann, R.; Ring, J.; Christophers, E.; Pavel, S.; Ziegler, J.; Gollnick, H. Topical Calcipotriol plus Oral Fumaric Acid Is More Effective and Faster Acting than Oral Fumaric Acid Monotherapy in the Treatment of Severe Chronic Plaque Psoriasis Vulgaris. Pharmacol. Treat. Dermatol. 2002, 205, 46–53. [Google Scholar] [CrossRef]
- Kokelj, F.; Torsello, P.; Plozzer, C. Calcipotriol Improves the Efficacy of Cyclosporine in the Treatment of Psoriasis Vulgaris. J. Eur. Acad. Dermatol. Venereol. 1998, 10, 143–146. [Google Scholar] [CrossRef]
- Rim, J.H.; Park, J.Y.; Choe, Y.B.; Youn, J. Il The Efficacy of Calcipotriol + Acitretin Combination Therapy for Psoriasis Comparison with Acitretin Monotherapy. Am. J. Clin. Dermatol. 2003, 4, 507–510. [Google Scholar] [CrossRef]
- Pinter, A.; Green, L.J.; Selmer, J.; Praestegaard, M.; Gold, L.S.; Augustin, M. A Pooled Analysis of Randomized, Controlled, Phase 3 Trials Investigating the Efficacy and Safety of a Novel, Fixed Dose Calcipotriene and Betamethasone Dipropionate Cream for the Topical Treatment of Plaque Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 228–236. [Google Scholar] [CrossRef]
- Miyachi, Y.; Ohkawara, A.; Ohkido, M.; Harada, S.; Tamaki, K.; Nakagawa, H.; Hori, Y.; Nishiyama, S. Long-Term Safety and Efficacy of High-Concentration (20 Microg/g) Tacalcitol Ointment in Psoriasis Vulgaris. Eur. J. Dermatol. 2002, 12, 463–468. [Google Scholar]
- Aggarwal, P.; Aggarwal, K.; Jain, V.K. Tacalcitol: A Useful Adjunct to Narrow Band Ultraviolet B Phototherapy in Psoriasis. J. Dermatol. Treat. 2016, 27, 546–551. [Google Scholar] [CrossRef]
- Barker, J.N.W.N.; Ashton, R.E.; Marks, R.; Harris, R.I.; Berth-Jones, J. Topical Maxacalcitol for the Treatment of Psoriasis Vulgaris: A Placebo-Controlled, Double-Blind, Dose-Finding Study with Active Comparator. Br. J. Dermatol. 1999, 141, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.; Morikawa, M.; Miyamoto, K.; Kaiho, S.-I.; Fukushima, M.; Miyaura, C.; Abe, E.; Suds, T.; Nishii, Y. Synthetic Analogues of Vitamin D3 with an Oxygen Atom in the Side Chain Skeleton. A Trial of the Development of Vitamin D Compounds Which Exhibit Potent Differentiation-Inducing Activity without Inducing Hypercalcemia. FEBS Lett. 1987, 226, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Takekoshi, T.; Asahina, A.; Komine, M.; Tamaki, K. Treatment of Psoriasis Vulgaris with Narrow-Band UVB and Topical Maxacalcitol. Acta Derm. Venereol. 2006, 86, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Furuhashi, T.; Matsumoto, K.; Morita, A. Safety Profiles of Topical Vitamin D3 in Psoriasis Patients: A Retrospective Large-Scale Study. Psoriasis Targets Ther. 2012, 2, 81–88. [Google Scholar] [CrossRef][Green Version]
- McLane, J.A.; Katz, M.; Abdelkader, N. Effect of 1,25-Dihydroxyvitamin D3 on Human Keratinocytes Grown under Different Culture Conditions. In Vitro Cell. Dev. Biol. 1990, 26, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Walworth, N.; Holick, M. Effect of 1 Alpha,25-Dihydroxyvitamin D3 on the Morphologic and Biochemical Differentiation of Cultured Human Epidermal Keratinocytes Grown in Serum-Free Conditions. J. Investig. Dermatol. 1986, 86, 709–714. [Google Scholar] [CrossRef]
- Chandrashekar, L.; Krishna Kumari, G.R.; Rajappa, M.; Revathy, G.; Munisamy, M.; Thappa, D.M. 25-Hydroxy Vitamin D and Ischaemia-Modified Albumin Levels in Psoriasis and Their Association with Disease Severity. Br. J. Biomed. Sci. 2015, 72, 56–60. [Google Scholar] [CrossRef]
- Pokharel, R.; Agrawal, S.; Pandey, P.; Lamsal, M. Assessment of Vitamin D Level in Patients with Psoriasis and Its Correlation with Disease Severity: A Case–Control Study. Psoriasis Targets Ther. 2022, 12, 251–258. [Google Scholar] [CrossRef]
- Disphanurat, W.; Viarasilpa, W.; Chakkavittumrong, P.; Pongcharoen, P. The Clinical Effect of Oral Vitamin D2 Supplementation on Psoriasis: A Double-Blind, Randomized, Placebo-Controlled Study. Dermatol. Res. Pract. 2019, 2019, 5237642. [Google Scholar] [CrossRef]
- Maleki, M.; Nahidi, Y.; Azizahari, S.; Meibodi, N.T.; Hadianfar, A. Serum 25-OH Vitamin D Level in Psoriatic Patients and Comparison With Control Subjects. J. Cutan. Med. Surg. 2016, 20, 207–210. [Google Scholar] [CrossRef]
- Bergler Czop, B.; Ligia, B.W. Serum Vitamin D Level—The Effect on the Clinical Course of Psoriasis. Adv. Dermatol. Allergol. 2016, 33, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Song, G.G. Association between Circulating 25-Hydroxyvitamin D Levels and Psoriasis, and Correlation with Disease Severity: A Meta-Analysis. Clin. Exp. Dermatol. 2018, 43, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Ingram, M.A.; Jones, M.B.; Stonehouse, W.; Jarrett, P.; Scragg, R.; Mugridge, O.; von Hurst, P.R. Oral Vitamin D3 Supplementation for Chronic Plaque Psoriasis: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Dermatol. Treat. 2018, 29, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, P.; Camargo, C.A.; Coomarasamy, C.; Scragg, R. A Randomized, Double-Blind, Placebo-Controlled Trial of the Effect of Monthly Vitamin D Supplementation in Mild Psoriasis. J. Dermatol. Treat. 2018, 29, 324–328. [Google Scholar] [CrossRef]
- Moosazadeh, M.; Damiani, G.; Khademloo, M.; Kheradmand, M.; Nabinezhad-Male, F.; Hessami, A. Comparing Vitamin D Level Between Patients with Psoriasis and Healthy Individuals: A Systematic Review and Meta-Analysis. J. Evid. Based Integr. Med. 2023, 28, 1–10. [Google Scholar] [CrossRef]
- Holland, D.B.; Wood, E.J.; Roberts, S.G.; West, M.R.; Cunliffe, W.J. Epidermal Keratin Levels during Oral 1-Alpha-Hydroxyvitamin D3 Treatment for Psoriasis. Skin. Pharmacol. Physiol. 1989, 2, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Prystowsky, J.H.; Muzio, P.J.; Sevran, S.; Clemens, T.L. Effect of UVB Phototherapy and Oral Calcitriol (1,25-Dihydroxyvitamin D3) on Vitamin D Photosynthesis in Patients with Psoriasis. J. Am. Acad. Dermatol. 1996, 35, 690–695. [Google Scholar] [CrossRef]
- Gumowski-Sunek, D.; Rizzoli, R.; Saurat, J.-H. Effects of Topical Calcipotriol on Calcium Metabolism in Psoriatic Patients: Comparison with Oral Calcitriol. Dermatologica 1991, 183, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Prtina, A.; Simović, N.R.; Milivojac, T.; Vujnić, M.; Grabež, M.; Djuric, D.; Stojiljković, M.P.; Stanković, V.S.; Čolić, M.J.; Škrbić, R. The Effect of Three-Month Vitamin D Supplementation on the Levels of Homocysteine Metabolism Markers and Inflammatory Cytokines in Sera of Psoriatic Patients. Biomolecules 2021, 11, 1865. [Google Scholar] [CrossRef]
- Torres, T.; Ferreira, E.O.; Gonçalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on Atopic Dermatitis. Acta Med. Port. 2019, 32, 606–613. [Google Scholar] [CrossRef]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef] [PubMed]
- Nutten, S. Atopic Dermatitis: Global Epidemiology and Risk Factors. Ann. Nutr. Metab. 2015, 66, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Novak, N. Pathogenesis of Atopic Dermatitis. Clin. Exp. Allergy 2015, 45, 566–574. [Google Scholar] [CrossRef]
- Grieco, T.; Paolino, G.; Moliterni, E.; Chello, C.; Sernicola, A.; Egan, C.G.; Morelli, M.; Nannipieri, F.; Battaglia, S.; Accoto, M.; et al. Differential Expression of Proteins Involved in Skin Barrier Maintenance and Vitamin D Metabolism in Atopic Dermatitis: A Cross-Sectional, Exploratory Study. Int. J. Mol. Sci. 2025, 26, 211. [Google Scholar] [CrossRef]
- Palmer, C.N.A.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common Loss-of-Function Variants of the Epidermal Barrier Protein Filaggrin Are a Major Predisposing Factor for Atopic Dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y.M. The Immunology of Atopic Dermatitis and Its Reversibility with Broad-Spectrum and Targeted Therapies. J. Allergy Clin. Immunol. 2017, 139, 65–76. [Google Scholar] [CrossRef]
- Borzutzky, A.; Camargo, C.A. Role of Vitamin D in the Pathogenesis and Treatment of Atopic Dermatitis. Expert. Rev. Clin. Immunol. 2013, 9, 751–760. [Google Scholar] [CrossRef]
- Wang, T.-T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting Edge: 1,25-Dihydroxyvitamin D3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression. J. Immunol. 2014, 173, 2909–2912. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.Y.; Ohtake, T.; Brandt, C.; Strickland, I.; Boguniewicz, M.; Ganz, T.; Gallo, R.L.; Leung, D.Y.M. Endogenous Antimicrobial Peptides and Skin Infections in Atopic Dermatitis. N. Engl. J. Med. 2002, 347, 1151–1160. [Google Scholar] [CrossRef]
- Chiu, Y.E.; Havens, P.L.; Siegel, D.H.; Ali, O.; Wang, T.; Holland, K.E.; Galbraith, S.S.; Lyon, V.B.; Drolet, B.A. Serum 25-Hydroxyvitamin D Concentration Does Not Correlate with Atopic Dermatitis Severity. J. Am. Acad. Dermatol. 2013, 69, 40–46. [Google Scholar] [CrossRef]
- Lucas, R.; Mihály, J.; Gericke, J.; Törocsik, D.; Rühl, R. Vitamin D Signaling in a Mouse Allergic Sensitization Model. Int. J. Vitam. Nutr. Res. 2020, 90, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Kongsbak, M.; Levring, T.B.; Geisler, C.; Von Essen, M.R.; Rode Von Essen, M. The Vitamin D Receptor and T Cell Function. Front. Immunol. 2013, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, L.; Mavragani, C.P.; Koutsilieris, M. The Immunomodulatory Properties of Vitamin D. Mediterr. J. Rheumatol. 2022, 33, 7–13. [Google Scholar] [CrossRef]
- White, J.H. Vitamin D Signaling, Infectious Diseases, and Regulation of Innate Immunity. Infect. Immun. 2008, 76, 3837–3843. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and the Immune System: New Perspectives on an Old Theme. Endocrinol. Metab. Clin. North. Am. 2010, 39, 365–379. [Google Scholar] [CrossRef]
- Bastyte, D.; Tamasauskiene, L.; Stakaitiene, I.; Briede, K.; Ugenskiene, R.; Valiukeviciene, S.; Gradauskiene, B. Relation of T Cell Profile with Vitamin D Receptor and Vitamin D-Binding Protein Gene Polymorphisms in Atopy. Int. J. Mol. Sci. 2024, 25, 9021. [Google Scholar] [CrossRef]
- Galvão, A.A.; De Araújo Sena, F.; De Andrade Belitardo, E.M.M.; De Santana, M.B.R.; Costa, G.N.D.O.; Cruz, Á.A.; Barreto, M.L.; Costa, R.D.S.; Alcantara-Neves, N.M.; Figueiredo, C.A. Genetic Polymorphisms in Vitamin D Pathway Influence 25(OH)D Levels and Are Associated with Atopy and Asthma. Allergy Asthma Clin. Immunol. 2020, 16, 62. [Google Scholar] [CrossRef]
- Bäck, O.; Blomquist, H.K.; Hernell, O.; Stenberg, B. Does Vitamin D Intake During Infancy Promote the Development of Atopic Allergy? Acta Derm. Venereol. 2009, 89, 28–32. [Google Scholar] [CrossRef]
- Schuessler, M.; Astecker, N.; Herzig, G.; Vorisek, G.; Schuster, I. Skin Is an Autonomous Organ in Synthesis, Two-Step Activation and Degradation of Vitamin D3: CYP27 in Epidermis Completes the Set of Essential Vitamin D 3-Hydroxylases. Steroids 2001, 66, 399–408. [Google Scholar] [CrossRef]
- Peroni, D.G.; Piacentini, G.L.; Cametti, E.; Chinellato, I.; Boner, A.L. Correlation between Serum 25-Hydroxyvitamin D Levels and Severity of Atopic Dermatitis in Children. Br. J. Dermatol. 2011, 164, 1078–1082. [Google Scholar] [CrossRef]
- Yamanaka, K.; Dimitroff, C.J.; Fuhlbrigge, R.C.; Kakeda, M.; Kurokawa, I.; Mizutani, H.; Kupper, T.S. Vitamins A and D Are Potent Inhibitors of Cutaneous Lymphocyte-Associated Antigen Expression. J. Allergy Clin. Immunol. 2008, 121, 148–157.e3. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.P.; Palmer, D.; Zhang, G.; Prescott, S.L. Cord Blood 25-Hydroxyvitamin D3 and Allergic Disease During Infancy. Pediatrics 2012, 130, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- El Taieb, M.A.; Fayed, H.M.; Aly, S.S.; Ibrahim, A.K. Assessment of Serum 25-Hydroxyvitamin D Levels in Children with Atopic Dermatitis: Correlation With SCORAD Index. Dermatitis 2013, 24, 296–301. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, E.; Barberi, S.; Cerri, A.; Boccardi, D.; Turati, F.; Sortino, S.; Banderali, G.; Ciprandi, G. Vitamin D Status and Body Mass Index in Children with Atopic Dermatitis: A Pilot Study in Italian Children. Immunol. Lett. 2017, 181, 31–35. [Google Scholar] [CrossRef]
- Han, T.Y.; Kong, T.S.; Kim, M.H.; Chae, J.D.; Lee, J.H.K.; Son, S.J. Vitamin D Status and Its Association with the SCORAD Score and Serum LL-37 Level in Korean Adults and Children with Atopic Dermatitis. Ann. Dermatol. 2015, 27, 10–14. [Google Scholar] [CrossRef]
- Wang, S.S.; Hon, K.L.; Kong, A.P.S.; Pong, H.N.H.; Wong, G.W.K.; Leung, T.F. Vitamin D Deficiency Is Associated with Diagnosis and Severity of Childhood Atopic Dermatitis. Pediatr. Allergy Immunol. 2014, 25, 30–35. [Google Scholar] [CrossRef]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Dairy Food, Calcium and Vitamin D Intake in Pregnancy, and Wheeze and Eczema in Infants. Eur. Respir. J. 2010, 35, 1228–1234. [Google Scholar] [CrossRef]
- Akan, A.; Azkur, D.; Ginis, T.; Toyran, M.; Kaya, A.; Vezir, E.; Özcan, C.; Ginis, Z.; Kocabas, C.N. Vitamin D Level in Children Is Correlated with Severity of Atopic Dermatitis but Only in Patients with Allergic Sensitizations. Pediatr. Dermatol. 2013, 30, 359–363. [Google Scholar] [CrossRef]
- Ng, J.C.; Yew, Y.W. Effect of Vitamin D Serum Levels and Supplementation on Atopic Dermatitis: A Systematic Review and Meta-Analysis. Am. J. Clin. Dermatol. 2022, 23, 267–275. [Google Scholar] [CrossRef]
- Yang, L.; Sato, M.; Saito-Abe, M.; Nishizato, M.; Mezawa, H.; Yamamoto-Hanada, K.; Ohya, Y. Serum 25-Hydroxyvitamin D Concentrations and Atopic Dermatitis in Early Childhood: Findings from the Japan Environment and Children’s Sstudy. Nutrients 2021, 13, 2761. [Google Scholar] [CrossRef]
- Munawwarah, L.; Evalina, R.; Sofyani, S. Serum 25-Hydroxyvitamin-D Level and Atopic Dermatitis Severity in Children. Paediatr. Indones. 2018, 57, 234–238. [Google Scholar] [CrossRef][Green Version]
- Çiçek, F.; Köle, M.T. Evaluation of the Impact of Serum Vitamin D Levels on the Scoring Atopic Dermatitis Index in Pediatric Atopic Dermatitis. Children 2023, 10, 1522. [Google Scholar] [CrossRef]
- Su, O.; Bahali, A.G.; Demir, A.D.; Ozkaya, D.B.; Uzuner, S.; Dizman, D.; Onsun, N. The Relationship between Severity of Disease and Vitamin D Levels in Children with Atopic Dermatitis. Adv. Dermatol. Allergol. 2017, 34, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Samochocki, Z.; Bogaczewicz, J.; Jeziorkowska, R.; Sysa-Jȩdrzejowska, A.; Glińska, O.; Karczmarewicz, E.; McCauliffe, D.P.; Woźniacka, A. Vitamin D Effects in Atopic Dermatitis. J. Am. Acad. Dermatol. 2013, 69, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, J.; Li, W.; Zheng, H.; Dai, H.; Qiu, G.; Yu, D.; Yao, D.; Yin, X. Causal Associations between Vitamin D Levels and Psoriasis, Atopic Dermatitis, and Vitiligo: A Bidirectional Two-Sample Mendelian Randomization Analysis. Nutrients 2022, 14, 5284. [Google Scholar] [CrossRef]
- Dębińska, A.; Sikorska-Szaflik, H.; Urbanik, M.; Boznański, A. The Role of Vitamin D in Atopic Dermatitis. Dermatitis 2015, 26, 155–161. [Google Scholar] [CrossRef]
- Mansour, N.O.; Mohamed, A.A.; Hussein, M.; Eldemiry, E.; Daifalla, A.; Hassanin, S.; Nassar, N.; Ghaith, D.; Mohamed Salah, E. The Impact of Vitamin D Supplementation as an Adjuvant Therapy on Clinical Outcomes in Patients with Severe Atopic Dermatitis: A Randomized Controlled Trial. Pharmacol. Res. Perspect. 2020, 8, e00679. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.N.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Vitamin D Status and Efficacy of Vitamin D Supplementation in Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrients 2016, 8, 789. [Google Scholar] [CrossRef]
- Camargo, C.A.; Ganmaa, D.; Sidbury, R.; Erdenedelger, K.; Radnaakhand, N.; Khandsuren, B. Randomized Trial of Vitamin D Supplementation for Winter-Related Atopic Dermatitis in Children. J. Allergy Clin. Immunol. 2014, 134, 831–835. [Google Scholar] [CrossRef]
- Amestejani, M.; Salehi, B.S.; Vasigh, M.; Sobhkhiz, A.; Karami, M.; Alinia, H.; Kamrava, S.K.; Shamspour, N.; Ghalehbaghi, B.; Behzadi, A.H. Vitamin D Supplementation in the Treatment of Atopic Dermatitis: A Clinical Trial Study. J. Drugs Dermatol. 2012, 11, 327–330. [Google Scholar]
- Sidbury, R.; Sullivan, A.F.; Thadhani, R.I.; Camargo, C.A. Randomized Controlled Trial of Vitamin D Supplementation for Winter-Related Atopic Dermatitis in Boston: A Pilot Study. Br. J. Dermatol. 2008, 159, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Roider, E.; Ruzicka, T.; Schauber, J. Vitamin D, the Cutaneous Barrier, Antimicrobial Peptides and Allergies: Is There a Link? Allergy Asthma Immunol. Res. 2013, 5, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years Lived with Disability (YLDs) for 1160 Sequelae of 289 Diseases and Injuries 1990-2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef] [PubMed]
- Gary, M. White Recent Findings in the Epidemiologic Evidence, Classification and Subtypes of Acne Vulgaris. J. Am. Acad. Dermatol. 1998, 39, S34–S37. [Google Scholar] [CrossRef]
- Brown, S.K.; Shalita, A.R. Acne Vulgaris. Lancet 1998, 351, 1871–1876. [Google Scholar] [CrossRef]
- Dréno, B.; Bettoli, V.; Araviiskaia, E.; Sanchez Viera, M.; Bouloc, A. The Influence of Exposome on Acne. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 812–819. [Google Scholar] [CrossRef]
- Pappas, A.; Johnsen, S.; Liu, J.-C.; Eisinger, M. Sebum Analysis of Individuals with and without Acne. Dermato-Endocrinol. 2009, 1, 157–161. [Google Scholar] [CrossRef]
- Harris, H.H.; Downing, D.T.; Stewart, E.; Strauss, J.S. Sustainable Rates of Sebum Secretion in Acne Patients and Matched Normal Control Subjects. J. Am. Acad. Dermatol. 1983, 8, 200–203. [Google Scholar] [CrossRef]
- Cong, T.X.; Hao, D.; Wen, X.; Li, X.H.; He, G.; Jiang, X. From Pathogenesis of Acne Vulgaris to Anti-Acne Agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef]
- Smith, T.M.; Gilliland, K.; Clawson, G.A.; Thiboutot, D. IGF-1 Induces SREBP-1 Expression and Lipogenesis in SEB-1 Sebocytes via Activation of the Phosphoinositide 3-Kinase/Akt Pathway. J. Investig. Dermatol. 2008, 128, 1286–1293. [Google Scholar] [CrossRef]
- Melnik, B.C. Linking Diet to Acne Metabolomics, Inflammation, and Comedogenesis: An Update. Clin. Cosmet. Investig. Dermatol. 2015, 8, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Mayslich, C.; Grange, P.A.; Dupin, N. Cutibacterium acnes as an Opportunistic Pathogen: An Update of Its Virulence-Associated Factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef]
- Kurokawa, I.; Danby, F.W.; Ju, Q.; Wang, X.; Xiang, L.F.; Xia, L.; Chen, W.C.; Nagy, I.; Picardo, M.; Suh, D.H.; et al. New Developments in Our Understanding of Acne Pathogenesis and Treatment. Exp. Dermatol. 2009, 18, 821–832. [Google Scholar] [CrossRef]
- Krämer, C.; Seltmann, H.; Seifert, M.; Tilgen, W.; Zouboulis, C.C.; Reichrath, J. Characterization of the Vitamin D Endocrine System in Human Sebocytes in Vitro. J. Steroid Biochem. Mol. Biol. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Lee, W.J.; Choi, Y.H.; Sohn, M.Y.; Lee, S.J.; Kim, D.W. Expression of Inflammatory Biomarkers from Cultured Sebocytes Was Influenced by Treatment with Vitamin D. Indian. J. Dermatol. 2013, 58, 327. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Imai, N.; Akimoto, N.; Sakiguchi, T.; Kitamura, K.; Ito, A. Epidermal Growth Factor and 1a,25-Dihydroxyvitamin D 3 Suppress Lipogenesis in Hamster Sebaceous Gland Cells In Vitro. J. Investig. Dermatol. 2001, 117, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M. Pathogenesis of Acne Vulgaris II. Histopathology of Comedones Induced in the Rabbit Ear by Human Sebum. Arch. Dermatol. 1968, 98, 58–66. [Google Scholar] [CrossRef]
- Guy, R.; Kealey, T. Modelling the Infundibulum in Acne. Dermatology 1998, 196, 32–37. [Google Scholar] [CrossRef]
- Guy, R.; Kealey, T. The Effects of Inflammatory Cytokines on the Isolated Human Sebaceous Infundibulum. J. Investig. Dermatol. 1998, 110, 410–415. [Google Scholar] [CrossRef][Green Version]
- Guy, R.; Green, M.R.; Kealey, T. Modeling Acne in Vitro. J. Investig. Dermatol. 1996, 106, 176–182. [Google Scholar] [CrossRef]
- Li, W.H.; Zhang, Q.; Flach, C.R.; Mendelsohn, R.; Southall, M.D.; Parsa, R. In Vitro Modeling of Unsaturated Free Fatty Acid-Mediated Tissue Impairments Seen in Acne Lesions. Arch. Dermatol. Res. 2017, 309, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D Regulated Keratinocyte Differentiation. J. Cell Biochem. 2004, 92, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Bikle, D.D.; Oda, Y. Reciprocal Role of Vitamin D Receptor on β-Catenin Regulated Keratinocyte Proliferation and Differentiation. J. Steroid Biochem. Mol. Biol. 2014, 144, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Watanabe, H.; Yasukawa, H.; Uratsuji, H.; Kanazawa, H.; Ishimaru, M.; Kotera, N.; Akatsuka, M.; Kawashima, M. Comedolytic Effect of Topically Applied Active Vitamin D3 Analogue on Pseudocomedones in the Rhino Mouse. Br. J. Dermatol. 2006, 155, 895–901. [Google Scholar] [CrossRef]
- Nagy, I.; Pivarcsi, A.; Koreck, A.; Széll, M.; Urbán, E.; Kemény, L. Distinct Strains of Propionibacterium Acnes Induce Selective Human B-Defensin-2 and Interleukin-8 Expression in Human Keratinocytes Through Toll-Like Receptors. J. Investig. Dermatol. 2005, 124, 931–938. [Google Scholar] [CrossRef]
- Jugeau, S.; Tenaud, I.; Knol, A.C.; Jarrousse, V.; Quereux, G.; Khammari, A.; Dreno, B. Induction of Toll-like Receptors by Propionibacterium Acnes. Br. J. Dermatol. 2005, 153, 1105–1113. [Google Scholar] [CrossRef]
- Zhang, B.; Choi, Y.M.; Lee, J.; An, I.S.; Li, L.; He, C.; Dong, Y.; Bae, S.; Meng, H. Toll-like Receptor 2 Plays a Critical Role in Pathogenesis of Acne Vulgaris. Biomed. Dermatol. 2019, 3, 4. [Google Scholar] [CrossRef]
- Kim, J.; Ochoa, M.-T.; Krutzik, S.R.; Takeuchi, O.; Uematsu, S.; Legaspi, A.J.; Brightbill, H.D.; Holland, D.; Cunliffe, W.J.; Akira, S.; et al. Activation of Toll-like Receptor 2 in Acne Triggers Inflammatory Cytokine Responses. J. Immunol. 2002, 169, 1535–1541. [Google Scholar] [CrossRef]
- Qin, M.; Pirouz, A.; Kim, M.H.; Krutzik, S.R.; Garbán, H.J.; Kim, J. Propionibacterium Acnes Induces IL-1β Secretion via the NLRP3 Inflammasome in Human Monocytes. J. Investig. Dermatol. 2014, 134, 381–388. [Google Scholar] [CrossRef]
- Li, Z.J.; Choi, D.K.; Sohn, K.C.; Seo, M.S.; Lee, H.E.; Lee, Y.; Seo, Y.J.; Lee, Y.H.; Shi, G.; Zouboulis, C.C.; et al. Propionibacterium Acnes Activates the NLRP3 Inflammasome in Human Sebocytes. J. Investig. Dermatol. 2014, 134, 2747–2756. [Google Scholar] [CrossRef]
- Kistowska, M.; Gehrke, S.; Jankovic, D.; Kerl, K.; Fettelschoss, A.; Feldmeyer, L.; Fenini, G.; Kolios, A.; Navarini, A.; Ganceviciene, R.; et al. IL-1β Drives Inflammatory Responses to Propionibacterium Acnes In Vitro and In Vivo. J. Investig. Dermatol. 2014, 134, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Dahlan, N.H.; Sitohang, I.B.S.; Indriatmi, W.; Wibowo, H.; Enggy, L.E. Correlation Between Reduced IL-1β Levels in Acne Lesions and the Decrease in Acne Inflammatory Lesions Following Topical Vitamin D Administration: A Double-Blind Randomized Controlled Trial. Clin. Cosmet. Investig. Dermatol. 2024, 17, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Rodriguez, E.V.; Napolitani, G.; Lanzavecchia, A.; Sallusto, F. Interleukins 1β and 6 but Not Transforming Growth Factor-β Are Essential for the Differentiation of Interleukin 17-Producing Human T Helper Cells. Nat. Immunol. 2007, 8, 942–949. [Google Scholar] [CrossRef]
- Agak, G.W.; Qin, M.; Nobe, J.; Kim, M.H.; Krutzik, S.R.; Tristan, G.R.; Elashoff, D.; Garbán, H.J.; Kim, J. Propionibacterium Acnes Induces an IL-17 Response in Acne Vulgaris That Is Regulated by Vitamin A and Vitamin D. J. Investig. Dermatol. 2014, 134, 366–373. [Google Scholar] [CrossRef]
- Kistowska, M.; Meier, B.; Proust, T.; Feldmeyer, L.; Cozzio, A.; Kuendig, T.; Contassot, E.; French, L.E. Propionibacterium Acnes Promotes Th17 and Th17/Th1 Responses in Acne Patients. J. Investig. Dermatol. 2015, 135, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, U.; Wakita, D.; Ohkuri, T.; Chamoto, K.; Kitamura, H.; Iwakura, Y.; Nishimura, T. 1α,25-Dihydroxyvitamin D3 and All-Trans Retinoic Acid Synergistically Inhibit the Differentiation and Expansion of Th17 Cells. Immunol. Lett. 2010, 134, 7–16. [Google Scholar] [CrossRef]
- Abd-Elmaged, W.M.; Nada, E.A.; Hassan, M.H.; Elsadek, B.E.M.; Abdelrahim, E.A.; Ahmed, N.S.; Toghan, R.; Ahmed, H.T.I. Lesional and Circulating Levels of Interleukin-17 and 25-Hydroxycholecalciferol in Active Acne Vulgaris: Correlation to Disease Severity. J. Cosmet. Dermatol. 2019, 18, 671–676. [Google Scholar] [CrossRef]
- Singh, A.; Khurana, A.; Sardana, K.; Dixit, N.; Chitkara, A. Correlation of Serum 25-Hydroxy Vitamin D and Interleukin-17 Levels with Disease Severity in Acne Vulgaris. Indian. J. Dermatol. 2021, 66, 291–296. [Google Scholar] [CrossRef]
- Lim, S.K.; Ha, J.M.; Lee, Y.H.; Lee, Y.; Seo, Y.J.; Kim, C.D.; Lee, J.H.; Im, M. Comparison of Vitamin D Levels in Patients with and without Acne: A Case-Control Study Combined with a Randomized Controlled Trial. PLoS ONE 2016, 11, e0161162. [Google Scholar] [CrossRef]
- Kemeriz, F.; Tuncer, S.Ç.; Acar, E.M.; Tuğrul, B. Evaluation of 25-Hydroxy Vitamin D Levels and Disease Severity in Patients with Acne Vulgaris. Dermatol. Ther. 2020, 33, e13393. [Google Scholar] [CrossRef]
- Alhetheli, G.; Elneam, A.I.A.; Alsenaid, A.; Al-Dhubaibi, M. Vitamin D Levels in Patients with and without Acne and Its Relation to Acne Severity: A Case-Control Study. Clin. Cosmet. Investig. Dermatol. 2020, 13, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Toossi, P.; Azizian, Z.; Yavari, H.; Fakhim, T.H.; Hadi, S.; Amini, S.; Enamzade, R. Serum 25-Hydroxy Vitamin D Levels in Patients with Acne Vulgaris and Its Association with Disease Severity. Clin. Cases Miner. Bone Metab. 2015, 12, 238–242. [Google Scholar] [CrossRef] [PubMed]
- El-Ramly, A.Z.; Fawzi, M.M.T.; Mahmoud, S.B.; Abdel Ghaffar, M.M.; Shaker, O.G. Assessment of Serum Levels of Cathelicidin and Vitamin D in Acne Vulgaris. J. Egypt. Women’s Dermatol. Soc. 2016, 13, 99–105. [Google Scholar] [CrossRef]
- Lehmann, H.P.; Robinson, K.A.; Andrews, J.S.; Holloway, V.; Goodman, S.N. Acne Therapy: A Methodologic Review. J. Am. Acad. Dermatol. 2002, 47, 231–240. [Google Scholar] [CrossRef]
- Ahmed Mohamed, A.; Salah Ahmed, E.M.; Abdel-Aziz, R.T.A.; Eldeeb Abdallah, H.H.; El-Hanafi, H.; Hussein, G.; Abbassi, M.M.; El Borolossy, R. The Impact of Active Vitamin D on the Clinical Outcomes of Acne. J. Dermatol. Treat. 2021, 32, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Ruikchuchit, T.; Juntongjin, P. Role of Vitamin D Supplement Adjunct to Topical Benzoyl Peroxide in Acne: A Randomized Double-Blinded Controlled Study. Int. J. Womens Dermatol. 2024, 10, e163. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Desai, N.; Emtestam, L.; Hunger, R.E.; Ioannides, D.; Juhász, I.; Lapins, J.; Matusiak, L.; Prens, E.P.; Revuz, J.; et al. European S1 Guideline for the Treatment of Hidradenitis Suppurativa/Acne Inversa. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 619–644. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Bechara, F.G.; Benhadou, F.; Bettoli, V.; Bukvić Mokos, Z.; Del Marmol, V.; Dolenc-Voljč, M.; Giamarellos-Bourboulis, E.J.; Grimstad; Guillem, P.; et al. European S2k Guidelines for Hidradenitis Suppurativa/Acne Inversa Part 2: Treatment. J. Eur. Acad. Dermatol. Venereol. 2024, 39, 899–941. [Google Scholar] [CrossRef]
- Cosmatos, I.; Matcho, A.; Weinstein, R.; Montgomery, M.O.; Stang, P. Analysis of Patient Claims Data to Determine the Prevalence of Hidradenitis Suppurativa in the United States. J. Am. Acad. Dermatol. 2013, 68, 412–419. [Google Scholar] [CrossRef]
- E Jemec, G.B.; Heidenheim, M.; Henrik Nielsen, N. The Prevalence of Hidradenitis Suppurativa and Its Potential Precursor Lesions. J. Am. Acad. Dermatol. 1996, 35 Pt 1, 191–194. [Google Scholar] [CrossRef]
- Revuz, J.E.; Canoui-Poitrine, F.; Wolkenstein, P.; Viallette, C.; Gabison, G.; Pouget, F.; Poli, F.; Faye, O.; Roujeau, J.C.; Bonnelye, G.; et al. Prevalence and Factors Associated with Hidradenitis Suppurativa: Results from Two Case-Control Studies. J. Am. Acad. Dermatol. 2008, 59, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Markota Čagalj, A.; Marinović, B.; Bukvić Mokos, Z. New and Emerging Targeted Therapies for Hidradenitis Suppurativa. Int. J. Mol. Sci. 2022, 23, 3753. [Google Scholar] [CrossRef] [PubMed]
- Thorlacius, L.; Garg, A.; Riis, P.T.; Nielsen, S.M.; Bettoli, V.; Ingram, J.R.; Del Marmol, V.; Matusiak, L.; Pascual, J.C.; Revuz, J.; et al. Inter-Rater Agreement and Reliability of Outcome Measurement Instruments and Staging Systems Used in Hidradenitis Suppurativa. Br. J. Dermatol. 2019, 181, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Daoud, M.; Suppa, M.; Benhadou, F.; Daxhelet, M.; Njimi, H.; White, J.; Jemec, G.; Del Marmol, V. Overview and Comparison of the Clinical Scores in Hidradenitis Suppurativa: A Real-Life Clinical Data. Front. Med. 2023, 10, 1145152. [Google Scholar] [CrossRef]
- Hamzavi, I.H.; Sundaram, M.; Nicholson, C.; Zivkovic, M.; Parks-Miller, A.; Lee, J.; Yi, J.; Gu, Y.; Okun, M.M.; Ganguli, A.; et al. Uncovering Burden Disparity: A Comparative Analysis of the Impact of Moderate-to-Severe Psoriasis and Hidradenitis Suppurativa. J. Am. Acad. Dermatol. 2017, 77, 1038–1046. [Google Scholar] [CrossRef]
- Alya, A.S.; Saleh, A.M.; Sawsan, A.S.; Louai, S.; AlSiyoufi, A.M. Psychosocial Implications and Quality of Life in Patients with Hidradenitis Suppurativa Compared to Those With Atopic Dermatitis and Psoriasis: A Cross-Sectional Case-Control Study. Dermatol. Pract. Concept. 2023, 13, e2023076. [Google Scholar] [CrossRef]
- Kirby, J.S.; Thorlacius, L.; Villumsen, B.; Ingram, J.R.; Garg, A.; Christensen, K.B.; Butt, M.; Esmann, S.; Tan, J.; Jemec, G.B.E. The Hidradenitis Suppurativa Quality of Life (HiSQOL) Score: Development and Validation of a Measure for Clinical Trials. Br. J. Dermatol. 2020, 183, 340–348. [Google Scholar] [CrossRef]
- Fitzsimmons, J.S.; Guilbert, P.R. A Family Study of Hidradenitis Suppurativa. J. Med. Genet. 1985, 22, 367–373. [Google Scholar] [CrossRef]
- Pink, A.E.; Simpson, M.A.; Desai, N.; Dafou, D.; Hills, A.; Mortimer, P.; Smith, C.H.; Trembath, R.C.; Barker, J.N.W. Mutations in the γ-Secretase Genes NCSTN, PSENEN, and PSEN1 Underlie Rare Forms of Hidradenitis Suppurativa (Acne Inversa). J. Investig. Dermatol. 2012, 132, 2459–2461. [Google Scholar] [CrossRef]
- Von Laffert, M.; Helmbold, P.; Wohlrab, J.; Fiedler, E.; Stadie, V.; Marsch, W.C. Hidradenitis Suppurativa (Acne Inversa): Early Inflammatory Events at Terminal Follicles and at Interfollicular Epidermis. Exp. Dermatol. 2010, 19, 533–537. [Google Scholar] [CrossRef]
- Vossen, A.R.J.V.; van der Zee, H.H.; Prens, E.P. Hidradenitis Suppurativa: A Systematic Review Integrating Inflammatory Pathways Into a Cohesive Pathogenic Model. Front. Immunol. 2018, 9, 2965. [Google Scholar] [CrossRef] [PubMed]
- Scala, E.; Cacciapuoti, S.; Garzorz-Stark, N.; Megna, M.; Marasca, C.; Seiringer, P.; Volz, T.; Eyerich, K.; Fabbrocini, G. Hidradenitis Suppurativa: Where We Are and Where We Are Going. Cells 2021, 10, 2094. [Google Scholar] [CrossRef] [PubMed]
- Bukvić Mokos, Z.; Miše, J.; Balić, A.; Marinović, B. Understanding the Relationship Between Smoking and Hidradenitis Suppurativa. Acta Dermatovenerol. Croat. 2020, 28, 9–13. [Google Scholar]
- Langan, E.A.; Recke, A.; Bokor-billmann, T.; Billmann, F.; Kahle, B.K.; Zillikens, D. The Role of the Cutaneous Microbiome in Hidradenitis Suppurativa—Light at the End of the Microbiological Tunnel. Int. J. Mol. Sci. 2020, 21, 1205. [Google Scholar] [CrossRef]
- Lackner, L.; Zyriax, B.C.; Stephan, B. To What Extent Does Vitamin D and Its Serum Levels Influence the Severity of Hidradenitis Suppurativa: A Literature Review. Acta Derm. Venereol. 2024, 104, adv40321. [Google Scholar] [CrossRef]
- Osman, A.; Ralston, M.J.; Povelaitis, M.; Handler, M.Z. Relationship of Vitamin D to Pathogenesis and Outcomes of Hidradenitis Suppurativa: A Systematic Review. Arch. Dermatol. Res. 2025, 317, 29. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Agrawal, D.K. Vitamin D and Inflammatory Diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar]
- Ojaimi, S.; Skinner, N.A.; Strauss, B.J.G.; Sundararajan, V.; Woolley, I.; Visvanathan, K. Vitamin D Deficiency Impacts on Expression of Toll-like Receptor-2 and Cytokine Profile: A Pilot Study. J. Transl. Med. 2013, 11, 176. [Google Scholar] [CrossRef]
- Lee, W.J.; Cha, H.W.; Sohn, M.Y.; Lee, S.-J.; Kim, D.W. Vitamin D Increases Expression of Cathelicidin in Cultured Sebocytes. Arch. Dermatol. Res. 2012, 304, 627–632. [Google Scholar] [CrossRef]
- Brandao, L.; Moura, R.; Tricarico, P.M.; Gratton, R.; Genovese, G.; Moltrasio, C.; Garcovich, S.; Boniotto, M.; Crovella, S.; Marzano, A.V. Altered Keratinization and Vitamin D Metabolism May Be Key Pathogenetic Pathways in Syndromic Hidradenitis Suppurativa: A Novel Whole Exome Sequencing Approach. J. Dermatol. Sci. 2020, 99, 17–22. [Google Scholar] [CrossRef]
- Yu, C.C.; Cook, M.G. Hidradenitis Suppurativa: A Disease of Follicular Epithelium, Rather than Apocrine Glands. Br. J. Dermatol. 1990, 122, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Vural, S.; Baskurt, D.; Yıldırıcı, Ş.; Rasulova, G.; Danacı, S.; Botsalı, A. Evaluating Dietary Considerations in Hidradenitis Suppurativa: A Critical Examination of Existing Knowledge. Int. J. Dermatol. 2024, 63, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Fabbrocini, G.; Marasca, C.; Luciano, M.A.; Guarino, M.; Poggi, S.; Fontanella, G.; Cacciapuoti, S. Vitamin D Deficiency and Hidradenitis Suppurativa: The Impact on Clinical Severity and Therapeutic Responsivity. J. Dermatol. Treat. 2021, 32, 843–844. [Google Scholar] [CrossRef]
- Moltrasio, C.; Tricarico, P.M.; Genovese, G.; Gratton, R.; Marzano, A.V.; Crovella, S. 25-Hydroxyvitamin D Serum Levels Inversely Correlate to Disease Severity and Serum C-Reactive Protein Levels in Patients with Hidradenitis Suppurativa. J. Dermatol. 2021, 48, 715–717. [Google Scholar] [CrossRef]
- Seetan, K.; Eldos, B.; Saraireh, M.; Omari, R.; Rubbai, Y.; Jayyusi, A.; Jubran, M.A. Prevalence of Low Vitamin D Levels in Patients with Hidradenitis Suppurativa in Jordan: A Comparative Cross-Sectional Study. PLoS ONE 2022, 17, e0265672. [Google Scholar] [CrossRef]
- Koumaki, D.; Gregoriou, S.; Evangelou, G.; Rovithi, E.; Koumaki, V.; Petrou, D.; Solia Apokidou, E.; Ioannou, P.; Katoulis, A.; Papadakis, M.; et al. Vitamin D Deficiency as a Predictor of Hidradenitis Suppurativa Severity. Int. J. Dermatol. 2024, 64, 571–574. [Google Scholar] [CrossRef]
- Lee, J.W.; Heo, Y.W.; Lee, J.H.; Lee, S. Epidemiology and Comorbidity of Hidradenitis Suppurativa in Korea for 17 Years: A Nationwide Population-Based Cohort Study. J. Dermatol. 2023, 50, 778–786. [Google Scholar] [CrossRef]
- Navarro, I.; González-López, M.A.; Sierra, I.; Olmos, J.M.; Blanco, R.; Hernández, J.L. Bone Metabolism in Patients with Hidradenitis Suppurativa: A Case-Control Study. Acta Derm. Venereol. 2022, 102, adv00825. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-díaz, M.; Salvador-rodríguez, L.; Montero-vílchez, T.; Martínez-lópez, A.; Arias-santiago, S.; Molina-leyva, A. Cumulative Inflammation and Hba1c Levels Correlate with Increased Intima-media Thickness in Patients with Severe Hidradenitis Suppurativa. J. Clin. Med. 2021, 10, 5222. [Google Scholar] [CrossRef]
- Guillet, A.; Brocard, A.; Bach Ngohou, K.; Graveline, N.; Leloup, A.G.; Ali, D.; Nguyen, J.M.; Loirat, M.J.; Chevalier, C.; Khammari, A.; et al. Verneuil’s Disease, Innate Immunity and Vitamin D: A Pilot Study. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1347–1353. [Google Scholar] [CrossRef]
- Toker, M.; Ch’en, P.Y.; Rangu, S.; Campton, K.L.; Cohen, S.R. Vitamin D Deficiency May Be Associated with Severity of Hidradenitis Suppurativa: A Retrospective Cohort Analysis of a Racially and Ethnically Diverse Patient Population. Int. J. Dermatol. 2024, 63, e43–e44. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.; Sweeney, C.M.; Fitzgerald, R.; O’Keane, M.P.; Kilbane, M.; Lally, A.; Tobin, A.M.; McKenna, M.J.; Kirby, B. Vitamin D Status in Hidradenitis Suppurativa. Br. J. Dermatol. 2014, 170, 1379–1380. [Google Scholar] [CrossRef] [PubMed]
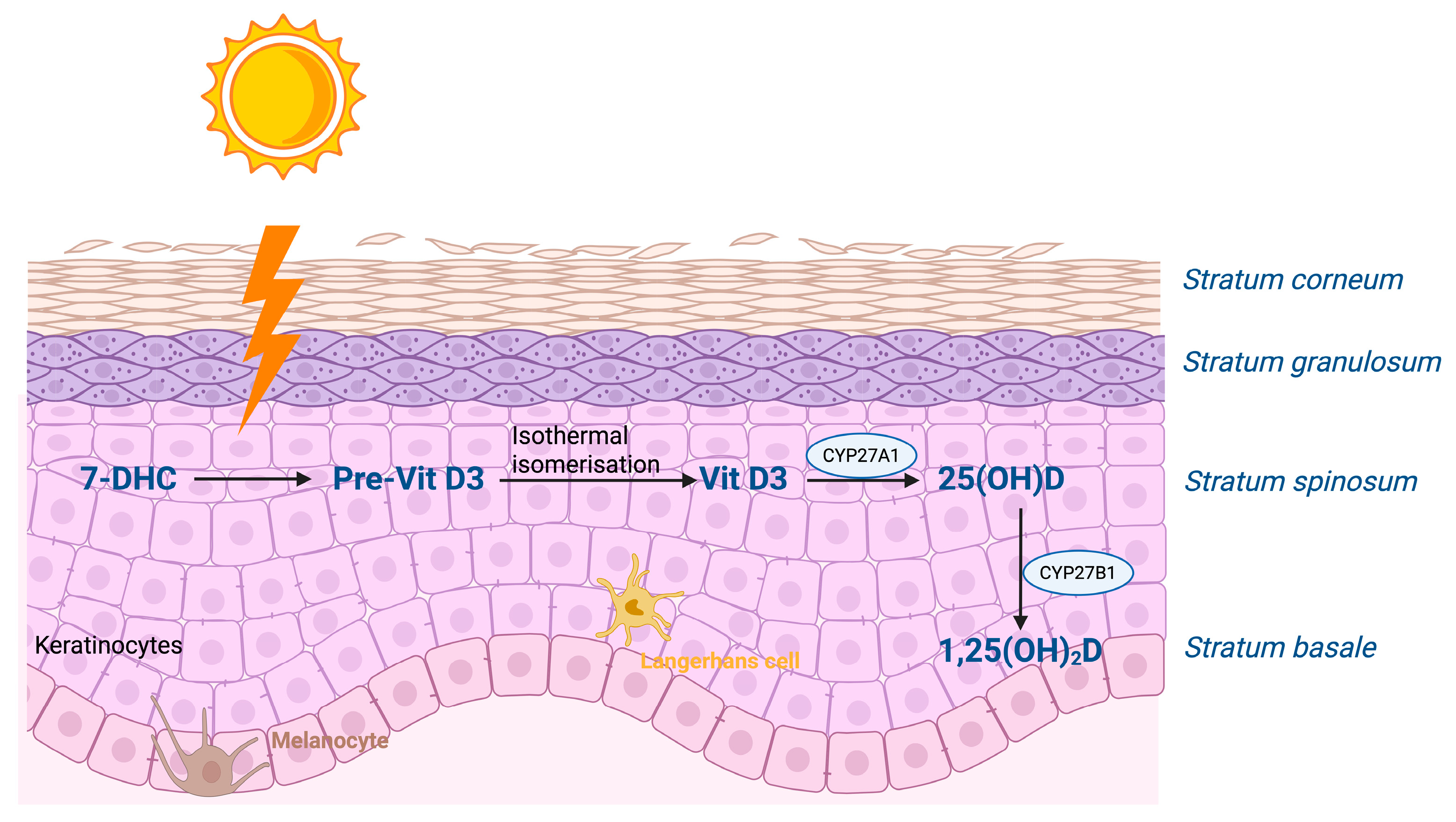
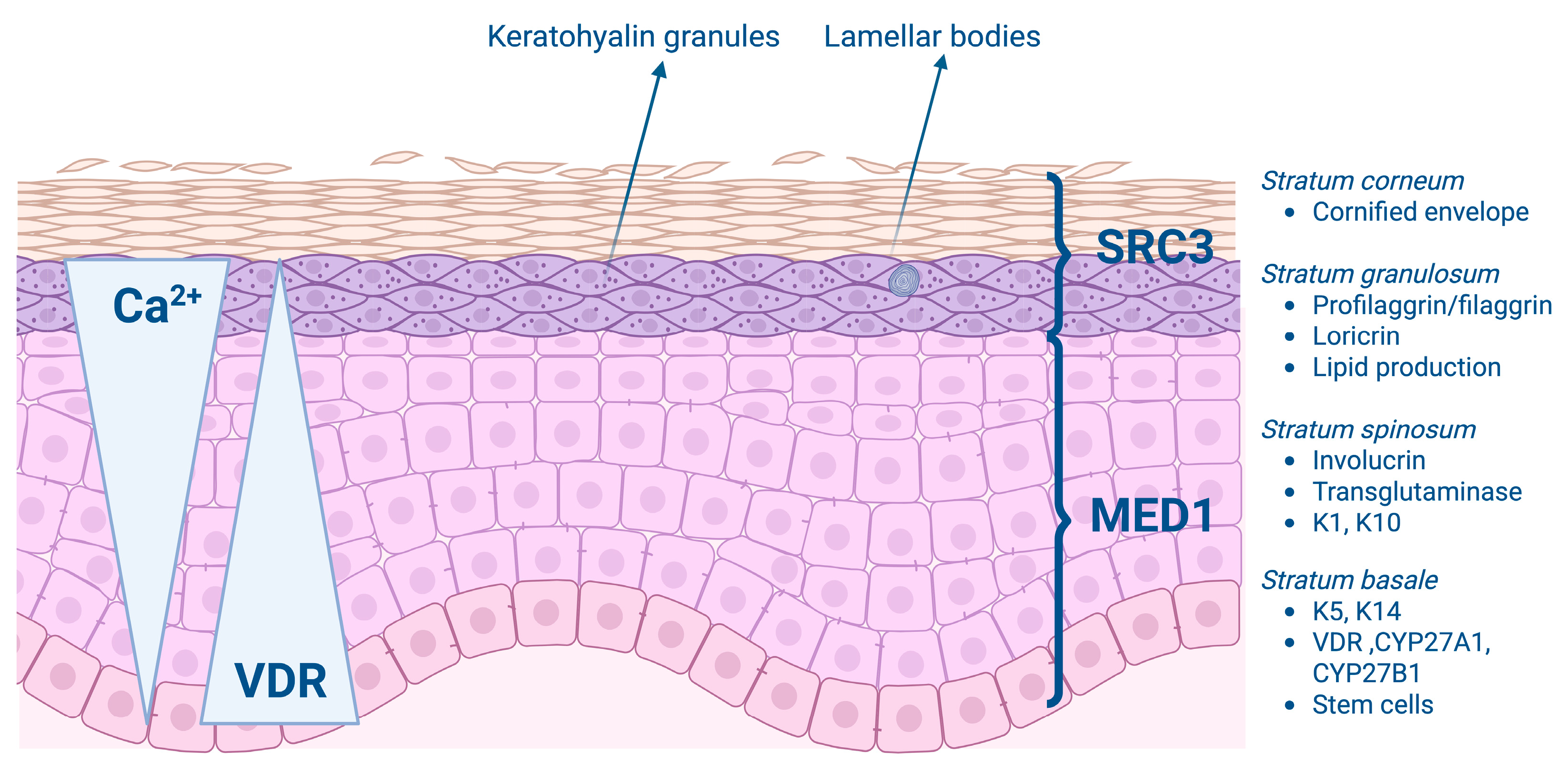
| Author, Year [Reference Number] | Study Design | N | Country | Serum Vitamin D Levels in Psoriasis Compared to Controls | Correlation Between Serum Vitamin D Levels and PASI Scores Without Vitamin D Supplementation | Vitamin D Supplementation: Outcomes |
|---|---|---|---|---|---|---|
| Chandrashekar et al., 2015. [57] | Cross-sectional | 43 | India | Significantly lower (p = 0.004) |
| N/A |
| Maleki et al., 2015. [60] | Case-control | 50 | Iran | Not statistically significant (p = 0.06) |
| N/A |
| Bergler-Czop et. al., 2016. [61] | Case-control | 40 | Poland | Significantly lower (p = 0.048) |
| N/A |
| Pokharel R. et al., 2022. [58] | Case- control | 120 | Nepal | Significantly lower (p = 0.001) |
| N/A |
| Disphanurat et al., 2019. [59] | RCT | 45 | Thailand | N/A |
|
|
| Prtina et al., 2021. [69] | Prospective clinical study | 40 | Bosnia and Herzegovina | N/A |
|
|
| Ingram et al., 2018. [63] | RCT | 101 | New Zealand | N/A |
|
|
| Jarrett et al., 2017. [64] | RCT | 65 | New Zealand | N/A |
|
|
| Author, Year [Reference Number] | Study Design | N | Country | Serum Vitamin D Levels in AD Compared to Controls | Vitamin D Levels and HS Severity Correlation |
|---|---|---|---|---|---|
| El Taieb et al., 2013 [93] | Case-control Study | 29 children | Egypt |
|
|
| D’Auria et al., 2017 [94] | Case-control Study | 52 children | Italy |
|
|
| Su et al., 2014 [103] | RCT | 60 children | Turkey |
|
|
| Samochocki et al., 2013 [104] | Cross-sectional Study | 95 adults | Poland |
|
|
| Han et al., 2015 [95] | Cross-sectional Study | 72 | Korea |
|
|
| Wang et al., 2014 [96] | Cross-sectional Study | 498 children | Hong Kong |
|
|
| Munawwarah et al., 2018 [101] | Cross-sectional Study | 26 children | Indonesia | N/A |
|
| Peroni et al., 2011 [90] | Cross-sectional Study | 37 children | Italy | N/A |
|
| Author, Year [Reference Number] | Study Design | N | Country | Serum Vitamin D Levels in AD Compared to Controls | Vitamin D Supplementation (Dosage) | Vitamin D Supplementation Outcomes |
|---|---|---|---|---|---|---|
| Camargo et al., 2014 [109] | RCT | 107 children | Mongolia |
|
|
|
| Mansour et al., 2020 [107] | RCT | 86 | Egypt | N/A |
|
|
| Amestejani et al., 2012 [110] | RCT | 60 | Iran | N/A |
|
|
| Sidbury et al., 2008 [111] | RCT | 11 children | USA |
|
|
|
| Samochocki et al., 2013 [104] | Cross-sectional Study | 95 adults | Poland |
|
|
|
| Author, Year [Reference Number] | Study Design | N | Country | Significant Findings | Correlation Between Vitamin D Levels and Acne Severity |
|---|---|---|---|---|---|
| Lim et al., 2016 [149] | Case-control study | 80 | Korea |
|
|
| Singh et al., 2021 [148] | Cross-sectional observational study | 50 | India |
|
|
| Kemeriz et al., 2019 [150] | Single-centered, prospective, and controlled study | 134 | Turkey |
|
|
| Abd-Elmaged et al., 2020 [147] | Case-control, hospital-based study | 135 | Egypt |
|
|
| Alhetheli et al., 2020 [151] | Cross-sectional study | 68 | Saudi Arabia |
|
|
| Toossi et al., 2015 [152] | Cross-sectional study | 39 | Iran |
|
|
| El-Ramly et al., 2016 [153] | Cross-sectional study | 60 | Egypt |
|
|
| Author, Year [Rference Number] | Study Design | N | Country | Significant Findings | Correlation Between Vitamin D Levels and HS Severity |
|---|---|---|---|---|---|
| Fabbrocini et al., 2021 [183] | controlled cohort study | 40 | Italy |
|
|
| Navarro et al., 2022 [188] | prospective case-control study | 81 | Spain |
|
|
| Seetan et al., 2022 [185] | comparative cross-sectional study | 110 | Jordan |
|
|
| Moltrasio et al., 2021 [184] | retrospective cross-sectional study | 250 | Italy |
|
|
| Koumaki et al., 2024 [186] | cross-sectional study | 136 | Greece |
|
|
| Toker et al., 2024 [191] | retrospective chart review | 198 | USA |
|
|
| Sánchez-Díaz et al., 2021 [189] | cross-sectional study | 50 | Spain |
|
|
| Guillet et al. 2015 [190] | pilot study | 22 | France |
|
|
| Brandao et al., 2020 [180] | case series | 5 | Italy |
| N/A |
| Kelly et al., 2014 [192] | cross-sectional study | 16 | Ireland |
| N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukvić Mokos, Z.; Tomić Krsnik, L.; Harak, K.; Marojević Tomić, D.; Tešanović Perković, D.; Vukojević, M. Vitamin D in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2025, 26, 5005. https://doi.org/10.3390/ijms26115005
Bukvić Mokos Z, Tomić Krsnik L, Harak K, Marojević Tomić D, Tešanović Perković D, Vukojević M. Vitamin D in the Prevention and Treatment of Inflammatory Skin Diseases. International Journal of Molecular Sciences. 2025; 26(11):5005. https://doi.org/10.3390/ijms26115005
Chicago/Turabian StyleBukvić Mokos, Zrinka, Lucija Tomić Krsnik, Kristijan Harak, Danijela Marojević Tomić, Deša Tešanović Perković, and Marija Vukojević. 2025. "Vitamin D in the Prevention and Treatment of Inflammatory Skin Diseases" International Journal of Molecular Sciences 26, no. 11: 5005. https://doi.org/10.3390/ijms26115005
APA StyleBukvić Mokos, Z., Tomić Krsnik, L., Harak, K., Marojević Tomić, D., Tešanović Perković, D., & Vukojević, M. (2025). Vitamin D in the Prevention and Treatment of Inflammatory Skin Diseases. International Journal of Molecular Sciences, 26(11), 5005. https://doi.org/10.3390/ijms26115005






