Disease Activity-Dependent Siglec-1 Expression on Monocyte Subsets of Patients with Idiopathic Inflammatory Myopathies
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics of Study Participants
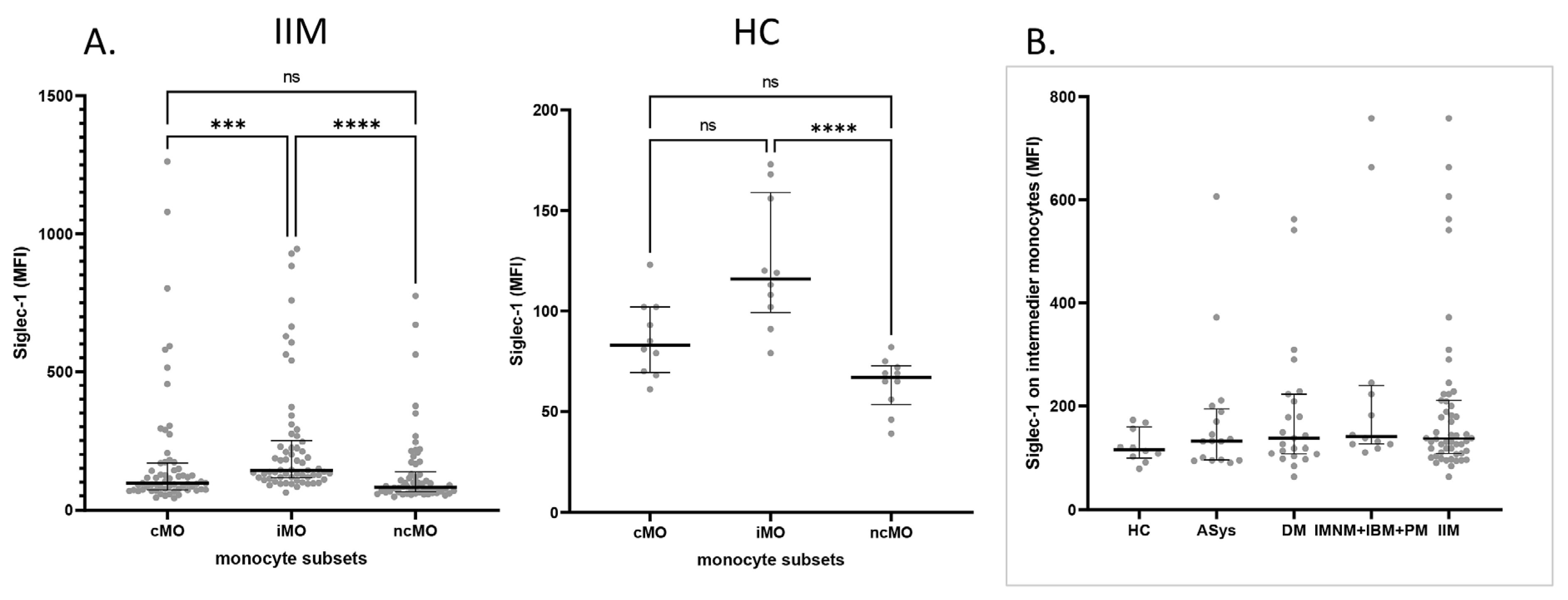
2.2. Siglec-1 Expression Across Monocyte Subsets in IIM Patients and Healthy Controls
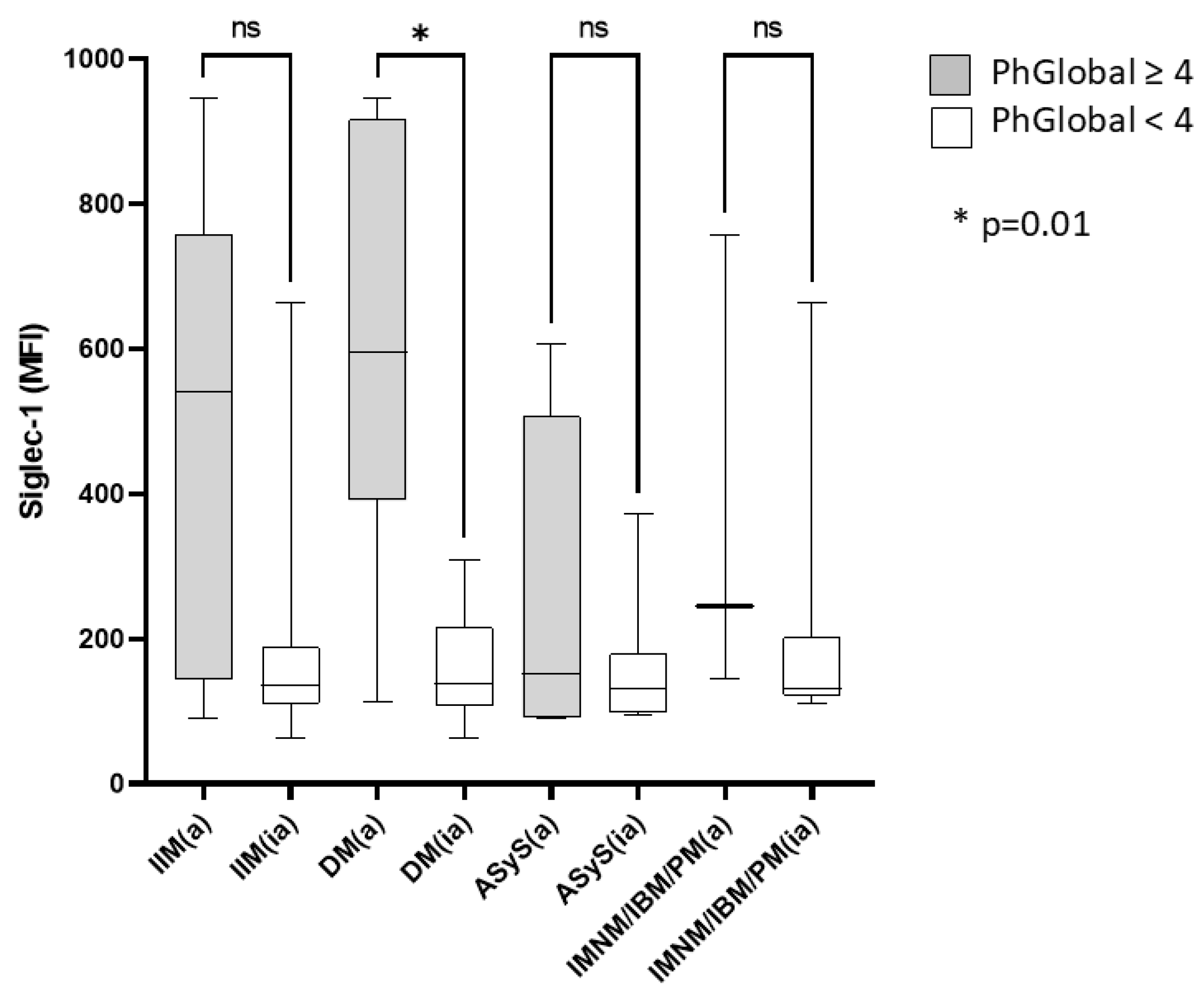
2.3. Increased Siglec-1 Expression in Active Dermatomyositis
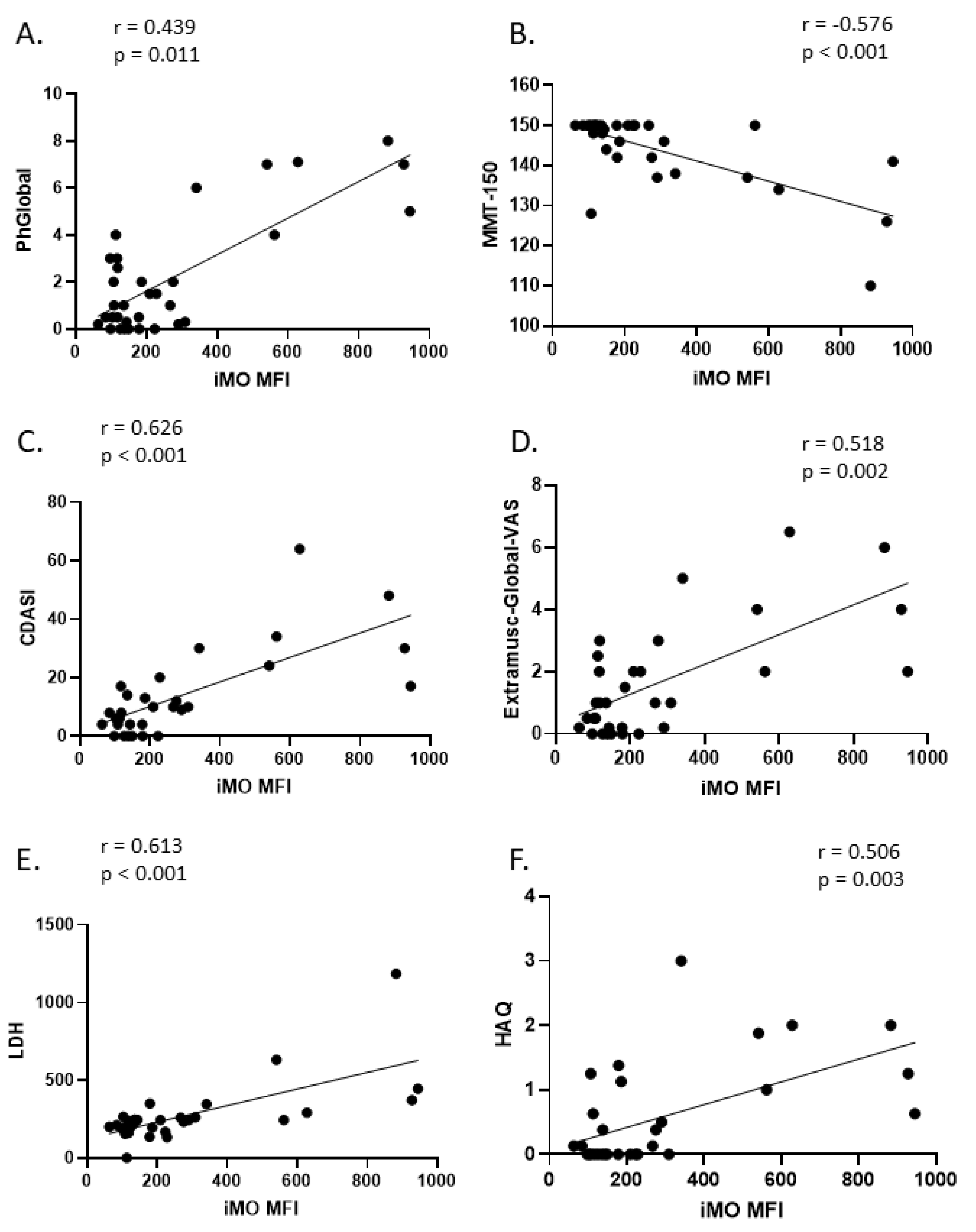
2.4. Correlation of Intermediate Monocyte Siglec-1 Expression with Disease Activity Parameters in DM
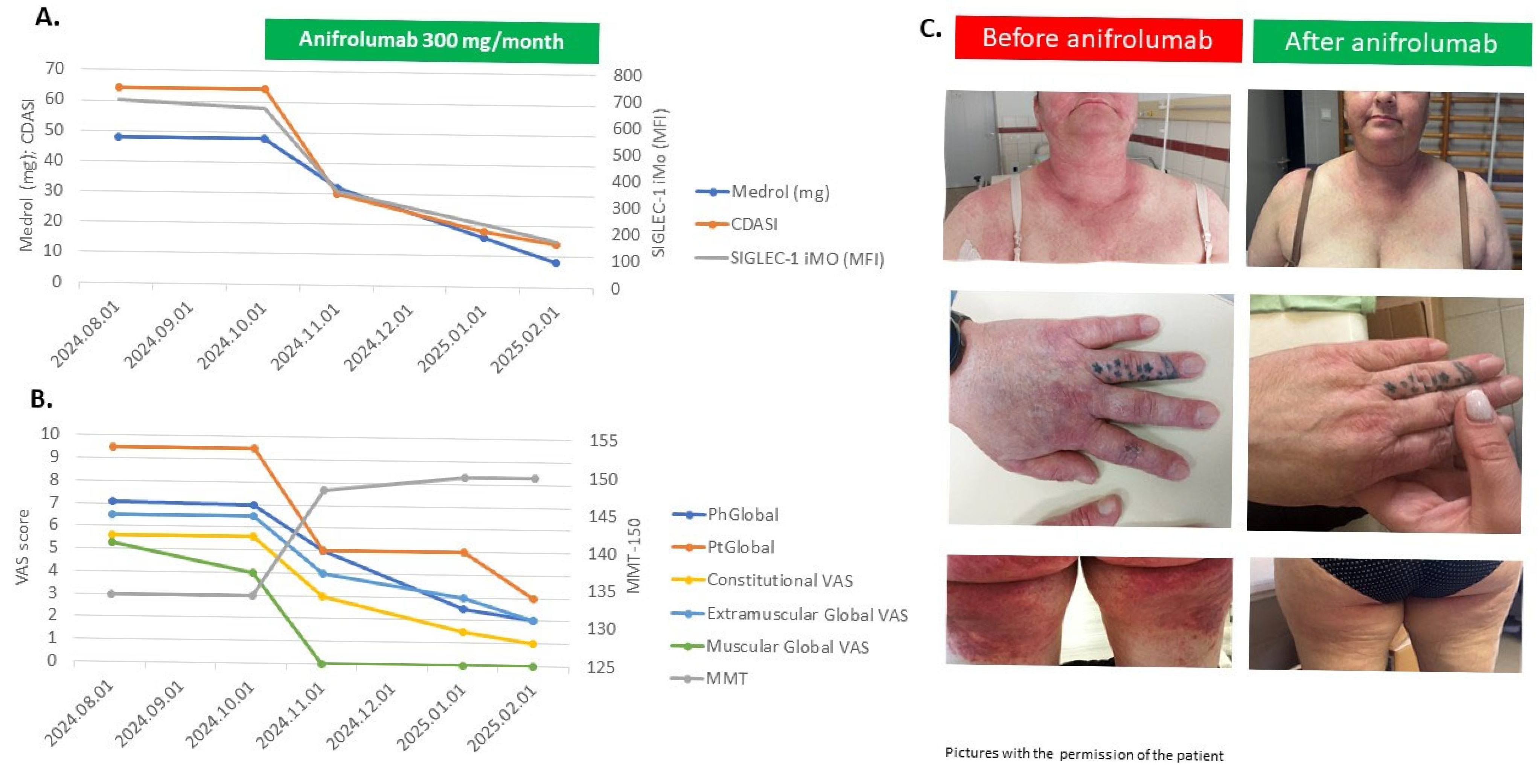
2.5. Siglec-1 Expressions with Disease Activity Parameters of Treatment-Refractory Patients Treated with Individualized Medications, Including Anifrolumab
3. Discussion
4. Materials and Methods
4.1. Evaluation of Myositis Disease Status
4.2. Flow Cytometry Analysis of Monocyte Subsets
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IIM | Idiopathic inflammatory myopathies |
| DM | Dermatomyositis |
| HC | Healthy Controls |
| IMACS | International Myositis Assessment and Clinical Studies |
| MFI | Mean fluorescence Intensity |
| CDASI | Cutaneous Dermatomyositis Disease Area and Severity Index |
| VAS | Visual Analog Scale |
| GIT | Gastrointestinal Tract |
| PM | Polymyositis |
| IBM | Inclusion Body Myositis |
| ASyS | Antisynthetase Syndrome |
| IMNM | Immune Mediated Necrotizing Myopathy |
| SLE | Systemic Lupus Erythematosus |
| IFN | Interferon |
| JDM | Juvenile Dermatomyositis |
| PhGA | Physician Global Activity |
| MMT | Manual Muscle Testing |
| CD | Cluster of Differentiaition |
| cMO | Classical monocytes |
| iMO | Intermediate monocytes |
| ncMO | Non-classical monocytes |
| IQR | Interquartile range |
| anti-Jo-1 | Anti-histydil-transfer ribonucleic acid synthetase antibody |
| anti-TIF1γ | Anti-transcriptional intermediary factor 1-gamma |
| anti-NXP2: | Anti-nuclear matrix protein 2 |
| anti-Mi2: | Anti-Mi2 antibody |
| anti-SAE | Anti-small ubiquitin-like modifier-1 (SUMO-1) activating enzyme |
| anti-MDA5 | Anti-melanoma differentiation-associated gene 5 |
| anti-SRP | Anti-Signal recognition particle |
| anti-PL7 | Anti-threonyl transfer ribonucleic acid synthetase antibody |
| anti-HMGCR | Anti-3-hydroxy-3-methyl-glutaryl-coenzyme A reductase |
| anti-Ku | Anti-Ku protein antibody |
| anti-SM-RNP | Anti-Smith- Ribonuclear Protein antibody |
| anti-PM-SCL | Anti-polymyositis/scl antibody |
| anti-Ro-52 | Anti-Ro52 protein antibody |
| PtGA | Patient Global Disease Activity |
| CK | Creatine kinase |
| CRP | C reactive protein |
| LDH | Lactate dehydrogenase |
| HAQ | Health Assessment Questionnaire |
| IVIG | Intravenous Immunoglobulin |
| TIS | Total Improvement Score |
| NA | Not Applicable |
| IFNAR1 | Type-I interferon receptor |
| BSL | Baseline |
| Mo | Month |
| GC | Glucocorticoid |
| MTX | Methotrexate |
| AZA | Azathioprin |
| MMF | Mycofenolate mofetil |
| RTX | Rituximab |
| IL | Interleukin |
| ISG | IFN-stimulated genes |
| HIV | Human immunodeficiency virus |
| CCL | Chemokine (C-C motif) ligand |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| CCR | Chemokine receptor |
| CVD | Cardiovascular disease |
| EULAR | European Alliance of Associations for Rheumatology |
| ACR | American College of Rheumatology |
| MDAAT | Myositis Disease Activity Assessment Tool |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Lundberg, I.E.; Fujimoto, M.; Vencovsky, J.; Aggarwal, R.; Holmqvist, M.; Christopher-Stine, L.; Mammen, A.L.; Miller, F.W. Idiopathic Inflammatory Myopathies. Nat. Rev. Dis. Primers 2021, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Selva-O’Callaghan, A.; Pinal-Fernandez, I.; Trallero-Araguás, E.; Milisenda, J.C.; Grau-Junyent, J.M.; Mammen, A.L. Classification and Management of Adult Inflammatory Myopathies. Lancet Neurol. 2018, 17, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Allenbach, Y.; Benveniste, O.; Stenzel, W.; Boyer, O. Immune-Mediated Necrotizing Myopathy: Clinical Features and Pathogenesis. Nat. Rev. Rheumatol. 2020, 16, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.A.; Pinkus, J.L.; Pinkus, G.S.; Burleson, T.; Sanoudou, D.; Tawil, R.; Barohn, R.J.; Saperstein, D.S.; Briemberg, H.R.; Ericsson, M.; et al. Interferon-Alpha/Beta-Mediated Innate Immune Mechanisms in Dermatomyositis. Ann. Neurol. 2005, 57, 664–678. [Google Scholar] [CrossRef]
- Amici, D.R.; Pinal-Fernandez, I.; Christopher-Stine, L.; Mammen, A.L.; Mendillo, M.L. A Network of Core and Subtype-Specific Gene Expression Programs in Myositis. Acta Neuropathol. 2021, 142, 887–898. [Google Scholar] [CrossRef]
- Gallay, L.; Mouchiroud, G.; Chazaud, B. Interferon-Signature in Idiopathic Inflammatory Myopathies. Curr. Opin. Rheumatol. 2019, 31, 634–642. [Google Scholar] [CrossRef]
- Huard, C.; Gullà, S.V.; Bennett, D.V.; Coyle, A.J.; Vleugels, R.A.; Greenberg, S.A. Correlation of Cutaneous Disease Activity with Type 1 Interferon Gene Signature and Interferon β in Dermatomyositis. Br. J. Dermatol. 2017, 176, 1224–1230. [Google Scholar] [CrossRef]
- Bilgic, H.; Ytterberg, S.R.; Amin, S.; McNallan, K.T.; Wilson, J.C.; Koeuth, T.; Ellingson, S.; Newman, B.; Bauer, J.W.; Peterson, E.J.; et al. Interleukin-6 and Type I Interferon-Regulated Genes and Chemokines Mark Disease Activity in Dermatomyositis. Arthritis Rheum. 2009, 60, 3436–3446. [Google Scholar] [CrossRef]
- Greenberg, S.A.; Higgs, B.W.; Morehouse, C.; Walsh, R.J.; Kong, S.W.; Brohawn, P.; Zhu, W.; Amato, A.; Salajegheh, M.; White, B.; et al. Relationship between Disease Activity and Type 1 Interferon- and Other Cytokine-Inducible Gene Expression in Blood in Dermatomyositis and Polymyositis. Genes Immun. 2012, 13, 207–213. [Google Scholar] [CrossRef]
- Baechler, E.C.; Bauer, J.W.; Slattery, C.A.; Ortmann, W.A.; Espe, K.J.; Novitzke, J.; Ytterberg, S.R.; Gregersen, P.K.; Behrens, T.W.; Reed, A.M. An Interferon Signature in the Peripheral Blood of Dermatomyositis Patients Is Associated with Disease Activity. Mol. Med. 2007, 13, 59–68. [Google Scholar] [CrossRef]
- O’Connor, K.A.; Abbott, K.A.; Sabin, B.; Kuroda, M.; Pachman, L.M. MxA Gene Expression in Juvenile Dermatomyositis Peripheral Blood Mononuclear Cells: Association with Muscle Involvement. Clin. Immunol. 2006, 120, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.J.; Kong, S.W.; Yao, Y.; Jallal, B.; Kiener, P.A.; Pinkus, J.L.; Beggs, A.H.; Amato, A.A.; Greenberg, S.A. Type I Interferon-Inducible Gene Expression in Blood Is Present and Reflects Disease Activity in Dermatomyositis and Polymyositis. Arthritis Rheum. 2007, 56, 3784–3792. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.M.; Peterson, E.; Bilgic, H.; Ytterberg, S.R.; Amin, S.; Hein, M.S.; Crowson, C.S.; Ernste, F.; Gillespie, E.B. Changes in Novel Biomarkers of Disease Activity in Juvenile and Adult Dermatomyositis Are Sensitive Biomarkers of Disease Course. Arthritis Rheum. 2012, 64, 4078–4086. [Google Scholar] [CrossRef]
- Greenberg, S.A. Dermatomyositis and Type 1 Interferons. Curr. Rheumatol. Rep. 2010, 12, 198–203. [Google Scholar] [CrossRef]
- Paik, J.J.; Casciola-Rosen, L.; Shin, J.Y.; Albayda, J.; Tiniakou, E.; Leung, D.G.; Gutierrez-Alamillo, L.; Perin, J.; Florea, L.; Antonescu, C.; et al. Study of Tofacitinib in Refractory Dermatomyositis: An Open-Label Pilot Study of Ten Patients. Arthritis Rheumatol. 2021, 73, 858–865. [Google Scholar] [CrossRef]
- Fiorentino, D.; Mangold, A.R.; Werth, V.P.; Christopher-Stine, L.; Femia, A.; Chu, M.; Musiek, A.C.M.; Sluzevich, J.C.; Graham, L.V.; Fernandez, A.P.; et al. Efficacy, Safety, and Target Engagement of Dazukibart, an IFNβ Specific Monoclonal Antibody, in Adults with Dermatomyositis: A Multicentre, Double-Blind, Randomised, Placebo-Controlled, Phase 2 Trial. Lancet 2025, 405, 137–146. [Google Scholar] [CrossRef]
- Lerkvaleekul, B.; Veldkamp, S.R.; van der Wal, M.M.; Schatorjé, E.J.H.; Kamphuis, S.S.M.; van den Berg, J.M.; Hissink Muller, P.C.E.; Armbrust, W.; Vastert, S.J.; Wienke, J.; et al. Siglec-1 Expression on Monocytes Is Associated with the Interferon Signature in Juvenile Dermatomyositis and Can Predict Treatment Response. Rheumatology 2022, 61, 2144–2155. [Google Scholar] [CrossRef]
- Kamperman, R.G.; Veldkamp, S.R.; Evers, S.W.; Lim, J.; van Schaik, I.; van Royen-Kerkhof, A.; van Wijk, F.; van der Kooi, A.J.; Jansen, M.; Raaphorst, J. Type I Interferon Biomarker in Idiopathic Inflammatory Myopathies: Associations of Siglec-1 with Disease Activity and Treatment Response. Rheumatology 2024, 64, 2979–2986. [Google Scholar] [CrossRef]
- Graf, M.; von Stuckrad, S.L.; Uruha, A.; Klotsche, J.; Zorn-Pauly, L.; Unterwalder, N.; Buttgereit, T.; Krusche, M.; Meisel, C.; Burmester, G.R.; et al. SIGLEC1 Enables Straightforward Assessment of Type I Interferon Activity in Idiopathic Inflammatory Myopathies. RMD Open 2022, 8, e001934. [Google Scholar] [CrossRef]
- Herzog, S.; Fragkou, P.C.; Arneth, B.M.; Mkhlof, S.; Skevaki, C. Myeloid CD169/Siglec1: An Immunoregulatory Biomarker in Viral Disease. Front. Med. 2022, 9, 979373. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Bonaguro, L.; Gemünd, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef] [PubMed]
- Rider, L.G.; Aggarwal, R.; Machado, P.M.; Hogrel, J.-Y.; Reed, A.M.; Christopher-Stine, L.; Ruperto, N. Update on Outcome Assessment in Myositis. Nat. Rev. Rheumatol. 2018, 14, 303–318. [Google Scholar] [CrossRef]
- Castellini, C.; Scotti, C.; Navarini, L.; Fu, Q.; Qian, J.; Giacomelli, R.; Cavagna, L.; Ruscitti, P. The Evaluation of Type I Interferon Score in Dermatomyositis, a Systematic Review and a Meta-Analysis. Autoimmun. Rev. 2024, 23, 103686. [Google Scholar] [CrossRef]
- Fasano, S.; Milone, A.; Nicoletti, G.F.; Isenberg, D.A.; Ciccia, F. Precision Medicine in Systemic Lupus Erythematosus. Nat. Rev. Rheumatol. 2023, 19, 331–342. [Google Scholar] [CrossRef]
- Paik, J.J.; Vencovský, J.; Charles-Schoeman, C.; Wright, G.C.; Vleugels, R.A.; Goriounova, A.S.; Mudd, P.N.; Aggarwal, R. Brepocitinib, a Potent and Selective TYK2/JAK1 Inhibitor: Scientific and Clinical Rationale for Dermatomyositis. Clin. Exp. Rheumatol. 2025, 43, 354–363. [Google Scholar] [CrossRef]
- Shayegan, L.H.; Shaw, K.S.; Wieschhoff, G.G.; Ezeh, N.; Kim, Y.J.; Shahriari, N.; Anshelevich, E.E.; Acevedo, L.A.; LaChance, A.; Dedeoglu, F.; et al. Improvement in Dermatomyositis-Associated Muscle Disease with Anifrolumab. Br. J. Dermatol. 2025, 192, 1126–1128. [Google Scholar] [CrossRef]
- Helm, M.F.; Maglakelidze, N.; Shamloul, N.; Foulke, G. Successful Treatment of Refractory Cutaneous Dermatomyositis With Anifrolumab. Int. J. Dermatol. 2025. [Google Scholar] [CrossRef]
- Chaudhary, S.; Baumer, M.; Upton, L.; Ong, S.; Smith, K.; West, D.; Kilian, A. Recalcitrant Dermatomyositis Treated With Anifrolumab. ACR Open Rheumatol. 2025, 7, e11792. [Google Scholar] [CrossRef]
- Claudio-Oliva, A.; Viedma-Martínez, M.; Gallo-Pineda, G.; Prieto-Rozados, M.; Villegas-Romero, I.; Jiménez-Gallo, D.; Linares-Barrios, M. Anifrolumab Use in Refractory Dermatomyositis: A Case Report and Literature Review. Clin. Exp. Dermatol. 2025, 50, 874–876. [Google Scholar] [CrossRef]
- Wong, K.L.; Tai, J.J.-Y.; Wong, W.-C.; Han, H.; Sem, X.; Yeap, W.-H.; Kourilsky, P.; Wong, S.-C. Gene Expression Profiling Reveals the Defining Features of the Classical, Intermediate, and Nonclassical Human Monocyte Subsets. Blood 2011, 118, e16–e31. [Google Scholar] [CrossRef]
- Lee, J.; Tam, H.; Adler, L.; Ilstad-Minnihan, A.; Macaubas, C.; Mellins, E.D. The MHC Class II Antigen Presentation Pathway in Human Monocytes Differs by Subset and Is Regulated by Cytokines. PLoS ONE 2017, 12, e0183594. [Google Scholar] [CrossRef] [PubMed]
- Cros, J.; Cagnard, N.; Woollard, K.; Patey, N.; Zhang, S.-Y.; Senechal, B.; Puel, A.; Biswas, S.K.; Moshous, D.; Picard, C.; et al. Human CD14dim Monocytes Patrol and Sense Nucleic Acids and Viruses via TLR7 and TLR8 Receptors. Immunity 2010, 33, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Belge, K.-U.; Dayyani, F.; Horelt, A.; Siedlar, M.; Frankenberger, M.; Frankenberger, B.; Espevik, T.; Ziegler-Heitbrock, L. The Proinflammatory CD14+CD16+DR++ Monocytes Are a Major Source of TNF. J. Immunol. 2002, 168, 3536–3542. [Google Scholar] [CrossRef]
- Szaflarska, A.; Baj-Krzyworzeka, M.; Siedlar, M.; Węglarczyk, K.; Ruggiero, I.; Hajto, B.; Zembala, M. Antitumor Response of CD14+/CD16+ Monocyte Subpopulation. Exp. Hematol. 2004, 32, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Zawada, A.M.; Rogacev, K.S.; Rotter, B.; Winter, P.; Marell, R.-R.; Fliser, D.; Heine, G.H. SuperSAGE Evidence for CD14++CD16+ Monocytes as a Third Monocyte Subset. Blood 2011, 118, e50–e61. [Google Scholar] [CrossRef]
- Weber, C.; Belge, K.-U.; von Hundelshausen, P.; Draude, G.; Steppich, B.; Mack, M.; Frankenberger, M.; Weber, K.S.C.; Ziegler-Heitbrock, H.W.L. Differential Chemokine Receptor Expression and Function in Human Monocyte Subpopulations. J. Leukoc. Biol. 2000, 67, 699–704. [Google Scholar] [CrossRef]
- Ellery, P.J.; Tippett, E.; Chiu, Y.-L.; Paukovics, G.; Cameron, P.U.; Solomon, A.; Lewin, S.R.; Gorry, P.R.; Jaworowski, A.; Greene, W.C.; et al. The CD16+ Monocyte Subset Is More Permissive to Infection and Preferentially Harbors HIV-1 In Vivo. J. Immunol. 2007, 178, 6581–6589. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, H.W.L.; Fingerle, G.; Ströbel, M.; Schraut, W.; Stelter, F.; Schütt, C.; Passlick, B.; Pforte, A. The Novel Subset of CD14 +/CD16 + Blood Monocytes Exhibits Features of Tissue Macrophages. Eur. J. Immunol. 1993, 23, 2053–2058. [Google Scholar] [CrossRef]
- Nockher, W.A.; Scherberich, J.E. Expanded CD14+ CD16+ Monocyte Subpopulation in Patients with Acute and Chronic Infections Undergoing Hemodialysis. Infect. Immun. 1998, 66, 2782–2790. [Google Scholar] [CrossRef]
- Radzyukevich, Y.V.; Kosyakova, N.I.; Prokhorenko, I.R. Participation of Monocyte Subpopulations in Progression of Experimental Endotoxemia (EE) and Systemic Inflammation. J. Immunol. Res. 2021, 2021, 1762584. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Yu, C.; Yang, X.-F.; Wang, H. Monocyte and Macrophage Differentiation: Circulation Inflammatory Monocyte as Biomarker for Inflammatory Diseases. Biomark. Res. 2014, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, I.E.; Tjärnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; de Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and Their Major Subgroups. Arthritis Rheumatol. 2017, 69, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.W. Proposed Preliminary Core Set Measures for Disease Outcome Assessment in Adult and Juvenile Idiopathic Inflammatory Myopathies. Rheumatology 2001, 40, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Rider, L.G.; Werth, V.P.; Huber, A.M.; Alexanderson, H.; Rao, A.P.; Ruperto, N.; Herbelin, L.; Barohn, R.; Isenberg, D.; Miller, F.W. Measures of Adult and Juvenile Dermatomyositis, Polymyositis, and Inclusion Body Myositis: Physician and Patient/Parent Global Activity, Manual Muscle Testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ), Childhood Myositis Assessment Scale (CMAS), Myositis Disease Activity Assessment Tool (MDAAT), Disease Activity Score (DAS), Short Form 36 (SF-36), Child Health Questionnaire (CHQ), physician global damage, Myositis Damage Index (MDI), Quantitative Muscle Testing (QMT), Myositis Functional Index-2 (FI-2), Myositis Activities Profile (MAP), Inclusion Body Myositis Functional Rating Scale (IBMFRS), Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), Cutaneous Assessment Tool (CAT), Dermatomyositis Skin Severity Index (DSSI), Skindex, and Dermatology Life Quality Index (DLQI). Arthritis Care Res. 2011, 63 (Suppl. S11), S118–S157. [Google Scholar] [CrossRef]
- Klein, R.Q.; Bangert, C.A.; Costner, M.; Connolly, M.K.; Tanikawa, A.; Okawa, J.; Rose, M.; Fakharzadeh, S.S.; Fiorentino, D.; Lee, L.A.; et al. Comparison of the Reliability and Validity of Outcome Instruments for Cutaneous Dermatomyositis. Br. J. Dermatol. 2008, 159, 887–894. [Google Scholar] [CrossRef]
- Aggarwal, R.; Rider, L.G.; Ruperto, N.; Bayat, N.; Erman, B.; Feldman, B.M.; Oddis, C.V.; Amato, A.A.; Chinoy, H.; Cooper, R.G.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Criteria for Minimal, Moderate, and Major Clinical Response in Adult Dermatomyositis and Polymyositis: An International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann. Rheum. Dis. 2017, 76, 792–801. [Google Scholar] [CrossRef]
- Selimoglu-Buet, D.; Wagner-Ballon, O.; Saada, V.; Bardet, V.; Itzykson, R.; Bencheikh, L.; Morabito, M.; Met, E.; Debord, C.; Benayoun, E.; et al. Characteristic Repartition of Monocyte Subsets as a Diagnostic Signature of Chronic Myelomonocytic Leukemia. Blood 2015, 125, 3618–3626. [Google Scholar] [CrossRef]
| Total IIM n = 62 | DM n = 33 | ASyS n = 17 | IMNM/PM/IBM n = 5/3/4 | HC n = 10 | |
|---|---|---|---|---|---|
| Female, n (%) | 34 (54.8) | 17 (51.5) | 11 (64.7) | 6 (50) | 8 (80) |
| Age (mean ± SD) | 55.27 ± 14.5 | 52.97 ± 15.4 | 55.35 ± 10.1 | 61.5 ± 16.13 | 45.9 ± 16.6 |
| MMT-150, median (IQR) | 147 (141–150) | 150 (141.5–150) | 146 (142–149.5) | 142.5 (116–148) | NA |
| PhGA, median (IQR) | 2 (0.5–3.57) | 1 (0.25–3.5) | 2 (1.75–3.58) | 3 (1–4.5) | NA |
| PtGA, median (IQR) | 2 (1–5) | 1 (0.5–3) | 3 (1.5–5) | 3 (0.625–5.75) | NA |
| Extramuscular Global VAS, median (IQR) | 1 (0.18–3) | 1 (0.2–2.75) | 3 (0.5–4) | 0 (0–2) | NA |
| CK (U/L) median (IQR) | 116 (66–223) | 96.5 (57.3–178) | 110 (65–273) | 212 (113–723) | NA |
| CRP (mg/L) median (IQR) | 3.1 (1.3–7.9) | 2.62 (1.26–7.7) | 4.4 (1.4–20.9) | 2.73 (1.07–5.19) | NA |
| LDH U/L median (IQR) | 244 (204–278) | 236.5 (196–264 | 252 (230–318) | 243 (226–317) | NA |
| HAQ median (IQR) | 0.19 (0–1.25) | 0.13 (0–1.07) | 0.25 (0–0.63) | 1.13 (0–2.32) | NA |
| CDASI, median (IQR) | 4 (0–13.3) | 10 (4–17.5) | 0 (0–10.5) | 0 (0–0) | NA |
| No antibodies, n (%) | 11 (18) | 5 (15) | 0 | 6 (50) | NA |
| anti-TIF1γ | 10 | 10 (30) | 0 | 0 | NA |
| anti-Mi2 | 4 | 4 (12) | 0 | 0 | NA |
| anti-SAE | 4 | 4 (12) | 0 | 0 | NA |
| anti-NXP2 | 2 | 2 (6) | 0 | 0 | NA |
| anti-MDA5 | 3 | 3 (9) | 0 | 0 | NA |
| anti-Jo1 | 17 | 1 (3) | 16 (94) | 0 | NA |
| anti-PL7 | 1 | 0 | 1 (6) | 0 | NA |
| anti-SRP | 3 | 0 | 0 | 3 (25) | NA |
| anti-HMGCR | 1 | 0 | 0 | 1 (8) | NA |
| anti-Ku | 1 | 0 | 0 | 1 (8) | NA |
| anti-Pm-scl | 3 | 3 (9) | 0 | 0 | NA |
| anti-Ro52 | 7 | 6 (18) | 0 | 1 (8) | NA |
| anti-SM/RNP | 1 | 0 | 0 | 1 (8) | NA |
| Patient No | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Sex | Male | Female | Male | Male | Male |
| Age | 52 | 45 | 24 | 54 | 25 |
| Subtype | ASyS | DM | DM | ASyS | JDM |
| Antibody | Jo1, Ro52 | TIF1γ; Ku | Pm-Scl | Jo1 | None |
| Previous Therapy | GC, MMF, | GC, MTX, IVIG | GC, MTX | GC, AZA | GC, MTX |
| BSL PhGA | 4.8 | 7 | 7 | 4,0 | 7 |
| BSL CK (IU/L) | 2496 | 55 | 5241 | 1164 | 721 |
| BSL Siglec-1 (%)/MFI on iMO | 36/606 | 64/628 | 25/545 | 0/100 | 77/928 |
| BSL GC dose (mg) | 60 | 60 | 80 | 10 | 40 |
| Treatment | RTX, IVIG | Anifrolumab | IVIG | RTX | Anifrolumab |
| 3Mo PhGA | 2.5 | 2 | 1.5 | 2.0 | 2 |
| 3Mo CK (IU/L) | 144 | 98 | 82 | 242 | 519 |
| 3Mo Siglec-1 (%)/MFI on iMO | NA | 1.9/171 | NA | NA | 94/944 |
| 3Mo GC Dose (mg) | 15 | 10 | 15 | 5 | 15 |
| 3MoTIS | 55 | 72.5 | 70 | 35 | 75 |
| 3MoTIS Category | MODERATE | MAJOR | MAJOR | MINIMAL | MAJOR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baráth, S.; Nagy-Vincze, M.; Kun, Z.; Szinay, D.; Griger, Z., Jr.; Béldi, T.G.; Szabó, K.; Száraz-Széles, M.; Hevessy, Z.; Griger, Z. Disease Activity-Dependent Siglec-1 Expression on Monocyte Subsets of Patients with Idiopathic Inflammatory Myopathies. Int. J. Mol. Sci. 2025, 26, 4950. https://doi.org/10.3390/ijms26104950
Baráth S, Nagy-Vincze M, Kun Z, Szinay D, Griger Z Jr., Béldi TG, Szabó K, Száraz-Széles M, Hevessy Z, Griger Z. Disease Activity-Dependent Siglec-1 Expression on Monocyte Subsets of Patients with Idiopathic Inflammatory Myopathies. International Journal of Molecular Sciences. 2025; 26(10):4950. https://doi.org/10.3390/ijms26104950
Chicago/Turabian StyleBaráth, Sándor, Melinda Nagy-Vincze, Zsuzsanna Kun, Dorottya Szinay, Zoltán Griger, Jr., Tibor Gábor Béldi, Katalin Szabó, Marianna Száraz-Széles, Zsuzsanna Hevessy, and Zoltán Griger. 2025. "Disease Activity-Dependent Siglec-1 Expression on Monocyte Subsets of Patients with Idiopathic Inflammatory Myopathies" International Journal of Molecular Sciences 26, no. 10: 4950. https://doi.org/10.3390/ijms26104950
APA StyleBaráth, S., Nagy-Vincze, M., Kun, Z., Szinay, D., Griger, Z., Jr., Béldi, T. G., Szabó, K., Száraz-Széles, M., Hevessy, Z., & Griger, Z. (2025). Disease Activity-Dependent Siglec-1 Expression on Monocyte Subsets of Patients with Idiopathic Inflammatory Myopathies. International Journal of Molecular Sciences, 26(10), 4950. https://doi.org/10.3390/ijms26104950







