Ciliary G-Protein Coupled Receptor Signaling in Polycystic Kidney Disease
Abstract
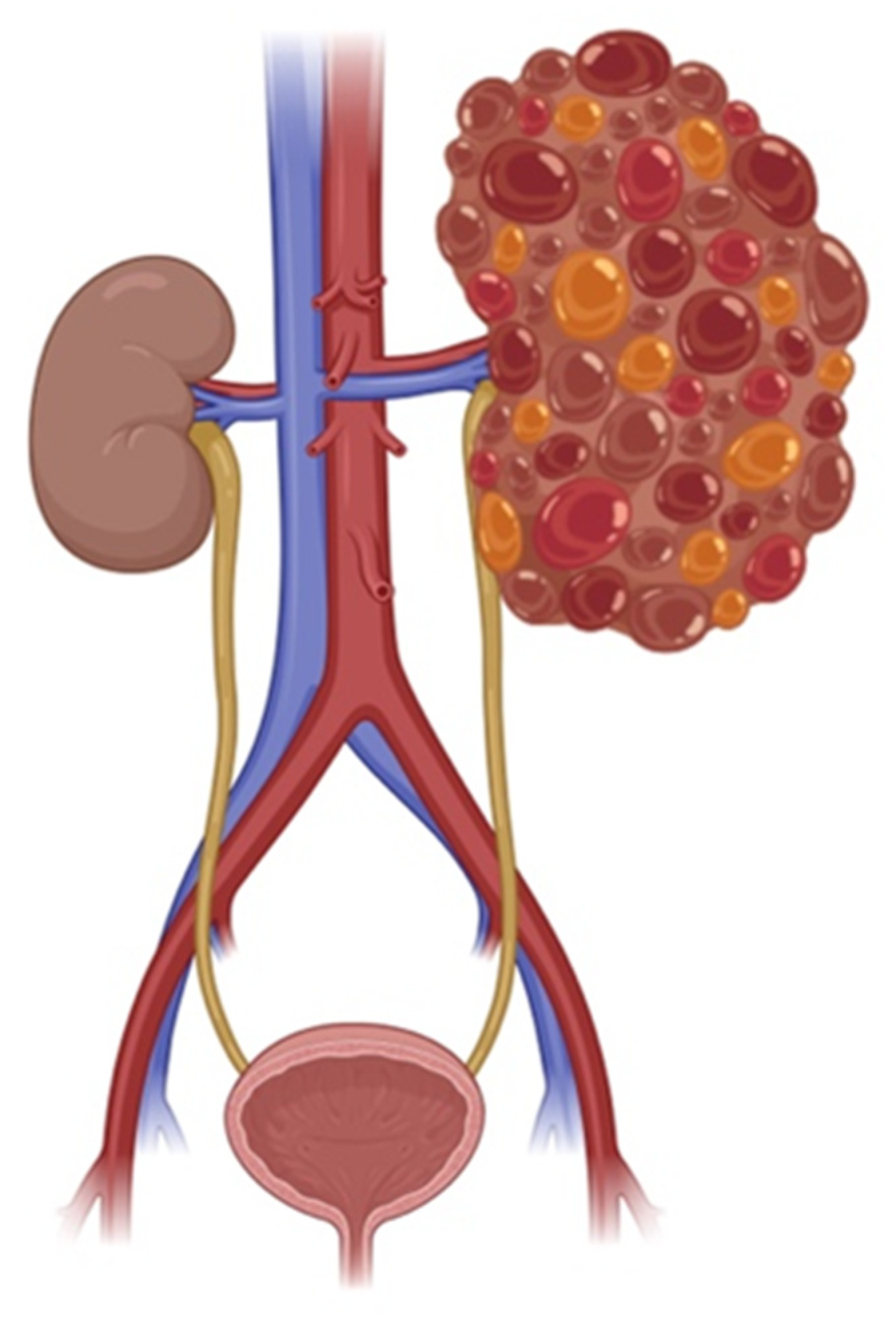
1. Introduction
2. Primary Cilia and Their Role in Cellular Signaling
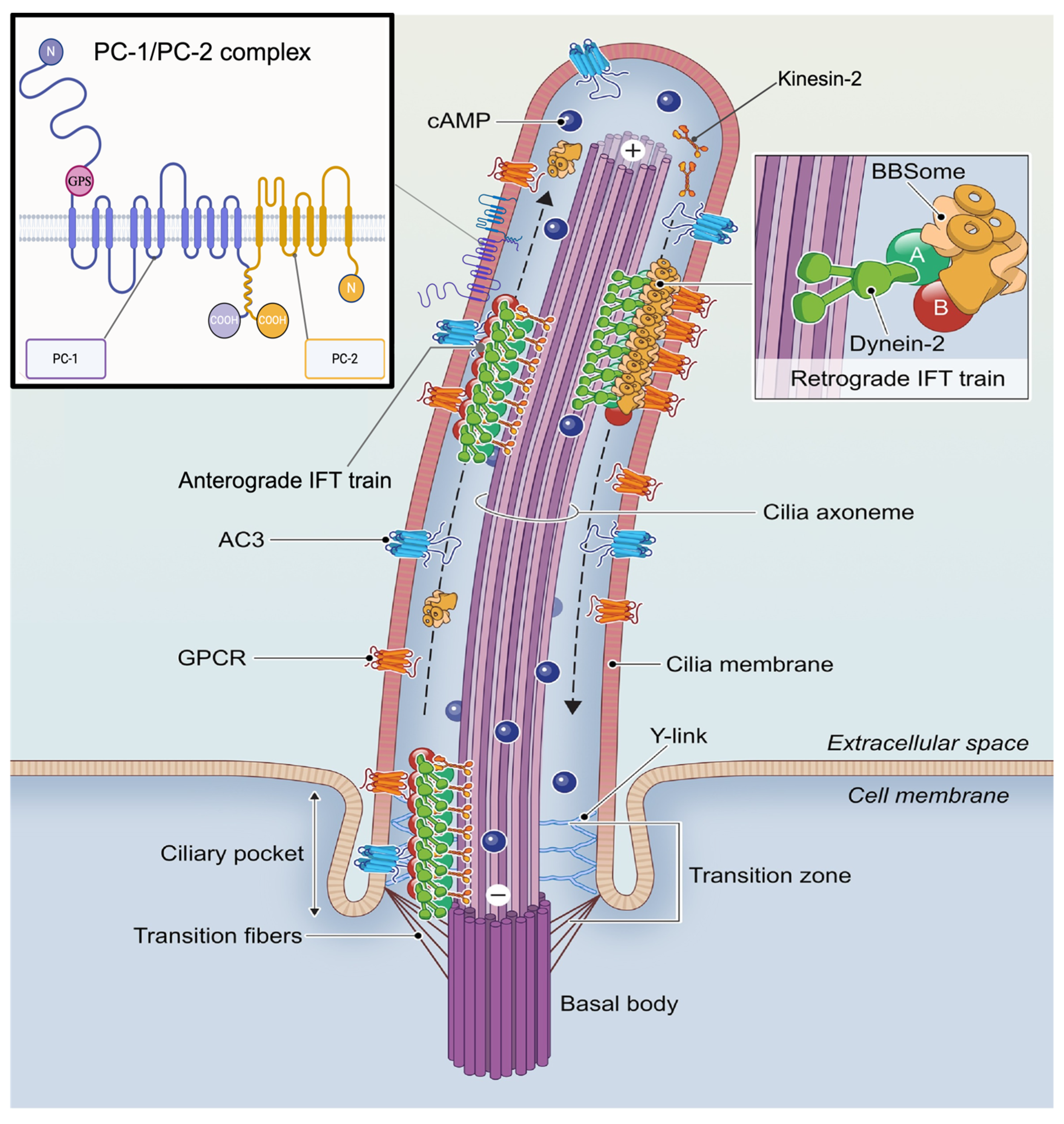
2.1. Ciliary GPCR Structure and Function
2.1.1. Physiological Roles of GPCRs
2.1.2. Role of GPCRs in Kidney Function
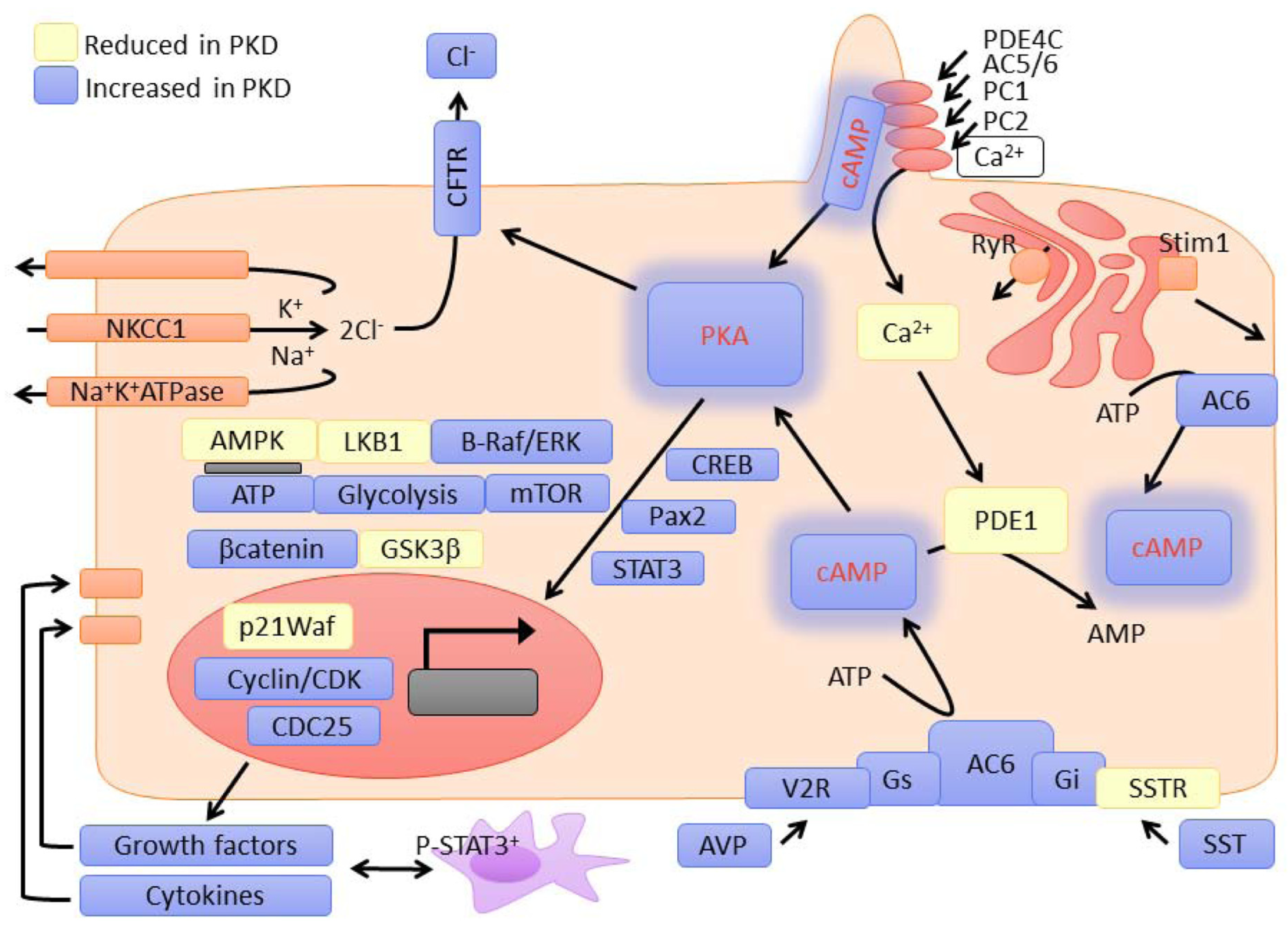
2.2. Mechanisms Linking Ciliary GPCR Dysfunction to PKD Pathogenesis
2.3. Mechanosensation and Fluid Flow Signaling in Ciliary GPCRs and PC1
3. Therapeutic Implications and Drug Development
4. Current Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADPKD | Autosomal Dominant Polycystic Kidney Disease |
| ADPKD1 | Autosomal Dominant Polycystic Kidney Disease Type 1 |
| ADPKD2 | Autosomal Dominant Polycystic Kidney Disease Type 2 |
| AT1R | Angiotensin II Type 1 Receptor |
| BMP | Bone Morphogenetic Protein |
| cAMP | Cyclic Adenosine Monophosphate |
| CFTR | Cystic Fibrosis Transmembrane Conductance Regulator |
| cGMP | Cyclic Guanosine Monophosphate |
| CKD | chronic kidney disease |
| COOH | Carboxy Terminus |
| DR5 | Dopamine Receptor 5 |
| eGFR | Estimated Glomerular Filtration Rate |
| EP1–EP4 | Prostaglandin E2 Receptors 1 to 4 |
| EP4 | Prostaglandin E2 receptor 4 |
| FDA | Food and Drug Administration |
| FFAR4 | Free fatty acid receptor 4 |
| GAIN | GPCR-Autoproteolytic Inducing Domain |
| GALR2 | Galanin receptor 2 |
| GBD | G protein-binding domain |
| GFR | Glomerular Filtration Rate |
| GPCR | G-Protein Coupled Receptor |
| GPS | GPCR Proteolysis Site |
| Gs, Gi, Gq, G12 | G Protein Classes |
| Gα, Gβ, Gγ | G Protein Subunits Alpha, Beta, Gamma |
| HDAC5 | Histone Deacetylase 5 |
| IC50 | Half Maximal Inhibitory Concentration |
| MDRD | Modification of Diet in Renal Disease |
| mGFR | measured Glomerular Filtration Rate |
| mTOR | Mammalian Target of Rapamycin |
| NDI | Nephrogenic Diabetes Insipidus |
| PC1 | Polycystin-1 |
| PC2 | Polycystin-2 |
| PKA | Protein Kinase A |
| PKD | Polycystic Kidney Disease |
| Pkd1 | Polycystic Kidney Disease gene 1. |
| Pkd2 | Polycystic Kidney Disease gene 2. |
| PLC | Phospholipase C |
| RAAS | Renin–Angiotensin–Aldosterone system |
| RTK | Receptor Tyrosine Kinase |
| SSRT2, SSRT3, SSRT5 | Somatostatin Receptor Subtypes 2, 3, and 5 |
| SST | Somatostatin |
| TGF-β | Transforming Growth Factor Beta |
| TSC2 | Tuberin (Tuberous Sclerosis Complex 2) |
| UT-A1 | Urea Transporter A1 |
| UT-A3 | Urea Transporter A3 |
| V2R | Vasopressin V2 Receptor |
| WNT | Wingless-related integration site |
| μm | Micrometer |
References
- Halvorson, C.R.; Bremmer, M.S.; Jacobs, S.C. Polycystic kidney disease: Inheritance, pathophysiology, prognosis, and treatment. Int. J. Nephrol. Renov. Dis. 2010, 3, 69–83. [Google Scholar]
- Chebib, F.T.; Torres, V.E. Autosomal Dominant Polycystic Kidney Disease: Core Curriculum 2016. Am. J. Kidney Dis. 2016, 67, 792–810. [Google Scholar] [CrossRef] [PubMed]
- Maser, R.L.; Calvet, J.P.; Parnell, S.C. The GPCR properties of polycystin-1 A new paradigm. Front. Mol. Biosci. 2022, 9, 1035507. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Harris, P.C. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J. Am. Soc. Nephrol. 2014, 25, 18–32. [Google Scholar] [CrossRef]
- Zhuang, J.; Aierken, A.; Yalikun, D.; Zhang, J.; Wang, X.; Ren, Y.; Tian, X.; Jiang, H. Case report: Genotype-phenotype characteristics of nine novel PKD1 mutations in eight Chinese patients with autosomal dominant polycystic kidney disease. Front. Med. 2023, 10, 1268307. [Google Scholar] [CrossRef]
- Yang, Y.; Ehrlich, B.E. Structural studies of the C-terminal tail of polycystin-2 (PC2) reveal insights into the mechanisms used for the functional regulation of PC2. J. Physiol. 2016, 594, 4141–4149. [Google Scholar] [CrossRef] [PubMed]
- Brill, A.L.; Fischer, T.T.; Walters, J.M.; Marlier, A.; Sewanan, L.R.; Wilson, P.C.; Johnson, E.K.; Moeckel, G.; Cantley, L.G.; Campbell, S.G.; et al. Polycystin 2 is increased in disease to protect against stress-induced cell death. Sci. Rep. 2020, 10, 386. [Google Scholar] [CrossRef]
- Leonhard, W.N.; Happe, H.; Peters, D.J. Variable Cyst Development in Autosomal Dominant Polycystic Kidney Disease: The Biologic Context. J. Am. Soc. Nephrol. 2016, 27, 3530–3538. [Google Scholar] [CrossRef]
- Nauli, S.M.; Alenghat, F.J.; Luo, Y.; Williams, E.; Vassilev, P.; Li, X.; Elia, A.E.; Lu, W.; Brown, E.M.; Quinn, S.J.; et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003, 33, 129–137. [Google Scholar] [CrossRef]
- Walker, R.V.; Keynton, J.L.; Grimes, D.T.; Sreekumar, V.; Williams, D.J.; Esapa, C.; Wu, D.; Knight, M.M.; Norris, D.P. Ciliary exclusion of Polycystin-2 promotes kidney cystogenesis in an autosomal dominant polycystic kidney disease model. Nat. Commun. 2019, 10, 4072. [Google Scholar] [CrossRef]
- Sussman, C.R.; Wang, X.; Chebib, F.T.; Torres, V.E. Modulation of polycystic kidney disease by G-protein coupled receptors and cyclic AMP signaling. Cell Signal. 2020, 72, 109649. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.J. To activate a G protein-coupled receptor permanently with cell surface photodynamic action in the gastrointestinal tract. World J. Gastroenterol. 2025, 31, 102423. [Google Scholar] [CrossRef]
- Mitchison, H.M.; Valente, E.M. Motile and non-motile cilia in human pathology: From function to phenotypes. J. Pathol. 2017, 241, 294–309. [Google Scholar] [CrossRef]
- Youn, Y.H.; Han, Y.-G. Primary cilia in brain development and diseases. Am. J. Pathol. 2018, 188, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Nachury, M.V.; Mick, D.U. Establishing and regulating the composition of cilia for signal transduction. Nat. Rev. Mol. Cell Biol. 2019, 20, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Fisch, C.; Dupuis-Williams, P. Ultrastructure of cilia and flagella–back to the future! Biol. Cell 2011, 103, 249–270. [Google Scholar] [CrossRef]
- Satir, P.; Pedersen, L.B.; Christensen, S.T. The primary cilium at a glance. J. Cell Sci. 2010, 123, 499–503. [Google Scholar] [CrossRef]
- Rosenbaum, J.L.; Witman, G.B. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 2002, 3, 813–825. [Google Scholar] [CrossRef]
- Macarelli, V.; Leventea, E.; Merkle, F.T. Regulation of the length of neuronal primary cilia and its potential effects on signalling. Trends Cell Biol. 2023, 33, 979–990. [Google Scholar] [CrossRef]
- Kaupp, U.B. Olfactory signalling in vertebrates and insects: Differences and commonalities. Nat. Rev. Neurosci. 2010, 11, 188–200. [Google Scholar] [CrossRef]
- Insel, P.A.; Snead, A.; Murray, F.; Zhang, L.; Yokouchi, H.; Katakia, T.; Kwon, O.; Dimucci, D.; Wilderman, A. GPCR expression in tissues and cells: Are the optimal receptors being used as drug targets? Br. J. Pharmacol. 2012, 165, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Flock, T.; Ravarani, C.N.J.; Sun, D.; Venkatakrishnan, A.J.; Kayikci, M.; Tate, C.G.; Veprintsev, D.B.; Babu, M.M. Universal allosteric mechanism for Galpha activation by GPCRs. Nature 2015, 524, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, Y.; Li, T.; Feng, X. G-Protein Coupled Receptors (GPCRs): Signaling Pathways, Characterization, and Functions in Insect Physiology and Toxicology. Int. J. Mol. Sci. 2021, 22, 5260. [Google Scholar] [CrossRef] [PubMed]
- Wacker, D.; Stevens, R.C.; Roth, B.L. How Ligands Illuminate GPCR Molecular Pharmacology. Cell 2017, 170, 414–427. [Google Scholar] [CrossRef]
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12. [Google Scholar] [CrossRef]
- Lohse, M.J.; Hein, P.; Hoffmann, C.; Nikolaev, V.O.; Vilardaga, J.P.; Bunemann, M. Kinetics of G-protein-coupled receptor signals in intact cells. Br. J. Pharmacol. 2008, 153, S125–S132. [Google Scholar] [CrossRef]
- Sutkeviciute, I.; Vilardaga, J.P. Structural insights into emergent signaling modes of G protein-coupled receptors. J. Biol. Chem. 2020, 295, 11626–11642. [Google Scholar] [CrossRef]
- Hoare, S.R.; Tewson, P.H.; Quinn, A.M.; Hughes, T.E.; Bridge, L.J. Analyzing kinetic signaling data for G-protein-coupled receptors. Sci. Rep. 2020, 10, 12263. [Google Scholar] [CrossRef]
- Hauser, A.S.; Chavali, S.; Masuho, I.; Jahn, L.J.; Martemyanov, K.A.; Gloriam, D.E.; Babu, M.M. Pharmacogenomics of GPCR Drug Targets. Cell 2018, 172, 41–54.e19. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Rasmussen, S.G.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef]
- Bateman, R.J.; Boychuk, C.R.; Philbin, K.E.; Mendelowitz, D. beta adrenergic receptor modulation of neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Neuroscience 2012, 210, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Bachelerie, F.; Graham, G.J.; Locati, M.; Mantovani, A.; Murphy, P.M.; Nibbs, R.; Rot, A.; Sozzani, S.; Thelen, M. New nomenclature for atypical chemokine receptors. Nat. Immunol. 2014, 15, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. Gut hormones as pharmaceuticals: From enteroglucagon to GLP-1 and GLP-2. Regul. Pept. 2000, 93, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Mykytyn, K.; Askwith, C. G-Protein-Coupled Receptor Signaling in Cilia. Cold Spring Harb. Perspect. Biol. 2017, 9, a028183. [Google Scholar] [CrossRef]
- Erdelyi, L.S.; Hunyady, L.; Balla, A. V2 vasopressin receptor mutations: Future personalized therapy based on individual molecular biology. Front Endocrinol 2023, 14, 1173601. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Carey, R.M. The intrarenal renin-angiotensin and dopaminergic systems: Control of renal sodium excretion and blood pressure. Hypertension 2013, 61, 673–680. [Google Scholar] [CrossRef]
- Zhang, F.; Armando, I.; Jose, P.A.; Zeng, C.; Yang, J. G protein-coupled receptor kinases in hypertension: Physiology, pathogenesis, and therapeutic targets. Hypertens. Res. 2024, 47, 2317–2336. [Google Scholar] [CrossRef]
- Lv, L.; Liu, Y.; Xiong, J.; Wang, S.; Li, Y.; Zhang, B.; Huang, Y.; Zhao, J. Role of G protein coupled receptors in acute kidney injury. Cell Commun. Signal. 2024, 22, 423. [Google Scholar] [CrossRef]
- Robben, J.H.; Kortenoeven, M.L.; Sze, M.; Yae, C.; Milligan, G.; Oorschot, V.M.; Klumperman, J.; Knoers, N.V.; Deen, P.M. Intracellular activation of vasopressin V2 receptor mutants in nephrogenic diabetes insipidus by nonpeptide agonists. Proc. Natl. Acad. Sci. USA 2009, 106, 12195–12200. [Google Scholar] [CrossRef]
- Dasgupta, C.; Zhang, L. Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discov. Today 2011, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Zhang, M.-Z. Dopamine, the kidney, and hypertension. Curr. Hypertens. Rep. 2012, 14, 138–143. [Google Scholar] [CrossRef]
- Schou, K.B.; Pedersen, L.B.; Christensen, S.T. Ins and outs of GPCR signaling in primary cilia. EMBO Rep. 2015, 16, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Saternos, H.C.; Forero, K.V.; Meqdad, M.A.; Buqaileh, R.; Sunderman, C.L.; Gallagher, G.; Messer, W.S., Jr.; Mohieldin, A.M.; Mucci, C.A.; Kumariya, S.; et al. Muscarinic acetylcholine receptor 3 localized to primary endothelial cilia regulates blood pressure and cognition. Sci. Rep. 2025, 15, 3745. [Google Scholar] [CrossRef]
- Nag, S.; Resnick, A. Biophysics and biofluid dynamics of primary cilia: Evidence for and against the flow-sensing function. Am. J. Physiol.-Ren. Physiol. 2017, 313, F706–F720. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.; Deen, P.M. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflügers Arch.-Eur. J. Physiol. 2008, 456, 1005–1024. [Google Scholar] [CrossRef]
- Juul, K.V.; Bichet, D.G.; Nielsen, S.; Nørgaard, J.P. The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am. J. Physiol.-Ren. Physiol. 2014, 306, F931–F940. [Google Scholar] [CrossRef]
- Bankir, L.; Bichet, D.G.; Morgenthaler, N.G. Vasopressin: Physiology, assessment and osmosensation. J. Intern. Med. 2017, 282, 284–297. [Google Scholar] [CrossRef]
- Bankir, L. Antidiuretic action of vasopressin: Quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc. Res. 2001, 51, 372–390. [Google Scholar] [CrossRef]
- Jin, D.; Zhong, T.P. Prostaglandin signaling in ciliogenesis and development. J. Cell. Physiol. 2022, 237, 2632–2643. [Google Scholar] [CrossRef]
- Abdul-Majeed, S.; Nauli, S.M. Dopamine receptor type 5 in the primary cilia has dual chemo-and mechano-sensory roles. Hypertension 2011, 58, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, V.S.; Muntean, B.S.; Kathem, S.H.; Hwang, J.J.; AbouAlaiwi, W.A.; Nauli, S.M. Roles of dopamine receptor on chemosensory and mechanosensory primary cilia in renal epithelial cells. Front. Physiol. 2014, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Ramachandran, S.; Xia, S.; Unruh, J.R.; Conkright-Fincham, J.; Li, R. Dopamine receptor antagonists as potential therapeutic agents for ADPKD. PLoS ONE 2019, 14, e0216220. [Google Scholar] [CrossRef] [PubMed]
- Messchendorp, A.L.; Casteleijn, N.F.; Meijer, E.; Gansevoort, R.T. Somatostatin in renal physiology and autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2020, 35, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Balster, D.A.; O’Dorisio, M.S.; Summers, M.A.; Turman, M.A. Segmental expression of somatostatin receptor subtypes sst1 and sst2 in tubules and glomeruli of human kidney. Am. J. Physiol.-Ren. Physiol. 2001, 280, F457–F465. [Google Scholar] [CrossRef]
- Bhandari, S.; Watson, N.; Long, E.; Sharpe, S.; Zhong, W.; Xu, S.-Z.; Atkin, S.L. Expression of somatostatin and somatostatin receptor subtypes 1–5 in human normal and diseased kidney. J. Histochem. Cytochem. 2008, 56, 733–743. [Google Scholar] [CrossRef]
- Winkler, S.; Torikai, S.; Levine, B.; Kurokawa, K. Effect of somatostatin on vasopressin-induced antidiuresis and renal cyclic AMP of rats. Miner. Electrolyte Metab. 1982, 7, 8–14. [Google Scholar]
- Bergmann, C. Genetics of autosomal recessive polycystic kidney disease and its differential diagnoses. Front. Pediatr. 2018, 5, 221. [Google Scholar] [CrossRef]
- Araç, D.; Boucard, A.A.; Bolliger, M.F.; Nguyen, J.; Soltis, S.M.; Südhof, T.C.; Brunger, A.T. A novel evolutionarily conserved domain of cell-adhesion GPCRs mediates autoproteolysis. EMBO J. 2012, 31, 1364–1378. [Google Scholar] [CrossRef]
- Ikeda, M.; Fong, P.; Cheng, J.; Boletta, A.; Qian, F.; Zhang, X.-M.; Cai, H.; Germino, G.G.; Guggino, W.B. A regulatory role of polycystin-1 on cystic fibrosis transmembrane conductance regulator plasma membrane expression. Cell. Physiol. Biochem. 2006, 18, 9–20. [Google Scholar] [CrossRef]
- Anvarian, Z.; Mykytyn, K.; Mukhopadhyay, S.; Pedersen, L.B.; Christensen, S.T. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 2019, 15, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Harris, P.C. Mechanisms of Disease: Autosomal dominant and recessive polycystic kidney diseases. Nat. Clin. Pract. Nephrol. 2006, 2, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Nagao, S.; Wallace, D.P.; Belibi, F.A.; Cowley, B.D.; Pelling, J.C.; Grantham, J.J. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int. 2003, 63, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Shillingford, J.M.; Murcia, N.S.; Larson, C.H.; Low, S.H.; Hedgepeth, R.; Brown, N.; Flask, C.A.; Novick, A.C.; Goldfarb, D.A.; Kramer-Zucker, A.; et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc. Natl. Acad. Sci. USA 2006, 103, 5466–5471. [Google Scholar] [CrossRef]
- Vasileva, V.Y.; Sultanova, R.F.; Sudarikova, A.V.; Ilatovskaya, D.V. Insights into the molecular mechanisms of polycystic kidney diseases. Front. Physiol. 2021, 12, 693130. [Google Scholar] [CrossRef]
- Ma, M.; Gallagher, A.-R.; Somlo, S. Ciliary mechanisms of cyst formation in polycystic kidney disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a028209. [Google Scholar] [CrossRef]
- Boehlke, C.; Kotsis, F.; Patel, V.; Braeg, S.; Voelker, H.; Bredt, S.; Beyer, T.; Janusch, H.; Hamann, C.; Gödel, M. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat. Cell Biol. 2010, 12, 1115–1122. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Y.; Zhou, J. Ciliary mechanosensation—Roles of polycystins and mastigonemes. J. Cell Sci. 2023, 136, jcs260565. [Google Scholar] [CrossRef]
- Prosseda, P.P.; Dannewitz Prosseda, S.; Tran, M.; Liton, P.B.; Sun, Y. Crosstalk between the mTOR pathway and primary cilia in human diseases. Curr. Top. Dev. Biol. 2023, 155, 1–37. [Google Scholar] [CrossRef]
- Lin, H.H.; Ng, K.F.; Chen, T.C.; Tseng, W.Y. Ligands and Beyond: Mechanosensitive Adhesion GPCRs. Pharmaceuticals 2022, 15, 219. [Google Scholar] [CrossRef]
- Di Mise, A.; Tamma, G.; Ranieri, M.; Centrone, M.; van den Heuvel, L.; Mekahli, D.; Levtchenko, E.N.; Valenti, G. Activation of Calcium-Sensing Receptor increases intracellular calcium and decreases cAMP and mTOR in PKD1 deficient cells. Sci. Rep. 2018, 8, 5704. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, S. Aggressive behavior and the Rosenzweig Picture-Frustration (P-F) Study. J. Clin. Psychol. 1976, 32, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Zittema, D.; Boertien, W.E.; van Beek, A.P.; Dullaart, R.P.; Franssen, C.F.; de Jong, P.E.; Meijer, E.; Gansevoort, R.T. Vasopressin, copeptin, and renal concentrating capacity in patients with autosomal dominant polycystic kidney disease without renal impairment. Clin. J. Am. Soc. Nephrol. 2012, 7, 906–913. [Google Scholar] [CrossRef]
- Bankir, L.; Bichet, D.G. An early urea-selective urine-concentrating defect in ADPKD. Nat. Rev. Nephrol. 2012, 8, 437–439. [Google Scholar] [CrossRef]
- Belibi, F.A.; Reif, G.; Wallace, D.P.; Yamaguchi, T.; Olsen, L.; Li, H.; Helmkamp Jr, G.M.; Grantham, J.J. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int. 2004, 66, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Boertien, W.E.; Meijer, E.; Zittema, D.; van Dijk, M.A.; Rabelink, T.J.; Breuning, M.H.; Struck, J.; Bakker, S.J.; Peters, D.J.; de Jong, P.E. Copeptin, a surrogate marker for vasopressin, is associated with kidney function decline in subjects with autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2012, 27, 4131–4137. [Google Scholar] [CrossRef]
- Meijer, E.; Gansevoort, R.; De Jong, P.; Van Der Wal, A.; Leonhard, W.; De Krey, S.; Van Den Born, J.; Mulder, G.; Van Goor, H.; Struck, J. Therapeutic potential of vasopressin V2 receptor antagonist in a mouse model for autosomal dominant polycystic kidney disease: Optimal timing and dosing of the drug. Nephrol. Dial. Transplant. 2011, 26, 2445–2453. [Google Scholar] [CrossRef]
- Wang, X.; Vincent Gattone, I.; Harris, P.C.; Torres, V.E. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J. Am. Soc. Nephrol. 2005, 16, 846–851. [Google Scholar] [CrossRef]
- Reif, G.A.; Yamaguchi, T.; Nivens, E.; Fujiki, H.; Pinto, C.S.; Wallace, D.P. Tolvaptan inhibits ERK-dependent cell proliferation, Cl− secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am. J. Physiol.-Ren. Physiol. 2011, 301, F1005–F1013. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Koch, G.; Ouyang, J.; McQuade, R.D.; Blais, J.D.; Czerwiec, F.S. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2017, 377, 1930–1942. [Google Scholar] [CrossRef] [PubMed]
- Perico, N.; Zoja, C.; Corna, D.; Rottoli, D.; Gaspari, F.; Haskell, L.; Remuzzi, G. V1/V2 Vasopressin receptor antagonism potentiates the renoprotection of renin–angiotensin system inhibition in rats with renal mass reduction. Kidney Int. 2009, 76, 960–967. [Google Scholar] [CrossRef]
- Strandhoy, J.; Zook, T. Antagonism of vasopressin-induced water flow by somatostatin. Res. Commun. Chem. Pathol. Pharmacol. 1979, 24, 599–602. [Google Scholar] [PubMed]
- Friedlander, G.; Amiel, C. Somatostatin and α2-adrenergic agonists selectively inhibit vasopressin-induced cyclic AMP accumulation in MDCK cells. FEBS Lett. 1986, 198, 38–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gong, A.-Y.; Tietz, P.S.; Muff, M.A.; Splinter, P.L.; Huebert, R.C.; Strowski, M.Z.; Chen, X.-M.; LaRusso, N.F. Somatostatin stimulates ductal bile absorption and inhibits ductal bile secretion in mice via SSTR2 on cholangiocytes. Am. J. Physiol.-Cell Physiol. 2003, 284, C1205–C1214. [Google Scholar] [CrossRef]
- Ferjoux, G.; Bousquet, C.; Cordelier, P.; Benali, N.; Lopez, F.; Rochaix, P.; Buscail, L.; Susini, C. Signal transduction of somatostatin receptors negatively controlling cell proliferation. J. Physiol.-Paris 2000, 94, 205–210. [Google Scholar] [CrossRef]
- Masyuk, T.V.; Masyuk, A.I.; Torres, V.E.; Harris, P.C.; Larusso, N.F. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′, 5′-cyclic monophosphate. Gastroenterology 2007, 132, 1104–1116. [Google Scholar] [CrossRef]
- Masyuk, T.V.; Radtke, B.N.; Stroope, A.J.; Banales, J.M.; Gradilone, S.A.; Huang, B.; Masyuk, A.I.; Hogan, M.C.; Torres, V.E.; LaRusso, N.F. Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology 2013, 58, 409–421. [Google Scholar] [CrossRef]
- Hopp, K.; Hommerding, C.J.; Wang, X.; Ye, H.; Harris, P.C.; Torres, V.E. Tolvaptan plus pasireotide shows enhanced efficacy in a PKD1 model. J. Am. Soc. Nephrol. 2015, 26, 39–47. [Google Scholar] [CrossRef]
- Perico, N.; Ruggenenti, P.; Perna, A.; Caroli, A.; Trillini, M.; Sironi, S.; Pisani, A.; Riccio, E.; Imbriaco, M.; Dugo, M. Octreotide-LAR in later-stage autosomal dominant polycystic kidney disease (ALADIN 2): A randomized, double-blind, placebo-controlled, multicenter trial. PLoS Med. 2019, 16, e1002777. [Google Scholar] [CrossRef]
- Pisani, A.; Sabbatini, M.; Imbriaco, M.; Riccio, E.; Rubis, N.; Prinster, A.; Perna, A.; Liuzzi, R.; Spinelli, L.; Santangelo, M. Long-term effects of octreotide on liver volume in patients with polycystic kidney and liver disease. Clin. Gastroenterol. Hepatol. 2016, 14, 1022–1030.e1024. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.; Mills, M.T.; Ong, A.C. Long-acting somatostatin analogue treatments in autosomal dominant polycystic kidney disease and polycystic liver disease: A systematic review and meta-analysis. BMJ Open 2020, 10, e032620. [Google Scholar] [CrossRef] [PubMed]
- Kathem, S.H.; Mohieldin, A.M.; Abdul-Majeed, S.; Ismail, S.H.; Altaei, Q.H.; Alshimmari, I.K.; Alsaidi, M.M.; Khammas, H.; Nauli, A.M.; Joe, B. Ciliotherapy: A novel intervention in polycystic kidney disease. J. Geriatr. Cardiol. JGC 2014, 11, 63. [Google Scholar] [PubMed]
| Drug | Target and MOA | Pre-Clinical Outcome | Clinical Trial Outcome | References |
|---|---|---|---|---|
| OPC 31260 | V2R antagonist | ↓ Cyst ratio and kidney weight after 3 weeks high-dose | [78] | |
| Tolvaptan | V2R antagonist | ↓ cAMP, ↓ proliferation, ↓ chloride secretion, ↓ cyst growth | ↓ Kidney volume growth (2.8% vs. 5.5% placebo), ↓ function decline | [80,81,82] |
| RWJ-676070 | V1a and V2 receptor antagonists | ↓ BP and proteinuria, partial glomerular/tubular restoration | ↓ eGFR decline (p < 0.001) | [83] |
| Octreotide | Somatostatin; SSRT2, 3, and 5 agonists | ↓ cAMP, ↓ liver/kidney cyst volume, ↓ mitotic index | ↓ Kidney growth and function loss in high-risk ADPKD patients | [88,91,92] |
| Pasireotide | Somatostatin; SSRT2, 3, and 5 agonists | ↓ Hepatorenal cystogenesis in rodent models | - | [89] |
| Tolvaptan and pasireotide -combination | V2R antagonist And SSRT2, 3, and 5 agonists | ↓ Cystic and fibrotic volume, normalized cAMP | - | [90] |
| Fenoldopam | Dopamine- DR5 agonist | ↑ Cilia function via NO production in response to shear | - | [93] |
| Levodopa | Non-specific dopamine agonist | - | ↓ BP in PKD patients with borderline hypertension | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buqaileh, R.; Alshriem, L.A.; AbouAlaiwi, W. Ciliary G-Protein Coupled Receptor Signaling in Polycystic Kidney Disease. Int. J. Mol. Sci. 2025, 26, 4971. https://doi.org/10.3390/ijms26114971
Buqaileh R, Alshriem LA, AbouAlaiwi W. Ciliary G-Protein Coupled Receptor Signaling in Polycystic Kidney Disease. International Journal of Molecular Sciences. 2025; 26(11):4971. https://doi.org/10.3390/ijms26114971
Chicago/Turabian StyleBuqaileh, Raghad, Lubna A. Alshriem, and Wissam AbouAlaiwi. 2025. "Ciliary G-Protein Coupled Receptor Signaling in Polycystic Kidney Disease" International Journal of Molecular Sciences 26, no. 11: 4971. https://doi.org/10.3390/ijms26114971
APA StyleBuqaileh, R., Alshriem, L. A., & AbouAlaiwi, W. (2025). Ciliary G-Protein Coupled Receptor Signaling in Polycystic Kidney Disease. International Journal of Molecular Sciences, 26(11), 4971. https://doi.org/10.3390/ijms26114971






