Targeting Aging Hallmarks with Monoclonal Antibodies: A New Era in Cancer Immunotherapy and Geriatric Medicine
Abstract
1. Introduction
1.1. MAbs: Mechanisms, Strategies, and Pathologies Addressing Aging Hallmarks Interpretive Meaning
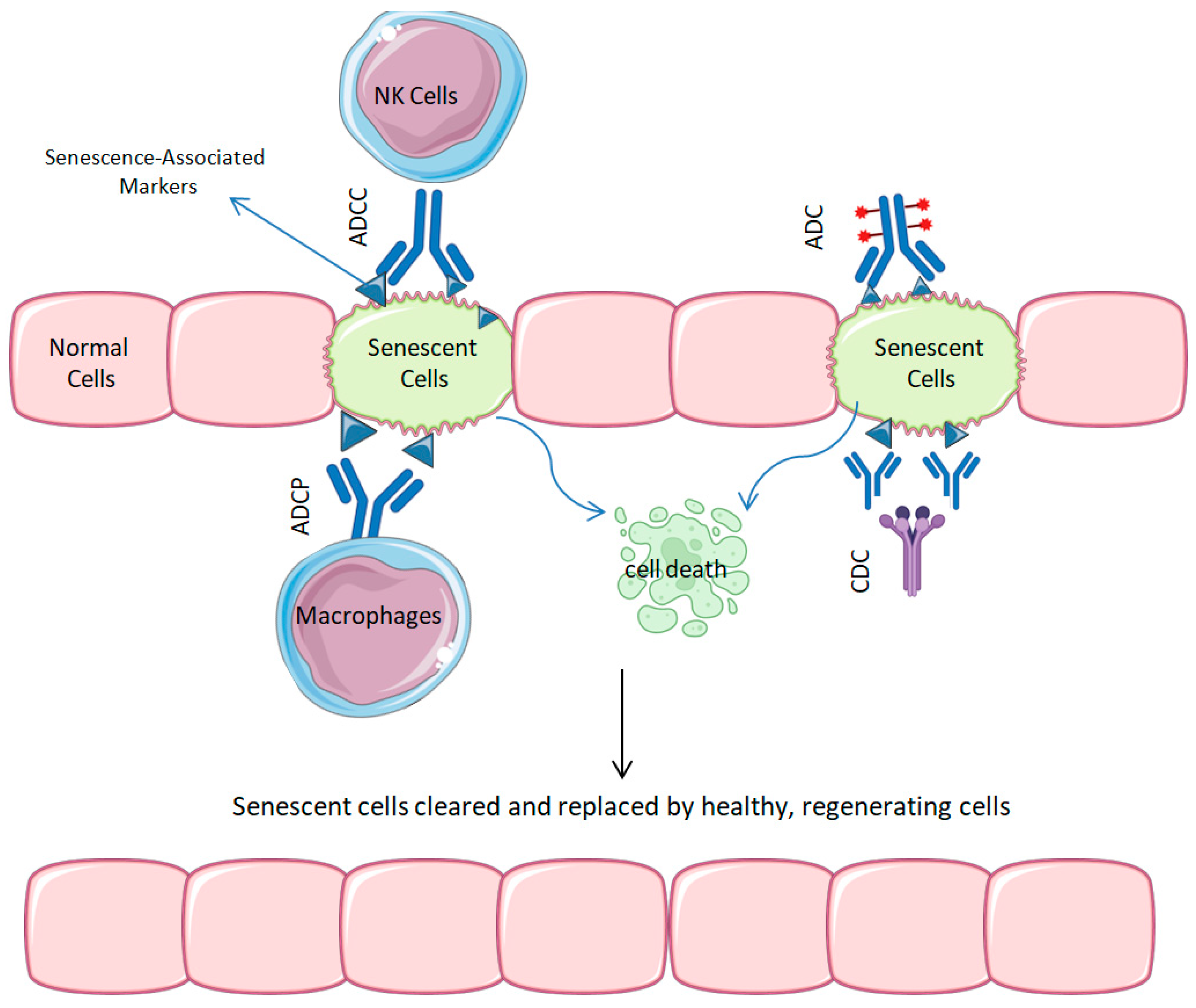
1.1.1. Cellular Senescence Mechanism
1.1.2. Senescence and Its Role in Aging and Oncology: Therapeutic Implications
- Senolytic ADCs: Certain mAbs are being developed as transporters for lethal medicines, mostly targeted at markers, like Apolipoprotein D (ApoD), that are common on the surfaces of senescent cells. Senolytic ADCs are designed to destroy harmful cells while maintaining healthy tissue; these senolytic ADCs help to restore the local tissue balance and thereby reduce the senescence-associated secretory phenotype [13].
- Neutralizing Antibodies Against SASP: Another approach is concentrating on particular components of the SASP, including IL-6, IL-8, and TNF-α; in other words, neutralizing antibodies against SASPs. By reducing local inflammation, neutralizing pro-inflammatory cytokines may help to either delay or stop tissue deterioration in aging organs [34,35,36].
- AD: Senescent glial and neuronal cells are thought to play a role in neuroinflammation and cognitive deterioration. Preliminary investigations of senolytic antibodies indicate their potential to diminish neurodegenerative disease in preclinical settings [37].
- Skin Aging: The accumulation of senescent fibroblasts and keratinocytes is associated with observable aging indicators, such as diminished skin suppleness and the formation of wrinkles. The precise removal of these cells may create new opportunities in dermatological rejuvenation therapy [41,42,43].
1.2. Inflammaging Mechanism
- Anti-IL-6 Receptor Antibodies: Therapeutic antibodies like Tocilizumab and Sarilumab inhibit the IL-6 receptor, thereby obstructing a crucial inflammatory signaling pathway. Initially formulated for rheumatoid arthritis, these antibodies are currently being explored for their potential in several inflammation-related illnesses linked to aging [47].
- Anti-TNF-α Antibodies: MAbs such as Infliximab and Adalimumab directly inhibit TNF-α, a principal regulator of systemic inflammation. Their immunomodulatory effects have been well established in the management of autoimmune disorders, and their significance in aging-related inflammation is becoming increasingly evident [48].
- Rheumatoid Arthritis (RA) exemplifies inflammaging, characterized by chronic cytokine activity that leads to joint deterioration and systemic inflammation. Anti-cytokine medications, such as mAbs, have revolutionized treatment, demonstrating their clinical effectiveness in reducing the inflammatory burden [49].
- Cardiovascular Disease (CVD): Chronic inflammation exacerbates endothelial dysfunction and plaque development, hastening atherosclerosis in the elderly. Recent results indicate that anti-cytokine mAbs may provide vascular protection by attenuating inflammatory pathways implicated in the etiology of cardiovascular disease [50].
- Sarcopenia: Muscle atrophy and weakness associated with aging, generally referred to as sarcopenia, have been correlated with increased inflammatory cytokines [51]. Despite the preliminary nature of clinical data, cytokine-targeting mAbs may offer potential in maintaining muscle mass and function in the aged.
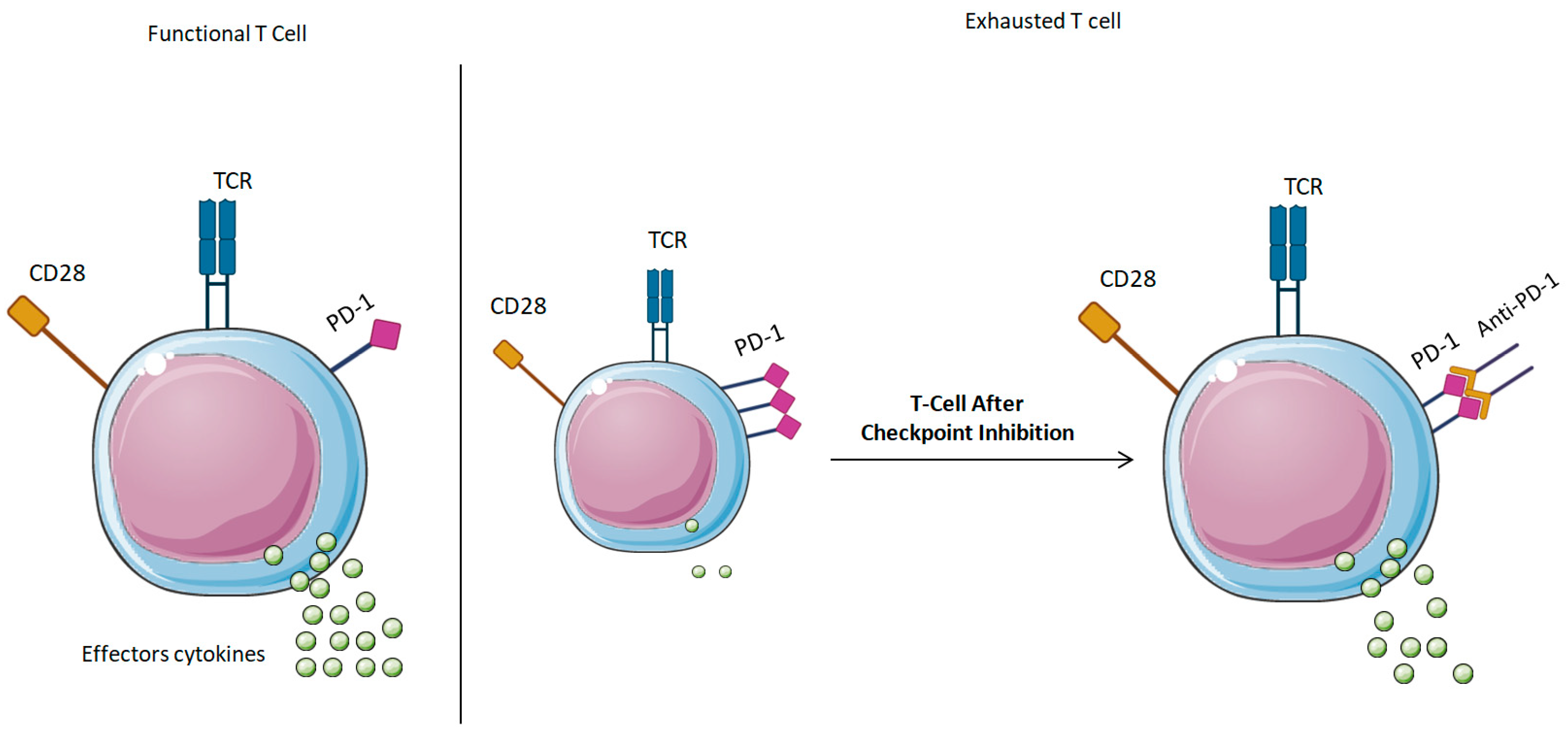
1.3. Immunosenescence Mechanism
- Checkpoint Inhibition: A promising strategy to mitigate immunosenescence is the application of mAbs that target immunological checkpoint proteins, including Programmed Death-1 (PD-1) and Cytotoxic T-Lymphocyte-Associated Protein 4 (CTLA-4). Inhibitory receptors are frequently increased on T-cells in elderly persons, leading to functional fatigue. Immunological checkpoint inhibitors, like Nivolumab (anti-PD-1) and Ipilimumab (anti-CTLA-4), have been shown to be effective in oncology by reactivating antitumor immunity and are currently being investigated for their potential to revitalize immunological responses in aging populations more generally [16,57].
- Cancer: The age-associated deterioration of immune surveillance promotes tumor evasion and advancement. By obstructing inhibitory checkpoints, mAbs can reinstate cytotoxic T-cell functionality and enhance tumor identification, rendering them a formidable choice for cancer therapy in elderly patients [16].
- Infectious Diseases: Elderly adults demonstrate diminished responses to infections and vaccinations. Preliminary studies indicate that altering checkpoint pathways may augment pathogen-specific T-cell responses and increase outcomes in viral and bacterial infections in the elderly [58].
1.4. Disruption of Proteostasis Mechanism:
- Anti-Amyloid MAbs: Numerous mAbs have been engineered to identify and attach to aggregated forms of amyloid-beta (Aβ), a peptide pivotal to the pathogenesis of AD. Aducanumab and Lecanemab are agents engineered to promote the immune-mediated removal of Aβ plaques from the brain, potentially alleviating subsequent neurodegeneration [63,64]. These antibodies operate by either enhancing phagocytosis via microglial activation or by directly obstructing plaque formation and dissemination.
- Alzheimer’s and Parkinson’s Diseases: Clinical Trials and Therapeutic mAbs:
- AD: The accumulation of amyloid-beta plaques is a characteristic hallmark of AD. Anti-Aβ antibodies seek to diminish amyloid accumulation and maintain cognitive function in the initial phases of the disease. Although clinical trials have shown reductions in the plaque burden, the degree of functional improvement is limited, and factors like patient selection, the timing of intervention, and safety concerns (e.g., amyloid-related imaging abnormalities, or ARIA) continue to influence their therapeutic application [70].
1.5. Advanced Glycation End-Products (AGEs)
- AGE-Targeting Antibodies: Innovative MAbs, such as SIWA318H, have been designed to preferentially identify and bind to AGE-modified proteins, facilitating their immune clearance. This strategy seeks to alleviate the pro-inflammatory and pro-fibrotic impacts of AGEs, thereby diminishing their role in chronic tissue damage. By mitigating the burden of AGEs, these antibodies may facilitate the restoration of homeostasis in aged tissues [74].
- Complications Associated with Diabetes: AGEs are pivotal in the development of diabetic microvascular and macrovascular problems, such as retinopathy, nephropathy, and atherosclerosis. Mitigating AGE buildup may reduce vascular damage and enhance long-term results in diabetic patients [75].
- Chronic Kidney Disease (CKD): AGE accumulation in renal tissues leads to fibrosis and deteriorating kidney function, particularly in elderly persons with comorbidities. MAb treatments aimed at advanced glycation end-products may present an innovative approach to decelerate chronic kidney disease progression by diminishing fibrotic signaling and maintaining nephron integrity [76].
1.6. Dysregulated Nutrient Sensing
- MAbs Against IGF-1R: Various MAbs, including Dalotuzumab, Cixutumumab, and Teprotumumab, have been engineered to obstruct IGF-1 receptor (IGF-1R) signaling [79,80,81]. Initially developed as anti-cancer medicines, these antibodies may be repurposed to mitigate excessive growth signals, decrease cellular proliferation, and potentially activate longevity-related mechanisms, such as autophagy, in the setting of aging.
- Antibodies Directed Against mTOR-Associated Nodes: Although direct mTOR inhibition using mAbs presents a technological hurdle, researchers are investigating indirect approaches that target upstream or downstream modulators of the mTOR system [82]. This involves targeting components of PI3K or S6 kinase (S6K), perhaps enabling the more precise modulation of metabolic activity without completely inhibiting critical mTOR functions.
- Metabolic Syndrome and Type 2 Diabetes: The modulation of IGF-1 signaling has demonstrated promise for enhancing insulin sensitivity and glucose management in metabolic illnesses that predominantly impact the elderly [85]. Antibodies directed at this axis may augment or improve current antidiabetic treatments.
- Neurodegenerative Diseases: mTOR inhibition is associated with the improved autophagic removal of neurotoxic aggregates, including amyloid-beta and tau [86], suggesting potential advantages in the treatment or prevention of Alzheimer’s and Parkinson’s diseases.
1.7. Side Effects
2. Senolytic MABs: Targeting Senescent Cells
2.1. ADCs for Senescent Cell Clearance
2.2. The Modulation of the SASP
2.3. Clinical Translation and Challenges
| Strategy | Target(s) | Mechanism | Preclinical/Clinical Status | Ref |
|---|---|---|---|---|
| ADCs | B2M, Senescent Cell Markers | Delivery of cytotoxic agents to induce apoptosis | Preclinical studies demonstrate senescent cell clearance and tissue rejuvenation. | [44] |
| SASP Modulation via Cytokine Targeting | IL-8 | Antagonism of pro-inflammatory signaling | Investigated in cancer models; potential application in aging under exploration. | [109,110] |
3. mAbs in Modulating Inflammaging
- Targeting IL-6:
- Inhibiting TNF-α:
- IL-1 Inhibition:
3.1. The Thbs Pathway Inhibition as a Novel Therapeutic Strategy
3.2. Clinical Translation and Challenges
| Target Cytokine | MAb | Mechanism of Action | Clinical Application and Status | Ref |
|---|---|---|---|---|
| IL-6 | Tocilizumab | IL-6 receptor antagonist | Approved for rheumatoid arthritis; potential in reducing systemic inflammation in the elderly. | [118] |
| TNF-α | Infliximab, Adalimumab | TNF-α neutralization | Approved for various inflammatory conditions; studies exploring effects on inflammaging ongoing. | [122,123] |
| IL-1 | Anakinra | IL-1 receptor antagonist | Used in rheumatoid arthritis; research into impact on age-related inflammation underway. | [129] |
| Thbs1 | Not yet developed | Inhibition of Thbs1-mediated inflammaging pathways | Preclinical studies indicate potential in preserving hematopoietic function during aging. | [131] |
4. Immune Checkpoint Modulation: Rejuvenating the Aging Immune System
4.1. Understanding Immune Checkpoints in Aging
4.2. Efficacy of Immune Checkpoint Inhibitors in the Elderly
4.3. Novel Targets: GD3 Ganglioside and NKG2D Ligands
4.4. Clinical Translation and Obstacles
| Target | Therapeutic Agent | Mechanism of Action | Clinical Implications | Ref |
|---|---|---|---|---|
| PD-1 | Nivolumab | PD-1 receptor blockade | Approved for various cancers; studies in elderly patients show variable outcomes. | [155] |
| PD-L1 | Atezolizumab | PD-L1 ligand blockade | Used in cancer therapy; potential in targeting senescent cells in aging tissues. | [156,157] |
| CTLA-4 | Ipilimumab | CTLA-4 receptor blockade | Combined with PD-1 inhibitors in cancer; limited data in aging immune modulation. | [158,159] |
| GD3 Ganglioside | Not yet developed | Targeting senescence-associated immune checkpoints | Preclinical studies suggest potential in enhancing clearance of senescent cells. | [148] |
5. Molecular and Structural Engineering of MAbs for Aging-Adapted Therapeutics
5.1. Fc Engineering for Improved Durability
5.2. Reducing Immunogenicity in Aged Immune Systems
5.3. Optimizing for Frail Physiology
5.4. Targeting Diverse Aging Tissues
5.5. Crossing the Blood–Brain Barrier (BBB) in Aging Neurodegeneration
5.6. Artificial Intelligence-Driven and pH-Sensitive mAb Design for Enhanced Therapeutic Precision
6. Progress in MAb Treatments
6.1. Improved Specificity and Diminished Adverse Effects
6.2. Combination Therapies
6.3. Expanding Applications Beyond Neurodegenerative Diseases
6.4. Personalized Medicine
7. Ethical Considerations
- Informed Consent
- The Evaluation of the Risks and Benefits
- The Allocation of Resources
- The Utilization of Animals in Research
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tenchov, R.; Sasso, J.M.; Wang, X.; Zhou, Q.A. Aging Hallmarks and Progression and Age-Related Diseases: A Landscape View of Research Advancement. ACS Chem. Neurosci. 2024, 15, 1–30. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- de Magalhães, J.P.; Passos, J.F. Stress, cell senescence and organismal ageing. Mech. Ageing Dev. 2018, 170, 2–9. [Google Scholar] [CrossRef]
- Tripathi, U.; Misra, A.; Tchkonia, T.; Kirkland, J.L. Impact of Senescent Cell Subtypes on Tissue Dysfunction and Repair: Importance and Research Questions. Mech. Ageing Dev. 2021, 198, 111548. [Google Scholar] [CrossRef] [PubMed]
- Goyani, P.; Christodoulou, R.; Vassiliou, E. Immunosenescence: Aging and Immune System Decline. Vaccines 2024, 12, 1314. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Ok, S.C. Insights into the Anti-Aging Prevention and Diagnostic Medicine and Healthcare. Diagnostics 2022, 12, 819. [Google Scholar] [CrossRef]
- Guarente, L.; Sinclair, D.A.; Kroemer, G. Human trials exploring anti-aging medicines. Cell Metab. 2024, 36, 354–376. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Niu, Y.; Wei, K.; Wang, X.; Niu, B.; Zhang, L. Research progress on aging mechanism and drugs and the role of stem cells in anti-aging process. Exp. Gerontol. 2023, 179, 112248. [Google Scholar] [CrossRef]
- Shepard, H.M.; Phillips, G.L.; Thanos, C.D.; Feldmann, M. Developments in therapy with monoclonal antibodies and related proteins. Clin. Med. 2017, 17, 220–232. [Google Scholar] [CrossRef]
- Jia, G.; Jiang, Y.; Li, X. Targeted drug conjugates in cancer therapy: Challenges and opportunities. Pharm. Sci. Adv. 2024, 2, 100048. [Google Scholar] [CrossRef]
- Takaya, K.; Asou, T.; Kishi, K. New Senolysis Approach via Antibody-Drug Conjugate Targeting of the Senescent Cell Marker Apolipoprotein D for Skin Rejuvenation. Int. J. Mol. Sci. 2023, 24, 5857. [Google Scholar] [CrossRef]
- Orjalo, A.V.; Bhaumik, D.; Gengler, B.K.; Scott, G.K.; Campisi, J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. USA 2009, 106, 17031–17036. [Google Scholar] [CrossRef]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef]
- Elias, R.; Karantanos, T.; Sira, E.; Hartshorn, K.L. Immunotherapy comes of age: Immune aging & checkpoint inhibitors. J. Geriatr. Oncol. 2017, 8, 229–235. [Google Scholar] [CrossRef]
- Kugel, C.H., 3rd; Douglass, S.M.; Webster, M.R.; Kaur, A.; Liu, Q.; Yin, X.; Weiss, S.A.; Darvishian, F.; Al-Rohil, R.N.; Ndoye, A.; et al. Age Correlates with Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations. Clin. Cancer Res. 2018, 24, 5347–5356. [Google Scholar] [CrossRef]
- Kasanen, H.; Hernberg, M.; Mäkelä, S.; Brück, O.; Juteau, S.; Kohtamäki, L.; Ilander, M.; Mustjoki, S.; Kreutzman, A. Age-associated changes in the immune system may influence the response to anti-PD1 therapy in metastatic melanoma patients. Cancer Immunol. Immunother. 2020, 69, 717–730. [Google Scholar] [CrossRef]
- Khatib, S.E.; Salla, M. The mosaic puzzle of the therapeutic monoclonal antibodies and antibody fragments—A modular transition from full-length immunoglobulins to antibody mimetics. Leuk. Res. Rep. 2022, 18, 100335. [Google Scholar] [CrossRef]
- Sawant, M.S.; Streu, C.N.; Wu, L.; Tessier, P.M. Toward Drug-Like Multispecific Antibodies by Design. Int. J. Mol. Sci. 2020, 21, 7496. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Berger, M.; Shankar, V.; Vafai, A. Therapeutic applications of monoclonal antibodies. Am. J. Med. Sci. 2002, 324, 14–30. [Google Scholar] [CrossRef]
- Mekala, J.R.; Nalluri, H.P.; Reddy, P.N.; Sainath, B.S.; Kumar, S.N.S.; Kiran, S.G.V.S.D.; Dhiman, R.; Chamarthy, S.; Komaragiri, R.R.; Manyam, R.R.; et al. Emerging trends and therapeutic applications of monoclonal antibodies. Gene 2024, 925, 148607. [Google Scholar] [CrossRef]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Ohtani, N. The roles and mechanisms of senescence-associated secretory phenotype (SASP): Can it be controlled by senolysis? Inflamm. Regen. 2022, 42, 11. [Google Scholar] [CrossRef]
- Yamauchi, S.; Takahashi, A. Cellular senescence: Mechanisms and relevance to cancer and aging. J. Biochem. 2025, 177, 163–169. [Google Scholar] [CrossRef]
- Dong, Z.; Luo, Y.; Yuan, Z.; Tian, Y.; Jin, T.; Xu, F. Cellular senescence and SASP in tumor progression and therapeutic opportunities. Mol. Cancer 2024, 23, 181. [Google Scholar] [CrossRef]
- Stoczynska-Fidelus, E.; Węgierska, M.; Kierasińska, A.; Ciunowicz, D.; Rieske, P. Role of Senescence in Tumorigenesis and Anticancer Therapy. J. Oncol. 2022, 2022, 5969536. [Google Scholar] [CrossRef]
- Jin, P.; Duan, X.; Li, L.; Zhou, P.; Zou, C.G.; Xie, K. Cellular senescence in cancer: Molecular mechanisms and therapeutic targets. MedComm 2024, 5, e542. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kang, D.; Shin, J.; Jung, J.; Ko, S.; Jung, K.H.; Hong, S.-S.; Park, J.E.; Oh, M.J.; An, H.J.; et al. Therapy-induced senescent cancer cells contribute to cancer progression by promoting ribophorin 1-dependent PD-L1 upregulation. Nat. Commun. 2025, 16, 353. [Google Scholar] [CrossRef]
- Zingoni, A.; Antonangeli, F.; Sozzani, S.; Santoni, A.; Cippitelli, M.; Soriani, A. The senescence journey in cancer immunoediting. Mol. Cancer 2024, 23, 68. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T.; Zhu, Y.; Niedernhofer, L.J.; Robbins, P.D. The Clinical Potential of Senolytic Drugs. J. Am. Geriatr. Soc. 2017, 65, 2297–2301. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Q.; Kirkland, J.L. Targeting senescent cells for a healthier longevity: The roadmap for an era of global aging. Life Med. 2022, 1, 103–119. [Google Scholar] [CrossRef]
- Herbstein, F.; Sapochnik, M.; Attorresi, A.; Pollak, C.; Senin, S.; Gonilski-Pacin, D.; Ciancio Del Giudice, N.; Fiz, M.; Elguero, B.; Fuertes, M.; et al. The SASP factor IL-6 sustains cell-autonomous senescent cells via a cGAS-STING-NFκB intracrine senescent noncanonical pathway. Aging Cell 2024, 23, e14258. [Google Scholar] [CrossRef]
- Ortiz-Montero, P.; Londoño-Vallejo, A.; Vernot, J.P. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun. Signal 2017, 15, 17. [Google Scholar] [CrossRef]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Misra Sen, J.; Gorospe, M.; et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 2019, 22, 719–728. [Google Scholar] [CrossRef]
- Astrike-Davis, E.M.; Coryell, P.; Loeser, R.F. Targeting cellular senescence as a novel treatment for osteoarthritis. Curr. Opin. Pharmacol. 2022, 64, 102213. [Google Scholar] [CrossRef]
- Chin, A.F.; Han, J.; Clement, C.C.; Choi, Y.; Zhang, H.; Browne, M.; Jeon, O.H.; Elisseeff, J.H. Senolytic treatment reduces oxidative protein stress in an aging male murine model of post-traumatic osteoarthritis. Aging Cell 2023, 22, e13979. [Google Scholar] [CrossRef]
- Appleman, L.J.; Beumer, J.H.; Jiang, Y.; Lin, Y.; Ding, F.; Puhalla, S.; Swartz, L.; Owonikoko, T.K.; Donald Harvey, R.; Stoller, R.; et al. Phase 1 study of veliparib (ABT-888), a poly (ADP-ribose) polymerase inhibitor, with carboplatin and paclitaxel in advanced solid malignancies. Cancer Chemother. Pharmacol. 2019, 84, 1289–1301. [Google Scholar] [CrossRef]
- Victorelli, S.; Lagnado, A.; Halim, J.; Moore, W.; Talbot, D.; Barrett, K.; Chapman, J.; Birch, J.; Ogrodnik, M.; Meves, A.; et al. Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. EMBO J. 2019, 38, e101982. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.; Song, M.J.; Park, C.-H.; Lee, D.H.; Lee, S.-H.; Chung, J.H. Inhibition of matrix metalloproteinase expression by selective clearing of senescent dermal fibroblasts attenuates ultraviolet-induced photoaging. Biomed. Pharmacother. 2022, 150, 113034. [Google Scholar] [CrossRef]
- Takaya, K.; Ishii, T.; Asou, T.; Kishi, K. Navitoclax (ABT-263) Rejuvenates Human Skin by Eliminating Senescent Dermal Fibroblasts in a Mouse/Human Chimeric Model. Rejuvenation Res. 2023, 26, 9–20. [Google Scholar] [CrossRef]
- Poblocka, M.; Bassey, A.L.; Smith, V.M.; Falcicchio, M.; Manso, A.S.; Althubiti, M.; Sheng, X.; Kyle, A.; Barber, R.; Frigerio, M.; et al. Targeted clearance of senescent cells using an antibody-drug conjugate against a specific membrane marker. Sci. Rep. 2021, 11, 20358. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular senescence: A key therapeutic target in aging and diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef]
- Tylutka, A.; Walas, Ł.; Zembron-Lacny, A. Level of IL-6, TNF, and IL-1β and age-related diseases: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1330386. [Google Scholar] [CrossRef]
- Avci, A.B.; Feist, E.; Burmester, G.R. Targeting IL-6 or IL-6 Receptor in Rheumatoid Arthritis: What Have We Learned? BioDrugs 2024, 38, 61–71. [Google Scholar] [CrossRef]
- Evangelatos, G.; Bamias, G.; Kitas, G.D.; Kollias, G.; Sfikakis, P.P. The second decade of anti-TNF-a therapy in clinical practice: New lessons and future directions in the COVID-19 era. Rheumatol. Int. 2022, 42, 1493–1511. [Google Scholar] [CrossRef]
- Bedeković, D.; Bošnjak, I.; Šarić, S.; Kirner, D.; Novak, S. Role of Inflammatory Cytokines in Rheumatoid Arthritis and Development of Atherosclerosis: A Review. Medicina 2023, 59, 1550. [Google Scholar] [CrossRef]
- Libby, P. Targeting Inflammatory Pathways in Cardiovascular Disease: The Inflammasome, Interleukin-1, Interleukin-6 and Beyond. Cells 2021, 10, 951. [Google Scholar] [CrossRef]
- Cacciatore, S.; Calvani, R.; Esposito, I.; Massaro, C.; Gava, G.; Picca, A.; Tosato, M.; Marzetti, E.; Landi, F. Emerging Targets and Treatments for Sarcopenia: A Narrative Review. Nutrients 2024, 16, 3271. [Google Scholar] [CrossRef]
- Hu, J.; Sun, Y.; Zuo, X.; Zou, Y. Assessment of adverse events related to anti-interleukin-6 receptor monoclonal antibodies using the FDA adverse event reporting system: A real-world pharmacovigilance study. Expert Opin. Drug Saf. 2024, 23, 1327–1339. [Google Scholar] [CrossRef]
- Heo, Y.A. Satralizumab: First Approval. Drugs 2020, 80, 1477–1482. [Google Scholar] [CrossRef]
- Padda, I.S.; Bhatt, R.; Parmar, M. Golimumab; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Abreu, M.T. Commentary: Golimumab in Moderate-to-Severe Ulcerative Colitis. Gastroenterol. Hepatol. 2014, 10, 455–456. [Google Scholar]
- Yang, Y.; Fan, L.; Li, M.; Wang, Z. Immune senescence: A key player in cancer biology. Semin. Cancer Biol. 2025, 108, 71–82. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Gustafson, C.E.; Kim, C.; Weyand, C.M.; Goronzy, J.J. Influence of immune aging on vaccine responses. J. Allergy Clin. Immunol. 2020, 145, 1309–1321. [Google Scholar] [CrossRef]
- Vucinic, D.; Skocilic, I.; Golcic, M.; Dobrila-Dintinjana, R.; Kolak, M.; Jerkovic, I.; Tesar, E.C.; Ferari, A.M.; Redjovic, A.; Marusic, J.; et al. Effectiveness and Safety of Immune Checkpoint Inhibitors in Older Cancer Patients. J. Pers. Med. 2024, 14, 278. [Google Scholar] [CrossRef]
- Yin, J.; Song, Y.; Fu, Y.; Jun, W.; Tang, J.; Zhang, Z.; Song, Q.; Zhang, B. The efficacy of immune checkpoint inhibitors is limited in elderly NSCLC: A retrospective efficacy study and meta-analysis. Aging 2023, 15, 15025–15049. [Google Scholar] [CrossRef]
- Labbadia, J.; Morimoto, R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef]
- Meller, A.; Shalgi, R. The aging proteostasis decline: From nematode to human. Exp. Cell Res. 2021, 399, 112474. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Johannesson, M.; Söderberg, L.; Zachrisson, O.; Fritz, N.; Kylefjord, H.; Gkanatsiou, E.; Button, E.; Svensson, A.-S.; Rachalski, A.; Nygren, P.; et al. Lecanemab demonstrates highly selective binding to Aβ protofibrils isolated from Alzheimer’s disease brains. Mol. Cell. Neurosci. 2024, 130, 103949. [Google Scholar] [CrossRef]
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef]
- Dyck, C.H.v.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Mintun, M.A.; Lo, A.C.; Evans, C.D.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef]
- Pagano, G.; Taylor, K.I.; Anzures Cabrera, J.; Simuni, T.; Marek, K.; Postuma, R.B.; Pavese, N.; Stocchi, F.; Brockmann, K.; Svoboda, H.; et al. Prasinezumab slows motor progression in rapidly progressing early-stage Parkinson’s disease. Nat. Med. 2024, 30, 1096–1103. [Google Scholar] [CrossRef]
- Lang, A.E.; Siderowf, A.D.; Macklin, E.A.; Poewe, W.; Brooks, D.J.; Fernandez, H.H.; Rascol, O.; Giladi, N.; Stocchi, F.; Tanner, C.M.; et al. Trial of Cinpanemab in Early Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 408–420. [Google Scholar] [CrossRef]
- Musiek, E.S.; Bennett, D.A. Aducanumab and the “post-amyloid” era of Alzheimer research? Neuron 2021, 109, 3045–3047. [Google Scholar] [CrossRef]
- Brockmann, R.; Nixon, J.; Love, B.L.; Yunusa, I. Impacts of FDA approval and Medicare restriction on antiamyloid therapies for Alzheimer’s disease: Patient outcomes, healthcare costs, and drug development. Lancet Reg. Health Am. 2023, 20, 100467. [Google Scholar] [CrossRef]
- Høilund-Carlsen, P.F.; Werner, T.J.; Alavi, A. The unsafe profile of lecanemab. J. Alzheimers Dis. 2025, 20, 13872877251325891. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.R.; Jensen, A.; Ng, S.; Yin, Z.; Li, A.; Misra, A.; Von Hoff, D.D.; Gruber, L.; Gruber, M.; Han, H. Advanced glycation end product (AGE) targeting antibody SIWA318H is efficacious in preclinical models for pancreatic cancer. Sci. Rep. 2023, 13, 16953. [Google Scholar] [CrossRef]
- Lee, J.; Yun, J.S.; Ko, S.H. Advanced Glycation End Products and Their Effect on Vascular Complications in Type 2 Diabetes Mellitus. Nutrients 2022, 14, 3086. [Google Scholar] [CrossRef]
- Dybiec, J.; Szlagor, M.; Młynarska, E.; Rysz, J.; Franczyk, B. Structural and Functional Changes in Aging Kidneys. Int. J. Mol. Sci. 2022, 23, 5435. [Google Scholar] [CrossRef] [PubMed]
- Wendel, U.; Persson, N.; Risinger, C.; Bengtsson, E.; Nodin, B.; Danielsson, L.; Welinder, C.; Nordin Fredrikson, G.; Jansson, B.; Blixt, O. A novel monoclonal antibody targeting carboxymethyllysine, an advanced glycation end product in atherosclerosis and pancreatic cancer. PLoS ONE 2018, 13, e0191872. [Google Scholar] [CrossRef]
- Johnson, S.C. Nutrient Sensing, Signaling and Ageing: The Role of IGF-1 and mTOR in Ageing and Age-Related Disease. Subcell. Biochem. 2018, 90, 49–97. [Google Scholar] [CrossRef]
- Huang, C.H.; Williamson, S.K.; Neupane, P.; Taylor, S.A.; Allen, A.; Smart, N.J.; Uypeckcuat, A.M.; Spencer, S.; Wick, J.; Smith, H.; et al. Impact Study: MK-0646 (Dalotuzumab), Insulin Growth Factor 1 Receptor Antibody Combined with Pemetrexed and Cisplatin in Stage IV Metastatic Non-squamous Lung Cancer. Front. Oncol. 2015, 5, 301. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; LoRusso, P.; Fu, S.; Hong, D.S.; Anderson, P.; Benjamin, R.S.; Ludwig, J.; Chen, H.X.; Doyle, L.A.; Kurzrock, R. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing’s sarcoma family tumors. Clin. Cancer Res. 2012, 18, 2625–2631. [Google Scholar] [CrossRef]
- Chen, H.; Mester, T.; Raychaudhuri, N.; Kauh, C.Y.; Gupta, S.; Smith, T.J.; Douglas, R.S. Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J. Clin. Endocrinol. Metab. 2014, 99, E1635–E1640. [Google Scholar] [CrossRef]
- Hong, J.Y.; Shin, M.H.; Douglas, I.S.; Chung, K.S.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Kim, S.K.; Chang, J.; Kim, Y.S.; et al. Inhibition of EphA2/EphrinA1 signal attenuates lipopolysaccharide-induced lung injury. Clin. Sci. 2016, 130, 1993–2003. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, P.; Wei, W. mTOR signaling in tumorigenesis. Biochim. Biophys. Acta 2014, 1846, 638–654. [Google Scholar] [CrossRef] [PubMed]
- Denduluri, S.K.; Idowu, O.; Wang, Z.; Liao, Z.; Yan, Z.; Mohammed, M.K.; Ye, J.; Wei, Q.; Wang, J.; Zhao, L.; et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes. Dis. 2015, 2, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A. Insulin-Like Growth Factor 1 (IGF-1) Signaling in Glucose Metabolism in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 6434. [Google Scholar] [CrossRef] [PubMed]
- Querfurth, H.; Lee, H.-K. Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol. Neurodegener. 2021, 16, 44. [Google Scholar] [CrossRef]
- Mao, K.; Quipildor, G.F.; Tabrizian, T.; Novaj, A.; Guan, F.; Walters, R.O.; Delahaye, F.; Hubbard, G.B.; Ikeno, Y.; Ejima, K.; et al. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat. Commun. 2018, 9, 2394. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, T.; Shen, H.; Cao, H.; Du, J. The adverse events profile of anti-IGF-1R monoclonal antibodies in cancer therapy. Br. J. Clin. Pharmacol. 2014, 77, 917–928. [Google Scholar] [CrossRef]
- Bagatell, R.; Herzog, C.E.; Trippett, T.M.; Grippo, J.F.; Cirrincione-Dall, G.; Fox, E.; Macy, M.; Bish, J.; Whitcomb, P.; Aikin, A.; et al. Pharmacokinetically guided phase 1 trial of the IGF-1 receptor antagonist RG1507 in children with recurrent or refractory solid tumors. Clin. Cancer Res. 2011, 17, 611–619. [Google Scholar] [CrossRef]
- Calabrò, A.; Accardi, G.; Aiello, A.; Caruso, C.; Galimberti, D.; Candore, G. Senotherapeutics to Counteract Senescent Cells Are Prominent Topics in the Context of Anti-Ageing Strategies. Int. J. Mol. Sci. 2024, 25, 1792. [Google Scholar] [CrossRef]
- Miyamoto, I.; Shimizu, T.; Hanamura, M.; Mizuno, Y.; Nakayama, R.; Kusahana, R.; Nomoto, M.; Nakagawa, Y.; Gon, Y. The Impact of Immune-Related Adverse Event Severity on Prognosis in Elderly Patients With Nonsmall-Cell Lung Cancer in First-Line Immune Checkpoint Inhibitor Treatment. Thorac. Cancer 2025, 16, e70006. [Google Scholar] [CrossRef]
- Raimondo, M.G.; Biggioggero, M.; Crotti, C.; Becciolini, A.; Favalli, E.G. Profile of sarilumab and its potential in the treatment of rheumatoid arthritis. Drug Des. Devel Ther. 2017, 11, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Crombag, M.R.; Joerger, M.; Thürlimann, B.; Schellens, J.H.; Beijnen, J.H.; Huitema, A.D. Pharmacokinetics of Selected Anticancer Drugs in Elderly Cancer Patients: Focus on Breast Cancer. Cancers 2016, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xu, W.; Liu, Q. Alterations of the blood-brain barrier during aging. J. Cereb. Blood Flow. Metab. 2024, 44, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.K.; Kodali, M.; Upadhya, R.; Madhu, L.N. Emerging Anti-Aging Strategies—Scientific Basis and Efficacy. Aging Dis. 2018, 9, 1165–1184. [Google Scholar] [CrossRef]
- Beerman, I.; Basisty, N.; de Cabo, R. Short-term senolytic treatment: A paradigm to promote fracture repair during aging. J. Clin. Invest. 2022, 132, e158871. [Google Scholar] [CrossRef]
- Mohamad Kamal, N.S.; Safuan, S.; Shamsuddin, S.; Foroozandeh, P. Aging of the cells: Insight into cellular senescence and detection Methods. Eur. J. Cell Biol. 2020, 99, 151108. [Google Scholar] [CrossRef]
- Lorenzo, E.C.; Torrance, B.L.; Haynes, L. Impact of senolytic treatment on immunity, aging, and disease. Front. Aging 2023, 4, 1161799. [Google Scholar] [CrossRef]
- Islam, M.T.; Tuday, E.; Allen, S.; Kim, J.; Trott, D.W.; Holland, W.L.; Donato, A.J.; Lesniewski, L.A. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age. Aging Cell 2023, 22, e13767. [Google Scholar] [CrossRef]
- Nambiar, A.; Kellogg, D., III; Justice, J.; Goros, M.; Gelfond, J.; Pascual, R.; Hashmi, S.; Masternak, M.; Prata, L.; LeBrasseur, N.; et al. Senolytics dasatinib and quercetin in idiopathic pulmonary fibrosis: Results of a phase I, single-blind, single-center, randomized, placebo-controlled pilot trial on feasibility and tolerability. eBioMedicine 2023, 90, 104481. [Google Scholar] [CrossRef]
- Sanborn, M.A.; Wang, X.; Gao, S.; Dai, Y.; Rehman, J. Unveiling the cell-type-specific landscape of cellular senescence through single-cell transcriptomics using SenePy. Nat. Commun. 2025, 16, 1884. [Google Scholar] [CrossRef]
- Xu, P.; Wang, M.; Song, W.-m.; Wang, Q.; Yuan, G.-C.; Sudmant, P.H.; Zare, H.; Tu, Z.; Orr, M.E.; Zhang, B. The landscape of human tissue and cell type specific expression and co-regulation of senescence genes. Mol. Neurodegener. 2022, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Suda, M.; Katsuumi, G.; Tchkonia, T.; Kirkland, J.L.; Minamino, T. Potential Clinical Implications of Senotherapies for Cardiovascular Disease. Circ. J. 2024, 88, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Bordeau, B.M.; Balthasar, J.P. Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers 2023, 15, 713. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, D.; Yang, J.; Salam, M.A.; Sengupta, S.; Al-Amin, M.Y.; Mustafa, S.; Khan, M.A.; Huang, X.; Pawar, J.S. Antibody-drug conjugates: The paradigm shifts in the targeted cancer therapy. Front. Immunol. 2023, 14, 1203073. [Google Scholar] [CrossRef]
- Wei, Q.; Li, P.; Yang, T.; Zhu, J.; Sun, L.; Zhang, Z.; Wang, L.; Tian, X.; Chen, J.; Hu, C.; et al. The promise and challenges of combination therapies with antibody-drug conjugates in solid tumors. J. Hematol. Oncol. 2024, 17, 1. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, K.; Wang, K.; Zhu, H. Treatment-related adverse events of antibody-drug conjugates in clinical trials: A systematic review and meta-analysis. Cancer 2023, 129, 283–295. [Google Scholar] [CrossRef]
- Prattichizzo, F.; Giuliani, A.; Recchioni, R.; Bonafè, M.; Marcheselli, F.; De Carolis, S.; Campanati, A.; Giuliodori, K.; Rippo, M.R.; Brugè, F.; et al. Anti-TNF-α treatment modulates SASP and SASP-related microRNAs in endothelial cells and in circulating angiogenic cells. Oncotarget 2016, 7, 11945–11958. [Google Scholar] [CrossRef]
- Huang, S.; Mills, L.; Mian, B.; Tellez, C.; McCarty, M.; Yang, X.D.; Gudas, J.M.; Bar-Eli, M. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am. J. Pathol. 2002, 161, 125–134. [Google Scholar] [CrossRef]
- Najdaghi, S.; Razi, S.; Rezaei, N. An overview of the role of interleukin-8 in colorectal cancer. Cytokine 2020, 135, 155205. [Google Scholar] [CrossRef]
- von Kobbe, C. Targeting senescent cells: Approaches, opportunities, challenges. Aging 2019, 11, 12844–12861. [Google Scholar] [CrossRef]
- Gardner, S.E.; Humphry, M.; Bennett, M.R.; Clarke, M.C. Senescent Vascular Smooth Muscle Cells Drive Inflammation Through an Interleukin-1α-Dependent Senescence-Associated Secretory Phenotype. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Abdeldaim, D.T.; Schindowski, K. Fc-Engineered Therapeutic Antibodies: Recent Advances and Future Directions. Pharmaceutics 2023, 15, 2402. [Google Scholar] [CrossRef] [PubMed]
- Mosch, R.; Guchelaar, H.J. Immunogenicity of Monoclonal Antibodies and the Potential Use of HLA Haplotypes to Predict Vulnerable Patients. Front. Immunol. 2022, 13, 885672. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Merz, M.; Büch, E.; Aigner, A. Antibody-Drug Conjugates as a Targeted Therapeutic Approach Across Entities in Oncology. Dtsch. Arztebl. Int. 2023, 120, 329–336. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Mihara, M. The roles of interleukin-6 in the pathogenesis of rheumatoid arthritis. Arthritis 2011, 2011, 765624. [Google Scholar] [CrossRef]
- Mihara, M.; Ohsugi, Y.; Kishimoto, T. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, for treatment of rheumatoid arthritis. Open Access Rheumatol. 2011, 3, 19–29. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Bruunsgaard, H.; Skinhøj, P.; Pedersen, A.N.; Schroll, M.; Pedersen, B.K. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin. Exp. Immunol. 2000, 121, 255–260. [Google Scholar] [CrossRef]
- Thilagar, S.; Theyagarajan, R.; Sudhakar, U.; Suresh, S.; Saketharaman, P.; Ahamed, N. Comparison of serum tumor necrosis factor-α levels in rheumatoid arthritis individuals with and without chronic periodontitis: A biochemical study. J. Indian. Soc. Periodontol. 2018, 22, 116–121. [Google Scholar] [CrossRef]
- Chen, H.C.; Shunyakova, J.; Reddy, A.K.; Pandiri, S.; Hassman, L. Therapeutic drug monitoring and neutralizing anti-drug antibody detection to optimize TNF-alpha inhibitor treatment for uveitis. Front. Ophthalmol. 2025, 5, 1432935. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Wadhwa, M. Therapeutic use of specific tumour necrosis factor inhibitors in inflammatory diseases including COVID-19. Biomed. Pharmacother. 2021, 140, 111785. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Quarta, S.; Massaro, M.; Carluccio, M.A.; Calabriso, N.; Bravo, L.; Sarria, B.; García-Conesa, M.-T. An Exploratory Critical Review on TNF-α as a Potential Inflammatory Biomarker Responsive to Dietary Intervention with Bioactive Foods and Derived Products. Foods 2022, 11, 2524. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef]
- Boraschi, D. What Is IL-1 for? The Functions of Interleukin-1 Across Evolution. Front. Immunol. 2022, 13, 872155. [Google Scholar] [CrossRef] [PubMed]
- Caiado, F.; Manz, M.G. IL-1 in aging and pathologies of hematopoietic stem cells. Blood 2024, 144, 368–377. [Google Scholar] [CrossRef]
- Cavalli, G.; Dinarello, C.A. Anakinra Therapy for Non-cancer Inflammatory Diseases. Front. Pharmacol. 2018, 9, 1157. [Google Scholar] [CrossRef]
- den Broeder, A.A.; de Jong, E.; Franssen, M.J.; Jeurissen, M.E.; Flendrie, M.; van den Hoogen, F.H. Observational study on efficacy, safety, and drug survival of anakinra in rheumatoid arthritis patients in clinical practice. Ann. Rheum. Dis. 2006, 65, 760–762. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Roberts, D.D. Thrombospondin-1 in maladaptive aging responses: A concept whose time has come. Am. J. Physiol. Cell Physiol. 2020, 319, C45–C63. [Google Scholar] [CrossRef]
- Yamashiro, Y.; Thang, B.Q.; Shin, S.J.; Lino, C.A.; Nakamura, T.; Kim, J.; Sugiyama, K.; Tokunaga, C.; Sakamoto, H.; Osaka, M.; et al. Role of Thrombospondin-1 in Mechanotransduction and Development of Thoracic Aortic Aneurysm in Mouse and Humans. Circ. Res. 2018, 123, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Baldo, B.A. Immune- and Non-Immune-Mediated Adverse Effects of Monoclonal Antibody Therapy: A Survey of 110 Approved Antibodies. Antibodies 2022, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.R.; Morton, L.D.; Spindeldreher, S.; Kiessling, A.; Allenspach, R.; Hey, A.; Muller, P.Y.; Frings, W.; Sims, J. Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. MAbs 2010, 2, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Cornes, P. The economic pressures for biosimilar drug use in cancer medicine. Target. Oncol. 2012, 7, 57–67. [Google Scholar] [CrossRef]
- Ray, J.C.; Allen, P.; Bacsi, A.; Bosco, J.J.; Chen, L.; Eller, M.; Kua, H.; Lim, L.L.; Matharu, M.S.; Monif, M.; et al. Inflammatory complications of CGRP monoclonal antibodies: A case series. J. Headache Pain 2021, 22, 121. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Han, Y.; Gu, Z.; Sun, C. Immunosenescence, aging and successful aging. Front. Immunol. 2022, 13, 942796. [Google Scholar] [CrossRef]
- Dong, Y.; Sun, Q.; Zhang, X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget 2017, 8, 2171–2186. [Google Scholar] [CrossRef]
- Lee, K.A.; Shin, K.S.; Kim, G.Y.; Song, Y.C.; Bae, E.A.; Kim, I.K.; Koh, C.H.; Kang, C.Y. Characterization of age-associated exhausted CD8⁺ T cells defined by increased expression of Tim-3 and PD-1. Aging Cell 2016, 15, 291–300. [Google Scholar] [CrossRef]
- Han, S.; Georgiev, P.; Ringel, A.E.; Sharpe, A.H.; Haigis, M.C. Age-associated remodeling of T cell immunity and metabolism. Cell Metab. 2023, 35, 36–55. [Google Scholar] [CrossRef]
- Srinivasan, P.; Wu, X.; Basu, M.; Rossi, C.; Sandler, A.D. PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease. PLoS Med. 2018, 15, e1002497. [Google Scholar] [CrossRef]
- Kim, C.M.; Lee, J.B.; Shin, S.J.; Ahn, J.B.; Lee, M.; Kim, H.S. The efficacy of immune checkpoint inhibitors in elderly patients: A meta-analysis and meta-regression. ESMO Open 2022, 7, 100577. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Bentley, J.P.; Bhattacharya, K.; Yang, Y.; Chang, Y.; Earl, S.; Ramachandran, S. Incidence and risk factors of immune-related adverse events induced by immune checkpoint inhibitors among older adults with non-small cell lung cancer. Cancer Med. 2024, 13, e6879. [Google Scholar] [CrossRef]
- Spassova, I.; Ugurel, S.; Terheyden, P.; Sucker, A.; Hassel, J.C.; Ritter, C.; Kubat, L.; Habermann, D.; Farahpour, F.; Saeedghalati, M.; et al. Predominance of Central Memory T Cells with High T-Cell Receptor Repertoire Diversity is Associated with Response to PD-1/PD-L1 Inhibition in Merkel Cell Carcinoma. Clin. Cancer Res. 2020, 26, 2257–2267. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Thompson, C.B. At the bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J. Leukoc. Biol. 2013, 94, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Malisan, F.; Testi, R. GD3 in cellular ageing and apoptosis. Exp. Gerontol. 2002, 37, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Iltis, C.; Moskalevska, I.; Debiesse, A.; Seguin, L.; Fissoun, C.; Cervera, L.; Moudombi, L.; Ardin, M.; Ferrari, A.; Eliott, C.; et al. A ganglioside-based immune checkpoint enables senescent cells to evade immunosurveillance during aging. Nat. Aging 2025, 5, 219–236. [Google Scholar] [CrossRef]
- Hashimoto, N.; Ito, S.; Harazono, A.; Tsuchida, A.; Mouri, Y.; Yamamoto, A.; Okajima, T.; Ohmi, Y.; Furukawa, K.; Kudo, Y.; et al. Bidirectional signals generated by Siglec-7 and its crucial ligand tri-sialylated T to escape of cancer cells from immune surveillance. iScience 2024, 27, 111139. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, X.; Su, D.; Zhang, Y.; Yu, L.; Jiang, F.; Zhou, X.; Feng, Y.; Ma, F. The Roles of Siglec7 and Siglec9 on Natural Killer Cells in Virus Infection and Tumour Progression. J. Immunol. Res. 2020, 2020, 6243819. [Google Scholar] [CrossRef]
- Zingoni, A.; Vulpis, E.; Loconte, L.; Santoni, A. NKG2D Ligand Shedding in Response to Stress: Role of ADAM10. Front. Immunol. 2020, 11, 447. [Google Scholar] [CrossRef]
- Suárez-Álvarez, B.; López-Vázquez, A.; Baltar, J.M.; Ortega, F.; López-Larrea, C. Potential Role of NKG2D and Its Ligands in Organ Transplantation: New Target for Immunointervention. Am. J. Transplant. 2009, 9, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Middha, P.; Thummalapalli, R.; Quandt, Z.; Balaratnam, K.; Cardenas, E.; Falcon, C.J.; Gubens, M.A.; Huntsman, S.; Khan, K.; Li, M.; et al. Germline prediction of immune checkpoint inhibitor discontinuation for immune-related adverse events. medRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Zhang, L.; Lian, X.; Wang, Y. Risk factors associated with immune-related severe adverse events in patients with cancer: A scoping review. Asia-Pac. J. Oncol. Nurs. 2025, 12, 100661. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Hans, H.; Lipson, E.J. Nivolumab: Targeting PD-1 to Bolster Antitumor Immunity. Future Oncol. 2015, 11, 1307–1326. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; Marinis, F.d.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Jimeno, A. Atezolizumab: A novel PD-L1 inhibitor in cancer therapy with a focus in bladder and non-small cell lung cancers. Drugs Today 2017, 53, 217–237. [Google Scholar] [CrossRef]
- Camacho, L.H. CTLA-4 blockade with ipilimumab: Biology, safety, efficacy, and future considerations. Cancer Med. 2015, 4, 661–672. [Google Scholar] [CrossRef]
- Grote, C.; Bohne, A.S.; Blome, C.; Kähler, K.C. Quality of life under treatment with the immune checkpoint inhibitors ipilimumab and nivolumab in melanoma patients. Real-world data from a prospective observational study at the Skin Cancer Center Kiel. J. Cancer Res. Clin. Oncol. 2024, 150, 454. [Google Scholar] [CrossRef]
- Foss, S.; Sakya, S.A.; Aguinagalde, L.; Lustig, M.; Shaughnessy, J.; Cruz, A.R.; Scheepmaker, L.; Mathiesen, L.; Ruso-Julve, F.; Anthi, A.K.; et al. Human IgG Fc-engineering for enhanced plasma half-life, mucosal distribution and killing of cancer cells and bacteria. Nat. Commun. 2024, 15, 2007. [Google Scholar] [CrossRef]
- Duchow, A.; Bellmann-Strobl, J. Satralizumab in the treatment of neuromyelitis optica spectrum disorder. Neurodegener. Dis. Manag. 2021, 11, 49–59. [Google Scholar] [CrossRef]
- Athanassiou, P.; Katsimbri, P.; Bounas, A.; Gazi, S.; Sarikoudis, T.; Syrigou, V.; Boumpas, D. Persistence on subcutaneous tocilizumab as monotherapy or in combination with synthetic disease-modifying anti-rheumatic drugs in rheumatoid arthritis patients in Greece (EMBRACE study): A multicenter, post-marketing, non-interventional, observational trial. Clin. Rheumatol. 2024, 43, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. Aging of the Immune System. Mechanisms and Therapeutic Targets. Ann. Am. Thorac. Soc. 2016, 13, S422–S428. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shao, C.; Li, J.; Zhu, F. Impact of Immunosenescence on Vaccine Immune Responses and Countermeasures. Vaccines 2024, 12, 1289. [Google Scholar] [CrossRef] [PubMed]
- Bartelds, G.M.; Krieckaert, C.L.; Nurmohamed, M.T.; van Schouwenburg, P.A.; Lems, W.F.; Twisk, J.W.; Dijkmans, B.A.; Aarden, L.; Wolbink, G.J. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 2011, 305, 1460–1468. [Google Scholar] [CrossRef]
- Radstake, T.R.; Svenson, M.; Eijsbouts, A.M.; van den Hoogen, F.H.; Enevold, C.; van Riel, P.L.; Bendtzen, K. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann. Rheum. Dis. 2009, 68, 1739–1745. [Google Scholar] [CrossRef]
- Jarvi, N.L.; Patel, M.; Shetty, K.A.; Nguyen, N.H.; Grasperge, B.F.; Mager, D.E.; Straubinger, R.M.; Balu-Iyer, S.V. Immune regulatory adjuvant approach to mitigate subcutaneous immunogenicity of monoclonal antibodies. Front. Immunol. 2024, 15, 1496169. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Biello, F.; Maggiora, P.M.; Bruna, R.; Burrafato, G.; Cappelli, M.; Varughese, F.; Martini, V.; Platini, F.; Deambrogi, C.; et al. A randomized clinical study on the impact of Comprehensive Geriatric Assessment (CGA) based interventions on the quality of life of elderly, frail, onco-hematologic patients candidate to anticancer therapy: Protocol of the ONCO-Aging study. BMC Geriatr. 2021, 21, 320. [Google Scholar] [CrossRef]
- Oberic, L.; Peyrade, F.; Puyade, M.; Bonnet, C.; Dartigues-Cuillères, P.; Fabiani, B.; Ruminy, P.; Maisonneuve, H.; Abraham, J.; Thieblemont, C.; et al. Subcutaneous Rituximab-MiniCHOP Compared With Subcutaneous Rituximab-MiniCHOP Plus Lenalidomide in Diffuse Large B-Cell Lymphoma for Patients Age 80 Years or Older. J. Clin. Oncol. 2021, 39, 1203–1213. [Google Scholar] [CrossRef]
- Firestone, R.; Lesokhin, A.M.; Usmani, S.Z. An Embarrassment of Riches: Three FDA-Approved Bispecific Antibodies for Relapsed Refractory Multiple Myeloma. Blood Cancer Discov. 2023, 4, 433–436. [Google Scholar] [CrossRef]
- Hedayati, E.; Fracheboud, L.; Srikant, V.; Greber, D.; Wallberg, S.; Linder Stragliotto, C. Economic benefits of subcutaneous trastuzumab administration: A single institutional study from Karolinska University Hospital in Sweden. PLoS ONE 2019, 14, e0211783. [Google Scholar] [CrossRef]
- Ouyang, Y.; Lee, H.Y.; Leong, F.L.; Tey, H.J.; Shih, V.; Lim, E.H.; Graves, N. Cost-minimization analysis comparing subcutaneous trastuzumab at home with intravenous trastuzumab for HER2-positive breast cancer in Singapore. Ther. Adv. Med. Oncol. 2024, 16, 17588359241293381. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Idda, M.L.; McClusky, W.G.; Lodde, V.; Munk, R.; Abdelmohsen, K.; Rossi, M.; Gorospe, M. Survey of senescent cell markers with age in human tissues. Aging 2020, 12, 4052–4066. [Google Scholar] [CrossRef]
- Gurkar, A.U.; Gerencser, A.A.; Mora, A.L.; Nelson, A.C.; Zhang, A.R.; Lagnado, A.B.; Enninful, A.; Benz, C.; Furman, D.; Beaulieu, D.; et al. Spatial mapping of cellular senescence: Emerging challenges and opportunities. Nat. Aging 2023, 3, 776–790. [Google Scholar] [CrossRef]
- Khalil, R.; Diab-Assaf, M.; Lemaitre, J.M. Emerging Therapeutic Approaches to Target the Dark Side of Senescent Cells: New Hopes to Treat Aging as a Disease and to Delay Age-Related Pathologies. Cells 2023, 12, 915. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Baghirov, H. Mechanisms of receptor-mediated transcytosis at the blood-brain barrier. J. Control. Release 2025, 381, 113595. [Google Scholar] [CrossRef]
- Grimm, H.P.; Schumacher, V.; Schäfer, M.; Imhof-Jung, S.; Freskgård, P.O.; Brady, K.; Hofmann, C.; Rüger, P.; Schlothauer, T.; Göpfert, U.; et al. Delivery of the Brainshuttle™ amyloid-beta antibody fusion trontinemab to non-human primate brain and projected efficacious dose regimens in humans. MAbs 2023, 15, 2261509. [Google Scholar] [CrossRef]
- Arroyo-Pacheco, N.; Sarmiento-Blanco, S.; Vergara-Cadavid, G.; Castro-Leones, M.; Contreras-Puentes, N. Monoclonal therapy with lecanemab in the treatment of mild Alzheimer’s disease: A systematic review and meta-analysis. Ageing Res. Rev. 2025, 104, 102620. [Google Scholar] [CrossRef]
- Dewaker, V.; Morya, V.K.; Kim, Y.H.; Park, S.T.; Kim, H.S.; Koh, Y.H. Revolutionizing oncology: The role of Artificial Intelligence (AI) as an antibody design, and optimization tools. Biomark. Res. 2025, 13, 52. [Google Scholar] [CrossRef]
- Kim, J.; McFee, M.; Fang, Q.; Abdin, O.; Kim, P.M. Computational and artificial intelligence-based methods for antibody development. Trends Pharmacol. Sci. 2023, 44, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; MacDonald, K.G.; Massi, E.; Chew, P.V.; Bee, C.; Perkins, P.; Chau, B.; Thudium, K.; Lohre, J.; Nandi, P.; et al. Improved therapeutic index of an acidic pH-selective antibody. MAbs 2022, 14, 2024642. [Google Scholar] [CrossRef] [PubMed]
- Howe, M.D.; Britton, K.J.; Joyce, H.E.; Pappas, G.J.; Faust, M.A.; Dawson, B.C.; Riddle, M.C.; Salloway, S.P. Initial Experiences with Amyloid-Related Imaging Abnormalities in Patients Receiving Aducanumab Following Accelerated Approval. J. Prev. Alzheimer’s Dis. 2023, 10, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Hampel, H.; Elhage, A.; Cho, M.; Apostolova, L.G.; Nicoll, J.A.R.; Atri, A. Amyloid-related imaging abnormalities (ARIA): Radiological, biological and clinical characteristics. Brain 2023, 146, 4414–4424. [Google Scholar] [CrossRef]
- Cogswell, P.M.; Barakos, J.A.; Barkhof, F.; Benzinger, T.S.; Jack, C.R., Jr.; Poussaint, T.Y.; Raji, C.A.; Ramanan, V.K.; Whitlow, C.T. Amyloid-Related Imaging Abnormalities with Emerging Alzheimer Disease Therapeutics: Detection and Reporting Recommendations for Clinical Practice. Am. J. Neuroradiol. 2022, 43, E19–E35. [Google Scholar] [CrossRef]
- Polsinelli, A.J.; Logan, P.E.; Lane, K.A.; Manchella, M.K.; Nemes, S.; Sanjay, A.B.; Gao, S.; Apostolova, L.G. APOE ε4 carrier status and sex differentiate rates of cognitive decline in early- and late-onset Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2023, 19, 1983–1993. [Google Scholar] [CrossRef]
- Wang, H.; Serap Monkul Nery, E.; Ardayfio, P.; Khanna, R.; Otero Svaldi, D.; Gueorguieva, I.; Shcherbinin, S.; Andersen, S.W.; Hauck, P.M.; Engle, S.E.; et al. Modified titration of donanemab reduces ARIA risk and maintains amyloid reduction. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2025, 21, e70062. [Google Scholar] [CrossRef]
- Kim, B.-H.; Kim, S.; Nam, Y.; Park, Y.H.; Shin, S.M.; Moon, M. Second-generation anti-amyloid monoclonal antibodies for Alzheimer’s disease: Current landscape and future perspectives. Transl. Neurodegener. 2025, 14, 6. [Google Scholar] [CrossRef]
- Marshall, M.J.E.; Stopforth, R.J.; Cragg, M.S. Therapeutic Antibodies: What Have We Learnt from Targeting CD20 and Where Are We Going? Front. Immunol. 2017, 8, 1245. [Google Scholar] [CrossRef]
- Marouf, B.H.; Iqbal, Z.; Mohamad, J.B.; Bashir, B.; Schofield, J.; Syed, A.; Kilpatrick, E.S.; Stefanutti, C.; Soran, H. Efficacy and Safety of PCSK9 Monoclonal Antibodies in Patients With Diabetes. Clin. Ther. 2022, 44, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Asahara, N.; Okada-Iwabu, M.; Iwabu, M.; Wada, K.; Oka, K.; Yamauchi, T.; Kadowaki, T. A monoclonal antibody activating AdipoR for type 2 diabetes and nonalcoholic steatohepatitis. Sci. Adv. 2023, 9, eadg4216. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef]
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells 2022, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y. The ethics of informed consent in Alzheimer disease research. Nat. Rev. Neurol. 2011, 7, 410–414. [Google Scholar] [CrossRef]
- Villain, N.; Planche, V.; Levy, R. High-clearance anti-amyloid immunotherapies in Alzheimer’s disease. Part 1: Meta-analysis and review of efficacy and safety data, and medico-economical aspects. Rev. Neurol. 2022, 178, 1011–1030. [Google Scholar] [CrossRef]
- Ross, E.L.; Weinberg, M.S.; Arnold, S.E. Cost-effectiveness of Aducanumab and Donanemab for Early Alzheimer Disease in the US. JAMA Neurol. 2022, 79, 478–487. [Google Scholar] [CrossRef]
- Ziogas, D.C.; Theocharopoulos, C.; Aravantinou, K.; Boukouris, A.E.; Stefanou, D.; Anastasopoulou, A.; Lialios, P.-P.; Lyrarakis, G.; Gogas, H. Clinical benefit of immune checkpoint inhibitors in elderly cancer patients: Current evidence from immunosenescence pathophysiology to clinical trial results. Crit. Rev. Oncol./Hematol. 2025, 208, 104635. [Google Scholar] [CrossRef]
- Keehan, S.P.; Fiore, J.A.; Poisal, J.A.; Cuckler, G.A.; Sisko, A.M.; Smith, S.D.; Madison, A.J.; Rennie, K.E. National Health Expenditure Projections, 2022–2031: Growth To Stabilize Once The COVID-19 Public Health Emergency Ends. Health Aff. 2023, 42, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Barocas, J.A. Cost-effectiveness of aducanumab to prevent Alzheimer’s disease progression at current list price. Alzheimer’s Dement. 2022, 8, e12256. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef] [PubMed]

| Disease | Antibody | Target | Phase | Key Outcomes | Status | Ref |
|---|---|---|---|---|---|---|
| AD | Aducanumab | Aβ aggregates | III | EMERGE demonstrated a 23% reduction in CDR-SB; ENGAGE failed to achieve the primary aim. | FDA-approved (2021); development discontinued in 2024. | [65] |
| AD | Lecanemab | Aβ protofibrils | III | A 27% reduction in clinical decline on CDR-SB at 18 months; incidence of ARIA-E observed. | FDA-approved (2023); EMA-approved (2025). | [66] |
| AD | Donanemab | N3pG-Aβ plaques | III | Cognitive decline was mitigated by 35%; elevated occurrence of ARIA-E; EMA declined approval due to risk–benefit apprehensions. | FDA-approved (2024); EMA rejected approval. | [67] |
| PD | Cinpanemab | α-synuclein | II | No notable impact on illness progression; development has been halted. | Development discontinued. | [69] |
| PD | Prasinezumab | α-synuclein | IIb | Failed to achieve the primary aim; demonstrated potential therapeutic efficacy in secondary endpoints. | Ongoing evaluation. | [68] |
| Hallmark of Aging | Key Molecular Targets | MAb Strategies | Representative Diseases | Development Status | Ref |
|---|---|---|---|---|---|
| Cellular Senescence | B2M, DPP4, IL-6, IL-8 | Senolytic ADCs; SASP-neutralizing mAbs | Alzheimer’s, osteoarthritis, skin aging | Preclinical to early phase | [13,14,15] |
| Inflammaging | IL-6, IL-1β, TNF-α | Cytokine-neutralizing mAbs (e.g., Tocilizumab, Infliximab) | RA, CVD, sarcopenia | Approved in inflammatory disease | [47,48,49,50,51] |
| Immunosenescence | PD-1, PD-L1, CTLA-4, GD3 | Checkpoint inhibitor mAbs (e.g., Nivolumab, Ipilimumab) | Cancer, infection susceptibility | Oncology-approved; aging under investigation | [16,57,58,59,60] |
| Loss of Proteostasis | Amyloid-β aggregates | Anti-amyloid mAbs (e.g., Aducanumab, Lecanemab) | AD | FDA-approved (limited efficacy) | [63,64,70,71,72] |
| Advanced Glycation End-products (AGEs) | AGE-modified proteins | Anti-AGE mAbs (e.g., SIWA318H) | Diabetic complications, skin aging | Preclinical | [74,75,76,77] |
| Dysregulated Nutrient Sensing | IGF-1R, PI3K, mTOR-associated nodes | Anti-IGF-1R mAbs (e.g., Dalotuzumab, Cixutumumab, Teprotumumab) | Cancer, metabolic syndrome, neurodegeneration | Early-phase oncology trials | [79,80,81,82,83,84,85,86,87,88,89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Bo, M.; Gambirasi, M.; Vruzhaj, I.; Cecchin, E.; Pishdadian, A.; Toffoli, G.; Safa, A. Targeting Aging Hallmarks with Monoclonal Antibodies: A New Era in Cancer Immunotherapy and Geriatric Medicine. Int. J. Mol. Sci. 2025, 26, 4982. https://doi.org/10.3390/ijms26114982
Dal Bo M, Gambirasi M, Vruzhaj I, Cecchin E, Pishdadian A, Toffoli G, Safa A. Targeting Aging Hallmarks with Monoclonal Antibodies: A New Era in Cancer Immunotherapy and Geriatric Medicine. International Journal of Molecular Sciences. 2025; 26(11):4982. https://doi.org/10.3390/ijms26114982
Chicago/Turabian StyleDal Bo, Michele, Marta Gambirasi, Idris Vruzhaj, Erika Cecchin, Abbas Pishdadian, Giuseppe Toffoli, and Amin Safa. 2025. "Targeting Aging Hallmarks with Monoclonal Antibodies: A New Era in Cancer Immunotherapy and Geriatric Medicine" International Journal of Molecular Sciences 26, no. 11: 4982. https://doi.org/10.3390/ijms26114982
APA StyleDal Bo, M., Gambirasi, M., Vruzhaj, I., Cecchin, E., Pishdadian, A., Toffoli, G., & Safa, A. (2025). Targeting Aging Hallmarks with Monoclonal Antibodies: A New Era in Cancer Immunotherapy and Geriatric Medicine. International Journal of Molecular Sciences, 26(11), 4982. https://doi.org/10.3390/ijms26114982







