Activation of the NALP3-CASP1-IL-1 β Inflammatory Pathway by Pesticide Exposure in Human Umbilical Vein Endothelial Cells
Abstract
1. Introduction
2. Results
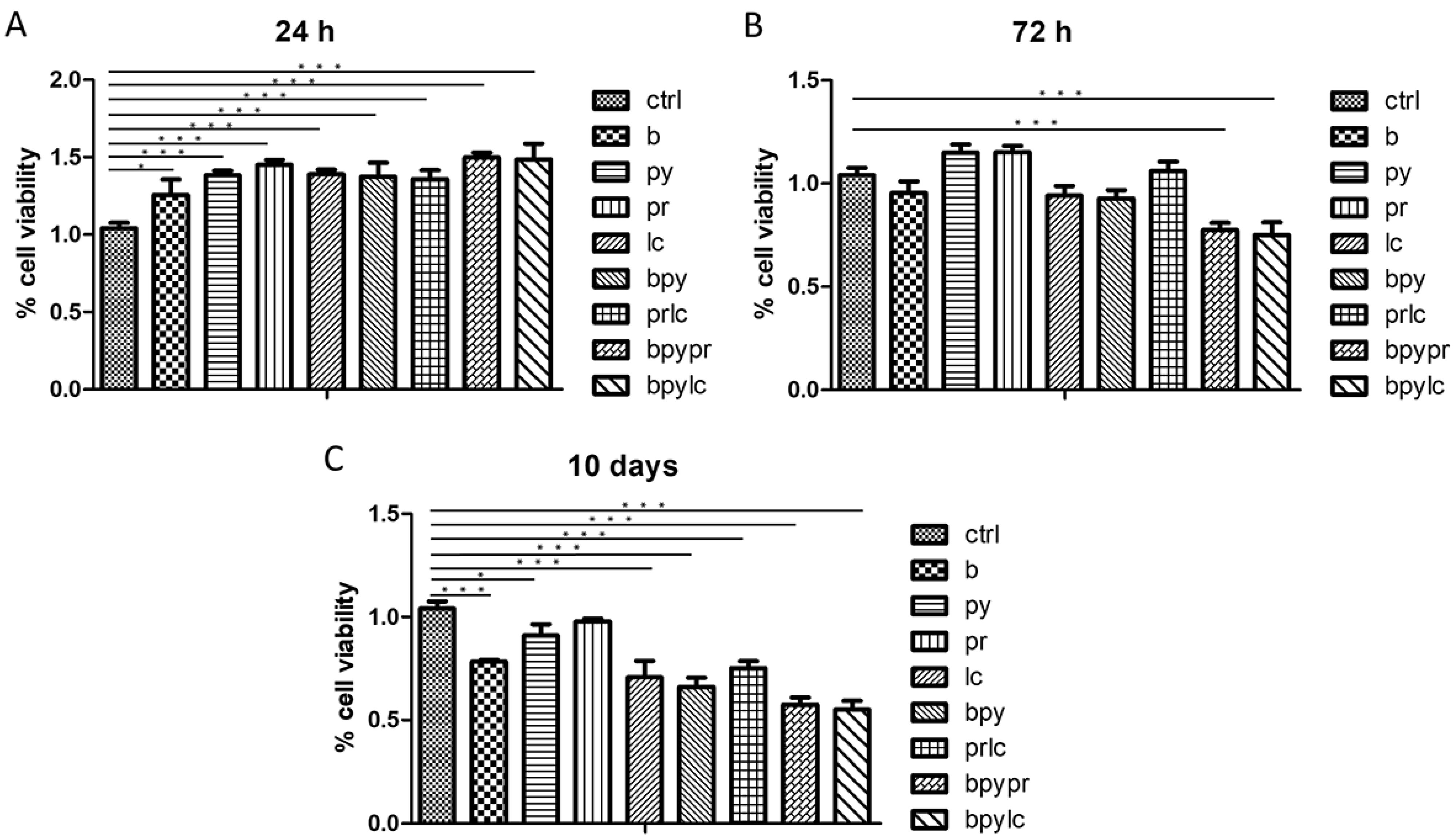
2.1. Cell Viability Evaluation (MTS)
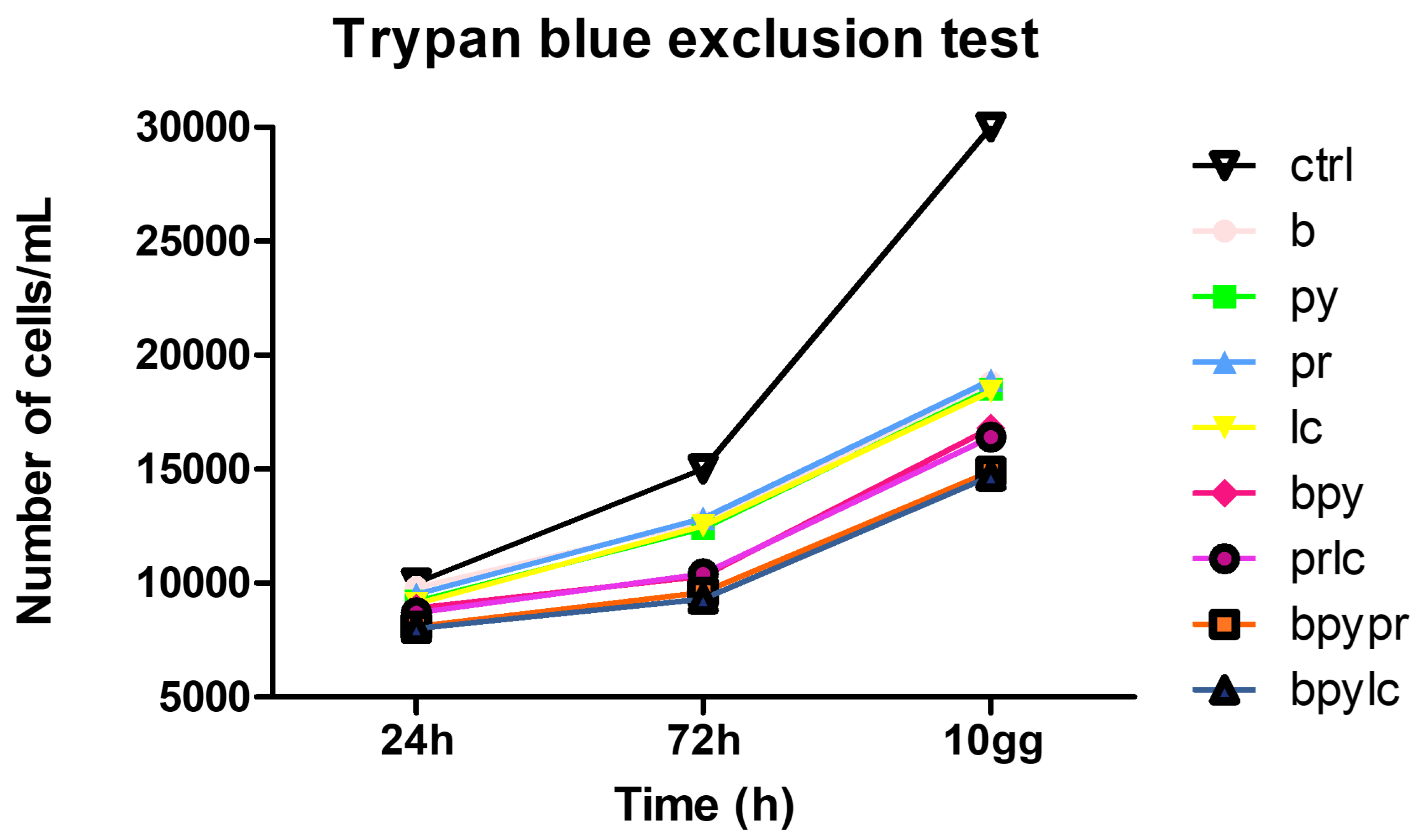
2.2. Trypan Blue Exclusion Test
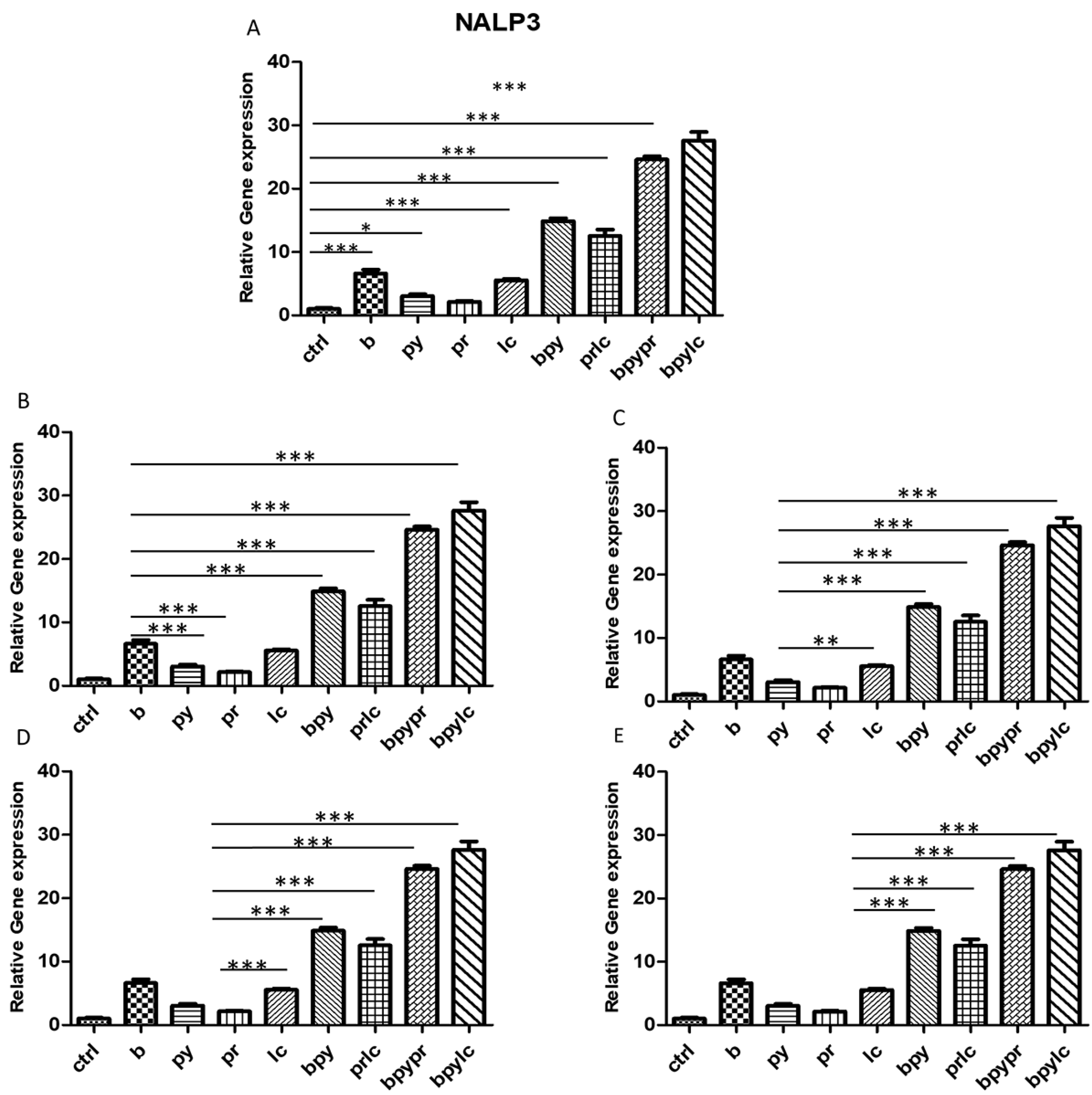
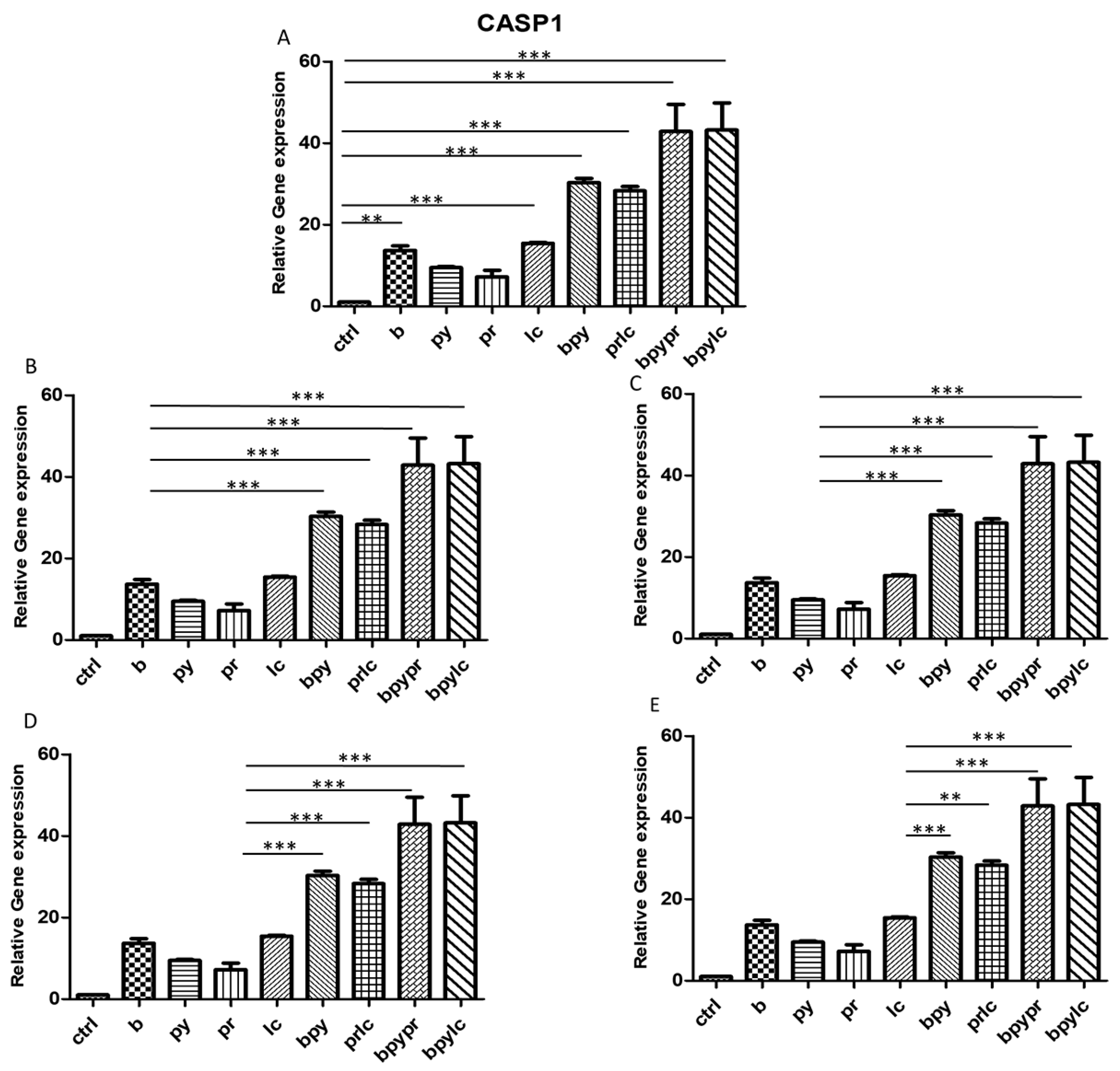
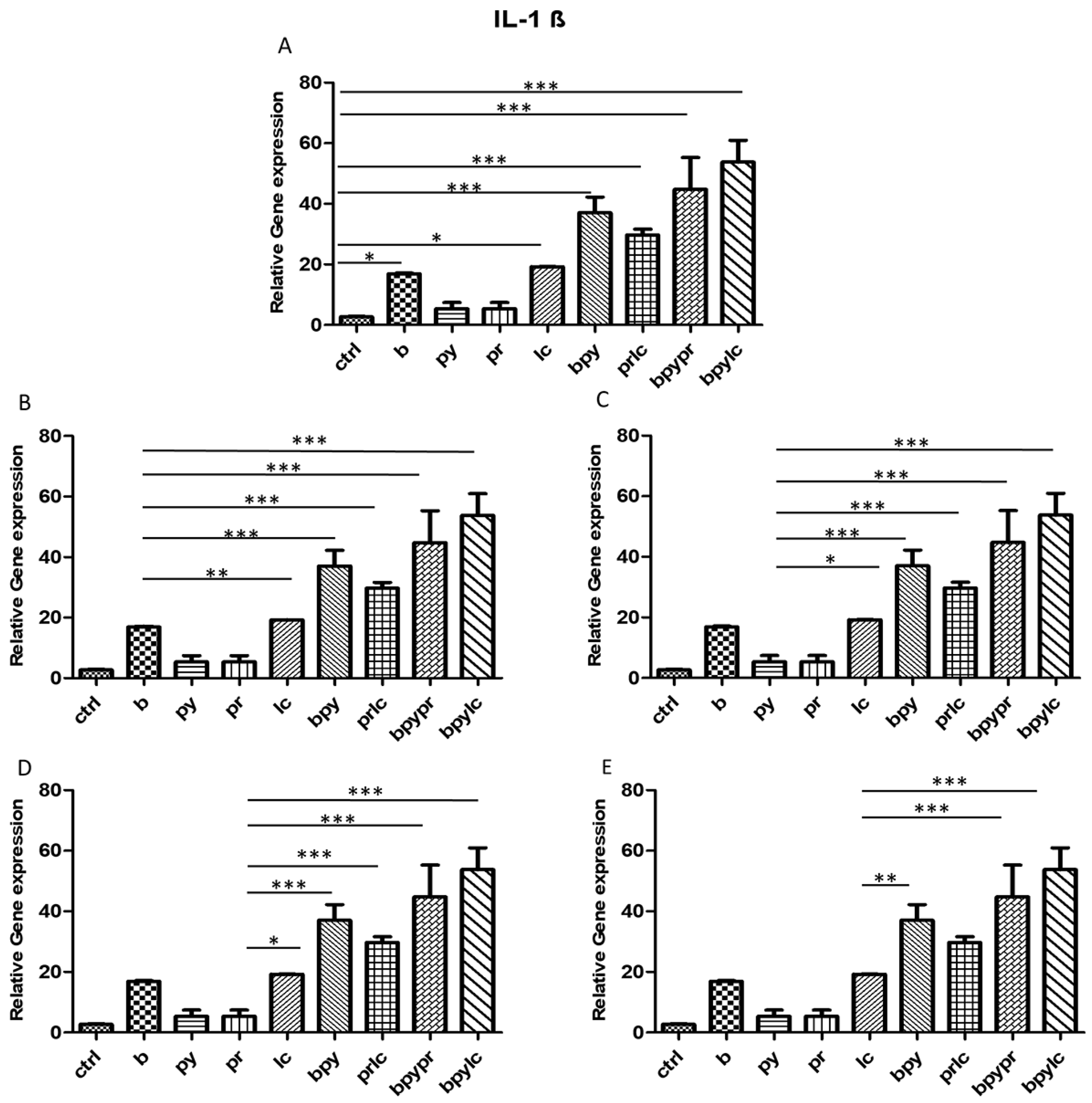
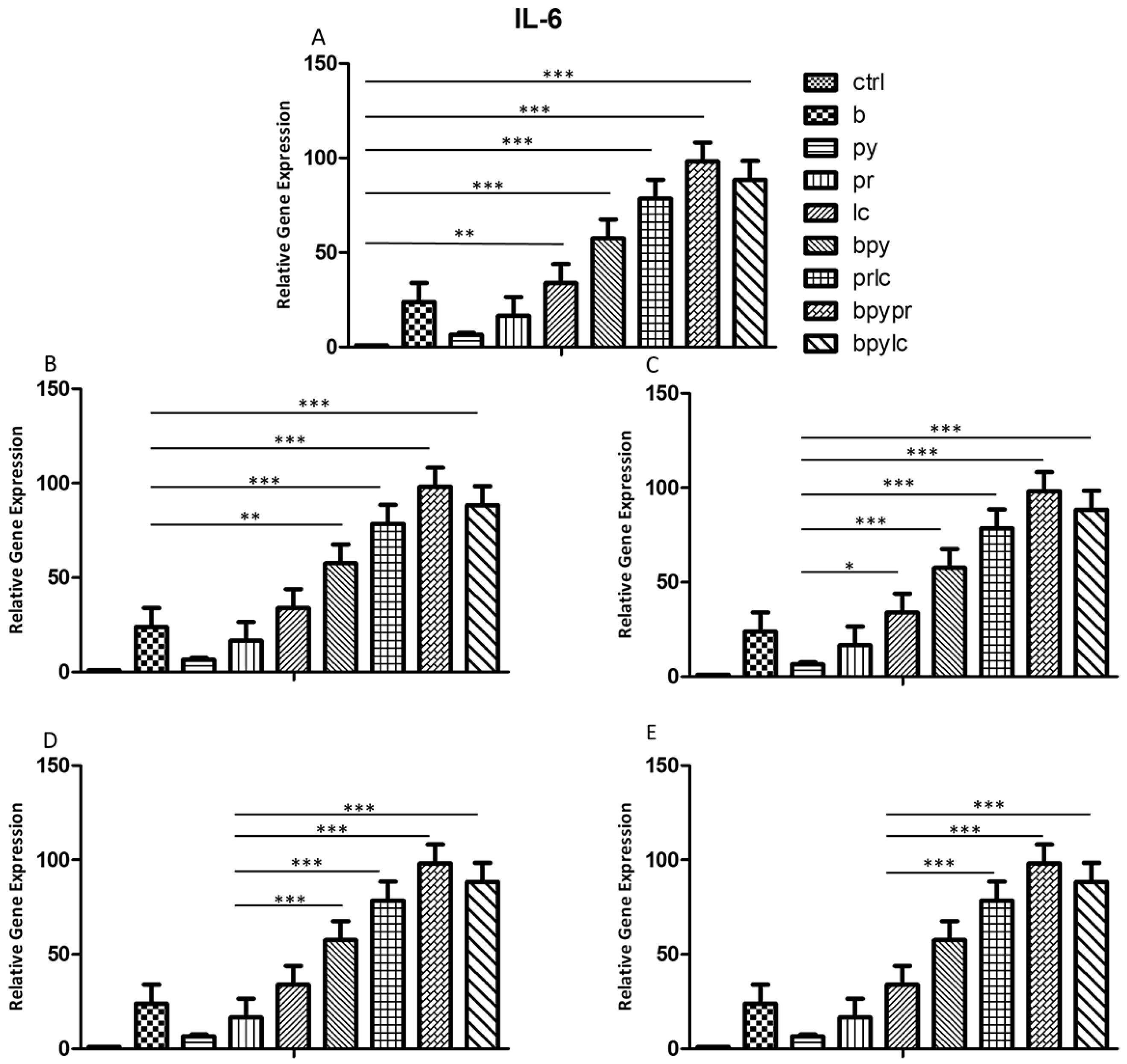
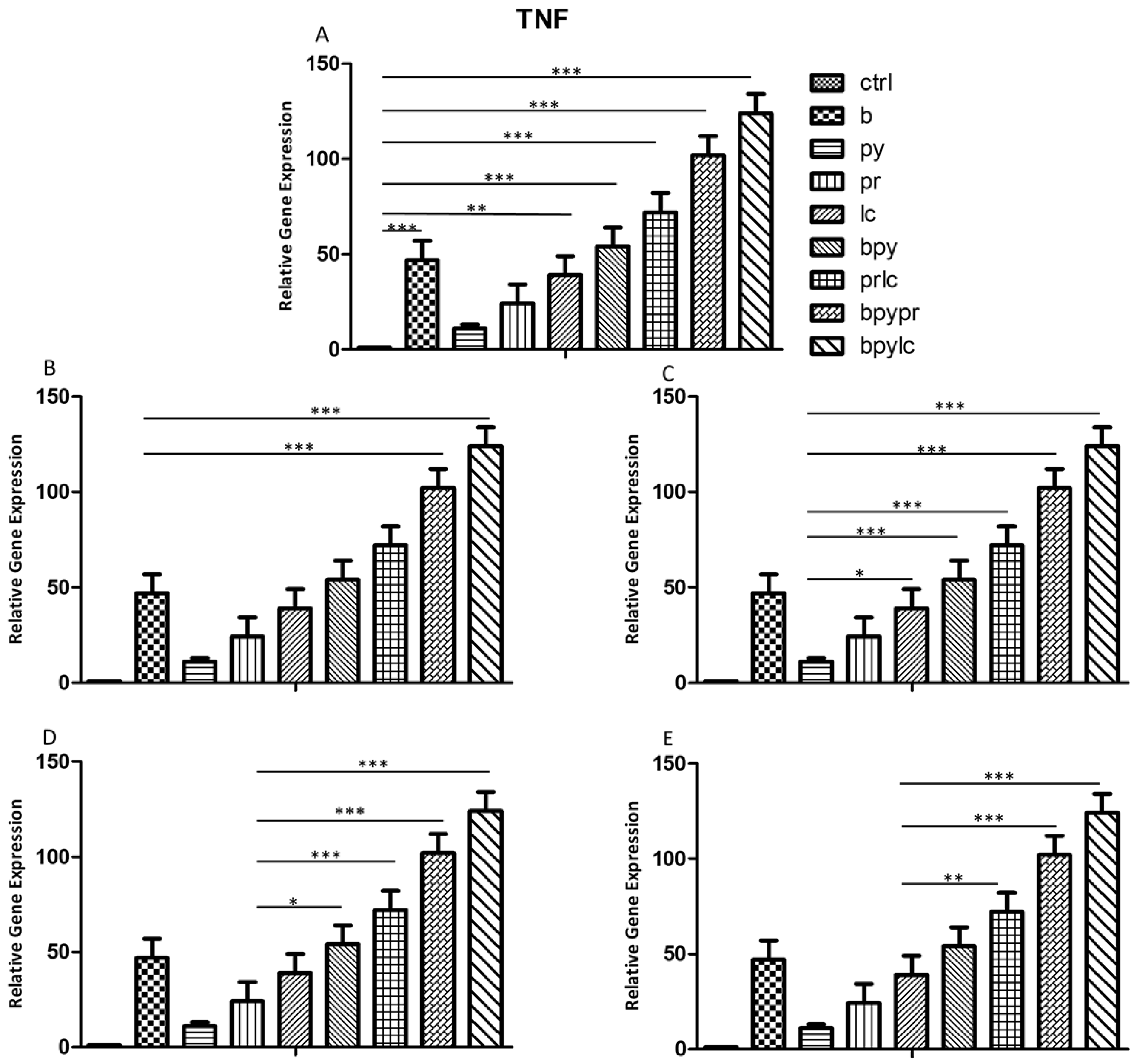
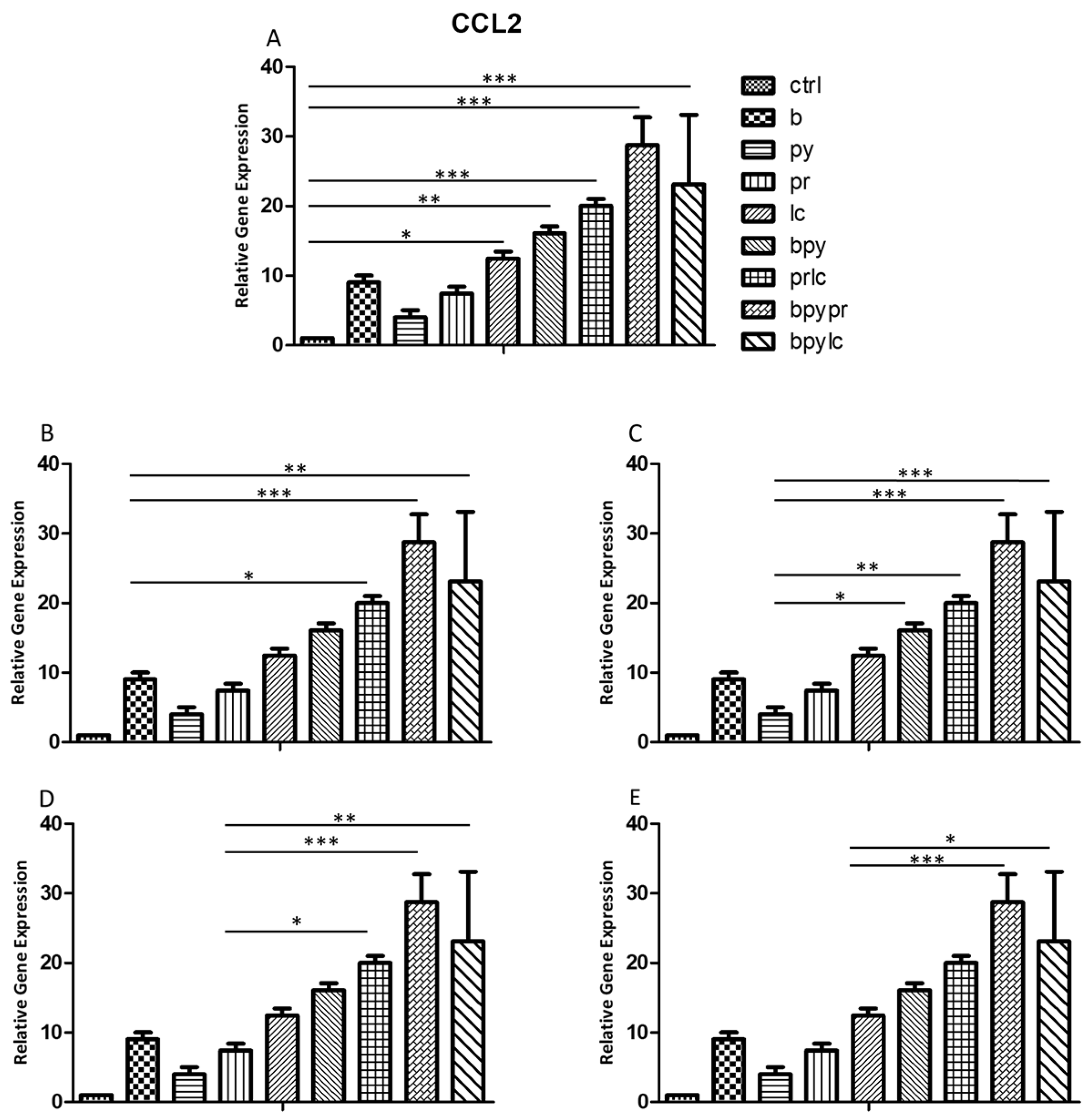
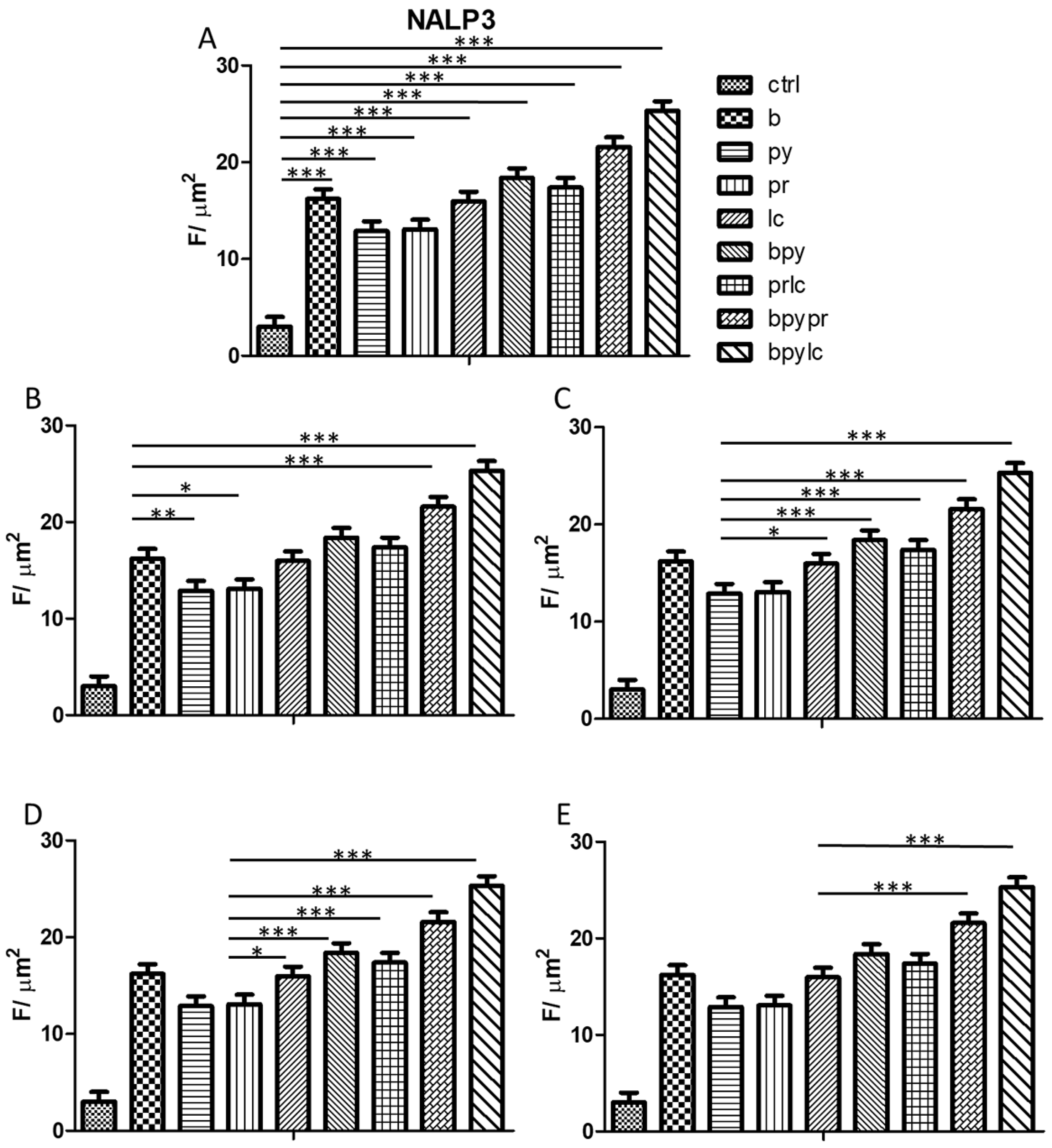
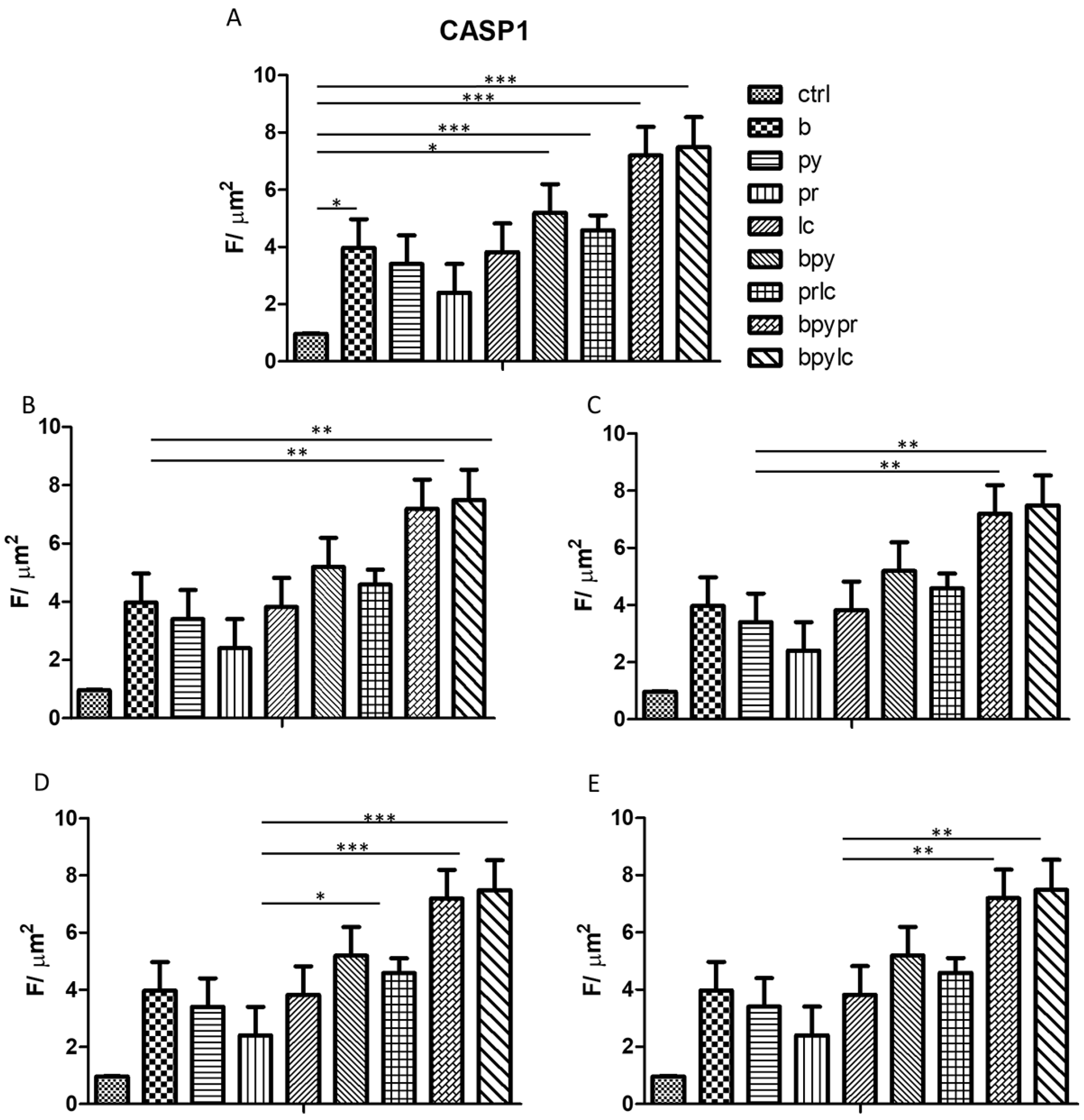
2.3. Genes Expression (RT-PCR)
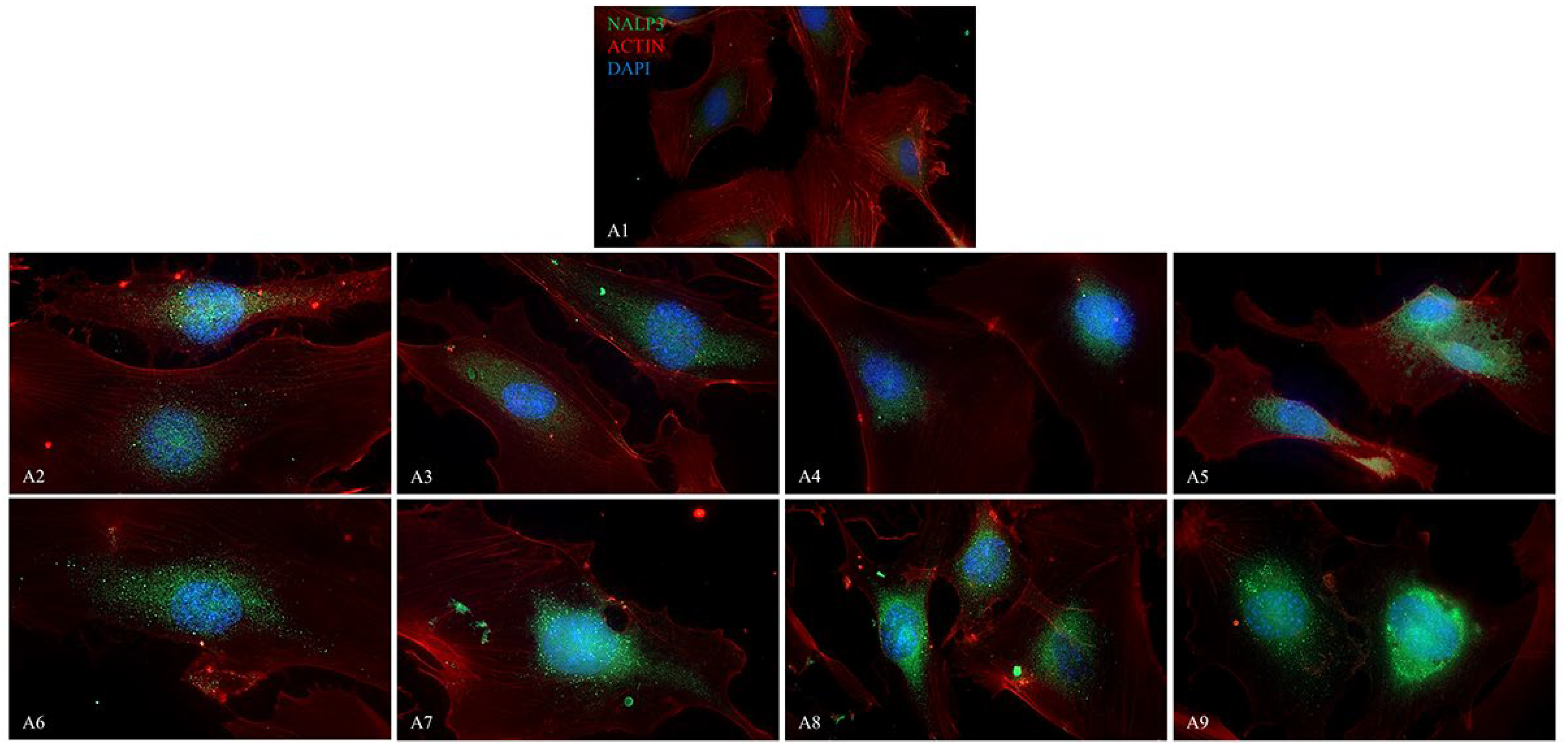
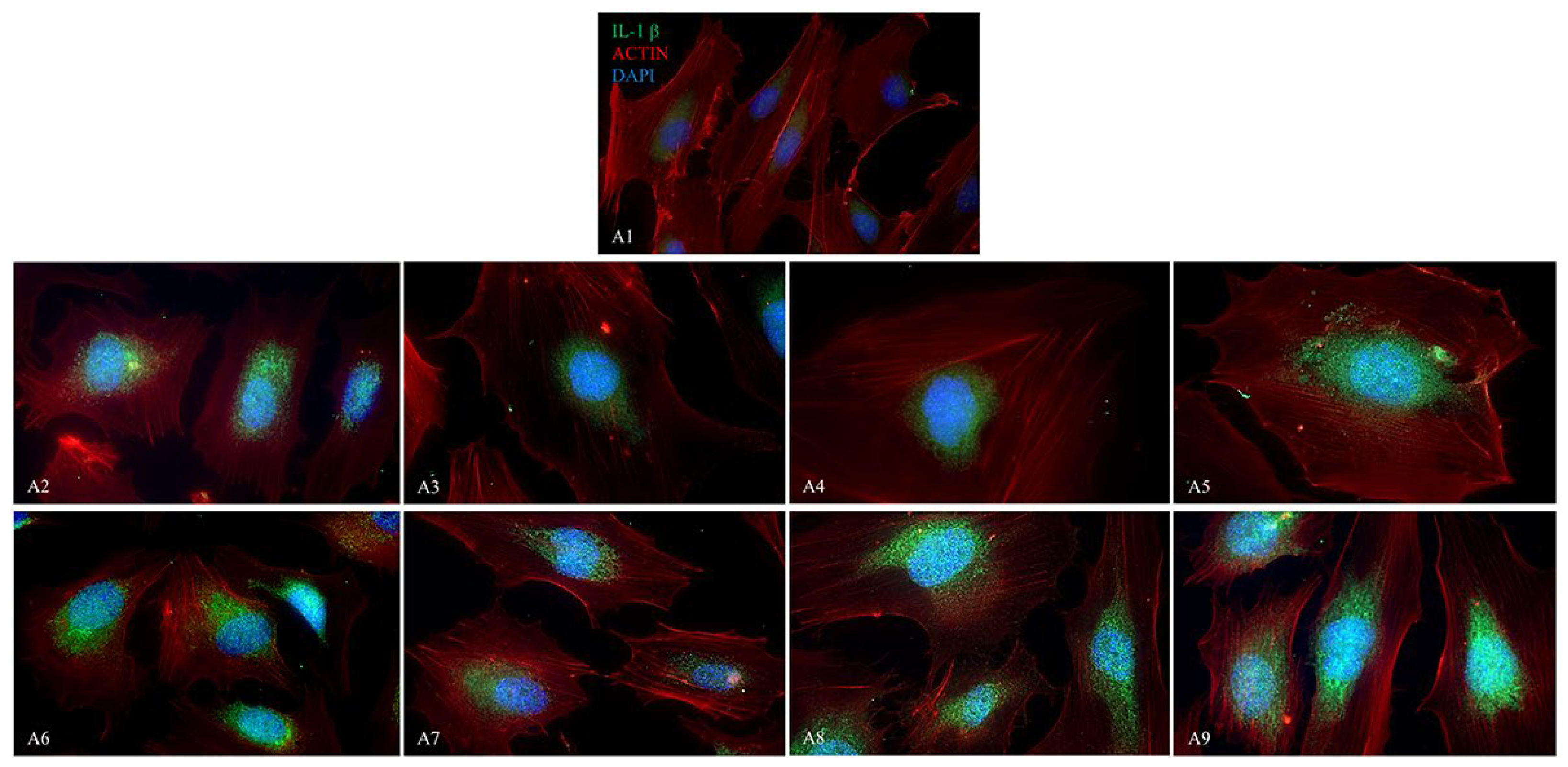
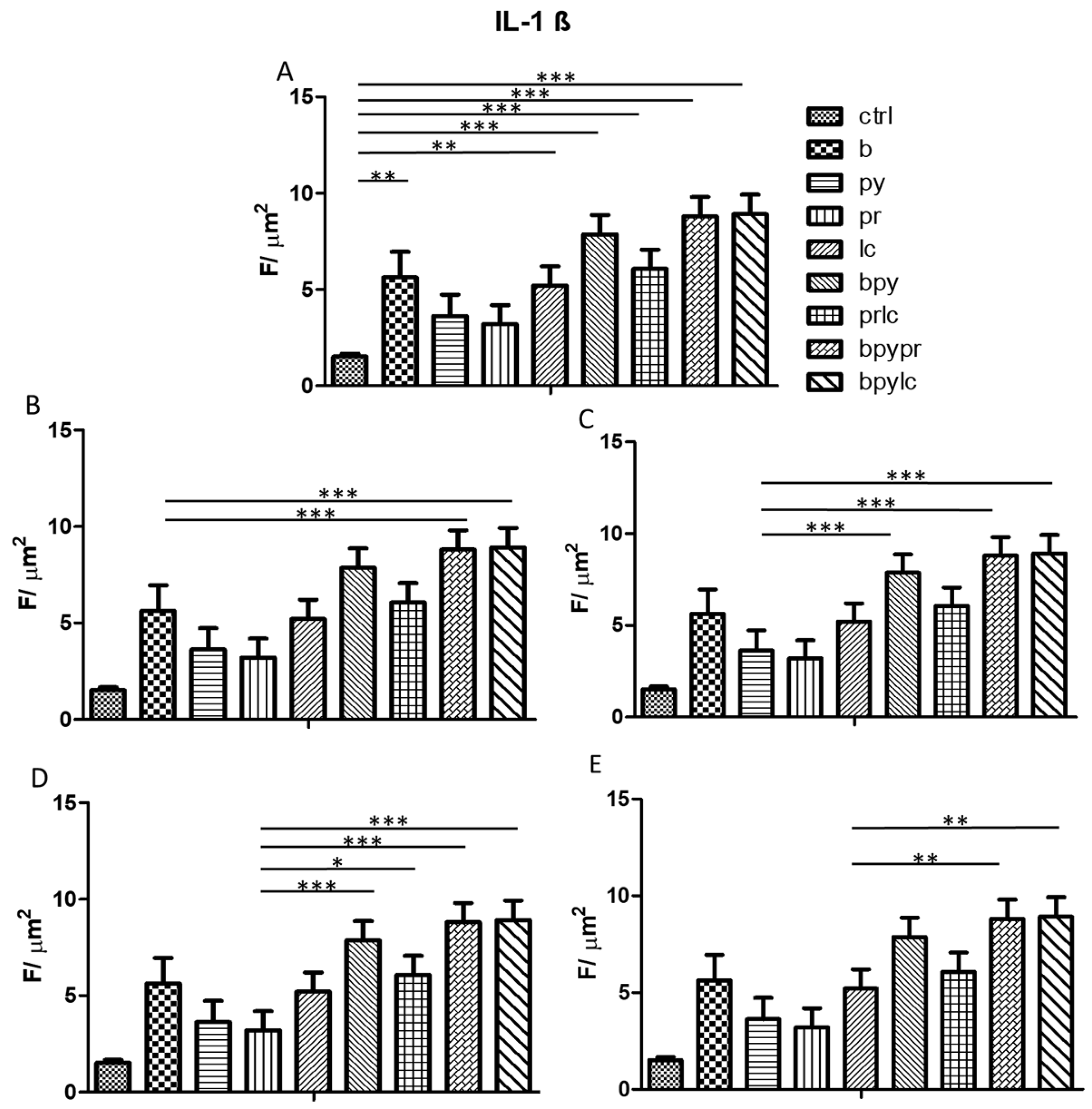
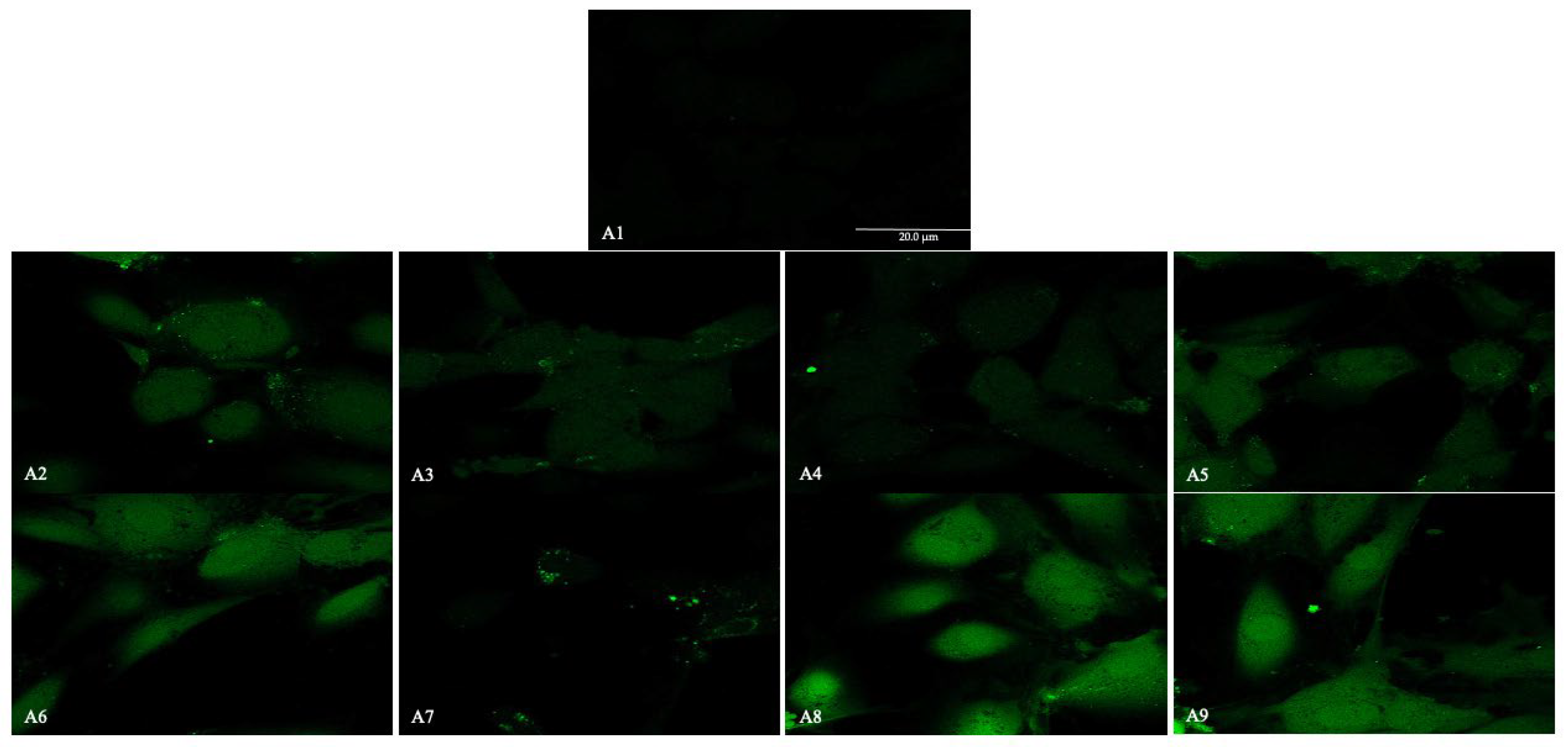
2.4. Immunofluorescence Analysis
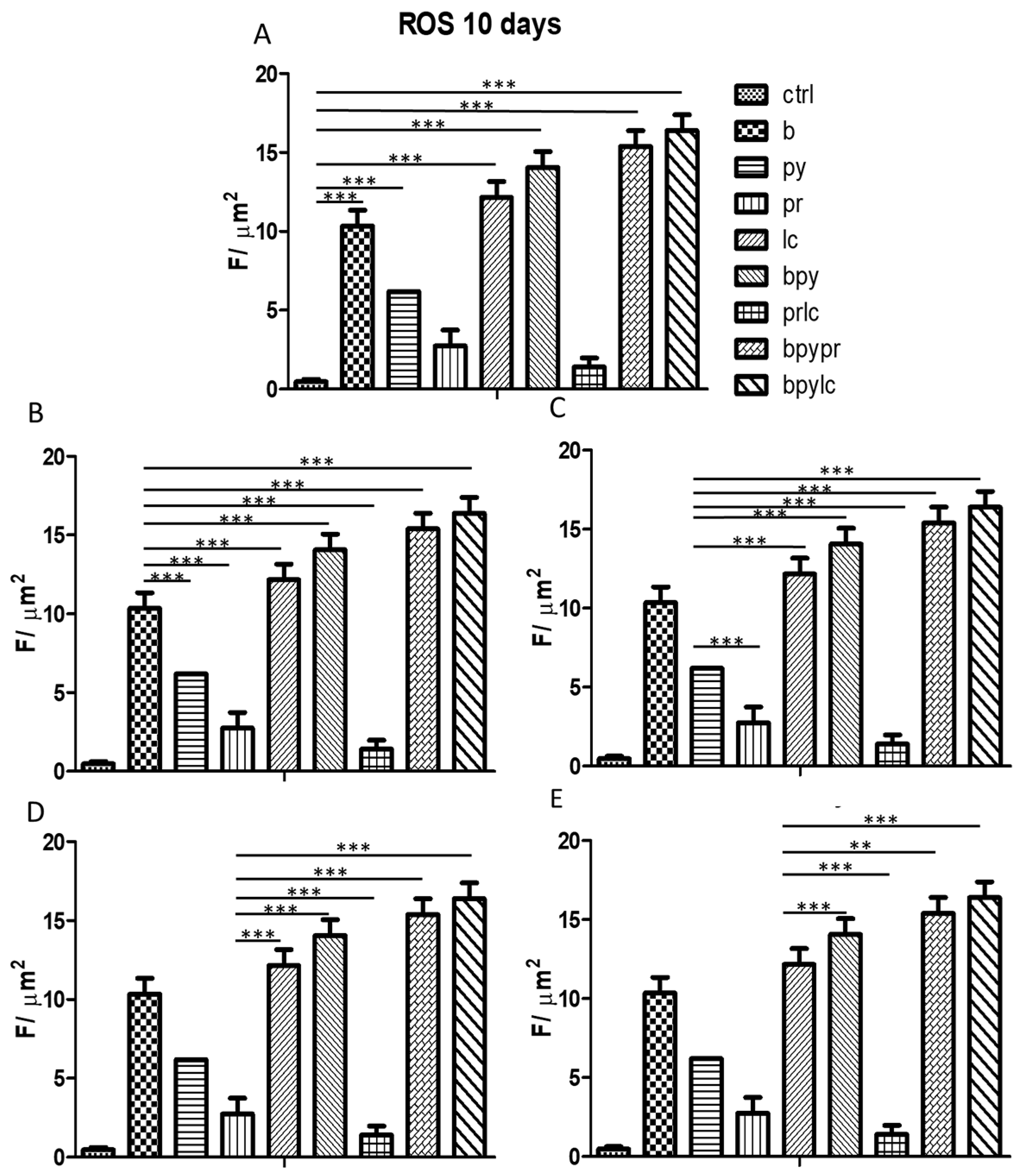
2.5. R0S (Reactive Oxidative Stress)
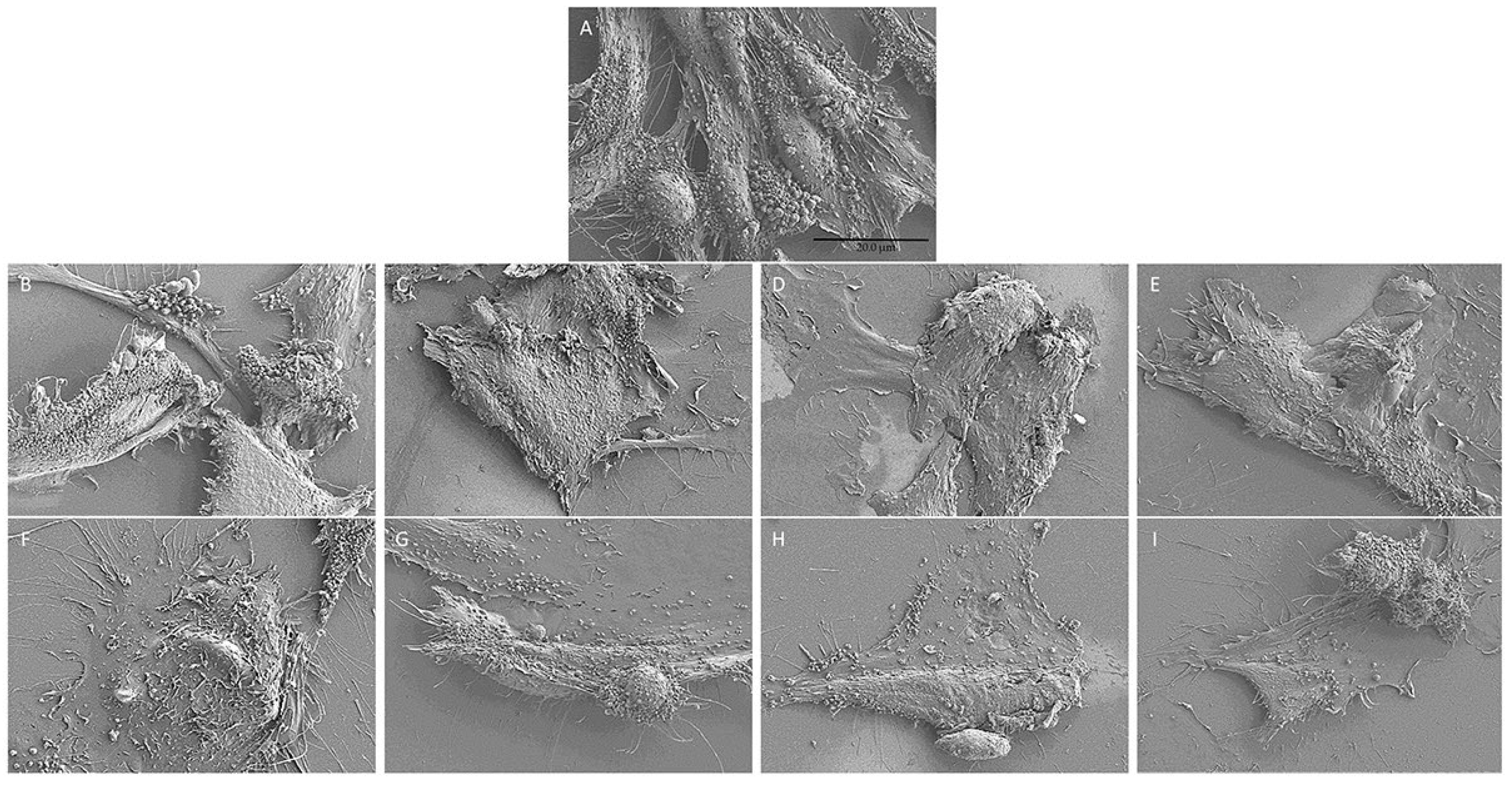
2.6. Cell Morphology
3. Discussion
4. Materials and Methods
4.1. Cell Culture Establishment
4.2. Experimental Study Design
- HUVECs cultured alone (ctrl);
- HUVECs cultured with Boscalid (b);
- HUVECs cultured with Pyraclostrobin (py);
- HUVECs cultured with Propamocarb (pr);
- HUVECs cultured with Lamba-cyhalothrin (lc);
- HUVECs cultured with the combination Boscalid+ Pyraclostrobin (b +py);
- HUVECs cultured with the combination Propamocarb+ Lambda-cyhalothrin (pr +lc);
- HUVECs cultured with the combination Boscalid+ Pyraclostrobin+ Propamocarb (b + py + pr);
- HUVEC cultured with the combination Boscalid+ Pyraclostrobin+ Lambda-cyhalothrin (b+ py + lc).
4.3. Calculation of Application Doses and Preparation of Pesticide Solutions
4.4. Metabolic Activity Evaluation(MTS)
4.5. Trypan Blue Exclusion Test
4.6. RNA Isolation and Real-Time RT-PCR Analysis
4.7. Immunofluorescence Analysis
4.8. Reactive Oxygen Species (ROS) Evaluation
4.9. Cell Morphology (Scanning Electron Microscopy)
4.10. Quantification of Fluorescence of Protein Expression and ROS
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; Alshahrani, A.M.; Muzammil, K.; Saati, A.A.; Wahab, S.; Elbendary, E.Y.; Kambal, N.; et al. Pesticides impacts on human health and the environment with their mechanisms of action and possible countermeasures. Heliyon 2024, 10, e29128. [Google Scholar] [CrossRef] [PubMed]
- Boobis, A.R.; Ossendorp, B.C.; Banasiak, U.; Hamey, P.Y.; Sebestyen, I.; Moretto, A. Cumulative risk assessment of pesticide residues in food. Toxicol. Lett. 2008, 180, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Streisand, J.B. Oral mucosal drug delivery: Clinical pharmacokinetics and therapeutic applications. Clin. Pharmacokinet. 2002, 41, 661–680. [Google Scholar] [CrossRef]
- Goswami, T.; Jasti, B.; Li, X. Sublingual drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2008, 25, 449–484. [Google Scholar] [CrossRef]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar] [CrossRef]
- Cabrera, L.C.; Di Piazza, G.; Dujardin, B.; Pastor, P.M. The 2021 European Union report on pesticide residues in food. EFSA J. 2023, 21, e07939. [Google Scholar] [CrossRef]
- Liao, L.H.; Wu, W.Y.; Dad, A.; Berenbaum, M.R. Fungicide suppression of flight performance in the honeybee and its amelioration by quercetin. Proc. R. Soc. B-Biol. Sci. 2019, 286, 20192041. [Google Scholar] [CrossRef]
- Zhang, F.; Xin, M.; Yu, S.Q.; Liu, D.; Zhou, X.Y.; Qin, Z.W. Expression and Functional Analysis of the Propamocarb-Related Gene in Cucumber. Front. Plant Sci. 2019, 10, 871. [Google Scholar] [CrossRef]
- Nieradko-Iwanicka, B.; Konopelko, M. Effect of Lambdacyhalothrin on Locomotor Activity, Memory, Selected Biochemical Parameters, Tumor Necrosis Factor α, and Interleukin 1ss in a Mouse Model. Int. J. Environ. Res. Public Health 2020, 17, 9240. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Domínguez-Pérez, M.; Mercado, I.; Villarreal-Molina, M.T.; Jacobo-Albavera, L. Use of Human Umbilical Vein Endothelial Cells (HUVEC) as a Model to Study Cardiovascular Disease: A Review. Appl. Sci. 2020, 10, 938. [Google Scholar] [CrossRef]
- Lau, S.; Gossen, M.; Lendlein, A.; Jung, F. Venous and Arterial Endothelial Cells from Human Umbilical Cords: Potential Cell Sources for Cardiovascular Research. Int. J. Mol. Sci. 2021, 22, 978. [Google Scholar] [CrossRef] [PubMed]
- Vailhé, B.; Vittet, D.; Feige, J.J. In vitro models of vasculogenesis and angiogenesis. Lab. Investig. 2001, 81, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Garbern, J.C.; Mummery, C.L.; Lee, R.T. Model Systems for Cardiovascular Regenerative Biology. Csh. Perspect. Med. 2013, 3, a014019. [Google Scholar] [CrossRef][Green Version]
- Trubiani, O.; Ballerini, P.; Murmura, G.; Pizzicannella, J.; Giuliani, P.; Buccella, S.; Caputi, S. Toll-Like Receptor 4 Expression, Interleukin-6, -8 and Ccl-20 Release, and Nf-Kb Translocation in Human Periodontal Ligament Mesenchymal Stem Cells Stimulated with LPS-P Gingivalis. Eur. J. Inflamm. 2012, 10, 81–89. [Google Scholar] [CrossRef]
- Della Rocca, Y.; Traini, E.M.; Trubiani, O.; Traini, T.; Mazzone, A.; Marconi, G.D.; Pizzicannella, J.; Diomede, F. Biological Effects of PMMA and Composite Resins on Human Gingival Fibroblasts: An In Vitro Comparative Study. Int. J. Mol. Sci. 2024, 25, 4880. [Google Scholar] [CrossRef]
- Fu, J.N.; Wu, H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef]
- Gaidt, M.M.; Hornung, V. The NLRP3 Inflammasome Renders Cell Death Pro-inflammatory. J. Mol. Biol. 2018, 430, 133–141. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.H.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Aglietti, R.A.; Dueber, E.C. Recent Insights into the Molecular Mechanisms Underlying Pyroptosis and Gasdermin Family Functions. Trends Immunol. 2017, 38, 261–271. [Google Scholar] [CrossRef]
- Kyriakopoulos, G.; Lambropoulou, M.; Valsami, G.; Kostomitsopoulos, N.; Konstandi, O.; Anagnostopoulos, K.; Tsalikidis, C.; Oikonomou, P.; Simopoulos, K.; Tsaroucha, A.K. Pro-inflammatory cytokines/chemokines, TNF-α, IL-6 and MCP-1, as biomarkers for the nephro- and pneumoprotective effect of silibinin after hepatic ischemia/reperfusion: Confirmation by immunohistochemistry and qRT-PCR. Basic. Clin. Pharmacol. 2022, 130, 457–467. [Google Scholar] [CrossRef]
- Eggestol, H.O.; Lunde, H.S.; Haugland, G.T. The proinflammatory cytokines TNF-α and IL-6 in lumpfish (L.) -identification, molecular characterization, phylogeny and gene expression analyses. Dev. Comp. Immunol. 2020, 105, 103608. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Tan, S.; Tan, S. NLRP3 inflammasome in sepsis (Review). Mol. Med. Rep. 2021, 24, 514. [Google Scholar] [CrossRef]
- Vora, S.M.; Lieberman, J.; Wu, H. Inflammasome activation at the crux of severe COVID-19. Nat. Rev. Immunol. 2021, 21, 694–703. [Google Scholar] [CrossRef]
- Al-Roub, A.; Al Madhoun, A.; Akhter, N.; Thomas, R.; Miranda, L.; Jacob, T.; Al-Ozairi, E.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. IL-1β and TNFα Cooperativity in Regulating IL-6 Expression in Adipocytes Depends on CREB Binding and H3K14 Acetylation. Cells 2021, 10, 3228. [Google Scholar] [CrossRef]
- Chen, W.; Gao, Q.; Han, S.Q.; Pan, F.; Fan, W. The CCL2/CCR2 axis enhances IL-6-induced epithelial-mesenchymal transition by cooperatively activating STAT3-Twist signaling. Tumor Biol. 2015, 36, 973–981. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interf. Cytok Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Strell, C.; Entschladen, F. Extravasation of leukocytes in comparison to tumor cells. Cell Commun. Signal. CCS 2008, 6, 10. [Google Scholar] [CrossRef]
- Dominic, A.; Le, N.T.; Takahashi, M. Loop Between NLRP3 Inflammasome and Reactive Oxygen Species. Antioxid. Redox Signal. 2022, 36, 784–796. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- van Hinsbergh, V.W.M. Endothelium-role in regulation of coagulation and inflammation. Semin. Immunopathol. 2012, 34, 93–106. [Google Scholar] [CrossRef]
- Petitjean, K.; Verres, Y.; Bristeau, S.; Ribault, C.; Aninat, C.; Olivier, C.; Leroyer, P.; Ropert, M.; Loreal, O.; Herault, O.; et al. Low concentrations of ethylene bisdithiocarbamate pesticides maneb and mancozeb impair manganese and zinc homeostasis to induce oxidative stress and caspase-dependent apoptosis in human hepatocytes. Chemosphere 2024, 346, 140535. [Google Scholar] [CrossRef] [PubMed]
- Leveque, X.; Hochane, M.; Geraldo, F.; Dumont, S.; Gratas, C.; Oliver, L.; Gaignier, C.; Trichet, V.; Layrolle, P.; Heymann, D.; et al. Low-Dose Pesticide Mixture Induces Accelerated Mesenchymal Stem Cell Aging In Vitro. Stem Cells 2019, 37, 1083–1094. [Google Scholar] [CrossRef]
- Wang, R.K.; Yang, X.; Wang, T.C.; Kou, R.R.; Liu, P.P.; Huang, Y.Q.; Chen, C. Synergistic effects on oxidative stress, apoptosis and necrosis resulting from combined toxicity of three commonly used pesticides on cells. Ecotoxicol. Environ. Saf. 2023, 263, 115237. [Google Scholar] [CrossRef]
- Desideri, E.; Ciccarone, F.; Ciriolo, M.R.; Fratantonio, D. Extracellular vesicles in endothelial cells: From mediators of cell-to-cell communication to cargo delivery tools. Free Radic. Bio. Med. 2021, 172, 508–520. [Google Scholar] [CrossRef]
- Mathiesen, A.; Hamilton, T.; Carter, N.; Brown, M.; McPheat, W.; Dobrian, A. Endothelial Extracellular Vesicles: From Keepers of Health to Messengers of Disease. Int. J. Mol. Sci. 2021, 22, 4640. [Google Scholar] [CrossRef]
- Cheng, L.S.; Hill, A.F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 2022, 21, 379–399. [Google Scholar] [CrossRef]
- Isola, A.L.; Chen, S.Z. Exosomes: The Messengers of Health and Disease. Curr. Neuropharmacol. 2017, 15, 157–165. [Google Scholar] [CrossRef]
- Trisko, J.; Fleck, J.; Kau, S.; Oesterreicher, J.; Holnthoner, W. Lymphatic and Blood Endothelial Extracellular Vesicles: A Story Yet to Be Written. Life 2022, 12, 654. [Google Scholar] [CrossRef]
- Hochane, M.; Trichet, V.; Pecqueur, C.; Avril, P.; Oliver, L.; Denis, J.; Brion, R.; Amiaud, J.; Pineau, A.; Naveilhan, P.; et al. Low-Dose Pesticide Mixture Induces Senescence in Normal Mesenchymal Stem Cells (MSC) and Promotes Tumorigenic Phenotype in Premalignant MSC. Stem Cells 2017, 35, 800–811. [Google Scholar] [CrossRef]
- Cabrera, L.C.; Di Piazza, G.; Dujardin, B.; Marchese, E.; Pastor, P.M. The 2022 European Union report on pesticide residues in food. EFSA J. 2024, 22, e8753. [Google Scholar] [CrossRef]
- Authority, E.F.S.; Anastassiadou, M.; Brancato, A.; Carrasco Cabrera, L.; Ferreira, L.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.; Magrans, J.O. Pesticide Residue Intake Model-EFSA PRIMo revision 3.1. EFSA J. 2019, 16, 43. [Google Scholar] [CrossRef]
- Cavalcanti, M.F.X.B.; Maria, D.A.; de Isla, N.; Leal, E.C.P.; Joensen, J.; Bjordal, J.M.; Lopes-Martins, R.A.M.B.; Diomede, F.; Trubiani, O.; Frigo, L. Evaluation of the Proliferative Effects Induced by Low-Level Laser Therapy in Bone Marrow Stem Cell Culture. Photomed. Laser Surg. 2015, 33, 610–616. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Della Rocca, Y.; Rajan, T.S.; Piattelli, A.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Human Periodontal Ligament Stem Cells Response to Titanium Implant Surface: Extracellular Matrix Deposition. Biology 2021, 10, 931. [Google Scholar] [CrossRef] [PubMed]

| Pesticide | Tipology | Mode of Action | hNDI (μM) |
|---|---|---|---|
| Boscalid | Fungicide | Succinate dehydrogenase (SDH) inhibition in the mitochondrial electron transport chain | 11.06 |
| Pyraclostrobin | Fungicide | Single-site mode of action, inhibition of mitochondrial respiration | 1.51 |
| Propamocarb | Fungicide | Antisporulating activity, interferes in the synthesis of fatty acids and phospholipids, damaging the formation of cell membranes. | 15.64 |
| Lambda-cyhalothrin | Insetticide | Action on the central and peripheral nervous system, at the level of axonal conduction with alteration of the permeability of the neuron membrane | 0.19 |
| Combination | Code |
|---|---|
| Boscalid Pyraclostrobin | b + py |
| Boscalid+ Pyraclostrobin + Propamocarb | b + py + pr |
| Propamocarb + Lambda-cyhalothrin | pr + lc |
| Boscalid+ Pyraclostrobin + Lambda cyhalothrin | b + py + lc |
| Gene | Forward Primer (5′-3′) | Revers Primer (5′-3′) |
|---|---|---|
| NALP3 | 5′-GAATGCCTTGG-GAGACTCAG-3′ | 5′-AGATTCTGATT-AGTGCTGAGTACC-3′ |
| CASP1 | 5′-TGCCTGTTCCTGTGATGTG-3′ | 5′-GTAGAAACATCTTGTCAAAGTCACT-3′ |
| IL-1β | 5′-CGTCCTAAAGA-CTCCATGATCTG-3′ | 5′-ACCAATCTTGT-AGGACTGACC-3′ |
| IL-6 | 5′-GCAGATGAGTACAAAAGTCCTGA3′ | 5′-TTCTGTGCCTGCAGCTTC-3′ |
| CCL2 | 5′-AGCAGCCACCTTCATTCC-3′ | 5′-GCCTCTGCACTGAGATCTTC3′ |
| TNF | 5′-TGCACTTTGGAGTGATCGG-3′ | 5′-TCAGCTTGAGGGTTTGCTAC-3′ |
| GAPDH | 5′-ACATCGCTCAGACACCATG-3′ | 5′-TGTAGTTGAGGTCAATGAAGGG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzone, A.; Della Rocca, Y.; Flamminii, F.; Guarnieri, S.; Bello, D.G.; Nanci, A.; Trubiani, O.; Diomede, F.; Pizzicannella, J. Activation of the NALP3-CASP1-IL-1 β Inflammatory Pathway by Pesticide Exposure in Human Umbilical Vein Endothelial Cells. Int. J. Mol. Sci. 2025, 26, 4947. https://doi.org/10.3390/ijms26104947
Mazzone A, Della Rocca Y, Flamminii F, Guarnieri S, Bello DG, Nanci A, Trubiani O, Diomede F, Pizzicannella J. Activation of the NALP3-CASP1-IL-1 β Inflammatory Pathway by Pesticide Exposure in Human Umbilical Vein Endothelial Cells. International Journal of Molecular Sciences. 2025; 26(10):4947. https://doi.org/10.3390/ijms26104947
Chicago/Turabian StyleMazzone, Antonella, Ylenia Della Rocca, Federica Flamminii, Simone Guarnieri, Dainelys Guadarrama Bello, Antonio Nanci, Oriana Trubiani, Francesca Diomede, and Jacopo Pizzicannella. 2025. "Activation of the NALP3-CASP1-IL-1 β Inflammatory Pathway by Pesticide Exposure in Human Umbilical Vein Endothelial Cells" International Journal of Molecular Sciences 26, no. 10: 4947. https://doi.org/10.3390/ijms26104947
APA StyleMazzone, A., Della Rocca, Y., Flamminii, F., Guarnieri, S., Bello, D. G., Nanci, A., Trubiani, O., Diomede, F., & Pizzicannella, J. (2025). Activation of the NALP3-CASP1-IL-1 β Inflammatory Pathway by Pesticide Exposure in Human Umbilical Vein Endothelial Cells. International Journal of Molecular Sciences, 26(10), 4947. https://doi.org/10.3390/ijms26104947







