Plasma Extracellular Vesicles from Preeclamptic Patients Trigger a Detrimental Crosstalk Between Glomerular Endothelial Cells and Podocytes Involving Endothelin-1
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Profiling of PE Patients
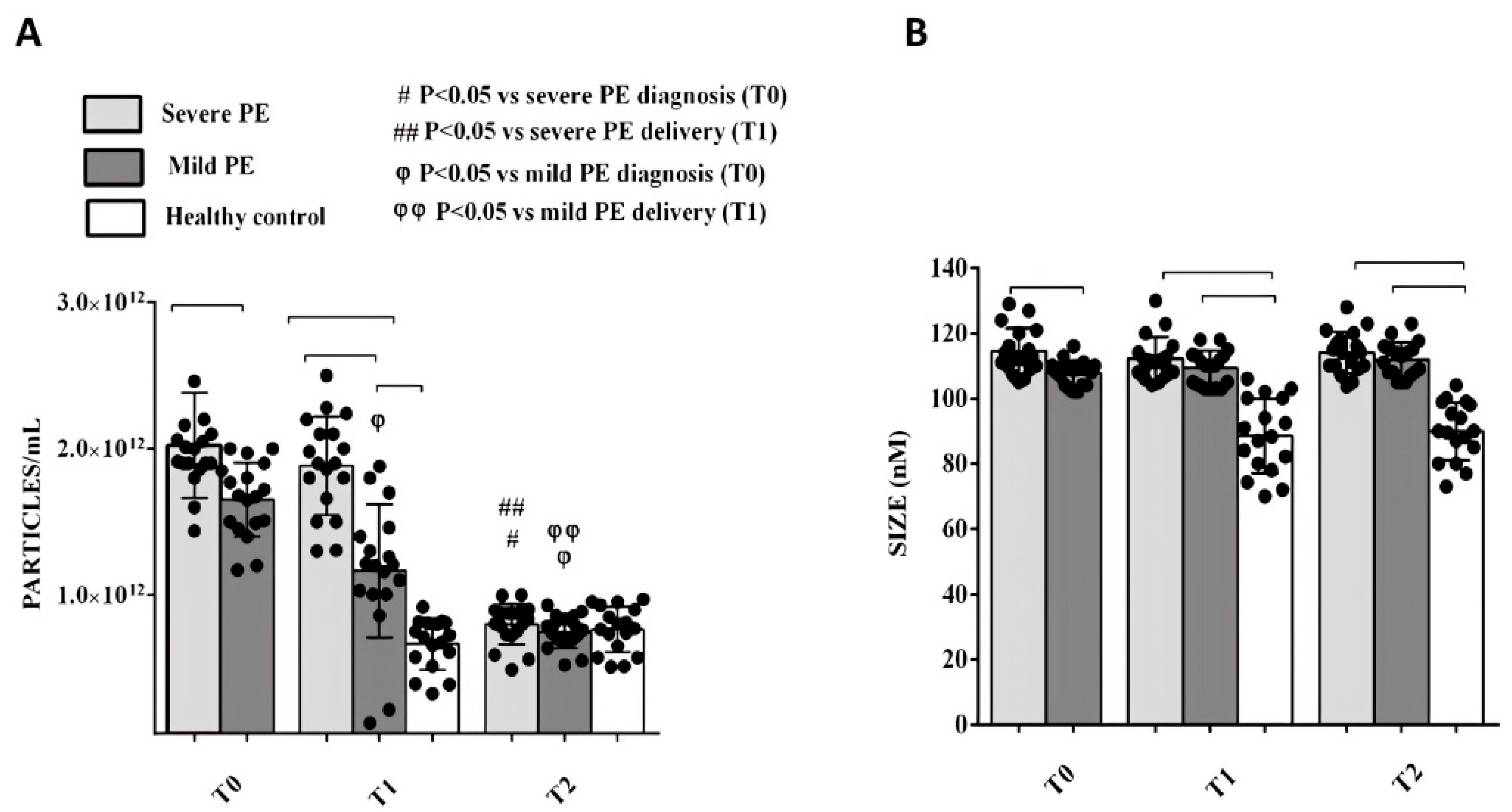
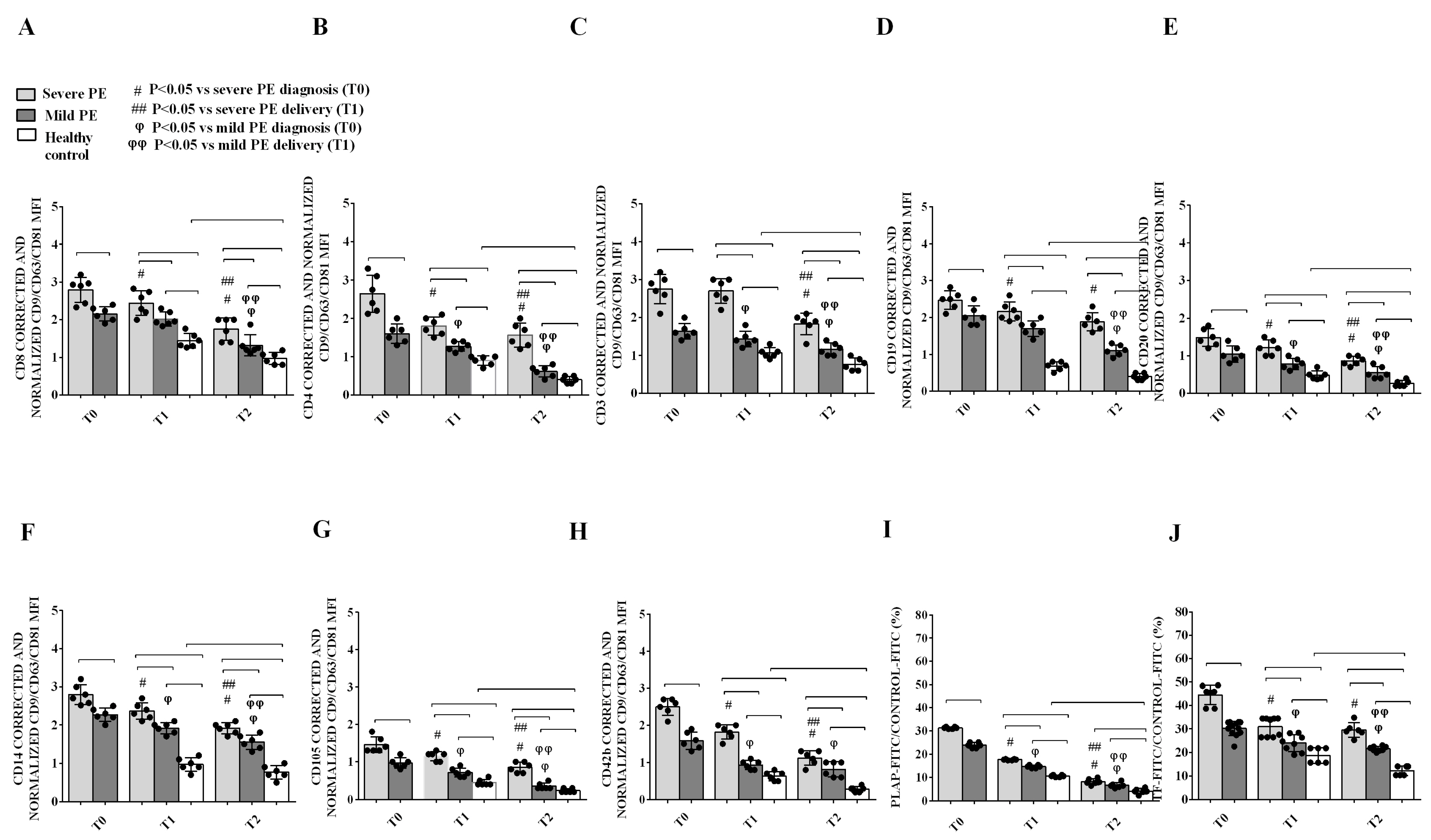
2.2. Phenotypic Characterization of Plasma EVs and Correlation with Clinical Parameters
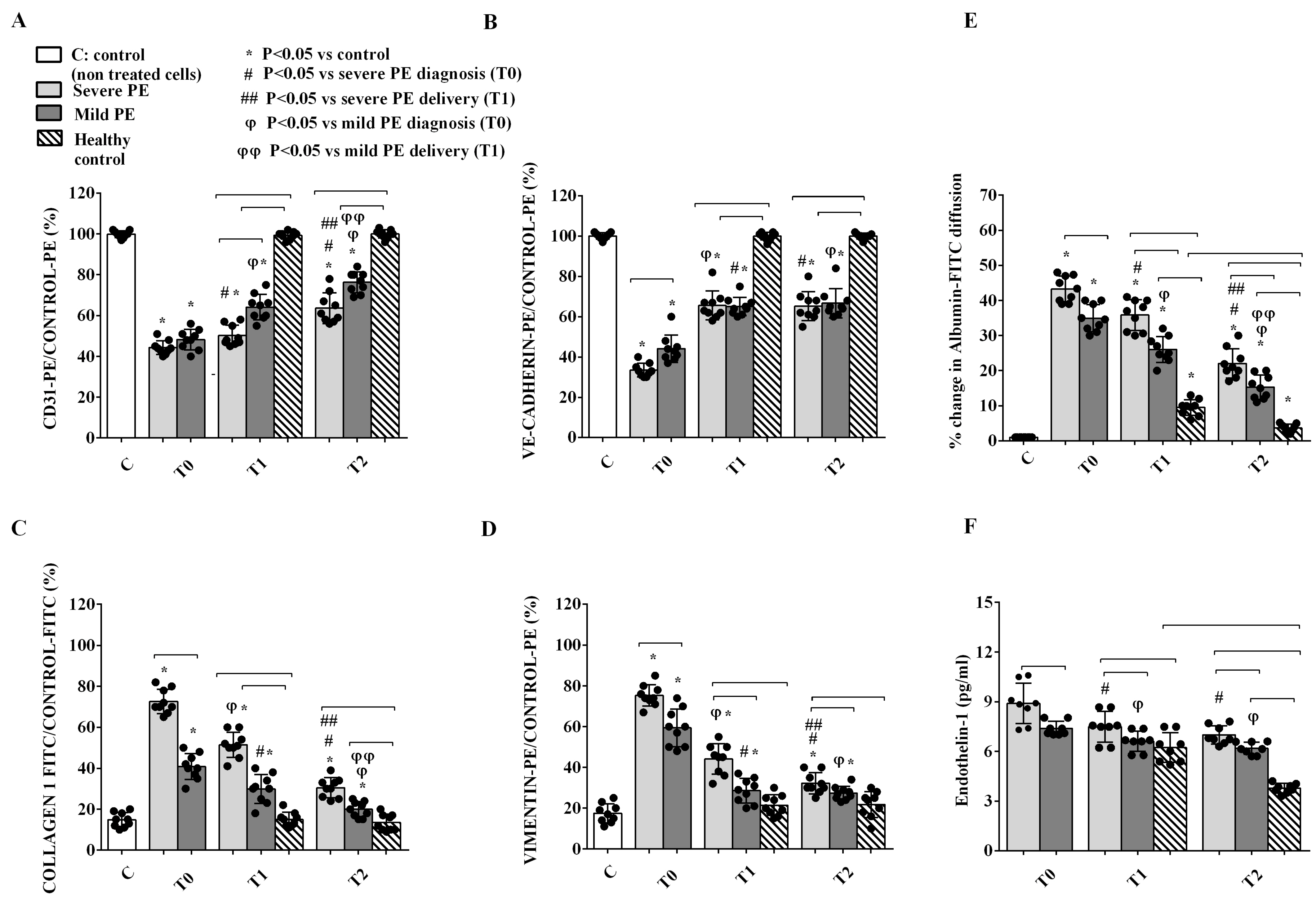
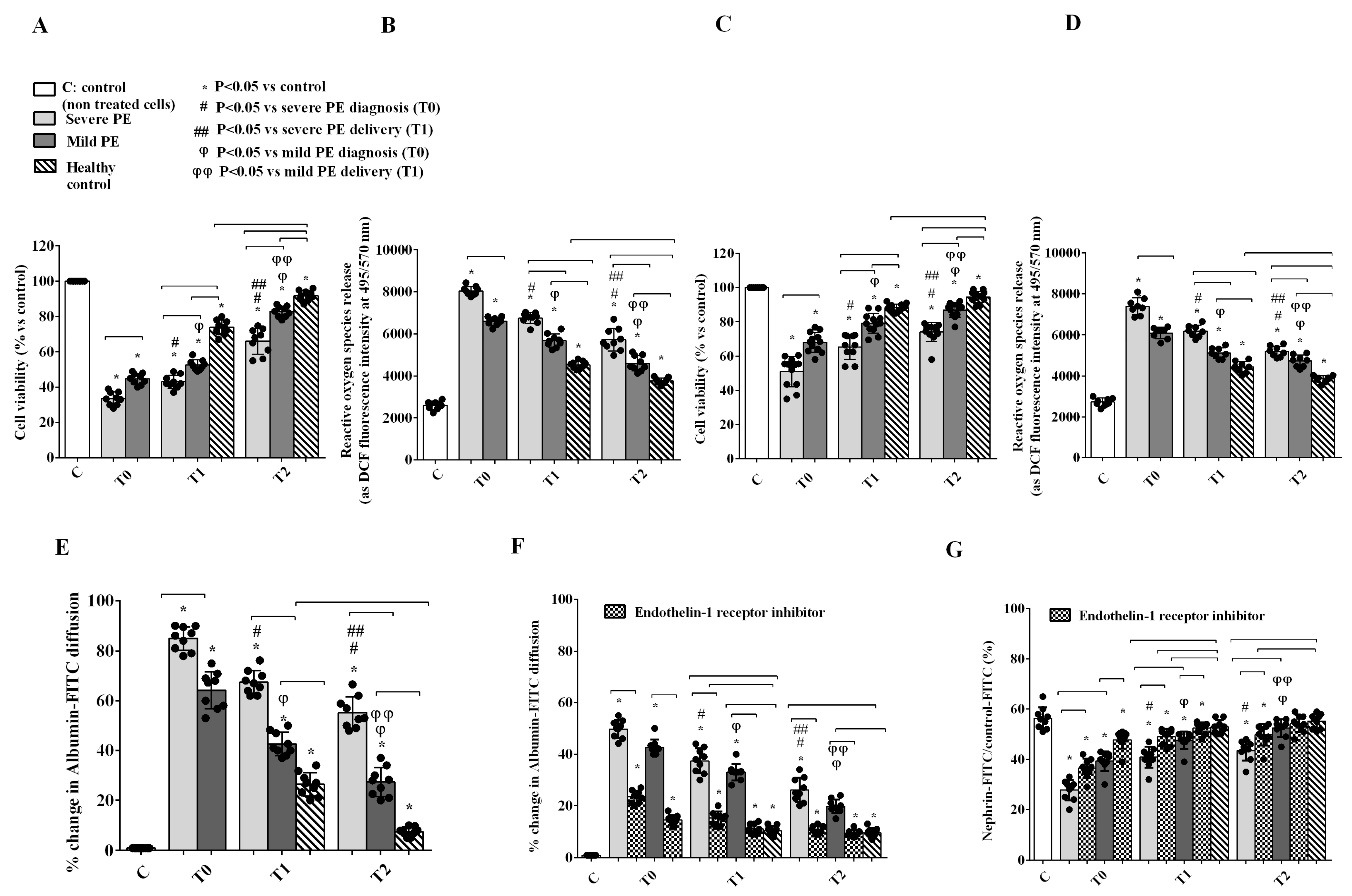
2.3. In Vitro Experiments on GEC and PODO Directly Stimulated by Plasma EVs
2.4. GEC-PODO Co-Culture Experiments
3. Discussion
4. Materials and Methods
4.1. Patients and Clinical Variables
4.2. Plasma EV Isolation and Characterization
4.3. In Vitro Studies
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, K.; McGrath, L.; Brookfield, K. Hypertension Management in Pregnancy. Annu. Rev. Med. 2025, 76, 315–326. [Google Scholar] [CrossRef]
- Friedman, S.A.; Schiff, E.; Kao, L.; Sibai, B.M. Neonatal outcome after preterm delivery for preeclampsia. Am. J. Obstet. Gynecol. 1995, 172, 1785–1788. [Google Scholar] [CrossRef]
- The World Health Report 2005. Make Every Mother and Child Count; World Health Organization: Geneva, Switzerland, 2005; Available online: https://www.who.int/publications/i/item/9241562900 (accessed on 1 May 2005).
- Gui, J.; Ling, Z.; Hou, X.; Fan, Y.; Xie, K.; Shen, R. In vitro fertilization is associated with the onset and progression of preeclampsia. Placenta 2020, 89, 50–57. [Google Scholar] [CrossRef]
- Roberts, J.M.; Hubel, C.A. The two stage model of preeclampsia: Variations on the theme. Placenta 2009, 30 (Suppl. A), S32–S37. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.V.; Gusar, V.A.; Kan, N.E.; Prozorovskaya, K.N.; Karapetyan, A.O.; Bayev, O.R.; Chagovets, V.V.; Kliver, S.F.; Iakovishina, D.Y.; Frankevich, V.E.; et al. Identification of potential early biomarkers of preeclampsia. Placenta 2018, 61, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Possomato-Vieira, J.S.; Khalil, R.A. Mechanisms of Endothelial Dysfunction in Hypertensive Pregnancy and Preeclampsia. Adv. Pharmacol. 2016, 77, 361–431. [Google Scholar] [CrossRef]
- Craici, I.M.; Wagner, S.J.; Weissgerber, T.L.; Grande, J.P.; Garovic, V.D. Advances in the pathophysiology of pre-eclampsia and related podocyte injury. Kidney Int. 2014, 86, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Tannetta, D.S.; Dragovic, R.A.; Gardiner, C.; Southcombe, J.H.; Collett, G.P.; Sargent, I.L. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta 2012, 33, S48–S54. [Google Scholar] [CrossRef]
- Adam, S.; Elfeky, O.; Kinhal, V.; Dutta, S.; Lai, A.; Jayabalan, N.; Nuzhat, Z.; Palma, C.; Rice, G.E.; Salomon, C. Review: Fetal-maternal communication via extracellular vesicles—Implications for complications of pregnancies. Placenta 2017, 54, 83–88. [Google Scholar] [CrossRef]
- Pardo, F.; Subiabre, M.; Fuentes, G.; Toledo, F.; Silva, L.; Villalobos-Labra, R.; Sobrevia, L. Altered foetoplacental vascular endothelial signalling to insulin in diabesity. Mol. Aspects Med. 2019, 66, 40–48. [Google Scholar] [CrossRef]
- Sáez, T.; de Vos, P.; Kuipers, J.; Sobrevia, L.; Faas, M.M. Fetoplacental endothelial exosomes modulate high d-glucose-induced endothelial dysfunction. Placenta 2018, 66, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Sáez, T.; de Vos, P.; Sobrevia, L.; Faas, M.M. Is there a role for exosomes in foetoplacental endothelial dysfunction in gestational diabetes mellitus? Placenta 2017, 61, 48–54. [Google Scholar] [CrossRef]
- Quesenberry, P.J.; Goldberg, L.R.; Aliotta, J.M.; Dooner, M.S.; Pereira, M.G.; Wen, S.; Camussi, G. Cellular phenotype and extracellular vesicles: Basic and clinical considerations. Stem Cells Dev. 2014, 23, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.I.; Weissgerber, T.L.; Garovic, V.D.; Jayachandran, M. Preeclampsia and Extracellular Vesicles. Curr. Hypertens. Rep. 2016, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Palma, C.; Jellins, J.; Lai, A.; Salas, A.; Campos, A.; Sharma, S.; Duncombe, G.; Hyett, J.; Salomon, C. Extracellular Vesicles and Preeclampsia: Current Knowledge and Future Research Directions. Subcell. Biochem. 2021, 97, 455–482. [Google Scholar] [CrossRef]
- Collino, F.; Bussolati, B.; Gerbaudo, E.; Marozio, L.; Pelissetto, S.; Benedetto, C.; Camussi, G. Preeclamptic sera induce nephrin shedding from podocytes through endothelin-1 release by endothelial glomerular cells. Am. J. Physiol. Renal Physiol. 2008, 294, F1185–F1194. [Google Scholar] [CrossRef]
- Williams, S.; Fernandez-Rhodes, M.; Law, A.; Peacock, B.; Lewis, M.P.; Davies, O.G. Comparison of extracellular vesicle isolation processes for therapeutic applications. J. Tissue Eng. 2023, 14, 20417314231174609. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Paradela, A.; Asunción Sánchez-Gil, M.; Rodriguez-Martin, S.; De León-Luis, J.A.; Pereda-Cerquella, C.; Bujan, J.; Guijarro, L.G.; et al. Unfolding the role of placental-derived Extracellular Vesicles in Pregnancy: From homeostasis to pathophysiology. Front. Cell Dev. Biol. 2022, 10, 1060850. [Google Scholar] [CrossRef]
- Mincheva-Nilsson, L.; Baranov, V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: Immune modulation for pregnancy success. Am. J. Reprod. Immunol. 2014, 72, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmed, A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ. Res. 2004, 95, 884–891. [Google Scholar] [CrossRef]
- Awoyemi, T.; Cerdeira, A.S.; Zhang, W.; Jiang, S.; Rahbar, M.; Logenthiran, P.; Redman, C.; Vatish, M. Preeclampsia and syncytiotrophoblast membrane extracellular vesicles (STB-EVs). Clin. Sci. 2022, 136, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Luttun, A.; Carmeliet, P. Soluble VEGF receptor Flt1: The elusive preeclampsia factor discovered? J. Clin. Investig. 2003, 111, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010, 78, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, C.; Dellepiane, S.; Fonsato, V.; Medica, D.; Marengo, M.; Migliori, M.; Quercia, A.D.; Pitino, A.; Formica, M.; Panichi, V.; et al. Online Hemodiafiltration Inhibits Inflammation-Related Endothelial Dysfunction and Vascular Calcification of Uremic Patients Modulating miR-223 Expression in Plasma Extracellular Vesicles. J. Immunol. 2019, 202, 2372–2383. [Google Scholar] [CrossRef]
- Kupper, N.; Huppertz, B. The endogenous exposome of the pregnant mother: Placental extracellular vesicles and their effect on the maternal system. Mol. Aspects Med. 2022, 87, 100955. [Google Scholar] [CrossRef]
- Zhaoer, Y.; Mingming, G.; Wei, Z.; Dan, Y.; Yating, Q.; Ruizhe, J. Extracellular vesicles for the treatment of preeclampsia. Tissue Cell 2022, 77, 101860. [Google Scholar] [CrossRef]
- Nakahara, A.; Nair, S.; Ormazabal, V.; Elfeky, O.; Garvey, C.E.; Longo, S.; Salomon, C. Circulating Placental Extracellular Vesicles and Their Potential Roles During Pregnancy. Ochsner J. 2020, 20, 439–445. [Google Scholar] [CrossRef]
- Matsubara, K.; Matsubara, Y.; Uchikura, Y.; Sugiyama, T. Pathophysiology of Preeclampsia: The Role of Exosomes. Int. J. Mol. Sci. 2021, 22, 2572. [Google Scholar] [CrossRef]
- Staff, A.C.; Braekke, K.; Johnsen, G.M.; Karumanchi, S.A.; Harsem, N.K. Circulating concentrations of soluble endoglin (CD105) in fetal and maternal serum and in amniotic fluid in preeclampsia. Am. J. Obstet. Gynecol. 2007, 197, 176.e1–176.e6. [Google Scholar] [CrossRef]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.I.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.H.; Yuan, H.T.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef]
- Aggarwal, P.K.; Chandel, N.; Jain, V.; Jha, V. The relationship between circulating endothelin-1, soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. J. Hum. Hypertens. 2012, 26, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Tersigni, C.; Furqan Bari, M.; Cai, S.; Zhang, W.; Kandzija, N.; Buchan, A.; Miranda, F.; Di Simone, N.; Redman, C.W.; Bastie, C.; et al. Syncytiotrophoblast-derived extracellular vesicles carry apolipoprotein-E and affect lipid synthesis of liver cells in vitro. J. Cell. Mol. Med. 2022, 26, 123–132. [Google Scholar] [CrossRef]
- Brien, M.E.; Duval, C.; Palacios, J.; Boufaied, I.; Hudon-Thibeault, A.A.; Nadeau-Vallée, M.; Vaillancourt, C.; Sibley, C.P.; Abrahams, V.M.; Jones, R.L.; et al. Uric Acid Crystals Induce Placental Inflammation and Alter Trophoblast Function via an IL-1-Dependent Pathway: Implications for Fetal Growth Restriction. J. Immunol. 2017, 198, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Shirasuna, K.; Karasawa, T.; Takahashi, M. Role of the NLRP3 Inflammasome in Preeclampsia. Front. Endocrinol. 2020, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Hisatome, I.; Kihara, Y.; Higashi, Y. Hyperuricemia and endothelial function: From molecular background to clinical perspectives. Atherosclerosis 2018, 278, 226–231. [Google Scholar] [CrossRef]
- Franzin, R.; Stasi, A.; Sallustio, F.; Bruno, S.; Merlotti, G.; Quaglia, M.; Grandaliano, G.; Pontrelli, P.; Thurman, J.M.; Camussi, G.; et al. Extracellular vesicles derived from patients with antibody-mediated rejection induce tubular senescence and endothelial to mesenchymal transition in renal cells. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2022, 22, 2139–2157. [Google Scholar] [CrossRef]
- Daehn, I.S.; Duffield, J.S. The glomerular filtration barrier: A structural target for novel kidney therapies. Nat. Rev. Drug Discov. 2021, 20, 770–788. [Google Scholar] [CrossRef]
- Wagner, S.J.; Craici, I.M.; Grande, J.P.; Garovic, V.D. From placenta to podocyte: Vascular and podocyte pathophysiology in preeclampsia. Clin. Nephrol. 2012, 78, 241–249. [Google Scholar] [CrossRef]
- Turner, R.J.; Bloemenkamp, K.W.M.; Penning, M.E.; Bruijn, J.A.; Baelde, H.J. From Glomerular Endothelium to Podocyte Pathobiology in Preeclampsia: A Paradigm Shift. Curr. Hypertens. Rep. 2015, 17, 54. [Google Scholar] [CrossRef]
- Qu, H.; Khalil, R.A. Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H661–H681. [Google Scholar] [CrossRef]
- Kwiatkowska, E.; Stefańska, K.; Zieliński, M.; Sakowska, J.; Jankowiak, M.; Trzonkowski, P.; Marek-Trzonkowska, N.; Kwiatkowski, S. Podocytes-The Most Vulnerable Renal Cells in Preeclampsia. Int. J. Mol. Sci. 2020, 21, 5051. [Google Scholar] [CrossRef] [PubMed]
- Bakrania, B.A.; Spradley, F.T.; Satchell, S.C.; Stec, D.E.; Rimoldi, J.M.; Gadepalli, R.S.; Granger, J.P. Heme oxygenase-1 is a potent inhibitor of placental ischemia-mediated endothelin-1 production in cultured human glomerular endothelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R427–R432. [Google Scholar] [CrossRef] [PubMed]
- Smeijer, J.D.; Kohan, D.E.; Dhaun, N.; Noronha, I.L.; Liew, A.; Heerspink, H.J.L. Endothelin receptor antagonists in chronic kidney disease. Nat. Rev. Nephrol. 2025, 21, 175–188. [Google Scholar] [CrossRef]
- Iannaccone, A.; Reisch, B.; Kimmig, R.; Schmidt, B.; Mavarani, L.; Darkwah Oppong, M.; Tyczynski, B.; Dzietko, M.; Jahn, M.; Gellhaus, A.; et al. Therapeutic Plasma Exchange in Early-Onset Preeclampsia: A 7-Year Monocentric Experience. J. Clin. Med. 2023, 12, 4289. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Kluivers, A.C.; Cruz-López, E.O.; Broekhuizen, M.; Chen, Z.; Neuman, R.I.; Schoenmakers, S.; Ruijgrok, L.; Van De Velde, D.; De Winter, B.C.; et al. Statins Prevent the Deleterious Consequences of Placental Chemerin Upregulation in Preeclampsia. Hypertension 2024, 81, 861–875. [Google Scholar] [CrossRef]
- Schröder, S.; Epple, R.; Fischer, A.; Schettler, V.J.J. Effective exosomes reduction in hypercholesterinemic patients suffering from cardiovascular diseases by lipoprotein apheresis: Exosomes apheresis. Ther. Apher. Dial. 2024, 28, 863–870. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W.; et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl. Med. 2015, 4, 513–522. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Zhu, Y.; Li, L.; Wu, Y.; Ying, J.; Li, Y.; Chen, J. Human Trophoblast Cell-Derived Extracellular Vesicles Facilitate Preeclampsia by Transmitting miR-1273d, miR-4492, and miR-4417 to Target HLA-G. Reprod. Sci. 2022, 29, 2685–2696. [Google Scholar] [CrossRef]
- Awoyemi, T.; Jiang, S.; Rahbar, M.; Logentherian, P.; Collett, G.; Zhang, W.; Cribbs, A.; Cerdeira, S.; Vatish, M. MicroRNA analysis of medium/large placenta extracellular vesicles in normal and preeclampsia pregnancies. Front. Cardiovasc. Med. 2024, 11, 1371168. [Google Scholar] [CrossRef]
- Yang, Z.; Shan, N.; Deng, Q.; Wang, Y.; Hou, Y.; Mei, J.; Wu, Z. Extracellular vesicle-derived microRNA-18b ameliorates preeclampsia by enhancing trophoblast proliferation and migration via Notch2/TIM3/mTORC1 axis. J. Cell Mol. Med. 2021, 25, 4583–4595. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Medica, D.; Franzin, R.; Stasi, A.; Castellano, G.; Migliori, M.; Panichi, V.; Figliolini, F.; Gesualdo, L.; Camussi, G.; Cantaluppi, V. Extracellular Vesicles Derived from Endothelial Progenitor Cells Protect Human Glomerular Endothelial Cells and Podocytes from Complement- and Cytokine-Mediated Injury. Cells 2021, 10, 1675. [Google Scholar] [CrossRef] [PubMed]
- Cantaluppi, V.; Medica, D.; Mannari, C.; Stiaccini, G.; Figliolini, F.; Dellepiane, S.; Quercia, A.D.; Migliori, M.; Panichi, V.; Giovannini, L.; et al. Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial injury in experimental anti-Thy1.1 glomerulonephritis. Nephrol. Dial. Transplant. 2015, 30, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Cantaluppi, V.; Assenzio, B.; Pasero, D.; Romanazzi, G.M.; Pacitti, A.; Lanfranco, G.; Puntorieri, V.; Martin, E.L.; Mascia, L.; Monti, G.; et al. Polymyxin-B hemoperfusion inactivates circulating proapoptotic factors. Intensive Care Med. 2008, 34, 1638–1645. [Google Scholar] [CrossRef]
- Mariano, F.; Cantaluppi, V.; Stella, M.; Romanazzi, G.M.; Assenzio, B.; Cairo, M.; Biancone, L.; Triolo, G.; Ranieri, V.; Camussi, G. Circulating plasma factors induce tubular and glomerular alterations in septic burns patients. Crit. Care Lond. Engl. 2008, 12, R42. [Google Scholar] [CrossRef] [PubMed]
- Bussolati, B.; Deregibus, M.C.; Fonsato, V.; Doublier, S.; Spatola, T.; Procida, S.; Di Carlo, F.; Camussi, G. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J. Am. Soc. Nephrol. JASN 2005, 16, 1936–1947. [Google Scholar] [CrossRef] [PubMed]
- Grossini, E.; Garhwal, D.; Venkatesan, S.; Ferrante, D.; Mele, A.; Saraceno, M.; Scognamiglio, A.; Mandrioli, J.; Amedei, A.; De Marchi, F.; et al. The Potential Role of Peripheral Oxidative Stress on the Neurovascular Unit in Amyotrophic Lateral Sclerosis Pathogenesis: A Preliminary Report from Human and In Vitro Evaluations. Biomedicines 2022, 10, 691. [Google Scholar] [CrossRef] [PubMed]
- Grossini, E.; De Marchi, F.; Venkatesan, S.; Mele, A.; Ferrante, D.; Mazzini, L. Effects of Acetyl-L-Carnitine on Oxidative Stress in Amyotrophic Lateral Sclerosis Patients: Evaluation on Plasma Markers and Members of the Neurovascular Unit. Antioxidants 2023, 12, 1887–2023. [Google Scholar] [CrossRef]
- Grossini, E.; Venkatesan, S.; Alkabes, M.; Toma, C.; de Cillà, S. Membrane Blue Dual Protects Retinal Pigment Epithelium Cells/Ganglion Cells-Like through Modulation of Mitochondria Function. Biomedicines 2022, 10, 2854. [Google Scholar] [CrossRef]
- De Cillà, S.; Farruggio, S.; Cocomazzi, G.; Mary, D.; Alkabes, M.; Rossetti, L.; Vujosevic, S.; Grossini, E. Aflibercept and Ranibizumab Modulate Retinal Pigment Epithelial Cells Function by Acting on Their Cross Talk with Vascular Endothelial Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2020, 54, 161–179. [Google Scholar] [CrossRef]
- Farruggio, S.; Cocomazzi, G.; Marotta, P.; Romito, R.; Surico, D.; Calamita, G.; Bellan, M.; Pirisi, M.; Grossini, E. Genistein and 17β-Estradiol Protect Hepatocytes from Fatty Degeneration by Mechanisms Involving Mitochondria, Inflammasome and Kinases Activation. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2020, 54, 401–416. [Google Scholar] [CrossRef]
- Surico, D.; Ercoli, A.; Farruggio, S.; Raina, G.; Filippini, D.; Mary, D.; Minisini, R.; Surico, N.; Pirisi, M.; Grossini, E. Modulation of Oxidative Stress by 17 β-Estradiol and Genistein in Human Hepatic Cell Lines In Vitro. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 42, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Savoia, P.; Raina, G.; Camillo, L.; Farruggio, S.; Mary, D.; Veronese, F.; Graziola, F.; Zavattaro, E.; Tiberio, R.; Grossini, E. Anti-oxidative effects of 17 β-estradiol and genistein in human skin fibroblasts and keratinocytes. J. Dermatol. Sci. 2018, 92, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Rotrosen, D.; Gallin, J.I. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J. Cell Biol. 1986, 103, 2379–2387. [Google Scholar] [CrossRef]


| Severe PE (n = 18) | Mild PE (n = 18) | HCs (n = 17) | P# | P£ | P$ | |
|---|---|---|---|---|---|---|
| Mean age | 33.82 | 33.14 | 30.40 | 0.820 | 1.000 | 0.710 |
| Range | (27–41) | (25–47) | (21–42) | |||
| SD | 4391 | 6562 | 8081 | |||
| Age < 35 | 10/18 | 13/18 | 11/17 | 1.000 | 0.527 | 0.553 |
| 35–40 | 6/18 | 3/18 | 5/17 | |||
| >40 | 2/18 | 2/18 | 1/17 | |||
| Mean BMI | 26.16 | 31.50 | 25.54 | 0.863 | 0.55 | 0.091 |
| Range | (17.6–41) | (21.7–42.6) | (23.4–27.7) | |||
| SD | 6175 | 6364 | 1902 | |||
| BMI < 25 | 8/18 | 3/18 | 4/17 | 0.400 | 0.797 | 0.020 |
| 25–30 | 4/18 | 5/18 | 8/17 | |||
| >30 | 6/18 | 10/18 | 5/17 | |||
| Ethnicity: Caucasian | 16/18 | 15/18 | 15/17 | 0.624 | 0.660 | 0.643 |
| African | 2/18 | 1/18 | 1/17 | |||
| Asian | 0/18 | 2/18 | 1/17 | |||
| Smoking: | ||||||
| Current | 1/18 | 1/18 | 1/17 | 1.000 | 0.017 | 0.170 |
| Previous | 5/18 | 0/18 | 0/17 | |||
| Absent | 12/18 | 17/18 | 16/17 |
| Severe PE (n = 18) | Mild PE (n = 18) | HCs (n = 17) | P# | P£ | P$ | ||
|---|---|---|---|---|---|---|---|
| Delivery (weeks) | 33.39 | 37.39 | 37.47 | ||||
| Range | (26–39) | (35–40) | (24–41) | ||||
| SD | 3773 | 1339 | 4354 | ||||
| Weeks | <32 | 7/18 | 0/18 | 2/17 | 0.890 | 0.004 | 0.002 |
| 32–37 | 8/18 | 9/18 | 4/17 | ||||
| >37 | 3/18 | 9/18 | 11/17 | ||||
| HELLP | 5/18 | 0 | 0 | <0.0001 | <0.0001 | ||
| Live newborns | 100% | 100% | 100% | ||||
| Mean weight | <1000 g | 3/18 | 0/18 | 0/17 | 0.076 | 0.002 | <0.001 |
| 1000–2500 g | 11/18 | 2/18 | 5/17 | ||||
| >2500 g | 4/18 | 16/18 | 12/17 | ||||
| APGAR 1 min | 7 | 0/18 | 1/18 | 0/17 | 0.371 | 0.003 | 0.090 |
| 8 | 4/18 | 0/18 | 0/17 | ||||
| 9 | 7/18 | 5/18 | 3/17 | ||||
| 10 | 7/18 | 12/18 | 14/17 | ||||
| NICU hospitalization | Yes | 12/18 | 3/18 | 2/17 | 0.689 | <0.001 | 0.002 |
| No | 6/18 | 15/18 | 15/17 | ||||
| Modality | CC | 17/18 | 7/18 | 7/17 | 0.335 | 0.006 | 0.002 |
| ID | 1/18 | 5/18 | 1/17 | ||||
| SP | 0/18 | 6/18 | 9/17 |
| Severe PE (n = 18) | Mild PE (n = 18) | HCs (n = 17) | P# | P£ | P$ | ||
|---|---|---|---|---|---|---|---|
| GE (weeks) | <32 | 9/18 | 3/18 | 2/17 | 0.240 | 0.041 | 0.023 |
| 32–37 | 7/18 | 9/18 | 4/17 | ||||
| >37 | 2/18 | 6/18 | 11/17 | ||||
| AST (U/L) | <20 | 4/18 | 9/18 | 12/17 | 0.164 | <0.001 | 0.010 |
| 20–100 | 7/18 | 9/18 | 5/17 | ||||
| >100 | 7/18 | 0/18 | 0/17 | ||||
| ALT (U/L) | <20 | 5/18 | 12/18 | 14/17 | 0.681 | <0.001 | 0.001 |
| 20–100 | 6/18 | 6/18 | 3/17 | ||||
| >100 | 7/18 | 0/18 | 0/17 | ||||
| PLTs | <100 × 103 | 3/18 | 0/18 | 0/17 | 0.073 | 0.005 | 0.065 |
| 100–50 × 103 | 5/18 | 3/18 | 0/17 | ||||
| >150 × 103 | 10/18 | 15/18 | 17/17 | ||||
| Uric acid (mg/dL) | <6 | 6/18 | 13/18 | 17/17 | 0.108 | 0.068 | 0.693 |
| >6 | 12/18 | 5/18 | 0/17 | ||||
| Hb (g/dL) | <10.5 | 2/18 | 3/18 | 9/17 | 0.116 | 0.001 | 0.232 |
| 10.5–12.5 | 7/18 | 10/18 | 7/17 | ||||
| >12.5 | 9/18 | 5/18 | 1/17 | ||||
| SAP (mmHg) | <140 | 3/18 | 9/18 | 17/17 | 0.016 | 0.006 | 0.525 |
| 140–160 | 7/18 | 7/18 | 0/17 | ||||
| >160 | 8/18 | 2/18 | 0/17 | ||||
| DAP (mmHg) | <90 | 4/18 | 10/18 | 17/17 | 0.002 | 0.022 | 0.418 |
| 90–110 | 11/18 | 5/18 | 0/17 | ||||
| >110 | 3/18 | 1/18 | 0/17 | ||||
| Proteinuria (mg/day) SD | 1589 | 1371 | / | / | / | 0.490 | |
| 1415 | 1152 | / | |||||
| sFlt-1 (pg/mL) | 5992 | 3902 | / | / | / | 0.0008 | |
| 1386 | 903 |
| Severe PE (n = 18) | Mild PE (n = 18) | HCs (n = 17) | P# | P£ | P$ | ||
|---|---|---|---|---|---|---|---|
| AST (U/L) | <20 | 4/18 | 8/18 | 12/17 | 0.081 | <0.001 | 0.018 |
| 20–100 | 8/18 | 10/18 | 5/17 | ||||
| >100 | 6/18 | 0/18 | 0/17 | ||||
| ALT (U/L) | <20 | 5/18 | 12/18 | 14/17 | 0.431 | <0.001 | 0.002 |
| 20–100 | 6/18 | 6/18 | 3/17 | ||||
| >100 | 7/18 | 0/18 | 0/17 | ||||
| PTLs | <100 × 103 | 3/18 | 0/18 | 0/17 | 0.154 | 0.005 | 0.031 |
| 100–150 × 103 | 5/18 | 3/18 | 0/17 | ||||
| >150 × 103 | 10/18 | 15/18 | 17/17 | ||||
| Uric acid (mg/dL) | <6 | 6/18 | 10/18 | 17/17 | 0.116 | 0.272 | 0.846 |
| >6 | 12/18 | 8/18 | 0/17 | ||||
| Hb (g/dL) | <10.5 | 9/18 | 9/18 | 9/17 | 0.894 | 0.469 | 0.412 |
| 10.5–12.5 | 6/18 | 8/18 | 7/17 | ||||
| >12.5 | 3/18 | 1/18 | 1/17 | ||||
| SAP (mmHg) | <140 | 4/18 | 10/18 | 17/17 | 0.200 | 0.079 | 0.485 |
| 140–160 | 8/18 | 8/18 | 0/17 | ||||
| >160 | 6/18 | 0/18 | 0/17 | ||||
| DAP (mmHg) | <90 | 4/18 | 9/18 | 17/17 | 0.870 | 0.055 | 0.152 |
| 90–110 | 10/18 | 9/18 | 0/17 | ||||
| >110 | 4/18 | 0/18 | 0/17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grossini, E.; Quaglia, M.; Prenna, S.; Stasi, A.; Franzin, R.; Castellano, G.; Remorgida, V.; Libretti, A.; Venkatesan, S.; Smirne, C.; et al. Plasma Extracellular Vesicles from Preeclamptic Patients Trigger a Detrimental Crosstalk Between Glomerular Endothelial Cells and Podocytes Involving Endothelin-1. Int. J. Mol. Sci. 2025, 26, 4962. https://doi.org/10.3390/ijms26114962
Grossini E, Quaglia M, Prenna S, Stasi A, Franzin R, Castellano G, Remorgida V, Libretti A, Venkatesan S, Smirne C, et al. Plasma Extracellular Vesicles from Preeclamptic Patients Trigger a Detrimental Crosstalk Between Glomerular Endothelial Cells and Podocytes Involving Endothelin-1. International Journal of Molecular Sciences. 2025; 26(11):4962. https://doi.org/10.3390/ijms26114962
Chicago/Turabian StyleGrossini, Elena, Marco Quaglia, Stefania Prenna, Alessandra Stasi, Rossana Franzin, Giuseppe Castellano, Valentino Remorgida, Alessandro Libretti, Sakthipriyan Venkatesan, Carlo Smirne, and et al. 2025. "Plasma Extracellular Vesicles from Preeclamptic Patients Trigger a Detrimental Crosstalk Between Glomerular Endothelial Cells and Podocytes Involving Endothelin-1" International Journal of Molecular Sciences 26, no. 11: 4962. https://doi.org/10.3390/ijms26114962
APA StyleGrossini, E., Quaglia, M., Prenna, S., Stasi, A., Franzin, R., Castellano, G., Remorgida, V., Libretti, A., Venkatesan, S., Smirne, C., Merlotti, G., Aquino, C. I., Bruno, S., Camussi, G., Surico, D., & Cantaluppi, V. (2025). Plasma Extracellular Vesicles from Preeclamptic Patients Trigger a Detrimental Crosstalk Between Glomerular Endothelial Cells and Podocytes Involving Endothelin-1. International Journal of Molecular Sciences, 26(11), 4962. https://doi.org/10.3390/ijms26114962







