RANKL Drives Bone Metastasis in Mammary Cancer: Protective Effects of Anti-Resorptive Treatments
Abstract
1. Introduction
2. Results
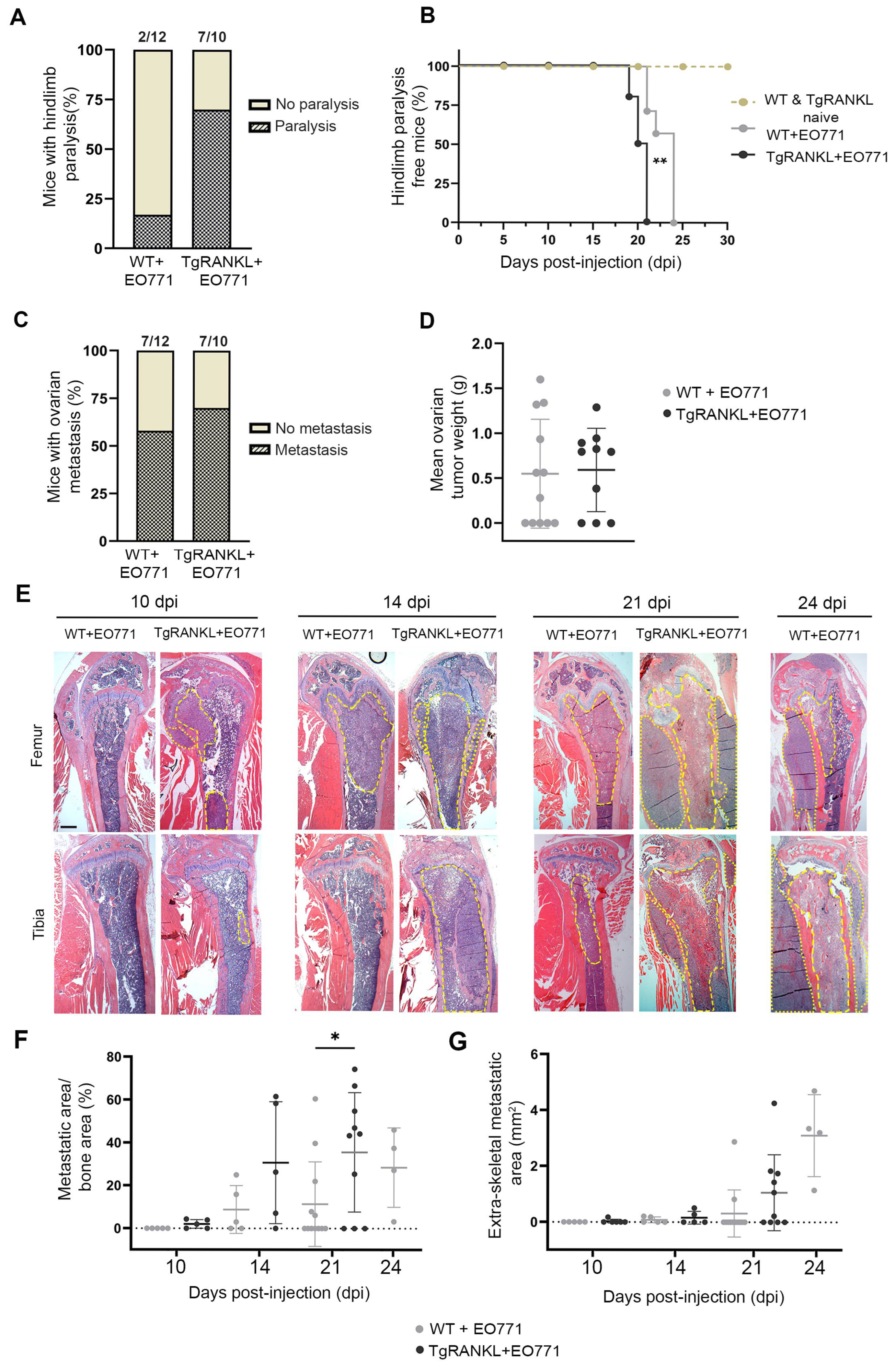
2.1. TgRANKL Mice Develop Earlier Onset and Increased Burden of Mammary-Cancer-Induced Bone Metastasis
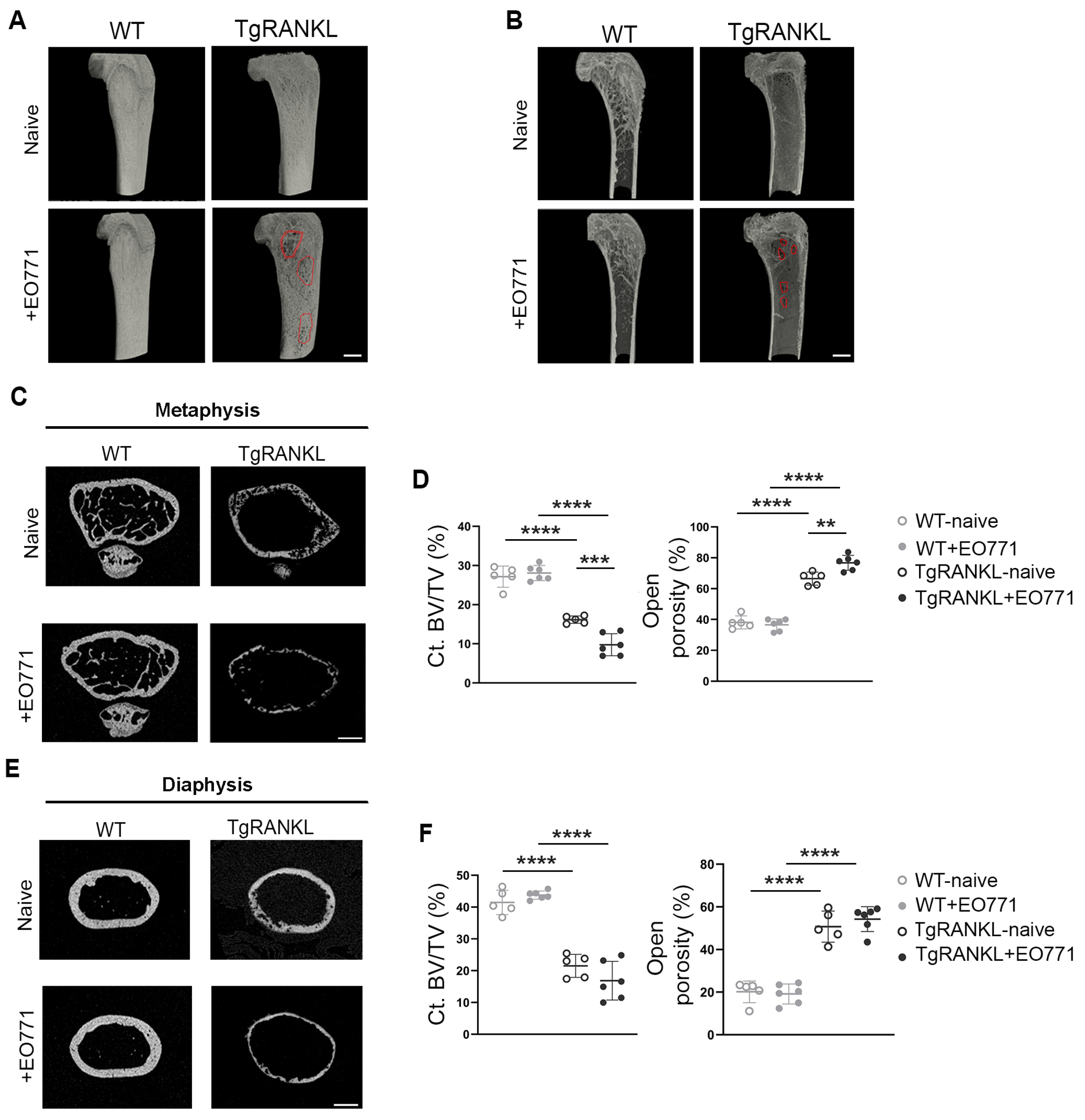
2.2. Severe Metastasis-Induced Osteolysis in TgRANKL Mice
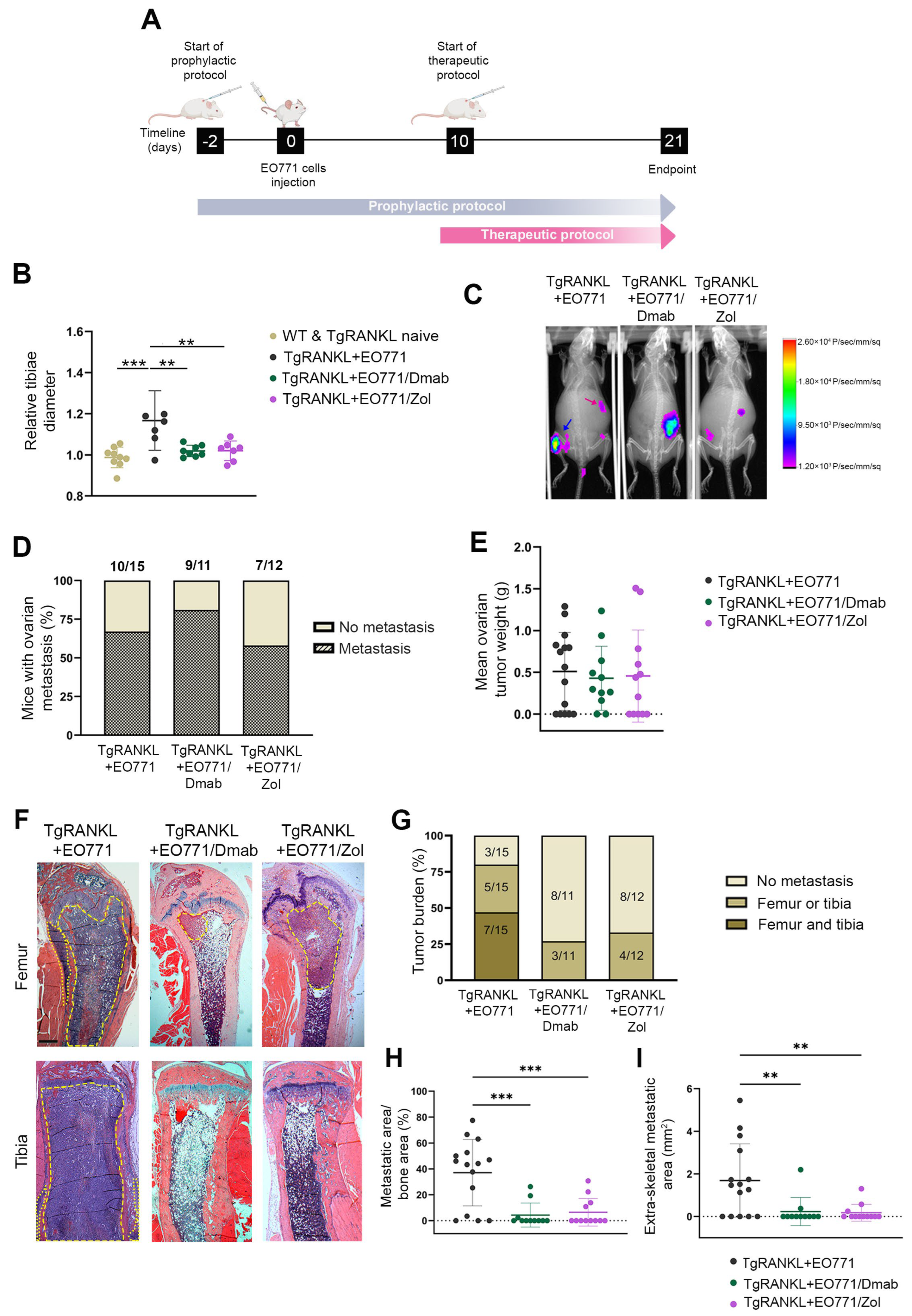
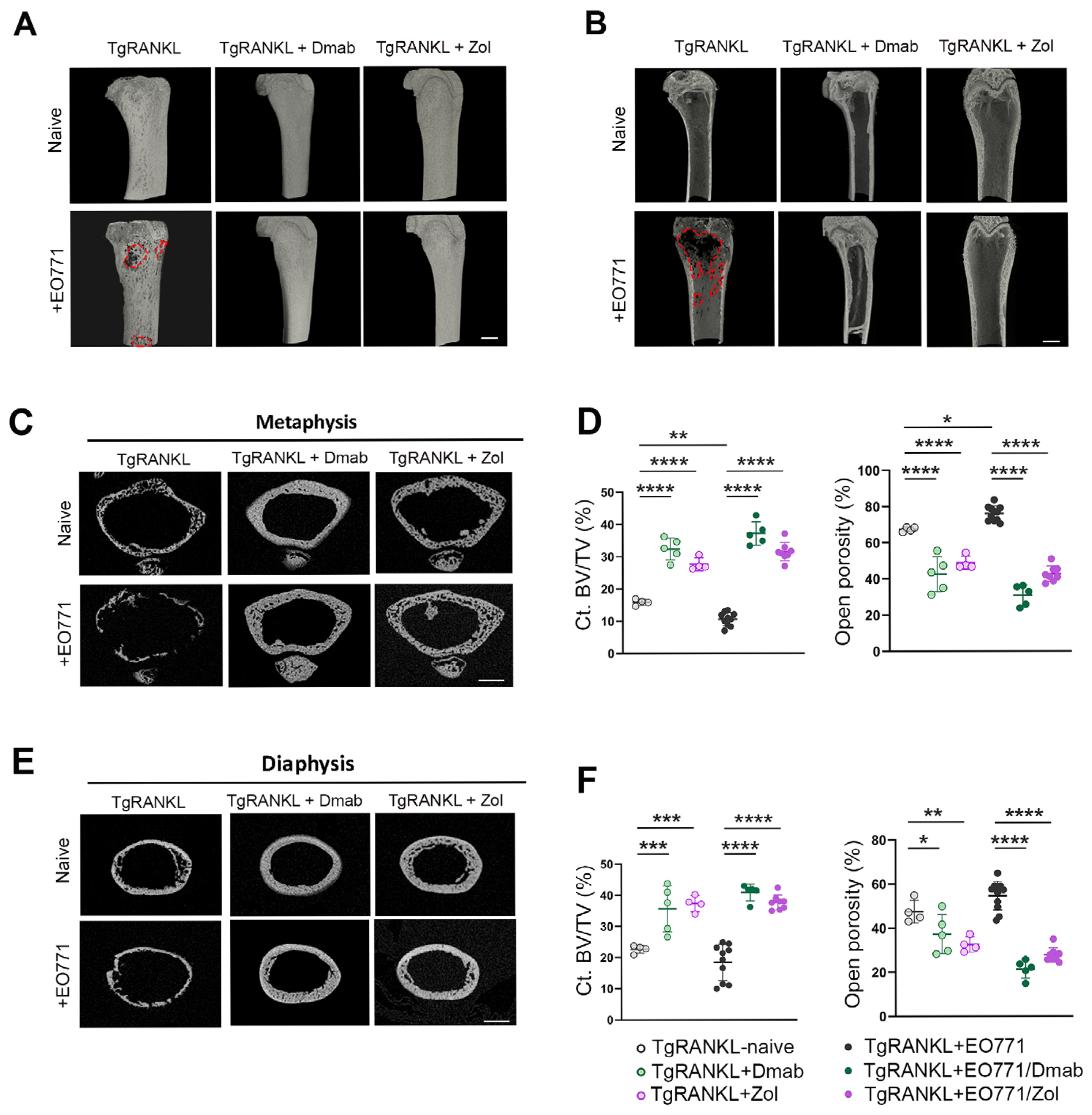
2.3. Prophylactic Anti-Resorptive Treatments Prevent Bone Metastasis and Osteolysis in TgRANKL Mice
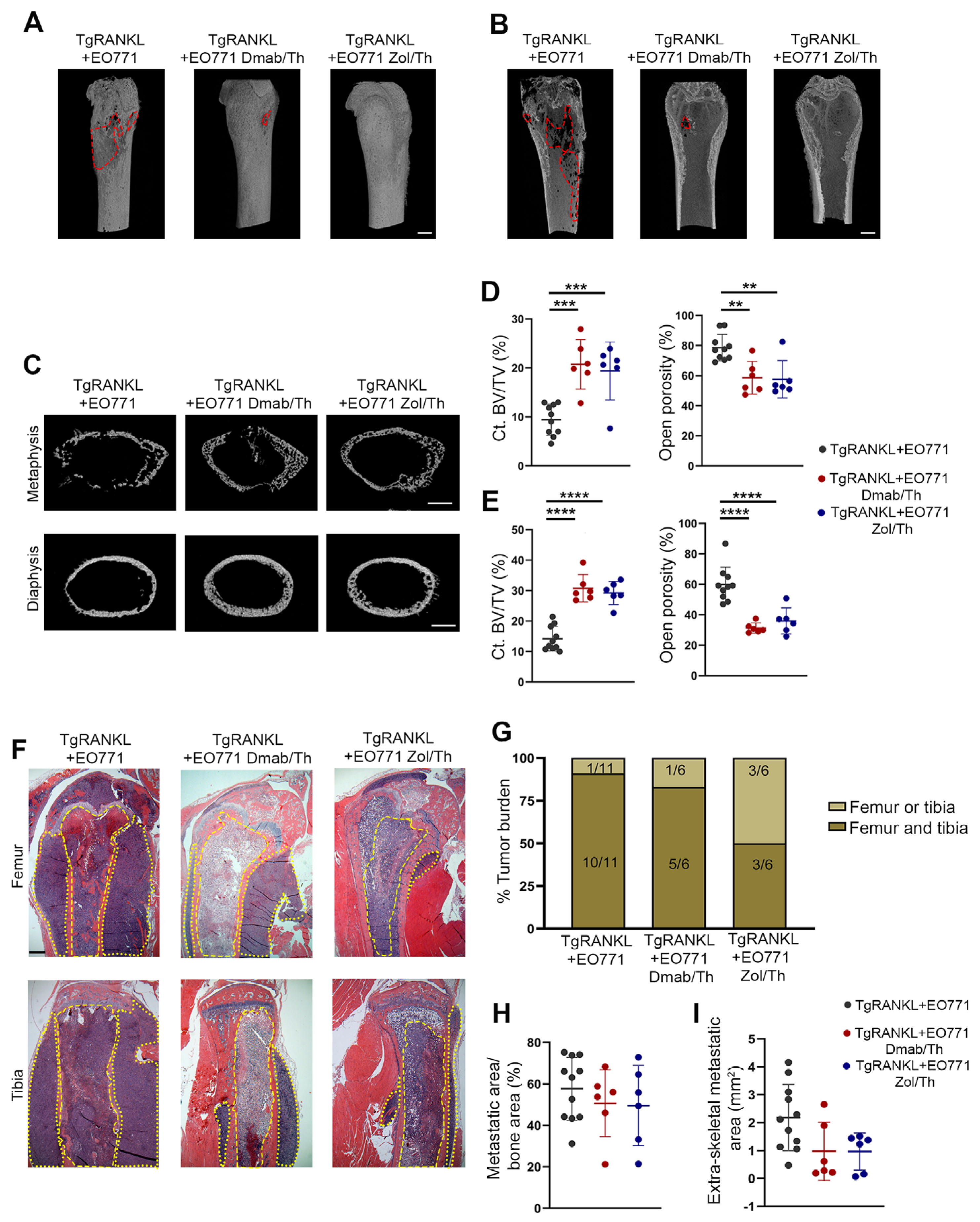
2.4. Effect of Therapeutic Anti-Resorptive Treatments in Bone Metastasis and Osteolysis
3. Discussion
4. Materials and Methods
4.1. Mouse Husbandry
4.2. EO771 Cell Line Culture
4.3. Bone Metastasis Model
4.4. Denosumab and Zoledronic Acid Treatments
4.5. In Vivo Imaging
4.6. Histological Examination
4.7. Micro-CT Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ct.BV/TV | Cortical bone volume/total volume |
| Dmab | Denosumab |
| dpi | Days post-injection |
| OPG | Osteoprotegerin |
| RANK | Receptor activator of nuclear factor-κB |
| RANK | Receptor activator of nuclear factor-κB ligand |
| SREs | Skeletal-related events |
| WT | Wild-type |
| Zol | Zoledronic acid |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-F.; Shen, J.; Li, X.; Rengan, R.; Silvestris, N.; Wang, M.; Derosa, L.; Zheng, X.; Belli, A.; Zhang, X.-L.; et al. Incidence of Patients with Bone Metastases at Diagnosis of Solid Tumors in Adults: A Large Population-Based Study. Ann. Transl. Med. 2020, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Deng, G.; Huang, X.; Li, X.; Xie, X.; Wang, J.; Shuang, Z.; Wang, X. Bone Metastasis Pattern in Initial Metastatic Breast Cancer: A Population-Based Study. Cancer Manag. Res. 2018, 10, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Brown, J.; Holen, I. Bone Metastases. In Abeloff’s Clinical Oncology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 809–830.e3. [Google Scholar]
- Jones, D.H.; Nakashima, T.; Sanchez, O.H.; Kozieradzki, I.; Komarova, S.V.; Sarosi, I.; Morony, S.; Rubin, E.; Sarao, R.; Hojilla, C.V.; et al. Regulation of Cancer Cell Migration and Bone Metastasis by RANKL. Nature 2006, 440, 692–696. [Google Scholar] [CrossRef]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for Osteocyte Regulation of Bone Homeostasis through RANKL Expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in Bone Modeling and Remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Lüthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Anandarajah, A.P. Role of RANKL in Bone Diseases. Trends Endocrinol. Metab. 2009, 20, 88–94. [Google Scholar] [CrossRef]
- Cummings, S.R.; Martin, J.S.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for Prevention of Fractures in Postmenopausal Women with Osteoporosis. Obstet. Gynecol. Surv. 2009, 64, 805–807. [Google Scholar] [CrossRef]
- Brown-Glaberman, U.; Stopeck, A.T. Role of Denosumab in the Management of Skeletal Complications in Patients with Bone Metastases from Solid Tumors. Biol. Targets Ther. 2012, 6, 89–99. [Google Scholar] [CrossRef]
- Kendler, D.L.; Cosman, F.; Stad, R.K.; Ferrari, S. Denosumab in the Treatment of Osteoporosis: 10 Years Later: A Narrative Review. Adv. Ther. 2022, 39, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Räkel, A.; Boucher, A.; Ste-Marie, L.G. Role of Zoledronic Acid in the Prevention and Treatment of Osteoporosis. Clin. Interv. Aging 2011, 6, 89–99. [Google Scholar] [CrossRef]
- Casimiro, S.; Vilhais, G.; Gomes, I.; Costa, L. The Roadmap of RANKL/RANK Pathway in Cancer. Cells 2021, 10, 1978. [Google Scholar] [CrossRef]
- Asselin-Labat, M.L.; Vaillant, F.; Sheridan, J.M.; Pal, B.; Wu, D.; Simpson, E.R.; Yasuda, H.; Smyth, G.K.; Martin, T.J.; Lindeman, G.J.; et al. Control of Mammary Stem Cell Function by Steroid Hormone Signalling. Nature 2010, 465, 798–802. [Google Scholar] [CrossRef]
- Zhang, L.; Teng, Y.; Zhang, Y.; Liu, J.; Xu, L.; Qu, J.; Hou, K.; Yang, X.; Liu, Y.; Qu, X. C-Src-Mediated RANKL-Induced Breast Cancer Cell Migration by Activation of the ERK and Akt Pathway. Oncol. Lett. 2012, 3, 395–400. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, H.; Song, X.; Yang, Q. Metastatic Heterogeneity of Breast Cancer: Molecular Mechanism and Potential Therapeutic Targets. Semin. Cancer Biol. 2020, 60, 14–27. [Google Scholar] [CrossRef]
- Lee, S.; Lorenzo, J.A. Parathyroid Hormone Stimulates TRANCE and Inhibits Osteoprotegerin Messenger Ribonucleic Acid Expression in Murine Bone Marrow Cultures: Correlation with Osteoclast-Like Cell Formation. Endocrinology. 1999, 140, 3552–3561. [Google Scholar] [CrossRef]
- Wang, M.; Xia, F.; Wei, Y.; Wei, X. Molecular Mechanisms and Clinical Management of Cancer Bone Metastasis. Bone Res. 2020, 8, 30. [Google Scholar] [CrossRef]
- Gnant, M.; Pfeiler, G.; Dubsky, P.C.; Hubalek, M.; Greil, R.; Jakesz, R.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger, E.; et al. Adjuvant Denosumab in Breast Cancer (ABCSG-18): A Multicentre, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2015, 386, 433–443. [Google Scholar] [CrossRef]
- Coleman, R.; Finkelstein, D.M.; Barrios, C.; Martin, M.; Iwata, H.; Hegg, R.; Glaspy, J.; Periañez, A.M.; Tonkin, K.; Deleu, I.; et al. Adjuvant Denosumab in Early Breast Cancer (D-CARE): An International, Multicentre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2020, 21, 60–72. [Google Scholar] [CrossRef]
- Rinotas, V.; Niti, A.; Dacquin, R.; Bonnet, N.; Stolina, M.; Han, C.-Y.; Kostenuik, P.; Jurdic, P.; Ferrari, S.; Douni, E. Novel Genetic Models of Osteoporosis by Overexpression of Human RANKL in Transgenic Mice. J. Bone Miner. Res. 2014, 29, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Rinotas, V.; Gkikopoulou, E.; Tzortzis, E.; Kritikos, K.; Siatra, P.; Papadopoulos, A.; Perivolidi, V.-I.; Douni, E. Interplay between Bone Marrow Adiposity and Bone Resorption in RANKL-Mediated Modelled Osteoporosis. J. Cell. Physiol. 2024, 239, e31434. [Google Scholar] [CrossRef] [PubMed]
- Blake, M.L.; Tometsko, M.; Miller, R.; Jones, J.C.; Dougall, W.C. RANK Expression on Breast Cancer Cells Promotes Skeletal Metastasis. Clin. Exp. Metastasis 2014, 31, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Ewens, A.; Mihich, E.; Ehrke, M.J. Distant Metastasis from Subcutaneously Grown E0771 Medullary Breast Adenocarcinoma. Anticancer Res. 2005, 25, 3905–3915. [Google Scholar]
- Johnstone, C.N.; Smith, Y.E.; Cao, Y.; Burrows, A.D.; Cross, R.S.N.; Ling, X.; Redvers, R.P.; Doherty, J.P.; Eckhardt, B.L.; Natoli, A.L.; et al. Functional and Molecular Characterisation of EO771.LMB Tumours, a New C57BL/6-Mouse-Derived Model of Spontaneously Metastatic Mammary Cancer. Dis. Model. Mech. 2015, 8, 237–251. [Google Scholar] [CrossRef]
- Hemmatian, H.; Conrad, S.; Furesi, G.; Mletzko, K.; Krug, J.; Faila, A.V.; Kuhlmann, J.D.; Rauner, M.; Busse, B.; Jähn-Rickert, K. Reorganization of the Osteocyte Lacuno-Canalicular Network Characteristics in Tumor Sites of an Immunocompetent Murine Model of Osteotropic Cancers. Bone 2021, 152, 116074. [Google Scholar] [CrossRef]
- Kuchimaru, T.; Kataoka, N.; Nakagawa, K.; Isozaki, T.; Miyabara, H.; Minegishi, M.; Kadonosono, T.; Kizaka-Kondoh, S. A Reliable Murine Model of Bone Metastasis by Injecting Cancer Cells through Caudal Arteries. Nat. Commun. 2018, 9, 2981. [Google Scholar] [CrossRef]
- Hanley, D.A.; Adachi, J.D.; Bell, A.; Brown, V. Denosumab: Mechanism of Action and Clinical Outcomes. Int. J. Clin. Pract. 2012, 66, 1139–1146. [Google Scholar] [CrossRef]
- Xiong, J.; Onal, M.; Jilka, R.L.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A. Matrix-Embedded Cells Control Osteoclast Formation. Nat. Med. 2011, 17, 1235–1241. [Google Scholar] [CrossRef]
- Vargas, G.; Bouchet, M.; Bouazza, L.; Reboul, P.; Boyault, C.; Gervais, M.; Kan, C.; Benetollo, C.; Brevet, M.; Croset, M.; et al. ERRα Promotes Breast Cancer Cell Dissemination to Bone by Increasing RANK Expression in Primary Breast Tumors. Oncogene 2019, 38, 950–964. [Google Scholar] [CrossRef]
- Dougall, W.C.; Chaisson, M. The RANK/RANKL/OPG Triad in Cancer-Induced Bone Diseases. Cancer Metastasis Rev. 2006, 25, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Ottewell, P.D.; Wang, N.; Brown, H.K.; Fowles, C.A.; Croucher, P.I.; Eaton, C.L.; Holen, I. OPG-Fc Inhibits Ovariectomy-Induced Growth of Disseminated Breast Cancer Cells in Bone. Int. J. Cancer 2015, 137, 968–977. [Google Scholar] [CrossRef]
- Kraemer, B.; Rothmund, R.; Banys, M.; Krawczyk, N.; Solomayer, E.F.; Mack, C.; Wallwiener, D.; Fehm, T. Impaired Bone Microenvironment: Correlation between Bone Density and Presence of Disseminated Tumor Cells. Anticancer Res. 2011, 31, 4423–4428. [Google Scholar]
- Chen, H.M.; Chen, F.P.; Yang, K.C.; Yuan, S.S. Association of Bone Metastasis with Early-Stage Breast Cancer in Women with and without Precancer Osteoporosis According to Osteoporosis Therapy Status. JAMA Netw. Open 2019, 2, e190429. [Google Scholar] [CrossRef]
- Canon, J.R.; Roudier, M.; Bryant, R.; Morony, S.; Stolina, M.; Kostenuik, P.J.; Dougall, W.C. Inhibition of RANKL Blocks Skeletal Tumor Progression and Improves Survival in a Mouse Model of Breast Cancer Bone Metastasis. Clin. Exp. Metastasis 2008, 25, 119–129. [Google Scholar] [CrossRef]
- Nakai, Y.; Okamoto, K.; Terashima, A.; Ehata, S.; Nishida, J.; Imamura, T.; Ono, T.; Takayanagi, H. Efficacy of an Orally Active Small-Molecule Inhibitor of RANKL in Bone Metastasis. Bone Res. 2019, 7, 1. [Google Scholar] [CrossRef]
- Nannuru, K.C.; Futakuchi, M.; Sadanandam, A.; Wilson, T.J.; Varney, M.L.; Myers, K.J.; Li, X.; Marcusson, E.G.; Singh, R.K. Enhanced Expression and Shedding of Receptor Activator of NF-ΚB Ligand during Tumor–Bone Interaction Potentiates Mammary Tumor-Induced Osteolysis. Clin. Exp. Metastasis 2009, 26, 797–808. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Rachner, T.D.; Coleman, R.E.; Jakob, F. Endocrine Aspects of Bone Metastases. Lancet Diabetes Endocrinol. 2014, 2, 500–512. [Google Scholar] [CrossRef]
- Hadji, P.; Aapro, M.S.; Body, J.J.; Gnant, M.; Brandi, M.L.; Reginster, J.Y.; Zillikens, M.C.; Glüer, C.C.; de Villiers, T.; Baber, R.; et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in Postmenopausal Women with Hormone Sensitive Breast Cancer: Joint Position Statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J. Bone Oncol. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Henry, D.H.; Costa, L.; Goldwasser, F.; Hirsh, V.; Hungria, V.; Prausova, J.; Scagliotti, G.V.; Sleeboom, H.; Spencer, A.; Vadhan-Raj, S.; et al. Randomized, Double-Blind Study of Denosumab versus Zoledronic Acid in the Treatment of Bone Metastases in Patients with Advanced Cancer (Excluding Breast and Prostate Cancer) or Multiple Myeloma. J. Clin. Oncol. 2011, 29, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Zhou, Y.; Jandial, D.; Cadieux, B.; Chan, A. Bone Health Outcomes from the International, Multicenter, Randomized, Phase 3, Placebo-Controlled D-CARE Study Assessing Adjuvant Denosumab in Early Breast Cancer. Adv. Ther. 2021, 38, 4569–4580. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ashton, R.; Hensel, J.A.; Lee, J.H.; Khattar, V.; Wang, Y.; Deshane, J.S.; Ponnazhagan, S. RANKL-Targeted Combination Therapy with Osteoprotegerin Variant Devoid of TRAIL Binding Exerts Biphasic Effects on Skeletal Remodeling and Antitumor Immunity. Mol. Cancer Ther. 2021, 19, 2585–2597. [Google Scholar] [CrossRef]
- Asano, T.; Okamoto, K.; Nakai, Y.; Tsutsumi, M.; Muro, R.; Suematsu, A.; Hashimoto, K.; Okamura, T.; Ehata, S.; Nitta, T.; et al. Soluble RANKL Is Physiologically Dispensable but Accelerates Tumour Metastasis to Bone. Nat. Metab. 2019, 1, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, T.; Williams, P.J.; Ueda, A.; Tamura, D.; Yoneda, T. Zoledronic Acid Inhibits Visceral Metastases in the 4T1/Luc Mouse Breast Cancer Model. Clin. Cancer Res. 2004, 10, 4559–4567. [Google Scholar] [CrossRef]
- Kohno, N.; Aogi, K.; Minami, H.; Nakamura, S.; Asaga, T.; Iino, Y.; Watanabe, T.; Goessl, C.; Ohashi, Y.; Takashima, S. Zoledronic Acid Significantly Reduces Skeletal Complications Compared with Placebo in Japanese Women with Bone Metastases from Breast Cancer: A Randomized, Placebo-Controlled Trial. J. Clin. Oncol. 2005, 23, 3314–3321. [Google Scholar] [CrossRef]
- Gnant, M.; Mlineritsch, B.; Stoeger, H.; Luschin-Ebengreuth, G.; Heck, D.; Menzel, C.; Jakesz, R.; Seifert, M.; Hubalek, M.; Pristauz, G.; et al. Adjuvant Endocrine Therapy plus Zoledronic Acid in Premenopausal Women with Early-Stage Breast Cancer: 62-Month Follow-up from the ABCSG-12 Randomised Trial. Lancet Oncol. 2011, 12, 631–641. [Google Scholar] [CrossRef]
- Stopeck, A.T.; Lipton, A.; Body, J.-J.; Steger, G.G.; Tonkin, K.; de Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M.; et al. Denosumab Compared with Zoledronic Acid for the Treatment of Bone Metastases in Patients with Advanced Breast Cancer: A Randomized, Double-Blind Study. J. Clin. Oncol. 2010, 28, 5132–5139. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Bozec, A.; Rauner, M.; Jakob, F.; Perner, S.; Pantel, K. Novel Approaches to Target the Microenvironment of Bone Metastasis. Nat. Rev. Clin. Oncol. 2021, 18, 488–505. [Google Scholar] [CrossRef]
- Grif, R.J.; Avery, E.; Xia, C.Q. Predicting Approximate Clinically Effective Doses in Oncology Using Preclinical Ef Fi Cacy and Body Surface Area Conversion: A Retrospective Analysis. Front. Pharmacol. 2022, 13, 830972. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kolokotroni, A.; Gkikopoulou, E.; Rinotas, V.; Ntari, L.; Zareifi, D.; Rouchota, M.; Sarpaki, S.; Lymperopoulos, I.; Alexopoulos, L.G.; Loudos, G.; et al. A Humanized RANKL Transgenic Mouse Model of Progestin-Induced Mammary Carcinogenesis for Evaluation of Novel Therapeutics. Cancers 2023, 15, 4006. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Holen, I.; Dear, T.N.; Hunter, K.; Brown, H.K. Modifying the Osteoblastic Niche with Zoledronic Acid in Vivo—Potential Implications for Breast Cancer Bone Metastasis. Bone 2014, 66, 240–250. [Google Scholar] [CrossRef]
- Wakchoure, S.; Merrell, M.A.; Aldrich, W.; Millender-swain, T.; Harris, K.W.; Triozzi, P.; Selander, K.S. Cancer Therapy: Preclinical Bisphosphonates Inhibit the Growth of Mesothelioma Cells In Vitro and In Vivo. Clin. Cancer Res. 2006, 12, 2862–2869. [Google Scholar] [CrossRef]
- Kozutsumi, R.; Kuroshima, S.; Kaneko, H.; Sasaki, M.; Ishisaki, A. Zoledronic Acid Deteriorates Soft and Hard Tissue Healing of Murine Tooth Extraction Sockets in a Dose-Dependent Manner. Calcif. Tissue Int. 2021, 110, 104–116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkikopoulou, E.; Syrigos, C.-C.; Mantogiannakou, I.; Petraki, C.-E.; Stathopoulou, M.; Dragolia, M.; Rinotas, V.; Ntafis, V.; Rauner, M.; Douni, E. RANKL Drives Bone Metastasis in Mammary Cancer: Protective Effects of Anti-Resorptive Treatments. Int. J. Mol. Sci. 2025, 26, 4990. https://doi.org/10.3390/ijms26114990
Gkikopoulou E, Syrigos C-C, Mantogiannakou I, Petraki C-E, Stathopoulou M, Dragolia M, Rinotas V, Ntafis V, Rauner M, Douni E. RANKL Drives Bone Metastasis in Mammary Cancer: Protective Effects of Anti-Resorptive Treatments. International Journal of Molecular Sciences. 2025; 26(11):4990. https://doi.org/10.3390/ijms26114990
Chicago/Turabian StyleGkikopoulou, Evi, Christos-Chrysovalantis Syrigos, Ioanna Mantogiannakou, Chrysa-Eleni Petraki, Melina Stathopoulou, Melina Dragolia, Vagelis Rinotas, Vasileios Ntafis, Martina Rauner, and Eleni Douni. 2025. "RANKL Drives Bone Metastasis in Mammary Cancer: Protective Effects of Anti-Resorptive Treatments" International Journal of Molecular Sciences 26, no. 11: 4990. https://doi.org/10.3390/ijms26114990
APA StyleGkikopoulou, E., Syrigos, C.-C., Mantogiannakou, I., Petraki, C.-E., Stathopoulou, M., Dragolia, M., Rinotas, V., Ntafis, V., Rauner, M., & Douni, E. (2025). RANKL Drives Bone Metastasis in Mammary Cancer: Protective Effects of Anti-Resorptive Treatments. International Journal of Molecular Sciences, 26(11), 4990. https://doi.org/10.3390/ijms26114990






