Back to the Wastes: The Potential of Agri-Food Residues for Extracting Valuable Plant Cell Wall Polysaccharides
Abstract
1. Introduction
2. Results
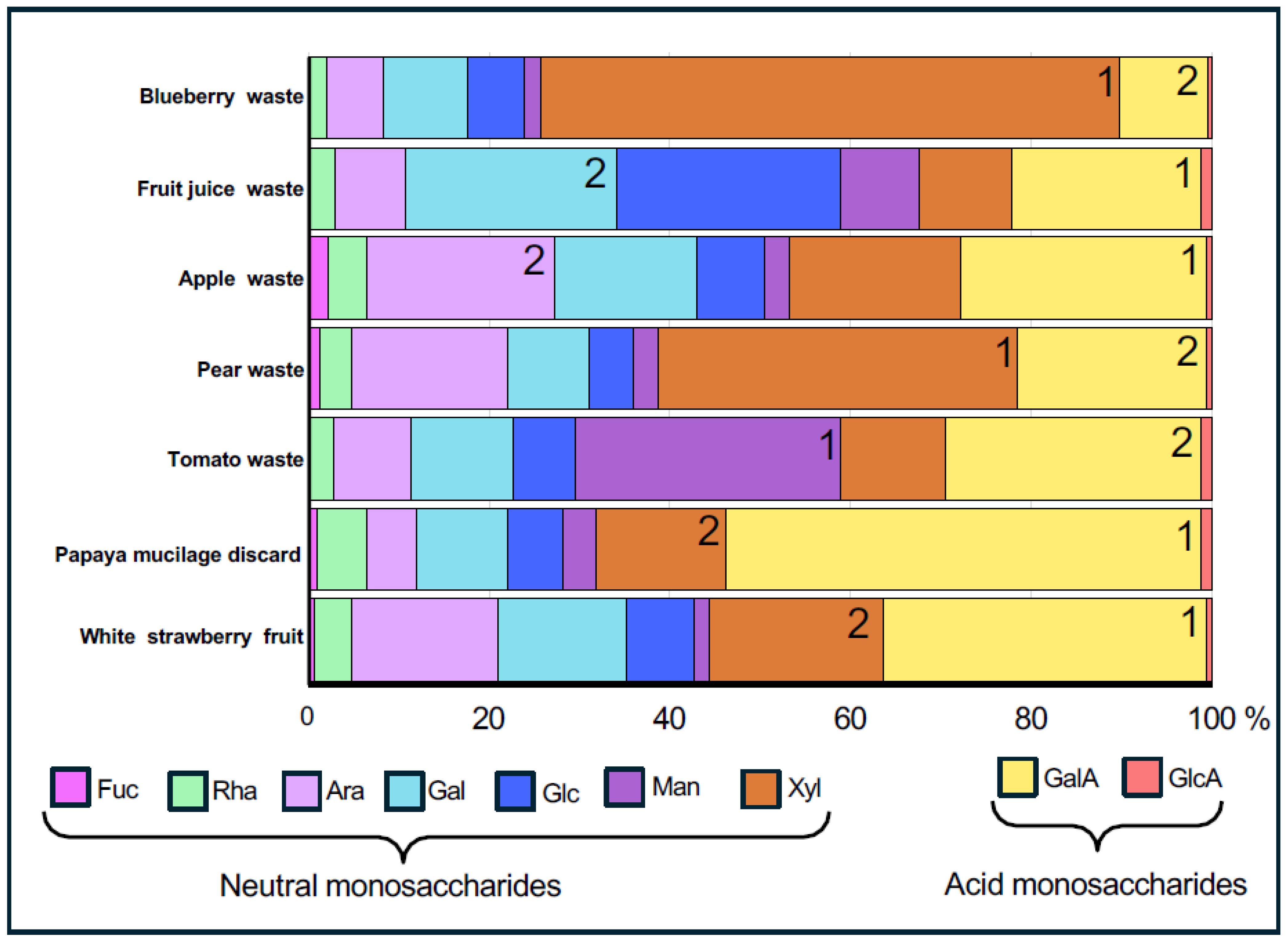
2.1. Analysis of Monosaccharide Composition in Alcohol-Insoluble Residues
2.2. Chemical Separation of Pectins and Hemicelluloses from Waste Samples
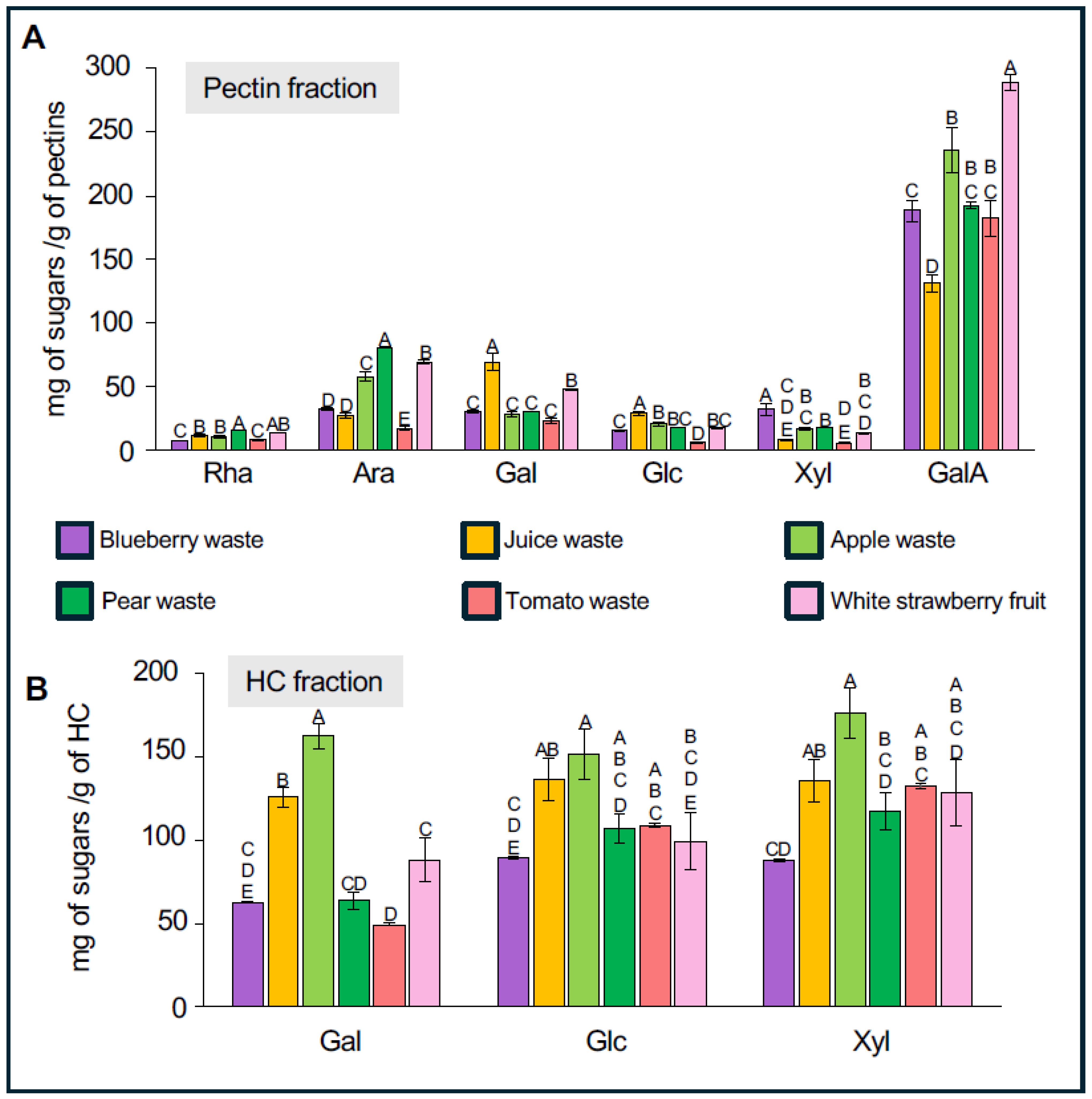
2.3. Analysis of the Monosaccharide Composition in Fractions Enriched in Pectin and Hemicellulose
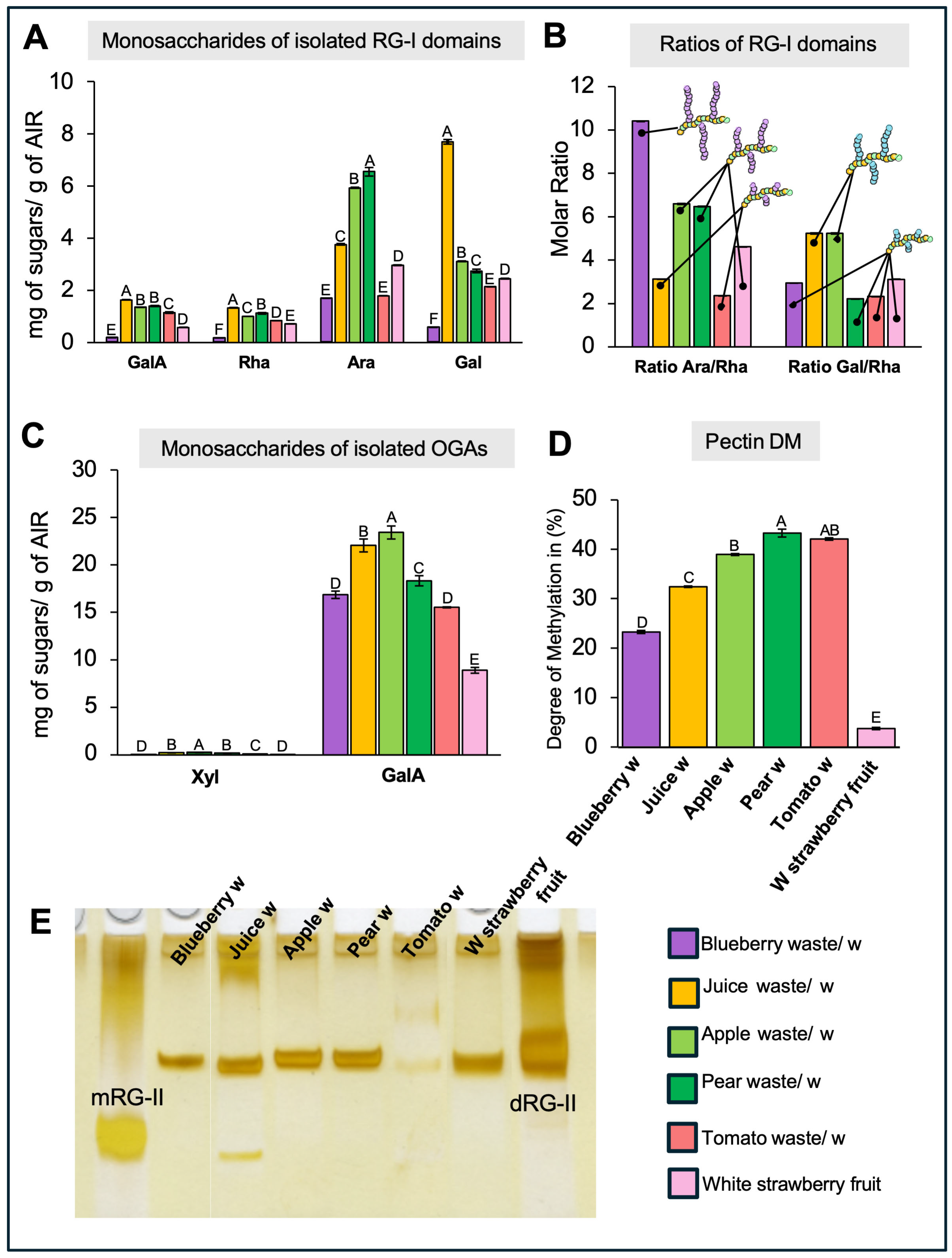
2.4. Analysis of the Isolated Rhamnogalacturonan-I Domain
2.5. Analysis of the Isolated Oligogalacturonans
2.6. Rhamnogalacturonan-II Detection and Quantification
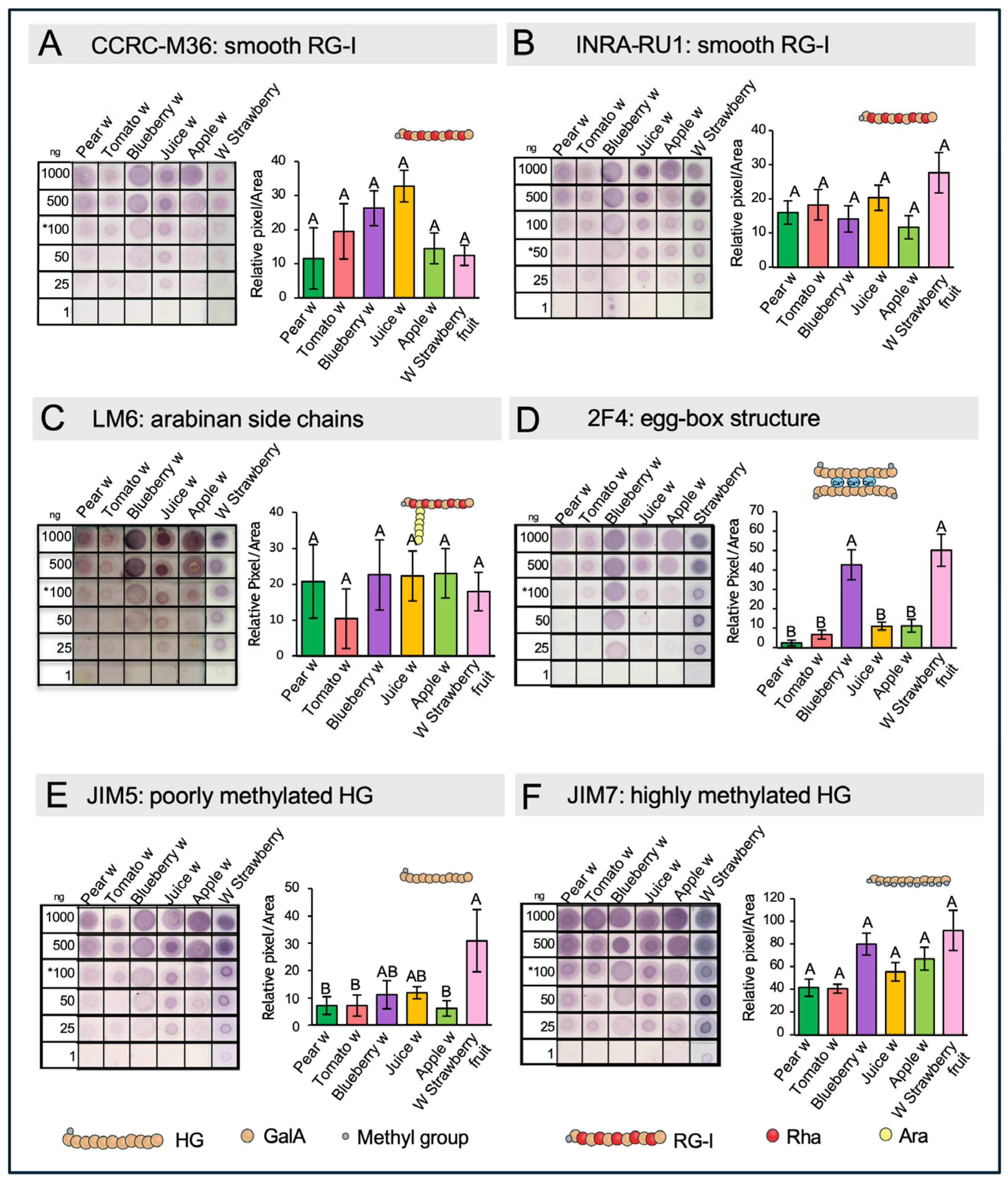
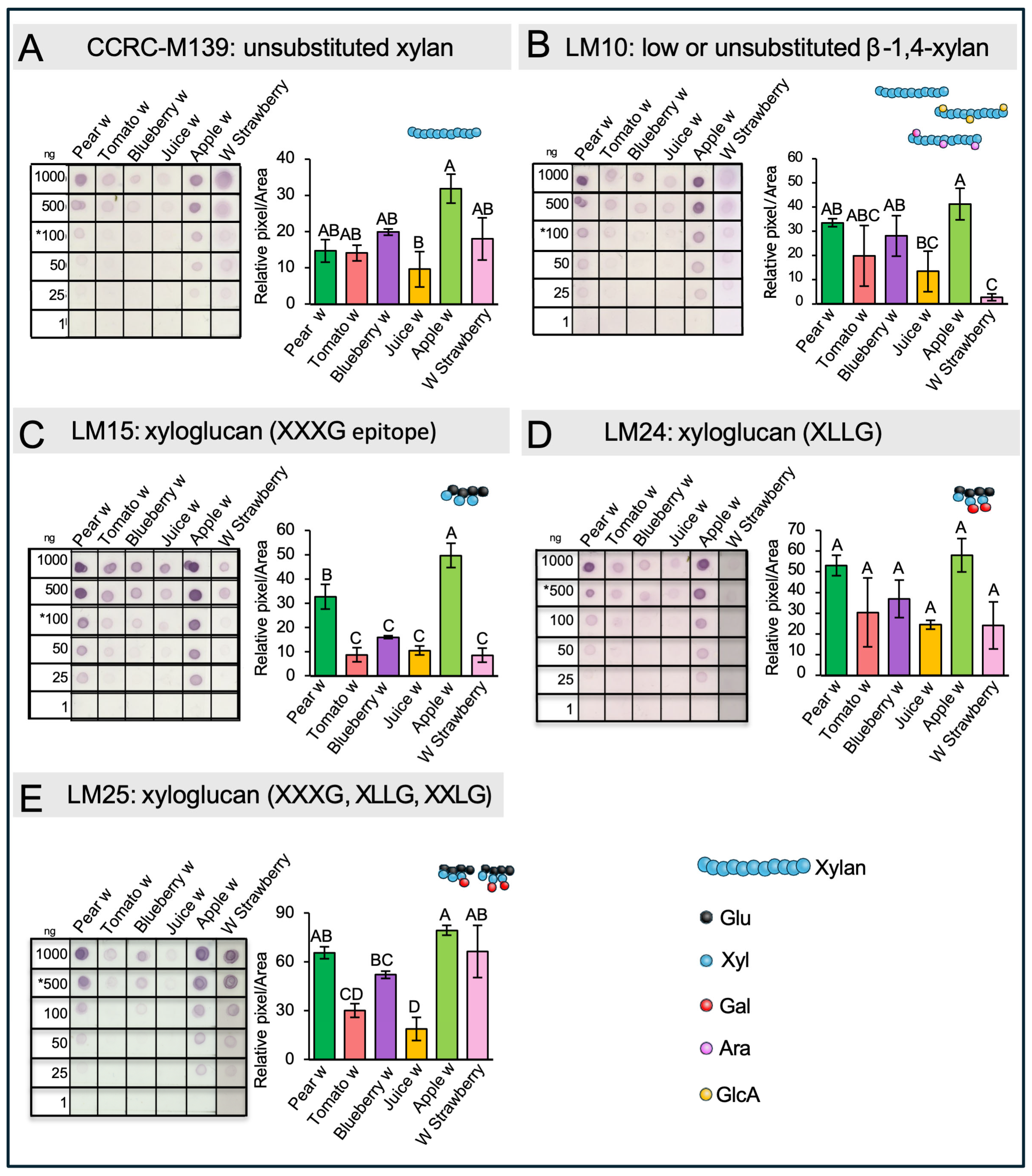
2.7. Dot Blot Assays of Hemicellulose
3. Discussion
3.1. Analysis of Fruit Waste Samples Revealed a High Carbohydrate Content with Diverse Polysaccharide Compositions
3.2. Chemical Analysis of Pectins- and HC-Enriched Fractions Suggest Variations in Pectins and Hemicellulose Compositions
3.3. The Amount and Structure of Rhamnogalacturonan-I (RG-I) Domains Vary Across Different Waste Types
3.4. The Isolation of OGAs Revealed Variations in HG Quantity and Degree of Methylation, as Well as the Presence of Xylogalacturonan Across Different Types of Waste
3.5. RG-II Detection and Quantification in Fruit Waste Samples Reveal Variations in Amount and Structure
3.6. Dot Blot Assays of Hemicellulose Fractions Reveal the Presence of Xyloglucans and Xylan in the Different Wastes
4. Methodology
4.1. Plant Residue Material
4.2. AIR Preparation
4.3. Cell Wall Fractionation
4.4. Acid Hydrolysis of Cell Wall Material (AIR, Pectin-, and Hemicellulose-Enriched Fractions) and Monosaccharide Quantification by HPAEC-PAD
4.5. Methylesterification Analysis
4.6. Uronic Acid Quantification
4.7. Pectin Domain Isolation
4.8. Electrophoretic Analysis of RG-II
4.9. Immunodot Blot Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Das, R.; Sangwan, S.; Rohatgi, B.; Khanam, R.; Peera, S.P.G.; Das, S.; Lyngdoh, Y.A.; Langyan, S.; Shukla, A.; et al. Utilisation of agro-industrial waste for sustainable green production: A review. Environ. Sustain. 2021, 4, 619–636. [Google Scholar] [CrossRef]
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorisation of food agro-industrial by-products: From the past to the present and perspectives. J. Environ. Manag. 2021, 299, 113571. [Google Scholar] [CrossRef]
- de Menezes, C.S.; Sousa, A.C.; Silva, L.M.; Rodrigues, T.H.; de Brito, E.S.; Ricardo, N.M. Extraction of cell wall pectins and hemicellulose from agro-industrial wastes: A sustainable alternative source. Carbohydr. Polym. 2025, 347, 122769. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Doblin, M.S.; Pettolino, F.; Bacic, A. Plant cell walls: The skeleton of the plant world. Funct. Plant Biol. 2010, 37, 357–381. [Google Scholar] [CrossRef]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, chemical modification, and application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Leiva-Candia, D.E.; Pinzi, S.; Redel-Macías, M.D.; Koutinas, A.; Webb, C.; Dorado, M.P. The potential for agro-industrial waste utilization using oleaginous yeast for the production of biodiesel. Fuel 2014, 123, 33–42. [Google Scholar] [CrossRef]
- Meseldzija, S.; Petrovic, J.; Onjia, A.; Volkov-Husovic, T.; Nesic, A.; Vukelic, N. Utilization of agro-industrial waste for removal of copper ions from aqueous solutions and mining-wastewater. J. Ind. Eng. Chem. 2019, 75, 246–252. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan production from agro-industrial waste and its applications in food industry: A review. Carbohydr. Polym. 2019, 217, 46–57. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. R. Soc. Chem. Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Montilla, A.; Villamiel, M. Role of pectin in the current trends towards low-glycaemic food consumption. Food Res. Int. 2021, 140, 109851. [Google Scholar] [CrossRef] [PubMed]
- Roman-Benn, A.; Contador, C.A.; Li, M.-W.; Lam, H.-M.; Ah-Hen, K.; Ulloa, P.E.; Ravanal, M.C. Pectin: An overview of sources, extraction and applications in food products, biomedical, pharmaceutical and environmental issues. Food Chem. Adv. 2023, 2, 100192. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Yildirim-Yalcin, M.; Tornuk, F.; Toker, O.S. Recent advances in the improvement of carboxymethyl cellulose-based edible films. Trends Food Sci. Technol. 2022, 129, 179–193. [Google Scholar] [CrossRef]
- Kong, P.; Rosnan, S.M.; Enomae, T. Carboxymethyl cellulose–chitosan edible films for food packaging: A review of recent advances. Carbohydr. Polym. 2024, 346, 122612. [Google Scholar] [CrossRef]
- Vityazev, F.V.; Fedyuneva, M.I.; Golovchenko, V.V.; Patova, O.A.; Ipatova, E.U.; Durnev, E.A.; Martinson, E.A.; Litvinets, S.G. Pectin-silica gels as matrices for controlled drug release in gastrointestinal tract. Carbohydr. Polym. 2017, 157, 9–20. [Google Scholar] [CrossRef]
- Murata, Y.; Maida, C.; Kofuji, K. Drug release profiles and disintegration properties of pectin films. Materials 2019, 12, 355. [Google Scholar] [CrossRef]
- Khotimchenko, M. Pectin polymers for colon-targeted antitumor drug delivery. Int. J. Biol. Macromol. 2020, 158, 1110–1124. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Blanco-Pérez, F.; Steigerwald, H.; Schülke, S.; Vieths, S.; Toda, M.; Scheurer, S. The dietary fiber pectin: Health benefits and potential for the treatment of allergies by modulation of gut microbiota. Curr. Allergy Asthma Rep. 2021, 21, 43. [Google Scholar] [CrossRef]
- Elshahed, M.S.; Miron, A.; Aprotosoaie, A.C.; Farag, M.A. Pectin in diet: Interactions with the human microbiome, role in gut homeostasis, and nutrient-drug interactions. Carbohydr. Polym. 2021, 255, 117388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, J.; Zhang, L.; Wang, Y.; Wang, J.; Gu, B.; Chen, J.; Hao, T.; Wang, C.; Wen, N. Preparation and characterization of calcium phosphate/pectin scaffolds for bone tissue engineering. R. Soc. Chem. Adv. 2016, 6, 62071–62082. [Google Scholar] [CrossRef]
- Dodd, D.; Cann, I.K. Enzymatic deconstruction of xylan for biofuel production. Glob. Changes Biol. Bioenergy 2009, 1, 2–17. [Google Scholar] [CrossRef]
- Dutta, S.K.; Chakraborty, S. Mixing effects on the kinetics and the dynamics of two-phase enzymatic hydrolysis of hemicellulose for biofuel production. Bioresour. Technol. 2018, 259, 276–285. [Google Scholar] [CrossRef]
- Dutta, S.K.; Chakraborty, S. Multiscale dynamics of hemicellulose hydrolysis for biofuel production. Ind. Eng. Chem. Res. 2019, 58, 8963–8978. [Google Scholar] [CrossRef]
- Huang, L.Z.; Ma, M.G.; Ji, X.X.; Choi, S.E.; Si, C. Recent developments and applications of hemicellulose from wheat straw: A review. Front. Bioeng. Biotechnol. 2021, 9, 690773. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Shaheen, H.; Wu, A.M. Cell wall hemicellulose for sustainable industrial utilization. Renew. Sustain. Energy Rev. 2021, 144, 110996. [Google Scholar] [CrossRef]
- Figueroa, C.R.; Pimentel, P.; Gaete-Eastman Carlos Moya, M.; Herrera, R.; Caligari, P.D.-S.; Moya-León, M.A. Softening rate of the Chilean strawberry (Fragaria chiloensis) fruit reflects the expression of polygalacturonase and pectate lyase genes. Postharvest Biol. Technol. 2008, 49, 210–220. [Google Scholar] [CrossRef]
- Sanhueza, D.; Sepúlveda-Orellana, P.; Salazar-Carrasco, A.; Zúñiga, S.; Herrera, R.; Moya-León, M.A.; Saez-Aguayo, S. Mucilage extracted from Chilean papaya seeds is enriched with homogalacturonan domains. Front. Plant Sci. 2024, 15, 1380533. [Google Scholar] [CrossRef]
- Sanhueza, D.; Balic-Norambuena, I.; Sepúlveda-Orellana, P.; Salazar-Carrasco, A.; Siña-López, S.; Moya-León, M.A.; Saez-Aguayo, S. Unraveling cell wall polysaccharides during blueberry ripening: Insights into the roles of rhamnogalacturonan-I and arabinogalactan proteins in fruit firmness. Front. Plant Sci. 2024, 15, 1422917. [Google Scholar] [CrossRef] [PubMed]
- Mattus-Araya, E.; Stappung, Y.; Herrera, R.; Moya-León, M.A. Molecular actors involved in the softening of Fragaria chiloensis fruit accelerated by ABA treatment. J. Plant Growth Regul. 2023, 42, 433–448. [Google Scholar] [CrossRef]
- Anthon, G.E.; Barrett, D.M. Comparison of three colorimetric reagents in the determination of methanol with alcohol oxidase. Application to the assay of pectin methylesterase. J. Agric. Food Chem. 2004, 52, 3749–3753. [Google Scholar] [CrossRef] [PubMed]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Sanhueza, D.; Ara Begum, R.; Albenne, C.; Jamet, E.; Fry, S.C. An Arabidopsis thaliana arabinogalactan-protein (AGP31) and several cationic AGP fragments catalyse the boron bridging of rhamnogalacturonan-II. Biochem. J. 2022, 479, 1967–1984. [Google Scholar] [CrossRef]
- Chormova, D.; Messenger, D.J.; Fry, S.C. Boron bridging of rhamnogalacturonan-II, monitored by gel electrophoresis, occurs during polysaccharide synthesis and secretion but not post-secretion. Plant J. 2014, 77, 534–546. [Google Scholar] [CrossRef]
- Babbar, N.; Dejonghe, W.; Gatti, M.; Sforza, S.; Elst, K. Pectic oligosaccharides from agricultural by-products: Production, characterization and health benefits. Crit. Rev. Biotechnol. 2015, 36, 594–606. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, J.; Mao, G.; Hu, W.; Ye, X.; Linhardt, R.J.; Chen, S. Rethinking the impact of RG-I mainly from fruits and vegetables on dietary health. Crit. Rev. Food Sci. Nutr. 2019, 60, 2938–2960. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef]
- Ralet, M.C.; Tranquet, O.; Poulain, D.; Moïse, A.; Guillon, F. Monoclonal antibodies to rhamnogalacturonan I backbone. Planta 2010, 231, 1373–1383. [Google Scholar] [CrossRef]
- Pattathil, S.; Avci, U.; Baldwin, D.; Swennes, A.G.; McGill, J.A.; Popper, Z.; Bootten, T.; Albert, A.; Davis, R.H.; Chennareddy, C.; et al. A Comprehensive Toolkit of Plant Cell Wall Glycan-Directed Monoclonal Antibodies. Plant Physiol 2010, 153, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.; Marcus, S.E.; Knox, J.P. Generation of monoclonal antibody specific to (1→5)-alpha-L-arabinan. Carbohydr. Res. 1998, 308, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.P.; Linstead, P.J.; King, J.; Cooper, C.; Roberts, K. Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 1990, 181, 512–521. [Google Scholar] [CrossRef]
- Clausen, M.H.; Willats, W.G.; Knox, J.P. Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr. Res. 2003, 338, 1797–1800. [Google Scholar] [CrossRef]
- Liners, F.; Letesson, J.J.; Didembourg, C.; Van Cutsem, P. Monoclonal antibodies against pectin: Recognition of a conformation induced by calcium. Plant Physiol. 1989, 91, 1419–1424. [Google Scholar] [CrossRef]
- McCartney, L.; Marcus, S.E.; Knox, J.P. Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J. Histochem. Cytochem. 2005, 53, 543–546. [Google Scholar] [CrossRef]
- Marcus, S.E.; Verhertbruggen, Y.; Hervé, C.; Ordaz-Ortiz, J.J.; Farkas, V.; Pedersen, H.L.; Willats, W.G.T.; Knox, J.P. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 2008, 8, 60. [Google Scholar] [CrossRef]
- Pedersen, H.L.; Fangel, J.U.; McCleary, B.; Ruzanski, C.; Rydahl, M.G.; Ralet, M.C.; Farkas, V.; Von Schantz, L.; Marcus, S.E.; Andersen, M.C.; et al. Versatile high resolution oligosaccharide microarrays for plant glycobiology and cell wall research. J. Biol. Chem. 2012, 287, 39429–39438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saez-Aguayo, S.; Sanhueza, D.; Fuenzalida, P.; Covarrubias, M.P.; Handford, M.; Herrera, R.; Moya-León, M.A. Back to the Wastes: The Potential of Agri-Food Residues for Extracting Valuable Plant Cell Wall Polysaccharides. Int. J. Mol. Sci. 2025, 26, 4942. https://doi.org/10.3390/ijms26104942
Saez-Aguayo S, Sanhueza D, Fuenzalida P, Covarrubias MP, Handford M, Herrera R, Moya-León MA. Back to the Wastes: The Potential of Agri-Food Residues for Extracting Valuable Plant Cell Wall Polysaccharides. International Journal of Molecular Sciences. 2025; 26(10):4942. https://doi.org/10.3390/ijms26104942
Chicago/Turabian StyleSaez-Aguayo, Susana, Dayan Sanhueza, Paloma Fuenzalida, María Paz Covarrubias, Michael Handford, Raúl Herrera, and María Alejandra Moya-León. 2025. "Back to the Wastes: The Potential of Agri-Food Residues for Extracting Valuable Plant Cell Wall Polysaccharides" International Journal of Molecular Sciences 26, no. 10: 4942. https://doi.org/10.3390/ijms26104942
APA StyleSaez-Aguayo, S., Sanhueza, D., Fuenzalida, P., Covarrubias, M. P., Handford, M., Herrera, R., & Moya-León, M. A. (2025). Back to the Wastes: The Potential of Agri-Food Residues for Extracting Valuable Plant Cell Wall Polysaccharides. International Journal of Molecular Sciences, 26(10), 4942. https://doi.org/10.3390/ijms26104942








