Nanosensors and Microsensors for Body Fluid Monitoring: Various Analyte Detection and Construction Solutions
Abstract
1. Introduction
2. Types of Body Fluids and Their Diagnostic Importance
3. Fundamentals and Classification of Microsensors and Nanosensors
3.1. Definition and Working Principles
- Electrochemical—measures electrical signals (e.g., current, voltage, impedance) produced by the interaction of the analyte with the sensor [35];
- Optical—uses fluorescence, absorbance, or other light-based methods to detect biomolecular interactions [17];
- Piezoelectric—detects changes in mass or mechanical forces by monitoring shifts in the resonant frequency [36];
- Field-effect transistor (FET)-based—uses semiconducting materials to modulate the sensor’s electrical conductivity in response to analyte binding [37].
3.2. Classification Based on Transduction Mechanism
3.2.1. Electrochemical Sensors
3.2.2. Optical Sensors
3.2.3. Piezoelectric Sensors
3.2.4. Field-Effect Transistor (FET)-Based Sensors
3.3. Classification Based on Materials Used
3.3.1. Carbon-Based Sensors
3.3.2. Metal Nanoparticles
3.3.3. Quantum Dots and Nanowires
3.3.4. Polymer-Based Sensors
4. Construction Solutions for Microsensors and Nanosensors
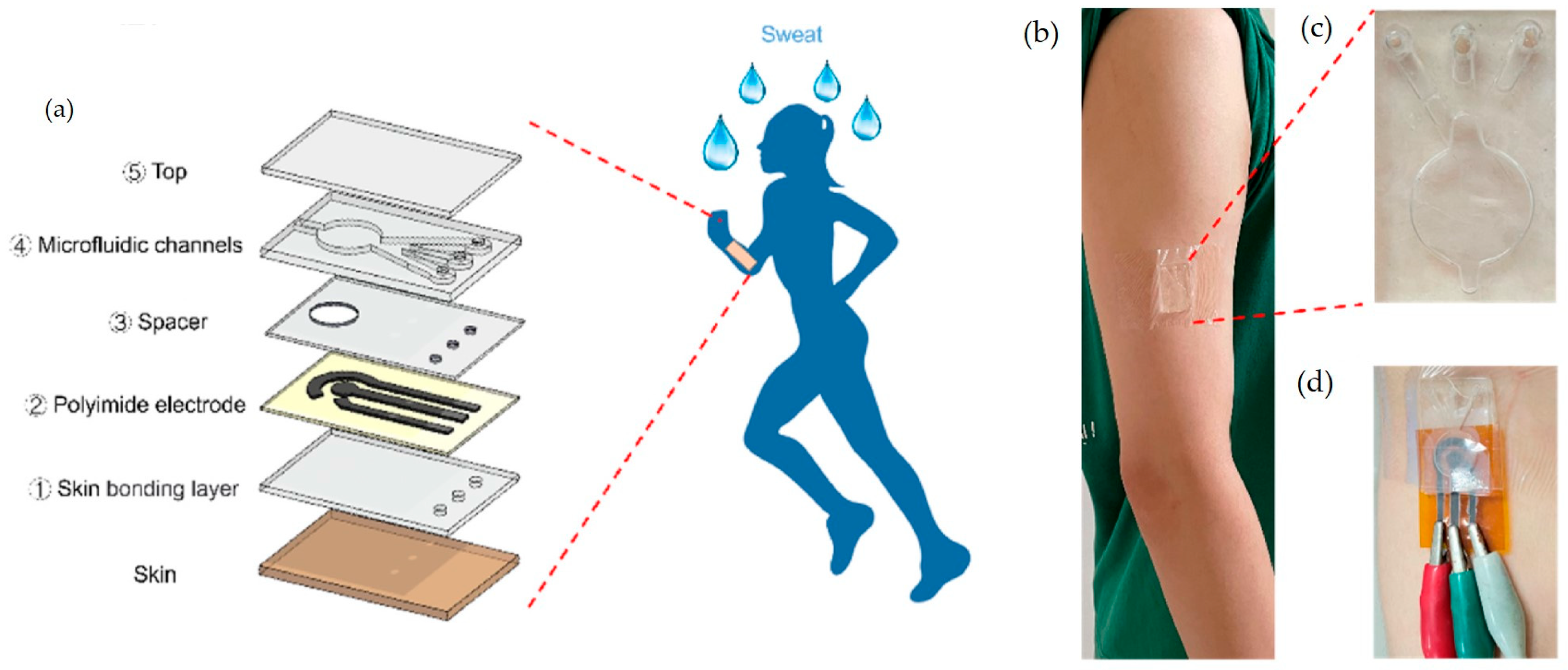
4.1. Microfluidic-Based Sensors
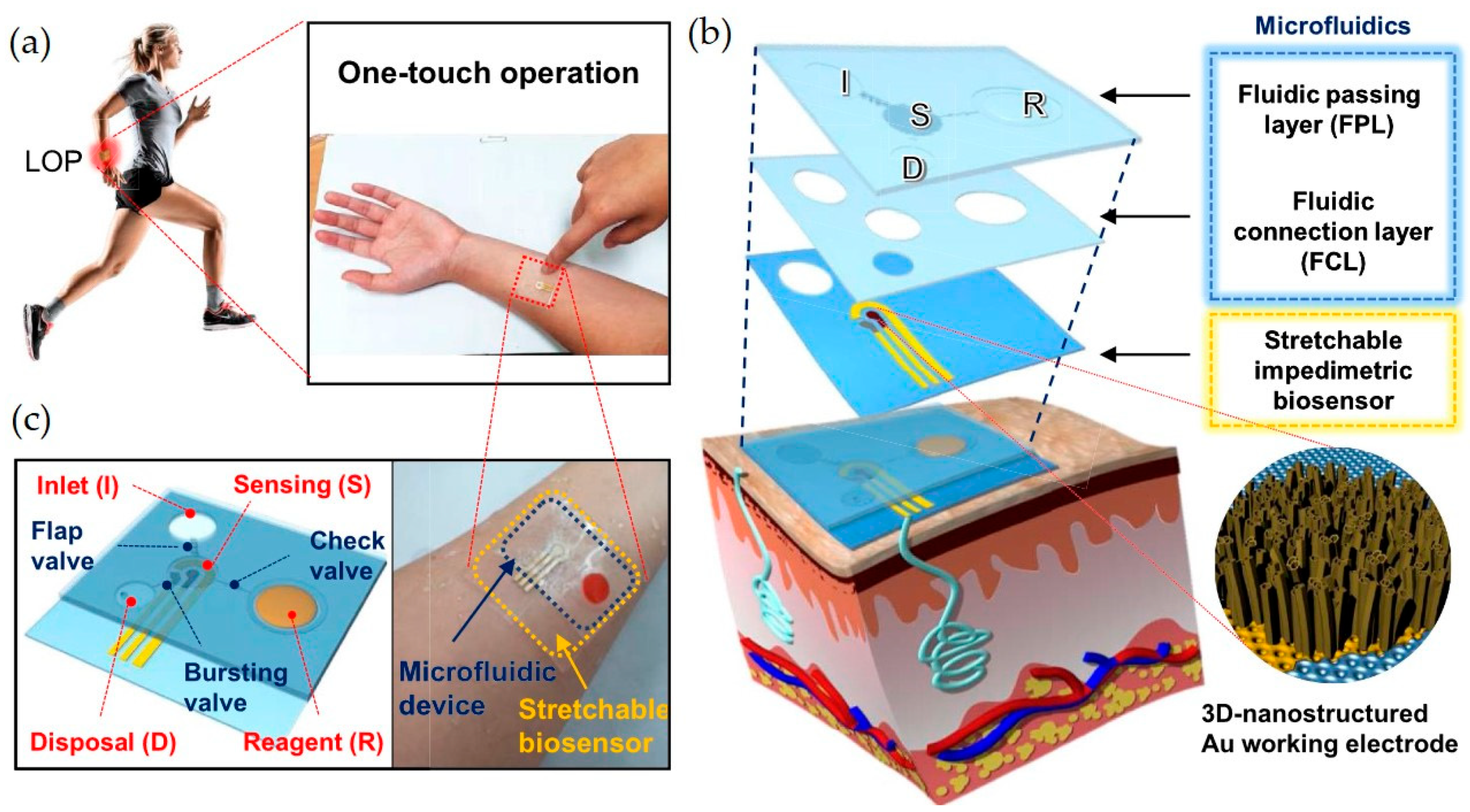
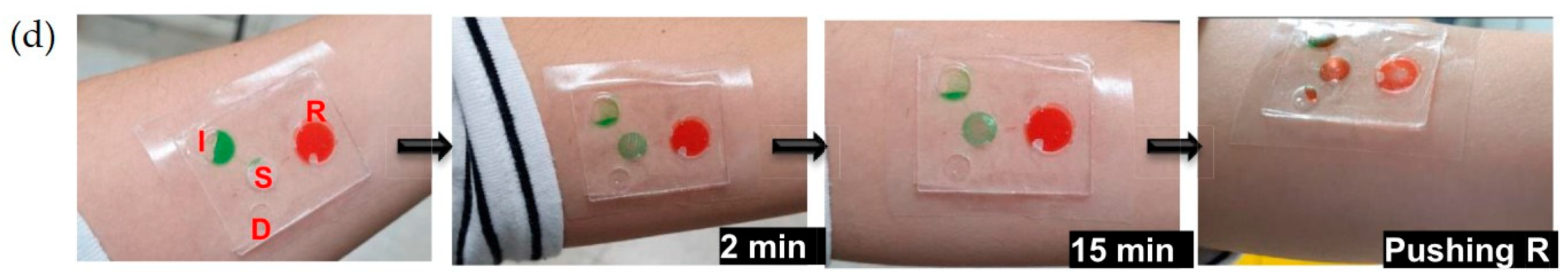
4.2. Lab-on-a-Chip (LoC) Systems
4.3. MEMS-Based Sensors
4.4. Integration with Smart Wearables and Implantables
5. Targeted Analytes for Microsensors and Nanosensors for Body Fluid Monitoring
5.1. Metabolites
| Analyte | Sensor Construction | Body Fluid | Method of Detection | Limit of Detection | Reference |
|---|---|---|---|---|---|
| Glucose | Wearable: microneedle (MN)-based assays | interstitial fluid (ISF) | electrochemical | 7.44 mM | [100] |
| Glucose | Wearable platform | sweat | electrochemical | - | [116] |
| Glucose | Thin-film holographic sensor | blood serum | optical | 3 mM | [99] |
| Glucose | Wearable sensor array | sweat | electrochemical | 2.35 nA/μM (sensitivity) | [117] |
| Lactate | Wearable: microneedle (MN)-based array | interstitial fluid (ISF) | electrochemical | 4.43 mM | [100] |
| Lactate | Eyeglasses-based wireless sensor platform | sweat | electrochemical | 0.39 mM | [101] |
| Lactate | Microneedle Array | blood | electrochemical | - | [118] |
| Lactate | Microneedle sensor patch | interstitial fluid (ISF) | electrochemical | 0.25 mM | [102] |
| Lactate | Wearable sensing interface | sweat | electrochemical | 1 mM | [119] |
| Lactate | Wearable sensor array | sweat | electrochemical | 220 nA/mM (sensitivity) | [117] |
| Lactate | Temporary-transfer tattoo | sweat | electrochemical | 1 mM | [103] |
| Uric acid | Microfluidic-based plasmonic microneedle | interstitial fluid (ISF) | Surface-Enhanced Raman Spectroscopy | 0.51 µM | [109] |
| Uric acid | Screen-printed electrode modified with gold nanoparticles (SPE-AuNps) | saliva | electrochemical | 11.91 μM | [110] |
| Uric acid | green synthesized silver nanoparticles (Ag NPs) | blood | colorimetric | 0.004 μM | [120] |
| Uric acid | Laser-engraved wearable sensor | sweat | electrochemical | 0.74 μM | [111] |
| Creatinine | Self-powered piezoelectric biosensor | sweat | piezoelectric | 1 × 10−5 mM | [112] |
| Cholesterol | Skin-worn microneedle sensor | sweat | electrochemical | 0.5 μM | [114] |
| Cholesterol | Smart contact lens | tears | electrochemical | 9.91 μm | [29] |
5.2. Electrolytes
| Analyte | Sensor Construction | Body Fluid | Method of Detection | Limit of Detection | Reference |
|---|---|---|---|---|---|
| Potassium | Eyeglasses-based wireless sensor platform | sweat | electrochemical | 10−3.9 M | [101] |
| Potassium | Wearable sensor array | sweat | electrochemical | 1 mM | [117] |
| Sodium | Wearable sensor array | sweat | electrochemical | 10 mM | [117] |
| Sodium | Epidermal tattoo | sweat | electrochemical | - | [122] |
| Sodium | Wearable platform | sweat | electrochemical | - | [18] |
| Sodium | Fluorescent dermal tattoo | interstitial fluid | optical | 100 mmol/L | [128] |
| Potassium | Fluorescent dermal tattoo | interstitial fluid | optical | 2 mmol/L | [128] |
| Hydrogen | Fluorescent dermal tattoo | interstitial fluid | optical | 6.6 (pH) | [128] |
| Hydrogen | Wearable Electrochemical Platform | sweat, urine, tears | electrochemical | - | [124] |
| Calcium | Wearable Electrochemical Platform | sweat, urine, tears | electrochemical | - | [124] |
| Hydrogen | Potentiometric Nanosensor | interstitial fluid | electrochemical | 6.0 (pH) | [129] |
| Hydrogen | Graphene Field-Effect Transistor (GFET) | electrolyte artificial solution | FET | 5.3 (pH) | [130] |
| Hydrogen | Stretchable wireless system | sweat | electrochemical | 5.0 (pH) | [123] |
5.3. Proteins and Peptides
| Analyte | Sensor Construction | Body Fluid | Method of Detection | Limit of Detection | Reference |
|---|---|---|---|---|---|
| CRP | Microfluidic wireless patch | sweat | electrochemical | - | [139] |
| CRP | Microfluidic Chip | blood | optical | 1 μg/mL | [134] |
| PSA | PEG/PEDOT nanocomposite—based biosensors | serum | electrochemical | 0.035 pg/mL | [137] |
| PSA | optical biosensor | blood | optical | 0.145 fg/mL | [138] |
5.4. Hormones
| Analyte | Sensor Construction | Body Fluid | Method of Detection | Limit of Detection | Reference |
|---|---|---|---|---|---|
| Cortisol | Graphene flexible sensor array | saliva, sweat | electrochemical | 0.08 ng/mL | [142] |
| Cortisol | MIP stretchable sensor | sweat | electrochemical | 0.2 × 10−9 M | [143] |
| Cortisol | Wearable microcapillary channel array | sweat | electrochemical | - | [144] |
| Cortisol | Field-Effect Transistor sensor | sweat | FET | 1 ng/mL | [145] |
| Interleukin-6 | Room Temperature Ionic Liquids (RTILs)—based sensor | sweat | optical | 0.2 pg/mL | [146] |
5.5. Nucleic Acids
| Analyte | Sensor Construction | Body Fluid | Method of Detection | Limit of Detection | Reference |
|---|---|---|---|---|---|
| SARS-CoV-2 | Electrochemical microsensor | blood serum | electrochemical | 0.01 ng/mL | [148] |
| SARS-CoV-2 | Microcavity-based Optical Fiber sensor | blood serum | optical | Single ng/mL | [149] |
| SARS-CoV-2 | nanobody-based photonic nanosensor | blood serum | optical | 598 FFU/mL | [151] |
| SARS-CoV-2 | Field-Fffect Transistor (FET)-based biosensing device | blood serum | FET | 2.42 × 102 copies/mL | [150] |
5.6. Drugs and Therapeutic Monitoring
| Analyte | Sensor Construction | Body Fluid | Method of Detection | Limit of Detection | Reference |
|---|---|---|---|---|---|
| Levodopa | Wearable sweat band on a nanodendritic platform | sweat | electrochemical | 1.25 μM | [155] |
| Levodopa | Wearable patch | sweat | electrochemical | - | [156] |
| Levodopa | Wearable Electrochemical Microneedle Sensor | interstitial fluid (ISF) | electrochemical | 0.5 μM | [157] |
| Caffeine | Wearable sensor | sweat | electrochemical | 3 × 10−6 M | [158] |
6. Emerging Technologies and Trends
6.1. Artificial Intelligence and Machine Learning for Sensor Data Processing
6.2. Internet of Things (IoT) and Wireless Sensing
6.3. Flexible and Stretchable Sensors
7. Challenges and Future Perspectives
7.1. Biocompatibility, Stability and Reversibility Issues
7.2. Calibration and Interferences
7.3. Real-Time Data Processing and Interpretation
7.4. Regulatory and Ethical Considerations
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MEMS | microelectromechanical systems |
| LOC | lab-on-a-chip |
| LOP | lab-on-a-patch |
| SoC | systems-on-chip |
| SiP | system-in-package |
| MOEMS | micro-optoelectromechanical systems |
| IoT | internet of things |
| GMC | continuous glucose monitoring |
| PSA | prostate-specific antigen |
| ISF | interstitial fluid |
| FET | field-effect transistor |
| CQDs | carbon quantum dots |
| QDs | quantum dots |
| SiNW | silicon nanowires |
| NFC | near-field communication |
| CNTs | carbon nanotubes |
| GO | graphene oxide |
| DNA | deoxyribonucleic acid |
| SERS | surface-enhanced raman spectroscopy |
| PANI | polyaniline |
| PPy | polypyrrole |
| PT | polythiophene |
| PDMS | polydimethylsiloxane |
| PEDOT | poly(3,4-ethylenedioxythiophene |
| MIPs | molecularly imprinted polymers |
| µTAS | miniaturized total analysis system |
| POC | point-of-care |
| AuNR | gold nanorods |
| GFR | glomerular filtration rate |
| CKD | chronic kidney disease |
| MN | microneedle |
| NPs | nanoparticles |
| SPE | Screen-printed electrode |
| CRP | C-reactive protein |
| IL-6 | interleukin-6 |
| RNA | ribonucleic acid |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| TDM | therapeutic drug monitoring |
| AI | artificial intelligence |
| PEG | polyethylene glycol |
| FDA | Food and Drug Administration |
References
- Xing, Z.; Hui, J.; Lin, B.; Wu, Z.; Mao, H. Recent Advances in Wearable Sensors for the Monitoring of Sweat: A Comprehensive Tendency Summary. Chemosensors 2023, 11, 470. [Google Scholar] [CrossRef]
- Khazaei, M.; Hosseini, M.S.; Haghighi, A.M.; Misaghi, M. Nanosensors and Their Applications in Early Diagnosis of Cancer. Sens. Bio-Sens. Res. 2023, 41, 100569. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, S.; Qin, Y.; Xia, X.; Sun, Y.; Han, G.; Shu, T.; Hu, L.; Zhang, Q. Flexible and Wearable Biosensors for Monitoring Health Conditions. Biosensors 2023, 13, 630. [Google Scholar] [CrossRef] [PubMed]
- Aftab, S.; Koyyada, G.; Rubab, N.; Assiri, M.A.; Truong, N.T.N. Advances in Wearable Nanomaterial-Based Sensors for Environmental and Health Monitoring: A Comprehensive Review. J. Environ. Chem. Eng. 2025, 13, 115788. [Google Scholar] [CrossRef]
- Verma, D.; Singh, K.R.; Yadav, A.K.; Nayak, V.; Singh, J.; Solanki, P.R.; Singh, R.P. Internet of Things (IoT) in Nano-Integrated Wearable Biosensor Devices for Healthcare Applications. Biosens. Bioelectron. X 2022, 11, 100153. [Google Scholar] [CrossRef]
- Palanisamy, P.; Padmanabhan, A.; Ramasamy, A.; Subramaniam, S. Remote Patient Activity Monitoring System by Integrating IoT Sensors and Artificial Intelligence Techniques. Sensors 2023, 23, 5869. [Google Scholar] [CrossRef]
- Morris, A.S.; Langari, R. Chapter 13–Sensor Technologies. In Measurement and Instrumentation, 2nd ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 375–405. [Google Scholar] [CrossRef]
- Ghorbian, M.; Ghobaei-Arani, M.; Babaei, M.R.; Ghorbian, S. Nanotechnology and Nanosensors in Personalized Healthcare: A Comprehensive Review. Sens. Bio-Sens. Res. 2025, 47, 100740. [Google Scholar] [CrossRef]
- Peringeth, K.; Ganguly, A.; Pal, A.; Roy Chowdhury, J.; Kaswan, K.; Ho, H.Y.; Yu, J.H.; Kao, F.C.; Lin, Z.H. Self-Powered Microfluidic-Based Sensor for Noninvasive Sweat Analysis. Sens. Actuators B Chem. 2025, 423, 136859. [Google Scholar] [CrossRef]
- Abbasnia Tehrani, M.; Ahmadi, S.H.; Alimohammadi, S.; Sasanpour, P.; Batvani, N.; Kazemi, S.H.; Kiani, M.A. Continuous Glucose Monitoring Using Wearable Non-Enzymatic Sensors in a Physiological Environment. Biosens. Bioelectron. X 2024, 18, 100482. [Google Scholar] [CrossRef]
- Pour, S.R.S.; Calabria, D.; Emamiamin, A.; Lazzarini, E.; Pace, A.; Guardigli, M.; Zangheri, M.; Mirasoli, M. Microfluidic-Based Non-Invasive Wearable Biosensors for Real-Time Monitoring of Sweat Biomarkers. Biosensors 2024, 14, 29. [Google Scholar] [CrossRef]
- Premanode, B.; Toumazou, C. A Novel, Low Power Biosensor for Real Time Monitoring of Creatinine and Urea in Peritoneal Dialysis. Sens. Actuators B Chem. 2007, 120, 732–735. [Google Scholar] [CrossRef]
- Block, D.R.; Genzen, J.R. Chapter 27–Diagnostic Body Fluid Testing. In Contemporary Practice in Clinical Chemistry, 4th ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 469–486. [Google Scholar] [CrossRef]
- Stone, M.S.; Martyn, L.; Weaver, C.M. Potassium Intake, Bioavailability, Hypertension, and Glucose Control. Nutrients 2016, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chang, S.J.; Chen, C.J.; Liu, J.T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2022, 22, 638. [Google Scholar] [CrossRef]
- Ahmed, I.; Jiang, N.; Shao, X.; Elsherif, M.; Alam, F.; Salih, A.; Butt, H.; Yetisen, A.K. Recent Advances in Optical Sensors for Continuous Glucose Monitoring. Sens. Diagn. 2022, 1, 1098–1125. [Google Scholar] [CrossRef]
- McCaul, M.; Porter, A.; Barrett, R.; White, P.; Stroiescu, F.; Wallace, G.; Diamond, D. Wearable Platform for Real-Time Monitoring of Sodium in Sweat. ChemPhysChem 2018, 19, 1531–1536. [Google Scholar] [CrossRef]
- Cabalar, I.; Le, T.H.; Silber, A.; O’Hara, M.; Abdallah, B.; Parikh, M.; Busch, R. The Role of Blood Testing in Prevention, Diagnosis, and Management of Chronic Diseases: A Review. Am. J. Med. Sci. 2024, 368, 274–286. [Google Scholar] [CrossRef]
- Swetha, P.; Balijapalli, U.; Feng, S.-P. Wireless accessing of salivary biomarkers based wearable electrochemical sensors: A mini-review. Electrochem. Commun. 2022, 140, 107314. [Google Scholar] [CrossRef]
- Surdu, A.; Foia, L.G.; Luchian, I.; Trifan, D.; Tatarciuc, M.S.; Scutariu, M.M.; Ciupilan, C.; Budala, D.G. Saliva as a Diagnostic Tool for Systemic Diseases—A Narrative Review. Medicina 2025, 61, 243. [Google Scholar] [CrossRef]
- Childs, A.; Mayol, B.; Lasalde-Ramírez, J.A.; Song, Y.; Sempionatto, J.R.; Gao, W. Diving into Sweat: Advances, Challenges, and Future Directions in Wearable Sweat Sensing. ACS Nano 2024, 18, 24605–24616. [Google Scholar] [CrossRef]
- Jo, S.; Sung, D.; Kim, S.; Koo, J. A Review of Wearable Biosensors for Sweat Analysis. Biomed. Eng. Lett. 2021, 11, 117–129. [Google Scholar] [CrossRef]
- Ferrão, A.R.; Pestana, P.; Borges, L.; Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. Quantification of Ions in Human Urine—A Review for Clinical Laboratories. Biomedicines 2024, 12, 1848. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Robards, K.; Prenzler, P.D.; Kendall, M. Recent and Potential Developments in the Analysis of Urine: A Review. Anal. Chim. Acta 2011, 684, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.M.; Ajithaprasad, S.; N, M.; Pavithran M, S.; Chidangil, S.; Lukose, J. Raman Spectroscopy Assisted Tear Analysis: A Label Free, Optical Approach for Noninvasive Disease Diagnostics. Exp. Eye Res. 2024, 243, 109913. [Google Scholar] [CrossRef]
- Adigal, S.S.; Rizvi, A.; Rayaroth, N.V.; John, R.V.; Barik, A.; Bhandari, S.; George, S.D.; Lukose, J.; Kartha, V.B.; Chidangil, S. Human Tear Fluid Analysis for Clinical Applications: Progress and Prospects. Expert Rev. Mol. Diagn. 2021, 21, 767–787. [Google Scholar] [CrossRef]
- Shetty, K.H.; Desai, D.T.; Patel, H.P.; Shah, D.O.; Willcox, M.D.P.; Maulvi, F.A. Contact Lens as an Emerging Platform for Non-Invasive Bio-Sensing: A Review. Sens. Actuators A Phys. 2024, 376, 115617. [Google Scholar] [CrossRef]
- Song, H.; Shin, H.; Seo, H.; Park, W.; Joo, B.J.; Kim, J.; Kim, J.; Kim, H.K.; Kim, J.; Park, J.U. Wireless Non-Invasive Monitoring of Cholesterol Using a Smart Contact Lens. Adv. Sci. 2022, 9, 2203597. [Google Scholar] [CrossRef]
- Yin, S.; Yu, Z.; Song, N.; Guo, Z.; Li, W.; Ma, J.; Wang, X.; Liu, J.; Liang, M. A long lifetime and highly sensitive wearable microneedle sensor for the continuous real-time monitoring of glucose in interstitial fluid. Biosens. Bioelectron. 2024, 244, 115822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, W.; Wu, W.; Chen, J. Recent Advances in the Preparation of Microneedle Patches for Interstitial Fluid Extraction and Analysis. Microchem. J. 2023, 195, 109477. [Google Scholar] [CrossRef]
- Vaneev, A.N.; Timoshenko, R.V.; Gorelkin, P.V.; Klyachko, N.L.; Korchev, Y.E.; Erofeev, A.S. Nano- and Microsensors for In Vivo Real-Time Electrochemical Analysis: Present and Future Perspectives. Nanomaterials 2022, 12, 3736. [Google Scholar] [CrossRef]
- Han, D.; Hosamo, H.; Ying, C.; Nie, R. A Comprehensive Review and Analysis of Nanosensors for Structural Health Monitoring in Bridge Maintenance: Innovations, Challenges, and Future Perspectives. Appl. Sci. 2023, 13, 11149. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Rab, S.; Suman, R. Exploring the Potential of Nanosensors: A Brief Overview. Sens. Int. 2021, 2, 100130. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Shaukat, H.; Ali, A.; Bibi, S.; Altabey, W.A.; Noori, M.; Kouritem, S.A. A Review of the Recent Advances in Piezoelectric Materials, Energy Harvester Structures, and Their Applications in Analytical Chemistry. Appl. Sci. 2023, 13, 1300. [Google Scholar] [CrossRef]
- Hao, R.; Liu, L.; Yuan, J.; Wu, L.; Lei, S. Recent Advances in Field Effect Transistor Biosensors: Designing Strategies and Applications for Sensitive Assay. Biosensors 2023, 13, 426. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Hu, T. A Graphene Oxide-Modified Biosensor for Non-Invasive Glucose Monitoring in College Athletes. Alex. Eng. J. 2024, 95, 321–332. [Google Scholar] [CrossRef]
- Zhang, S.; He, Z.; Zhao, W.; Liu, C.; Zhou, S.; Ibrahim, O.O.; Wang, C.; Wang, Q. Innovative Material-Based Wearable Non-Invasive Electrochemical Sweat Sensors towards Biomedical Applications. Nanomaterials 2024, 14, 857. [Google Scholar] [CrossRef]
- Tai, J.; Fan, S.; Ding, S.; Ren, L. Gold Nanoparticles Based Optical Biosensors for Cancer Biomarker Proteins: A Review of the Current Practices. Front. Bioeng. Biotechnol. 2022, 10, 877193. [Google Scholar] [CrossRef]
- Grubisha, D.S.; Lipert, R.J.; Park, H.Y.; Driskell, J.; Porter, M.D. Femtomolar Detection of Prostate-Specific Antigen: An Immunoassay Based on Surface-Enhanced Raman Scattering and Immunogold Labels. Anal. Chem. 2003, 75, 5936–5943. [Google Scholar] [CrossRef]
- Kansay, V.; Dutt Sharma, V.; Srivastava, V.; Batra, N.; Chakrabarti, S.; Bera, M.K. Wearable, Disposable and Non-Enzymatic Fluorescence Nanosensor for Monitoring Sweat Glucose through Smartphone. Microchem. J. 2024, 201, 110624. [Google Scholar] [CrossRef]
- Cao, H.; Lin, R.; Long, Z.; Xing, L.; Xue, X. A Self-Powered Wireless Sweat-Analysis Patch for Real-Time Monitoring Physiological Status. Nano Energy 2024, 123, 109411. [Google Scholar] [CrossRef]
- Saleh, S.; Alkalamouni, H.; Antar, K.; Rahme, J.; Kazan, M.; Karam, P.; Muthuswamy, J.; Zaraket, H.; Khraiche, M.L. Quartz Crystal Microbalance-Based Biosensor for Rapid and Ultrasensitive SARS-CoV-2 Detection. J. Pharm. Biomed. Anal. Open 2025, 5, 100071. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Nguyen, C.M.; Huynh, M.A.; Vu, H.H.; Nguyen, T.K.; Nguyen, N.T. Field Effect Transistor Based Wearable Biosensors for Healthcare Monitoring. J. Nanobiotechnology 2023, 21, 411. [Google Scholar] [CrossRef] [PubMed]
- Bungon, T.; Haslam, C.; Damiati, S.; O’Driscoll, B.; Whitley, T.; Davey, P.; Siligardi, G.; Charmet, J.; Awan, S.A. Graphene FET Sensors for Alzheimer’s Disease Protein Biomarker Clusterin Detection. Front. Mol. Biosci. 2021, 8, 651232. [Google Scholar] [CrossRef]
- Yang, M.; Ye, Z.; Ren, Y.; Farhat, M.; Chen, P.Y. Recent Advances in Nanomaterials Used for Wearable Electronics. Micromachines 2023, 14, 603. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Mazzaracchio, V.; Scognamiglio, V.; Amine, A.; Moscone, D. Carbon Black as an Outstanding and Affordable Nanomaterial for Electrochemical (Bio)Sensor Design. Biosens. Bioelectron. 2020, 156, 112033. [Google Scholar] [CrossRef]
- Rdest, M.; Janas, D. Carbon Nanotube Wearable Sensors for Health Diagnostics. Sensors 2021, 21, 5847. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, W.; Chen, G.; Gao, T.; Gao, Z.; Peng, L.; Wang, L.; Cai, W. A High-Performance Wearable Microneedle Sensor Based on a Carboxylated Carbon Nanotube-Carbon Nanotube Composite Electrode for the Simultaneous Detection of Uric Acid and Dopamine. Microchem. J. 2024, 206, 111607. [Google Scholar] [CrossRef]
- Vicentini, F.C.; Raymundo-Pereira, P.A.; Janegitz, B.C.; Machado, S.A.S.; Fatibello-Filho, O. Nanostructured Carbon Black for Simultaneous Sensing in Biological Fluids. Sens. Actuators B Chem. 2016, 227, 610–618. [Google Scholar] [CrossRef]
- Islam, T.; Hasan, M.M.; Awal, A.; Nurunnabi, M.; Saleh Ahammad, A.J. Metal Nanoparticles for Electrochemical Sensing: Progress and Challenges in the Clinical Transition of Point-of-Care Testing. Molecules 2020, 25, 5787. [Google Scholar] [CrossRef]
- Beck, F.; Loessl, M.; Baeumner, A.J. Signaling Strategies of Silver Nanoparticles in Optical and Electrochemical Biosensors: Considering Their Potential for the Point-of-Care. Microchim. Acta 2023, 190, 91. [Google Scholar] [CrossRef] [PubMed]
- Vannoy, C.H.; Tavares, A.J.; Omair Noor, M.; Uddayasankar, U.; Krull, U.J. Biosensing with Quantum Dots: A Microfluidic Approach. Sensors 2011, 11, 9732–9763. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Shen, Y.; Tian, S.; Xue, Z.; Meng, X. Recent Advances in Nanowire-Based Wearable Physical Sensors. Biosensors 2023, 13, 1025. [Google Scholar] [CrossRef] [PubMed]
- Ambhorkar, P.; Wang, Z.; Ko, H.; Lee, S.; Koo, K.I.; Kim, K.; Cho, D.I.D. Nanowire-Based Biosensors: From Growth to Applications. Micromachines 2018, 9, 679. [Google Scholar] [CrossRef]
- Zhang, G.J.; Ning, Y. Silicon Nanowire Biosensor and Its Applications in Disease Diagnostics: A Review. Anal. Chim. Acta 2012, 749, 1–15. [Google Scholar] [CrossRef]
- Kim, A.; Ah, C.S.; Yu, H.Y.; Yang, J.H.; Baek, I.B.; Ahn, C.G.; Park, C.W.; Jun, M.S.; Lee, S. Ultrasensitive, Label-Free, and Real-Time Immunodetection Using Silicon Field-Effect Transistors. Appl. Phys. Lett. 2007, 91, 103901. [Google Scholar] [CrossRef]
- Terán-Alcocer, Á.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef]
- Mazzara, F.; Patella, B.; D’Agostino, C.; Bruno, M.G.; Carbone, S.; Lopresti, F.; Aiello, G.; Torino, C.; Vilasi, A.; O’Riordan, A.; et al. PANI-Based Wearable Electrochemical Sensor for Ph Sweat Monitoring. Chemosensors 2021, 9, 169. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, X.; Wang, P.; Qin, J. Application of Polypyrrole-Based Electrochemical Biosensor for the Early Diagnosis of Colorectal Cancer. Nanomaterials 2023, 13, 674. [Google Scholar] [CrossRef]
- Kadhem, A.J.; Gentile, G.J.; de Cortalezzi, M.M.F. Molecularly Imprinted Polymers (MIPs) in Sensors for Environmental and Biomedical Applications: A Review. Molecules 2021, 26, 6233. [Google Scholar] [CrossRef]
- Pilvenyte, G.; Ratautaite, V.; Boguzaite, R.; Ramanavicius, S.; Chen, C.F.; Viter, R.; Ramanavicius, A. Molecularly Imprinted Polymer-Based Electrochemical Sensors for the Diagnosis of Infectious Diseases. Biosensors 2023, 13, 620. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, L.; Kong, Y.; Li, Y.; Wang, Q.; Wang, M.; Li, Y.; Davenport, A.; Li, B. Recent Advances in Molecularly Imprinted Polymer-Based Electrochemical Sensors. Biosens. Bioelectron. 2024, 249, 116018. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guan, C.; Liu, C.; Li, Z.; Han, G. Disease Diagnosis and Application Analysis of Molecularly Imprinted Polymers (MIPs) in Saliva Detection. Talanta 2024, 269, 125394. [Google Scholar] [CrossRef] [PubMed]
- Saylan, Y.; Akgönüllü, S.; Yavuz, H.; Ünal, S.; Denizli, A. Molecularly Imprinted Polymer Based Sensors for Medical Applications. Sensors 2019, 19, 1279. [Google Scholar] [CrossRef]
- Wusiman, M.; Taghipour, F. Molecularly Imprinted Fluorescence Sensor Chip for Lactate Measurement. Microsyst. Nanoeng. 2024, 10, 175. [Google Scholar] [CrossRef]
- Brazaca, L.C.; Imamura, A.H.; Blasques, R.V.; Camargo, J.R.; Janegitz, B.C.; Carrilho, E. The Use of Biological Fluids in Microfluidic Paper-Based Analytical Devices (ΜPADs): Recent Advances, Challenges and Future Perspectives. Biosens. Bioelectron. 2024, 246, 115846. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X. Sensing of inorganic ions in microfluidic devices. Sens. Actuators B Chem. 2021, 329, 129171. [Google Scholar] [CrossRef]
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized Total Chemical Analysis Systems: A Novel Concept for Chemical Sensing. Sens. Actuators B Chem. 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Mogera, U.; Guo, H.; Namkoong, M.; Rahman, S.; Nguyen, T.; Tian, L. Wearable Plasmonic Paper–Based Microfluidics for Continuous Sweat Analysis. Sci. Adv. 2022, 8, eabn1736. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Y.; Lu, J.; Gong, T.; Ibáñez, E.; Cifuentes, A.; Lu, W. Microfluidic Biosensors for Biomarker Detection in Body Fluids: A Key Approach for Early Cancer Diagnosis. Biomark. Res. 2024, 12, 153. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, X.; Wang, C.; Liu, Y.; Jin, C.; Chen, J.; Hou, J.; Huo, D.; Hou, C. An Integrated Wearable Microfluidic Biosensor for Simultaneous Detection of Multiple Biomarkers in Sweat. Talanta 2025, 285, 127404. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.C.; Jerman, J.H.; Angell, J.B. A Gas Chromatographic Air Analyzer Fabricated on a Silicon Wafer. IEEE Trans. Electron Devices 1979, 26, 1880–1886. [Google Scholar] [CrossRef]
- Figeys, D.; Pinto, D. Lab-on-a-Chip: A Revolution in Biological and Medical Sciences. Anal. Chem. 2000, 72, 330A–335A. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Haeberle, S.; Roth, G.; von Stetten, F.; Zengerle, R. Microfluidic Lab-on-a-Chip Platforms: Requirements, Characteristics and Applications. Chem. Soc. Rev. 2010, 39, 1153–1182. [Google Scholar] [CrossRef]
- Gardeniers, J.G.E.; van den Berg, A. Lab-on-a-Chip Systems for Biomedical and Environmental Monitoring. Anal. Bioanal. Chem. 2004, 378, 1700–1703. [Google Scholar] [CrossRef]
- Lee, H.B.; Meeseepong, M.; Trung, T.Q.; Kim, B.Y.; Lee, N.E. A Wearable Lab-on-a-Patch Platform with Stretchable Nanostructured Biosensor for Non-Invasive Immunodetection of Biomarker in Sweat. Biosens. Bioelectron. 2020, 156, 112133. [Google Scholar] [CrossRef]
- Jung, D.G.; Jung, D.; Kong, S.H. A Lab-on-a-Chip-Based Non-Invasive Optical Sensor for Measuring Glucose in Saliva. Sensors 2017, 17, 2607. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Z.; Feng, S.; Gao, Z.; Chen, R.; Cai, G.; Bian, S. Wearable Microfluidic Sweat Chip for Detection of Sweat Glucose and PH in Long-Distance Running Exercise. Biosensors 2023, 13, 157. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Martinez-Hurtado, J.L.; Ünal, B.; Khademhosseini, A.; Butt, H. Wearables in Medicine. Adv. Mater. 2018, 30, 1706910. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Gutruf, P.; Choi, J.; Lee, K.; Sekine, Y.; Reeder, J.T.; Jeang, W.J.; Aranyosi, A.J.; Lee, S.P.; Model, J.B.; et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 2019, 5, eaav3294. [Google Scholar] [CrossRef]
- Silvestri, S.; Schena, E. Micromachined Flow Sensors in Biomedical Applications. Micromachines 2012, 3, 225–243. [Google Scholar] [CrossRef]
- Kiran Kolluri, S.S.; Ananiah Durai, S. Wearable micro-electro-mechanical systems pressure sensors in health care: Advancements and trends—A review. IET Wirel. Sens. Syst. 2024, 14, 233–247. [Google Scholar] [CrossRef]
- Algamili, A.S.; Khir, M.H.M.; Dennis, J.O.; Ahmed, A.Y.; Alabsi, S.S.; Ba Hashwan, S.S.; Junaid, M.M. A Review of Actuation and Sensing Mechanisms in MEMS-Based Sensor Devices. Nanoscale Res. Lett. 2021, 16, 16. [Google Scholar] [CrossRef]
- Mistry, K.K.; Mahapatra, A. Design and Simulation of a Thermo Transfer Type MEMS Based Micro Flow Sensor for Arterial Blood Flow Measurement. Microsyst. Technol. 2012, 18, 683–692. [Google Scholar] [CrossRef]
- Park, H.; Park, W.; Lee, C.H. Electrochemically Active Materials and Wearable Biosensors for the in Situ Analysis of Body Fluids for Human Healthcare. NPG Asia Mater. 2021, 13, 23. [Google Scholar] [CrossRef]
- Spinelli, J.C.; Suleski, B.J.; Wright, D.E.; Grow, J.L.; Fagans, G.R.; Buckley, M.J.; Yang, D.S.; Yang, K.; Beil, S.M.; Wallace, J.C.; et al. Wearable Microfluidic Biosensors with Haptic Feedback for Continuous Monitoring of Hydration Biomarkers in Workers. NPJ Digit. Med. 2025, 8, 76. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, S.; Zhang, S.; Li, Y.; Qu, Z.; Chen, Y.; Lu, B.; Wang, X.; Feng, X. Skin-like Biosensor System via Electrochemical Channels for Noninvasive Blood Glucose Monitoring. Sci. Adv. 2017, 3, e1701629. [Google Scholar] [CrossRef]
- Kownacka, A.E.; Vegelyte, D.; Joosse, M.; Anton, N.; Toebes, B.J.; Lauko, J.; Buzzacchera, I.; Lipinska, K.; Wilson, D.A.; Geelhoed-Duijvestijn, N.; et al. Clinical Evidence for Use of a Noninvasive Biosensor for Tear Glucose as an Alternative to Painful Finger-Prick for Diabetes Management Utilizing a Biopolymer Coating. Biomacromolecules 2018, 19, 4504–4511. [Google Scholar] [CrossRef]
- Ruckh, T.T.; Clark, H.A. Implantable Nanosensors: Toward Continuous Physiologic Monitoring. Anal. Chem. 2014, 86, 1314–1323. [Google Scholar] [CrossRef]
- Kim, S.; Malik, J.; Seo, J.M.; Cho, Y.M.; Bien, F. Subcutaneously Implantable Electromagnetic Biosensor System for Continuous Glucose Monitoring. Sci. Rep. 2022, 12, 17395. [Google Scholar] [CrossRef]
- Senf, B.; Yeo, W.H.; Kim, J.H. Recent Advances in Portable Biosensors for Biomarker Detection in Body Fluids. Biosensors 2020, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R.; Lasalde-Ramírez, J.A.; Mahato, K.; Wang, J.; Gao, W. Wearable Chemical Sensors for Biomarker Discovery in the Omics Era. Nat. Rev. Chem. 2022, 6, 899–915. [Google Scholar] [CrossRef]
- Baker, S.A.; Rutter, J. Metabolites as Signalling Molecules. Nat. Rev. Mol. Cell Biol. 2023, 24, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Kubihal, S.; Goyal, A.; Gupta, Y.; Khadgawat, R. Glucose Measurement in Body Fluids: A Ready Reckoner for Clinicians. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Saeed Darweesh, M.; Soltan, A. Wearable Devices for Glucose Monitoring: A Review of State-of-the-Art Technologies and Emerging Trends. Alex. Eng. J. 2024, 89, 224–243. [Google Scholar] [CrossRef]
- Worsley, G.J.; Tourniaire, G.A.; Medlock, K.E.S.; Sartain, F.K.; Harmer, H.E.; Thatcher, M.; Horgan, A.M.; Pritchard, J. Continuous Blood Glucose Monitoring with a Thin-Film Optical Sensor. Clin. Chem. 2007, 53, 1820–1826. [Google Scholar] [CrossRef]
- Bakhshandeh, F.; Zheng, H.; Barra, N.G.; Sadeghzadeh, S.; Ausri, I.; Sen, P.; Keyvani, F.; Rahman, F.; Quadrilatero, J.; Liu, J.; et al. Wearable Aptalyzer Integrates Microneedle and Electrochemical Sensing for In Vivo Monitoring of Glucose and Lactate in Live Animals. Adv. Mater. 2024, 36, e2313743. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Nakagawa, T.; Pavinatto, A.; Mensah, S.T.; Imani, S.; Mercier, P.; Wang, J. Eyeglasses Based Wireless Electrolyte and Metabolite Sensor Platform. Lab Chip 2017, 17, 1834–1842. [Google Scholar] [CrossRef]
- Wang, Q.; Molinero-Fernandez, Á.; Wei, Q.; Xuan, X.; Konradsson-Geuken, Å.; Cuartero, M.; Crespo, G.A. Intradermal Lactate Monitoring Based on a Microneedle Sensor Patch for Enhanced In Vivo Accuracy. ACS Sens. 2024, 9, 3115–3125. [Google Scholar] [CrossRef]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical Tattoo Biosensors for Real-Time Noninvasive Lactate Monitoring in Human Perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, J.L.; González, S.; Aibar, C.; Rivera, D.; Avilés, E.; Beunza, J.J. Continuous and Non-Invasive Lactate Monitoring Techniques in Critical Care Patients. Biosensors 2024, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Deulkar, P.; Singam, A.; Mudiganti, V.N.K.S.; Jain, A. Lactate Monitoring in Intensive Care: A Comprehensive Review of Its Utility and Interpretation. Cureus 2024, 16, e66356. [Google Scholar] [CrossRef]
- Al-Tamer, Y.Y.; Hadi, E.A.; al-Badrani, I.I. Sweat Urea, Uric Acid and Creatinine Concentrations in Uraemic Patients. Urol. Res. 1997, 25, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Aafria, S.; Kumari, P.; Sharma, S.; Yadav, S.; Batra, B.; Rana, J.S.; Sharma, M. Electrochemical Biosensing of Uric Acid: A Review. Microchem. J. 2022, 182, 107945. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H. Physiological Functions and Pathogenic Potential of Uric Acid: A Review. J. Adv. Res. 2017, 8, 487–493. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, S.; Liu, Q.; Xu, T.; Zhang, X. Microfluidic-Based Plasmonic Microneedle Biosensor for Uric Acid Ultrasensitive Monitoring. Sens. Actuators B Chem. 2024, 398, 134685. [Google Scholar] [CrossRef]
- Piedras, J.; Dominguez, R.B.; Gutiérrez, J.M. Determination of Uric Acid in Artificial Saliva with Compact AMP3291 Reader and Au Nanoparticles Modified Electrode. Chemosensors 2021, 9, 73. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A Laser-Engraved Wearable Sensor for Sensitive Detection of Uric Acid and Tyrosine in Sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef]
- Wang, M.; Zi, G.; Liu, J.; Song, Y.; Zhao, X.; Wang, Q.; Zhao, T. Self-Powered Biosensor for Specifically Detecting Creatinine in Real Time Based on the Piezo-Enzymatic-Reaction Effect of Enzyme-Modified ZnO Nanowires. Biosensors 2021, 11, 342. [Google Scholar] [CrossRef]
- Pundir, C.S.; Kumar, P.; Jaiwal, R. Biosensing Methods for Determination of Creatinine: A Review. Biosens. Bioelectron. 2019, 126, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kadian, S.; Mishra, R.K.; Huang, T.; Zhou, C.; Liu, S.; Wang, Z.; Narayan, R.; Zhu, Z. Electrochemical Detection of Cholesterol in Human Biofluid Using Microneedle Sensor. J. Mater. Chem. B 2023, 11, 6075–6081. [Google Scholar] [CrossRef] [PubMed]
- Warnick, G.R.; Remaley, A.T. Measurement of Cholesterol in Plasma and Other Body Fluids. Curr. Atheroscler. Rep. 2001, 3, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous Sweat Extraction and Analysis Applied to Cystic Fibrosis and Glucose Monitoring Using a Fully Integrated Wearable Platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully Integrated Wearable Sensor Arrays for Multiplexed in Situ Perspiration Analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Chien, M.N.; Fan, S.H.; Huang, C.H.; Wu, C.C.; Huang, J.T. Continuous Lactate Monitoring System Based on Percutaneous Microneedle Array. Sensors 2022, 22, 1468. [Google Scholar] [CrossRef]
- Xuan, X.; Chen, C.; Molinero-Fernandez, A.; Ekelund, E.; Cardinale, D.; Swarén, M.; Wedholm, L.; Cuartero, M.; Crespo, G.A. Fully Integrated Wearable Device for Continuous Sweat Lactate Monitoring in Sports. ACS Sens. 2023, 8, 2401–2409. [Google Scholar] [CrossRef]
- Nishan, U.; Khan, N.; Muhammad, N.; Afridi, S.; Badshah, A.; Shah, M.; Asad, M.; Ullah, R.; Niamat, H.; Ullah, R.; et al. Colorimetric Sensing of Uric Acid Based on Sawdust-Deposited Silver Nanoparticles via an Eco-Friendly and Cost-Effective Approach. Front. Mater. 2023, 10, 1298873. [Google Scholar] [CrossRef]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and Flexible Electrochemical Sensors for Sweat Analysis: A Review. Microsyst. Nanoeng. 2023, 9, 1. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Molinnus, D.; Mirza, O.; Guinovart, T.; Windmiller, J.R.; Valdés-Ramírez, G.; Andrade, F.J.; Schöning, M.J.; Wang, J. Epidermal Tattoo Potentiometric Sodium Sensors with Wireless Signal Transduction for Continuous Non-Invasive Sweat Monitoring. Biosens. Bioelectron. 2014, 54, 603–609. [Google Scholar] [CrossRef]
- Dang, W.; Manjakkal, L.; Navaraj, W.T.; Lorenzelli, L.; Vinciguerra, V.; Dahiya, R. Stretchable Wireless System for Sweat PH Monitoring. Biosens. Bioelectron. 2018, 107, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.C.; Ota, H.; Davis, R.W.; et al. A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and PH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef]
- Keefe, M.S.; Benjamin, C.L.; Casa, D.J.; Sekiguchi, Y. Importance of Electrolytes in Exercise Performance and Assessment Methodology After Heat Training: A Narrative Review. Appl. Sci. 2024, 14, 10103. [Google Scholar] [CrossRef]
- Benelam, B.; Wyness, L. Hydration and Health: A Review. Nutr. Bull. 2010, 35, 3–25. [Google Scholar] [CrossRef]
- Villiger, M.; Stoop, R.; Vetsch, T.; Hohenauer, E.; Pini, M.; Clarys, P.; Pereira, F.; Clijsen, R. Evaluation and Review of Body Fluids Saliva, Sweat and Tear Compared to Biochemical Hydration Assessment Markers within Blood and Urine. Eur. J. Clin. Nutr. 2018, 72, 69–76. [Google Scholar] [CrossRef]
- Jiang, N.; Yetisen, A.K.; Linhart, N.; Flisikowski, K.; Dong, J.; Dong, X.; Butt, H.; Jakobi, M.; Schnieke, A.; Koch, A.W. Fluorescent Dermal Tattoo Biosensors for Electrolyte Analysis. Sens. Actuators B Chem. 2020, 320, 128378. [Google Scholar] [CrossRef]
- Aref, M.; Ranjbari, E.; García-Guzmán, J.J.; Hu, K.; Lork, A.; Crespo, G.A.; Ewing, A.G.; Cuartero, M. Potentiometric PH Nanosensor for Intracellular Measurements: Real-Time and Continuous Assessment of Local Gradients. Anal. Chem. 2021, 93, 15744–15751. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, C.; Petrone, N.; Yu, J.; Nuckolls, C.; Hone, J.; Lin, Q. A Solid Dielectric Gated Graphene Nanosensor in Electrolyte Solutions. Appl. Phys. Lett. 2015, 106, 123503. [Google Scholar] [CrossRef]
- Uljon, S.N.; Mazzarelli, L.; Chait, B.T.; Wang, R. Analysis from Biological Fluids Analysis of Proteins and Peptides Directly from Biological Fluids by Immunoprecipitation/Mass Spectrometry. Methods Mol. Biol. 2000, 146, 439–452. [Google Scholar] [CrossRef]
- Salvo, P.; Dini, V.; Kirchhain, A.; Janowska, A.; Oranges, T.; Chiricozzi, A.; Lomonaco, T.; Di Francesco, F.; Romanelli, M. Sensors and Biosensors for C-Reactive Protein, Temperature and pH, and Their Applications for Monitoring Wound Healing: A Review. Sensors 2017, 17, 2952. [Google Scholar] [CrossRef]
- John, R.V.; Devasiya, T.; Nidheesh, V.R.; Adigal, S.; Lukose, J.; Kartha, V.B.; Chidangil, S. Cardiovascular Biomarkers in Body Fluids: Progress and Prospects in Optical Sensors. Biophys. Rev. 2022, 14, 1023–1050. [Google Scholar] [CrossRef] [PubMed]
- Tavakolidakhrabadi, A.; Stark, M.; Küenzi, A.; Carrara, S.; Bessire, C. Optimized Microfluidic Biosensor for Sensitive C-Reactive Protein Detection. Biosensors 2025, 15, 214. [Google Scholar] [CrossRef] [PubMed]
- Feine, I.; Gafny, R.; Pinkas, I. Combination of Prostate-Specific Antigen Detection and Micro-Raman Spectroscopy for Confirmatory Semen Detection. Forensic Sci. Int. 2017, 270, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. Prostatic Fluid Electrolyte Composition for the Screening of Prostate Cancer: A Potential Solution to a Major Problem. Prostate Cancer Prostatic Dis. 2009, 12, 17–24. [Google Scholar] [CrossRef]
- Hui, N.; Wang, J.; Wang, D.; Wang, P.; Luo, X.; Lv, S. An Ultrasensitive Biosensor for Prostate Specific Antigen Detection in Complex Serum Based on Functional Signal Amplifier and Designed Peptides with Both Antifouling and Recognizing Capabilities. Biosens. Bioelectron. 2022, 200, 113921. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Sheta, S.M.; Salem, S.R.; Abd-Elzaher, M.M.; Basaleh, A.S.; Labib, A.A. Prostate-Specific Antigen Monitoring Using Nano Zinc(II) Metal–Organic Framework-Based Optical Biosensor. Biosensors 2022, 12, 931. [Google Scholar] [CrossRef]
- Tu, J.; Min, J.; Song, Y.; Xu, C.; Li, J.; Moore, J.; Hanson, J.; Hu, E.; Parimon, T.; Wang, T.-Y.; et al. A Wireless Patch for the Monitoring of C-Reactive Protein in Sweat HHS Public Access Author Manuscript. Nat. Biomed. Eng. 2023, 7, 1293–1306. [Google Scholar] [CrossRef]
- Hiller-Sturmhöfel, S.; Bartke, A. The Endocrine System An Overview. Alcohol Health Res. World 1998, 22, 153–164. [Google Scholar]
- Karachaliou, C.E.; Koukouvinos, G.; Goustouridis, D.; Raptis, I.; Kakabakos, S.; Petrou, P.; Livaniou, E. Cortisol Immunosensors: A Literature Review. Biosensors 2023, 13, 285. [Google Scholar] [CrossRef]
- Torrente-Rodríguez, R.M.; Tu, J.; Yang, Y.; Min, J.; Wang, M.; Song, Y.; Yu, Y.; Xu, C.; Ye, C.; IsHak, W.W.; et al. Investigation of Cortisol Dynamics in Human Sweat Using a Graphene-Based Wireless MHealth System. Matter 2020, 2, 921–937. [Google Scholar] [CrossRef]
- Tang, W.; Yin, L.; Sempionatto, J.R.; Moon, J.M.; Teymourian, H.; Wang, J. Touch-Based Stressless Cortisol Sensing. Adv. Mater. 2021, 33, 2008465. [Google Scholar] [CrossRef] [PubMed]
- Parlak, O.; Keene, S.T.; Marais, A.; Curto, V.F.; Salleo, A. Molecularly Selective Nanoporous Membrane-Based Wearable Organic Electrochemical Device for Noninvasive Cortisol Sensing. Sci. Adv. 2018, 4, eaar2904. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Lee, T.; Song, J.; Russell, L.; Li, H.; Dailey, J.; Searson, P.C.; Katz, H.E. Electronic Cortisol Detection Using an Antibody-Embedded Polymer Coupled to a Field-Effect Transistor. ACS Appl. Mater. Interfaces 2018, 10, 16233–16237. [Google Scholar] [CrossRef]
- Munje, R.D.; Muthukumar, S.; Jagannath, B.; Prasad, S. A New Paradigm in Sweat Based Wearable Diagnostics Biosensors Using Room Temperature Ionic Liquids (RTILs). Sci. Rep. 2017, 7, 1950. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Z.; Zhou, J.; Jia, X.; Li, H.; Wang, X.; Chen, Y.; Sun, Z.; He, X.; Li, H.; et al. Nano-Biosensor for SARS-CoV-2/COVID-19 Detection: Methods, Mechanism and Interface Design. RSC Adv. 2023, 13, 17883–17906. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, X.; Zhang, Y.; Zhou, X.; Yin, Z.-Z. An Electrochemical Microsensor of the SARS-CoV-2 Nucleocapsid Protein Based on a Surface-Imprinted Acupuncture Needle. Analyst 2025, 150, 851–859. [Google Scholar] [CrossRef]
- Janik, M.; Gabler, T.; Koba, M.; Panasiuk, M.; Dashkevich, Y.; Łęga, T.; Dąbrowska, A.; Naskalska, A.; Żołędowska, S.; Nidzworski, D.; et al. Low-Volume Label-Free SARS-CoV-2 Detection with the Microcavity-Based Optical Fiber Sensor. Sci. Rep. 2023, 13, 1512. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.H.; Choi, M.; Ku, K.B.; Lee, C.S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef]
- Ruiz-Vega, G.; Soler, M.; Estevez, M.C.; Ramirez-Priego, P.; Pazos, M.D.; Noriega, M.A.; Margolles, Y.; Francés-Gómez, C.; Geller, R.; Matusali, G.; et al. Rapid and Direct Quantification of the SARS-CoV-2 Virus with an Ultrasensitive Nanobody-Based Photonic Nanosensor. Sens. Diagn. 2022, 1, 983–993. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, M.-H. Overview of Therapeutic Drug Monitoring. Korean J. Intern. Med. 2009, 24, 1–10. [Google Scholar] [CrossRef]
- Cafaro, A.; Conti, M.; Pigliasco, F.; Barco, S.; Bandettini, R.; Cangemi, G. Biological Fluid Microsampling for Therapeutic Drug Monitoring: A Narrative Review. Biomedicines 2023, 11, 1962. [Google Scholar] [CrossRef] [PubMed]
- Pichini, S.; Altieri, I.; Zuccaro, P.; Pacifici, R. Drug Monitoring in Nonconventional Biological Fluids and Matrices. Clin. Pharmacokinet. 1996, 30, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.C.; Liaw, T.S.; Lin, Y.; Nyein, H.Y.Y.; Bariya, M.; Ji, W.; Hettick, M.; Zhao, C.; Zhao, J.; Hou, L.; et al. Wearable Sweat Band for Noninvasive Levodopa Monitoring. Nano Lett. 2019, 19, 6346–6351. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Bariya, M.; Tran, B.; Ahn, C.H.; Brown, B.J.; Ji, W.; Davis, N.; Javey, A. A Wearable Patch for Continuous Analysis of Thermoregulatory Sweat at Rest. Nat. Commun. 2021, 12, 1823. [Google Scholar] [CrossRef]
- Goud, K.Y.; Moonla, C.; Mishra, R.K.; Yu, C.; Narayan, R.; Litvan, I.; Wang, J. Wearable Electrochemical Microneedle Sensor for Continuous Monitoring of Levodopa: Toward Parkinson Management. ACS Sens. 2019, 4, 2196–2204. [Google Scholar] [CrossRef]
- Tai, L.C.; Gao, W.; Chao, M.; Bariya, M.; Ngo, Q.P.; Shahpar, Z.; Nyein, H.Y.Y.; Park, H.; Sun, J.; Jung, Y.; et al. Methylxanthine Drug Monitoring with Wearable Sweat Sensors. Adv. Mater. 2018, 30, e1707442. [Google Scholar] [CrossRef]
- Wasilewski, T.; Kamysz, W.; Gębicki, J. AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring. Biosensors 2024, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E. Can Calibration-Free Sensors Be Realized? ACS Sens. 2016, 1, 838–841. [Google Scholar] [CrossRef]
- Singh, I.; Gupta, A.; Gupta, C.; Mani, A.; Basu, T. AI-Driven Improvements in Electrochemical Biosensors for Effective Pathogen Detection at Point-of-Care. Eng. Proc. 2024, 73, 5. [Google Scholar] [CrossRef]
- Flynn, C.D.; Chang, D. Artificial Intelligence in Point-of-Care Biosensing: Challenges and Opportunities. Diagnostics 2024, 14, 1100. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Hsu, W.S. Integrating Artificial Intelligence and Wearable IoT System in Long-Term Care Environments. Sensors 2023, 23, 5913. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.C.; Muthukumar, S.; Prasad, S. Flex-GO (Flexible Graphene Oxide) Sensor for Electrochemical Monitoring Lactate in Low-Volume Passive Perspired Human Sweat. Talanta 2020, 214, 120810. [Google Scholar] [CrossRef]
- Leogrande, E.; Filosa, M.; Ballanti, S.; De Cicco, L.; Mazzoleni, S.; Ackerley, R.; Oddo, C.M.; Dell’Olio, F. Electronic Skin Technologies: From Hardware Building Blocks and Tactile Sensing to Control Algorithms and Applications. Sens. Actuators Rep. 2025, 9, 100312. [Google Scholar] [CrossRef]
- Ma, S.; Li, J.; Pei, L.; Feng, N.; Zhang, Y. Microneedle-Based Interstitial Fluid Extraction for Drug Analysis: Advances, Challenges, and Prospects. J. Pharm. Anal. 2023, 13, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Qiao, Z.; Chen, S.; Fan, S.; Liu, Y.; Qi, J.; Lim, C.T. Interstitial Fluid-Based Wearable Biosensors for Minimally Invasive Healthcare and Biomedical Applications. Commun. Mater. 2024, 5, 33. [Google Scholar] [CrossRef]
- Gideon, O.; Samuel, H.S.; Okino, I.A. Biocompatible Materials for Next-Generation Biosensors. Discov. Chem. 2024, 1, 34. [Google Scholar] [CrossRef]
- Lu, T.; Ji, S.; Jin, W.; Yang, Q.; Luo, Q.; Ren, T.L. Biocompatible and Long-Term Monitoring Strategies of Wearable, Ingestible and Implantable Biosensors: Reform the Next Generation Healthcare. Sensors 2023, 23, 2991. [Google Scholar] [CrossRef]
- Delgado, A.; Briciu-Burghina, C.; Regan, F. Antifouling Strategies for Sensors Used in Water Monitoring: Review and Future Perspectives. Sensors 2021, 21, 389. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Antifouling (Bio)Materials for Electrochemical (Bio)Sensing. Int. J. Mol. Sci. 2019, 20, 423. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, S.; Wang, Q.; Li, J. Recent progress in biosensor regeneration techniques. Nanoscale 2024, 16, 2834–2846. [Google Scholar] [CrossRef] [PubMed]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- McCann, B.; Tipper, B.; Shahbeigi, S.; Soleimani, M.; Jabbari, M.; Esfahani, M.N. A Review on Perception of Binding Kinetics in Affinity Biosensors: Challenges and Opportunities. ACS Omega 2025, 10, 4197–4216. [Google Scholar] [CrossRef]
- Rouhi, N.; Akhgari, A.; Orouji, N.; Nezami, A.; Rahimzadegan, M.; Kamali, H. Recent Progress in the Graphene-Based Biosensing Approaches for the Detection of Alzheimer’s Biomarkers. J. Pharm. Biomed. Anal. 2023, 222, 115084. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour-Haratbar, A.; Boraei, S.B.A.; Zare, Y.; Rhee, K.Y.; Park, S.J. Graphene-Based Electrochemical Biosensors for Breast Cancer Detection. Biosensors 2023, 13, 80. [Google Scholar] [CrossRef]
- Hemdan, M.; Abuelhaded, K.; Shaker, A.A.S.; Ashour, M.M.; Abdelaziz, M.M.; Dahab, M.I.; Nassar, Y.A.; Sarguos, A.M.M.; Zakaria, P.S.; Fahmy, H.A.; et al. Recent Advances in Nano-Enhanced Biosensors: Innovations in Design, Applications in Healthcare, Environmental Monitoring, and Food Safety, and Emerging Research Challenges. Sens. Bio-Sens. Res. 2025, 48, 100783. [Google Scholar] [CrossRef]
- Vitabile, S.; Marks, M.; Stojanovic, D.; Pllana, S.; Molina, J.M.; Krzyszton, M.; Sikora, A.; Jarynowski, A.; Hosseinpour, F.; Jakobik, A.; et al. Medical Data Processing and Analysis for Remote Health and Activities Monitoring. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: Berlin, Germany, 2019; Volume 11400, pp. 186–220. [Google Scholar]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing Healthcare: The Role of Artificial Intelligence in Clinical Practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
- Canali, S.; De Marchi, B.; Aliverti, A. Wearable Technologies and Stress: Toward an Ethically Grounded Approach. Int. J. Environ. Res. Public Health 2023, 20, 6737. [Google Scholar] [CrossRef]
- Capulli, E.; Druda, Y.; Palmese, F.; Butt, A.H.; Domenicali, M.; Macchiarelli, A.G.; Silvani, A.; Bedogni, G.; Ingravallo, F. Ethical and legal implications of health monitoring wearable devices: A scoping review. Soc. Sci. Med. 2025, 370, 117685. [Google Scholar] [CrossRef]
- Amisha; Malik, P.; Pathania, M.; Rathaur, V. Overview of Artificial Intelligence in Medicine. J. Fam. Med. Prim. Care 2019, 8, 2328–2331. [Google Scholar] [CrossRef]
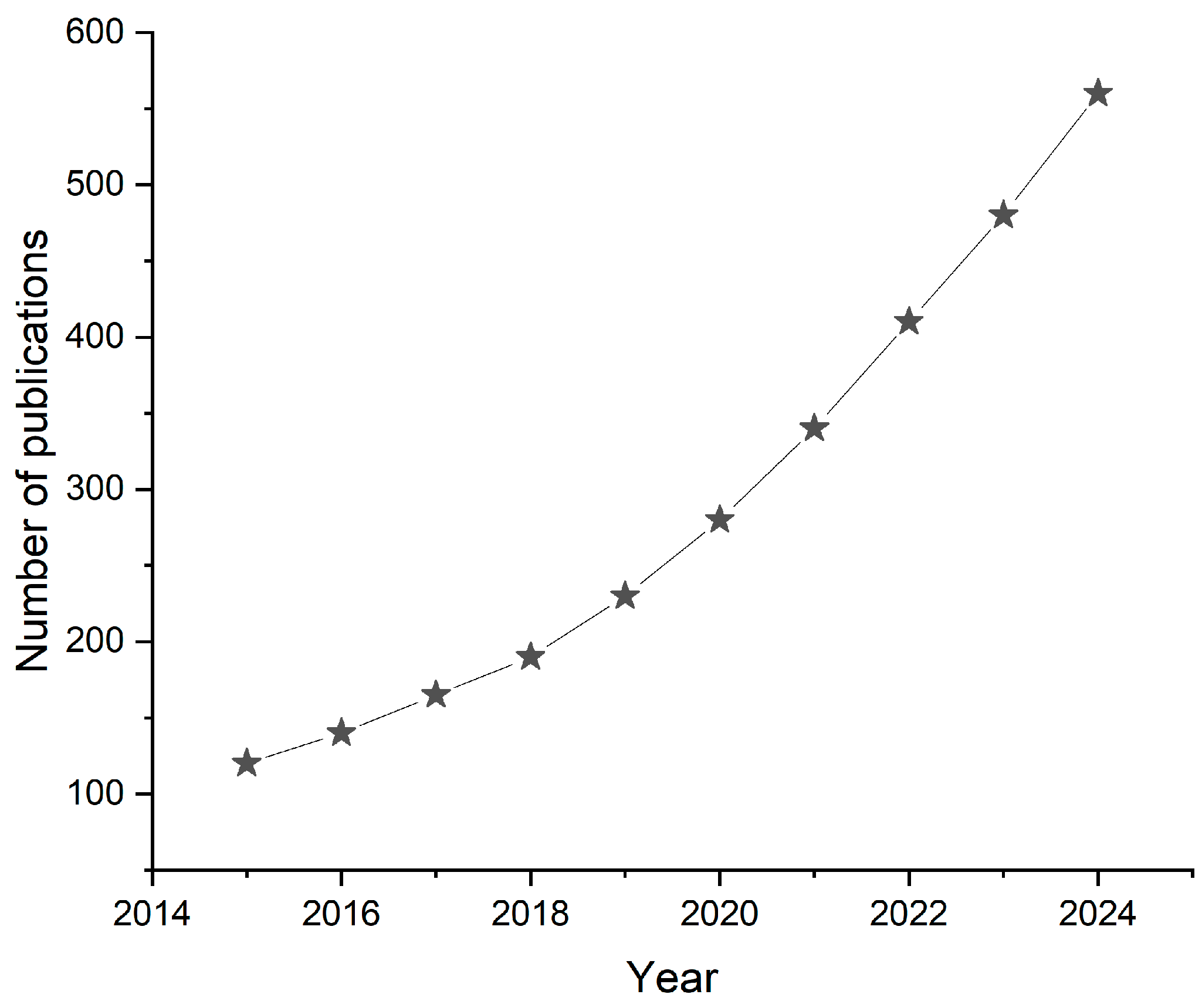

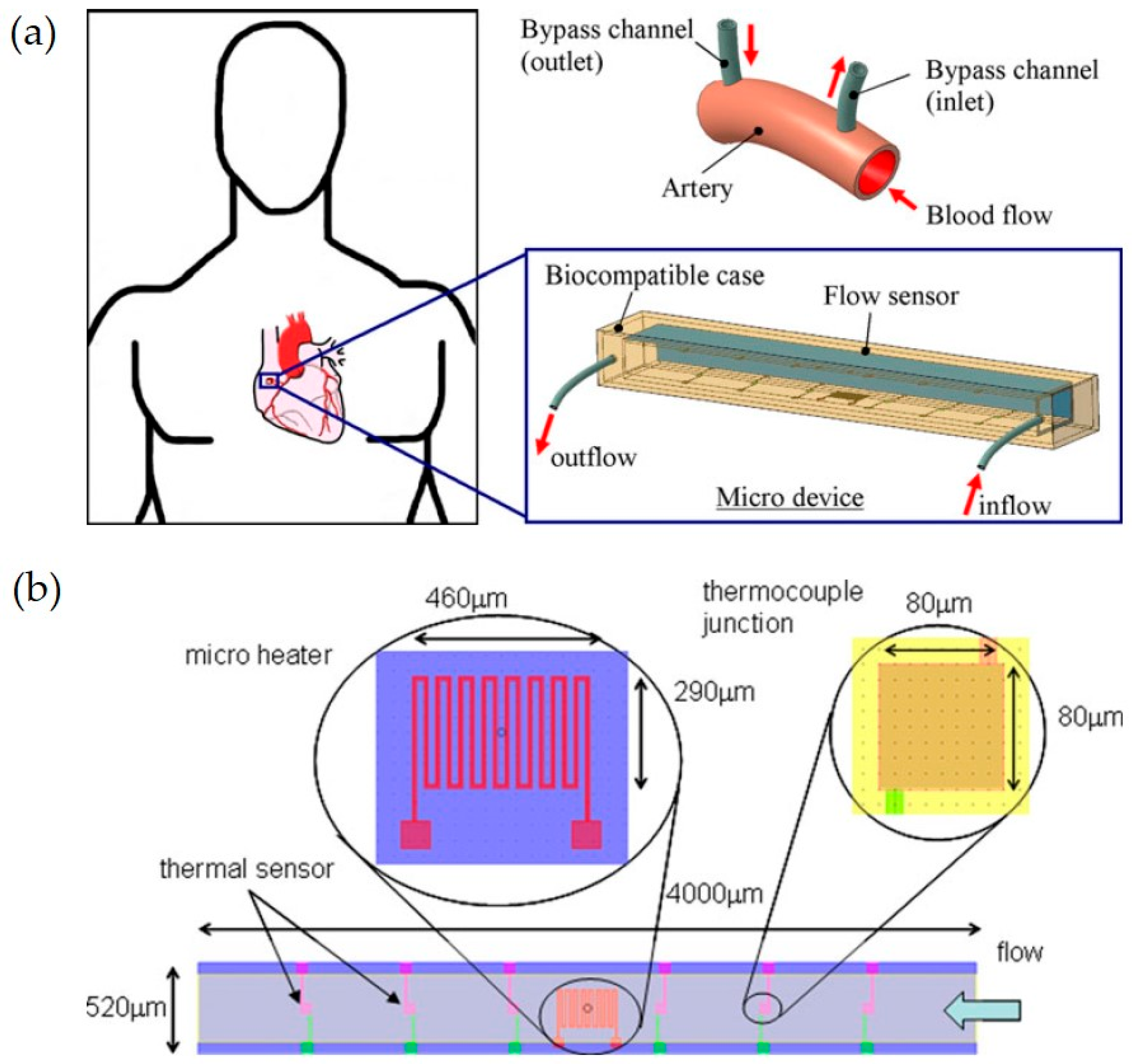
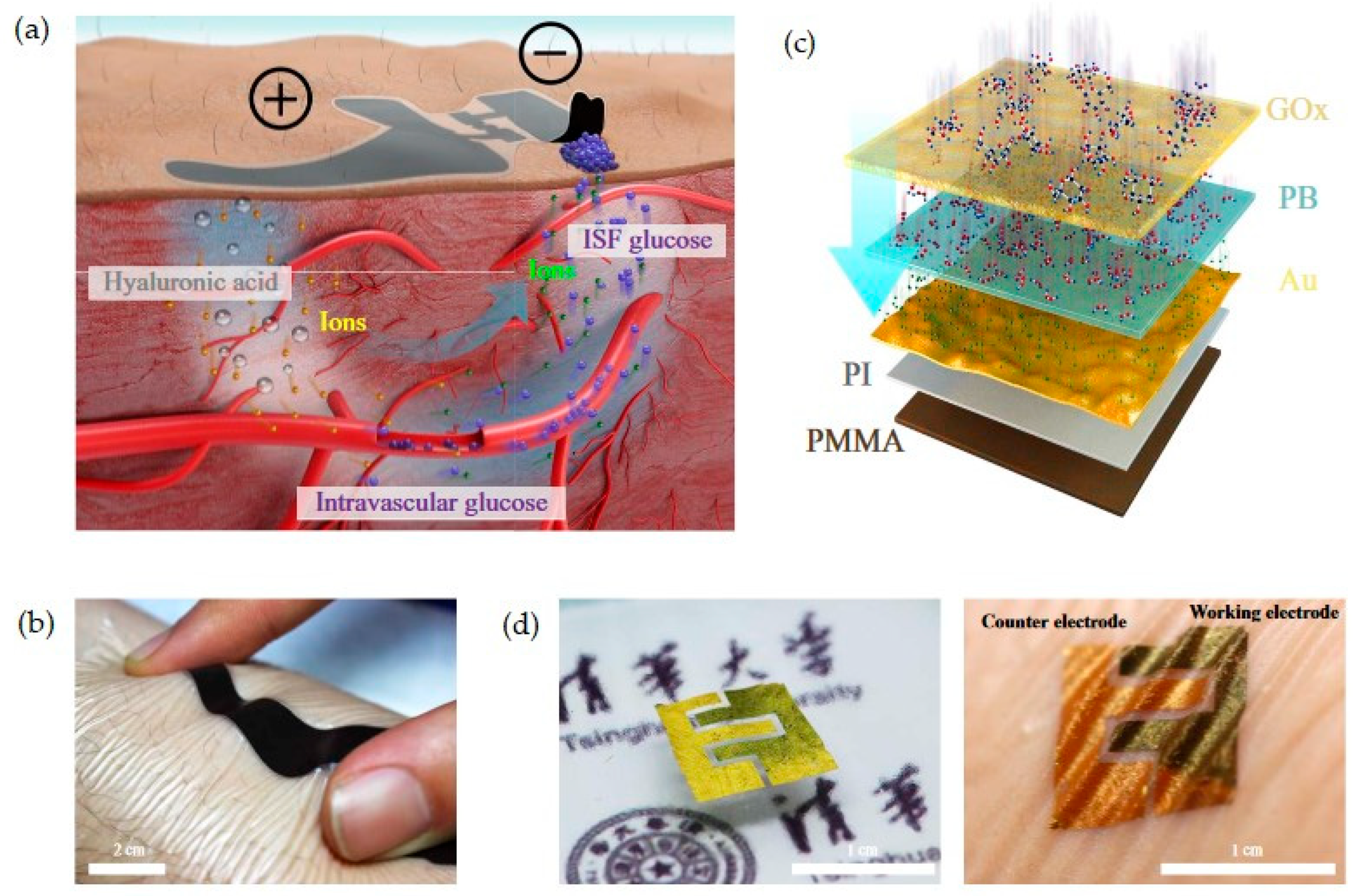
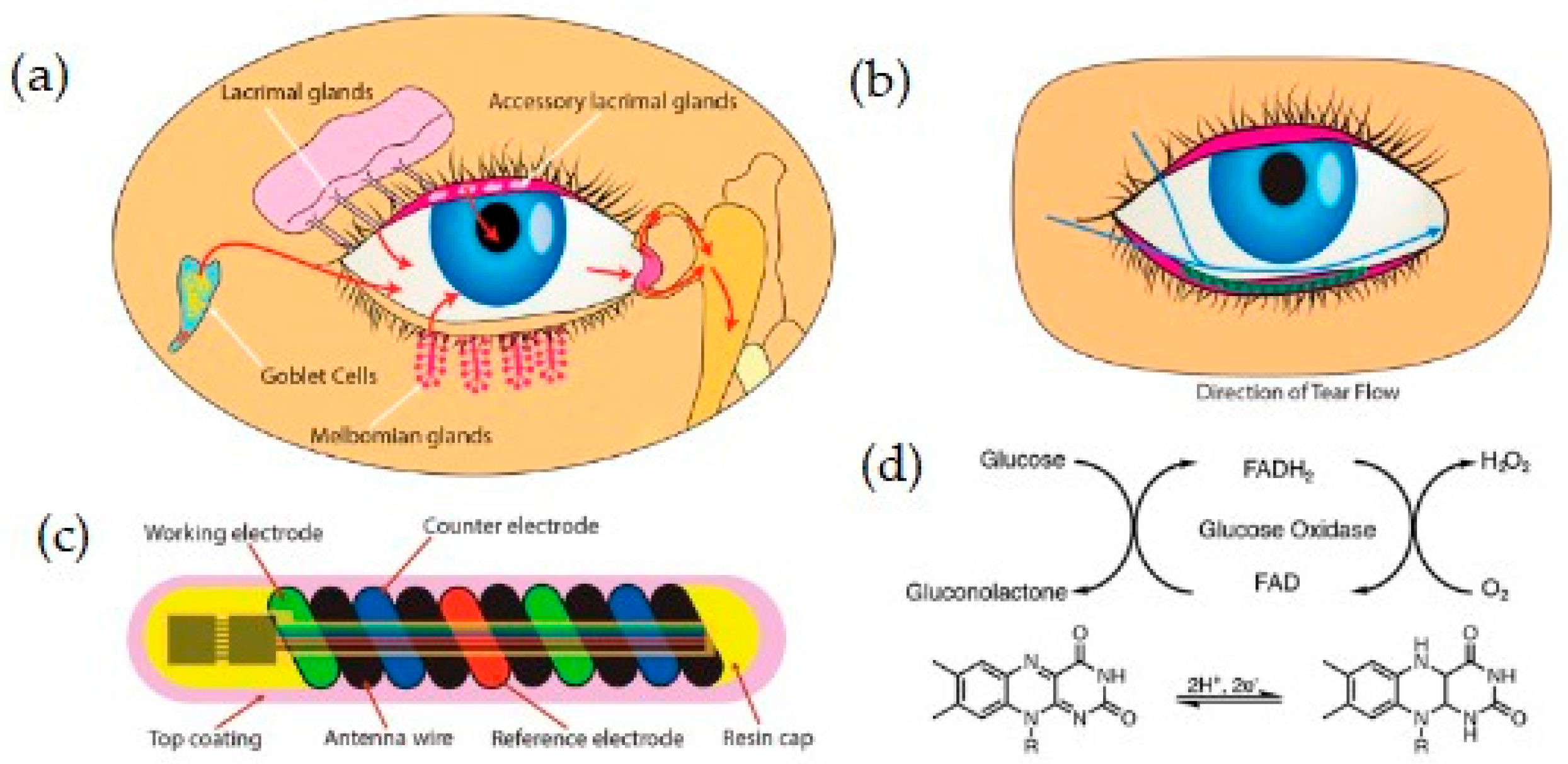
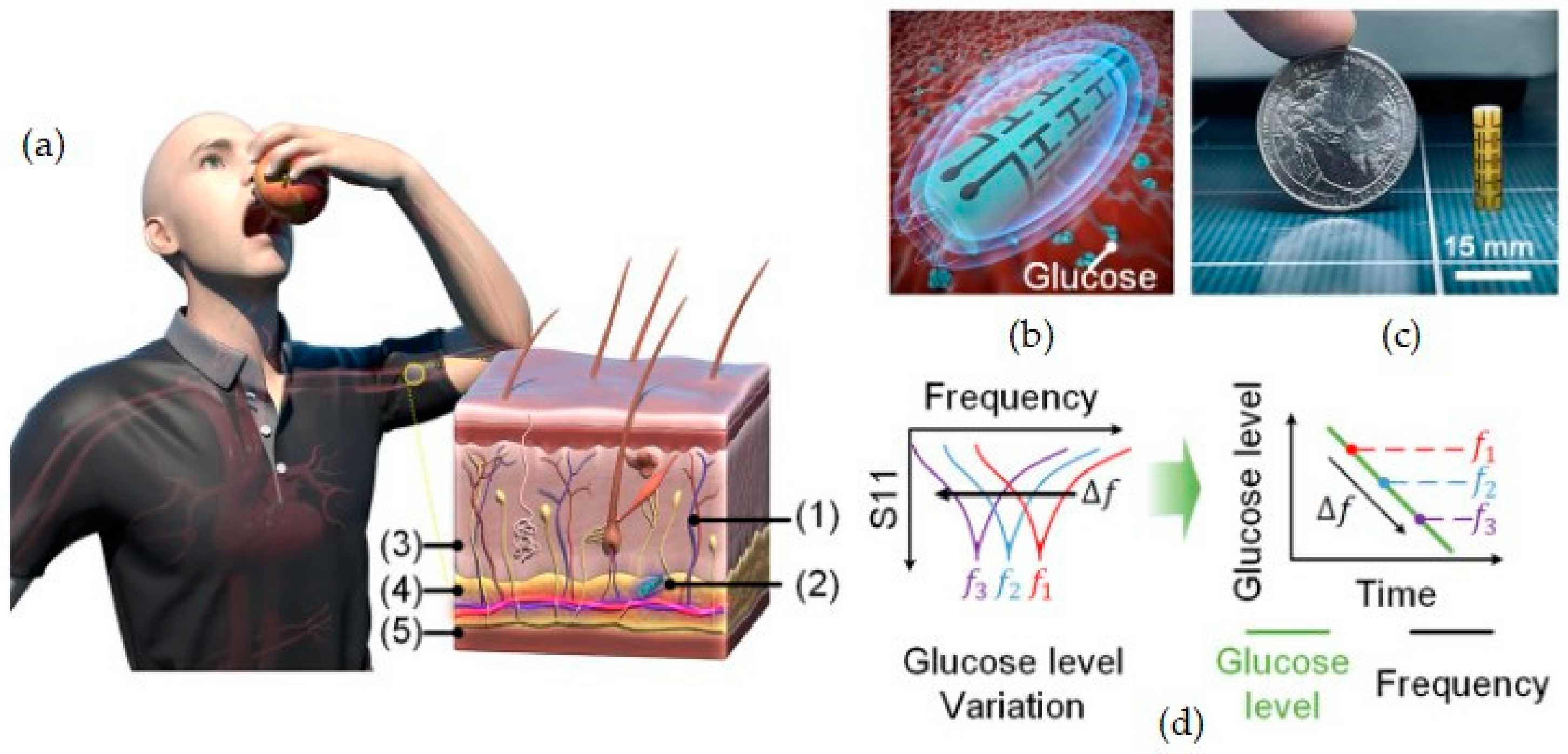
| Transduction Type | Sensitivity | Integration (Wearable) | Integration (Ingestible) | Cost | Real-Time Use | Miniaturization |
|---|---|---|---|---|---|---|
| Electrochemical | High | Excellent | Moderate | Low | Yes | Excellent |
| Optical | High | Moderate | Challenging | Medium | Yes | Good |
| Piezoelectric | Moderate | Moderate | Low | Medium | Limited | Moderate |
| FET-based | Very High | Emerging | Research-stage | High | Yes | Excellent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenar, N.; Paczosa-Bator, B. Nanosensors and Microsensors for Body Fluid Monitoring: Various Analyte Detection and Construction Solutions. Int. J. Mol. Sci. 2025, 26, 5001. https://doi.org/10.3390/ijms26115001
Lenar N, Paczosa-Bator B. Nanosensors and Microsensors for Body Fluid Monitoring: Various Analyte Detection and Construction Solutions. International Journal of Molecular Sciences. 2025; 26(11):5001. https://doi.org/10.3390/ijms26115001
Chicago/Turabian StyleLenar, Nikola, and Beata Paczosa-Bator. 2025. "Nanosensors and Microsensors for Body Fluid Monitoring: Various Analyte Detection and Construction Solutions" International Journal of Molecular Sciences 26, no. 11: 5001. https://doi.org/10.3390/ijms26115001
APA StyleLenar, N., & Paczosa-Bator, B. (2025). Nanosensors and Microsensors for Body Fluid Monitoring: Various Analyte Detection and Construction Solutions. International Journal of Molecular Sciences, 26(11), 5001. https://doi.org/10.3390/ijms26115001







