Transcriptome Sequencing and Metabolite Analysis Revealed the Single and Combined Effects of Microplastics and Di-(2-ethylhexyl) Phthalate on Mouse Liver
Abstract
1. Introduction
2. Results
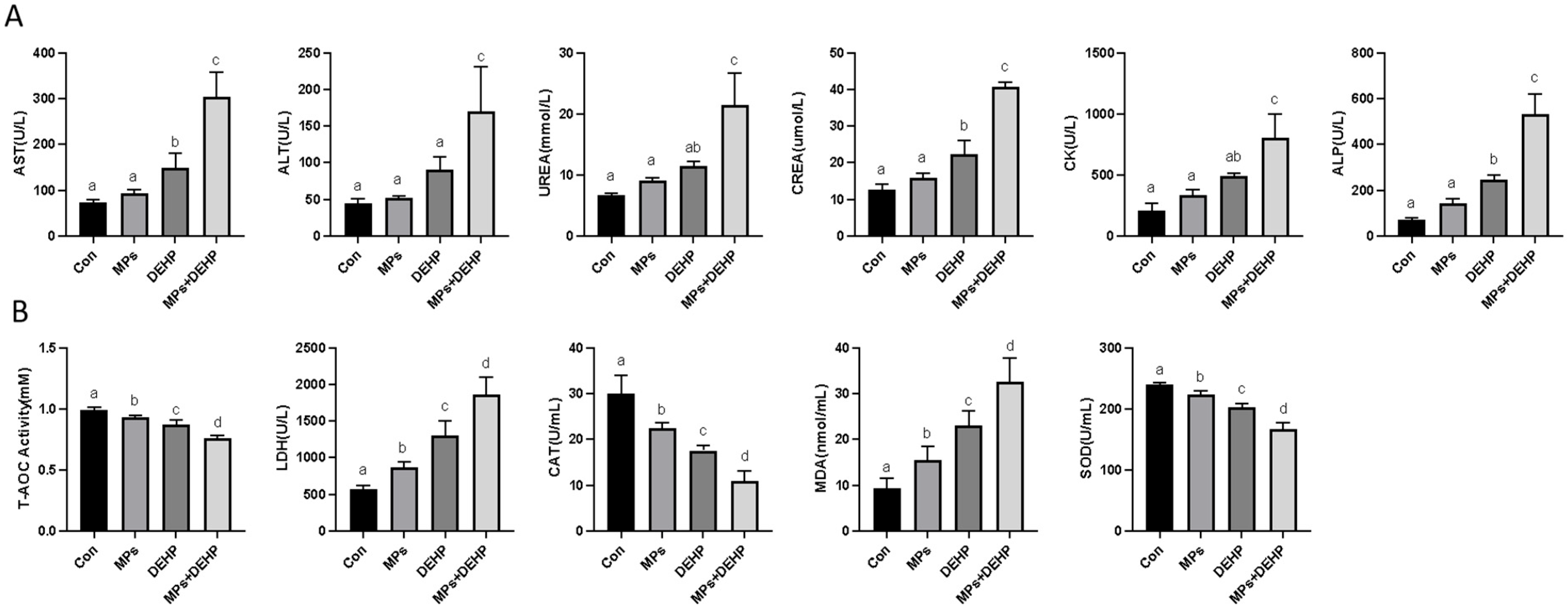
2.1. Effects of MPs, DEHP, and MPs + DEHP on Antioxidant Biomarkers in Mice
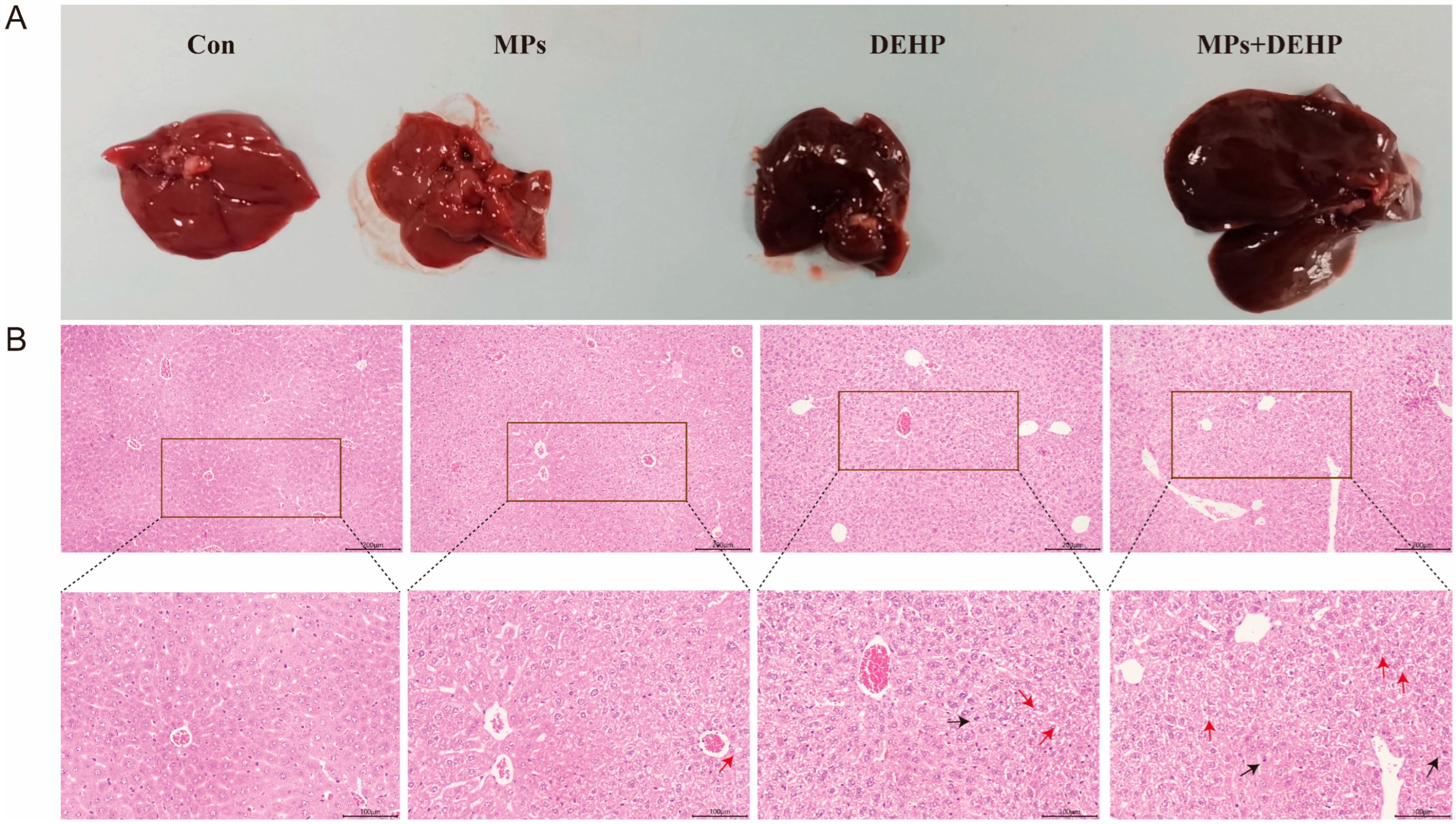
2.2. Effects of MPs, DEHP, and MPs + DEHP on Liver Tissue in Mice
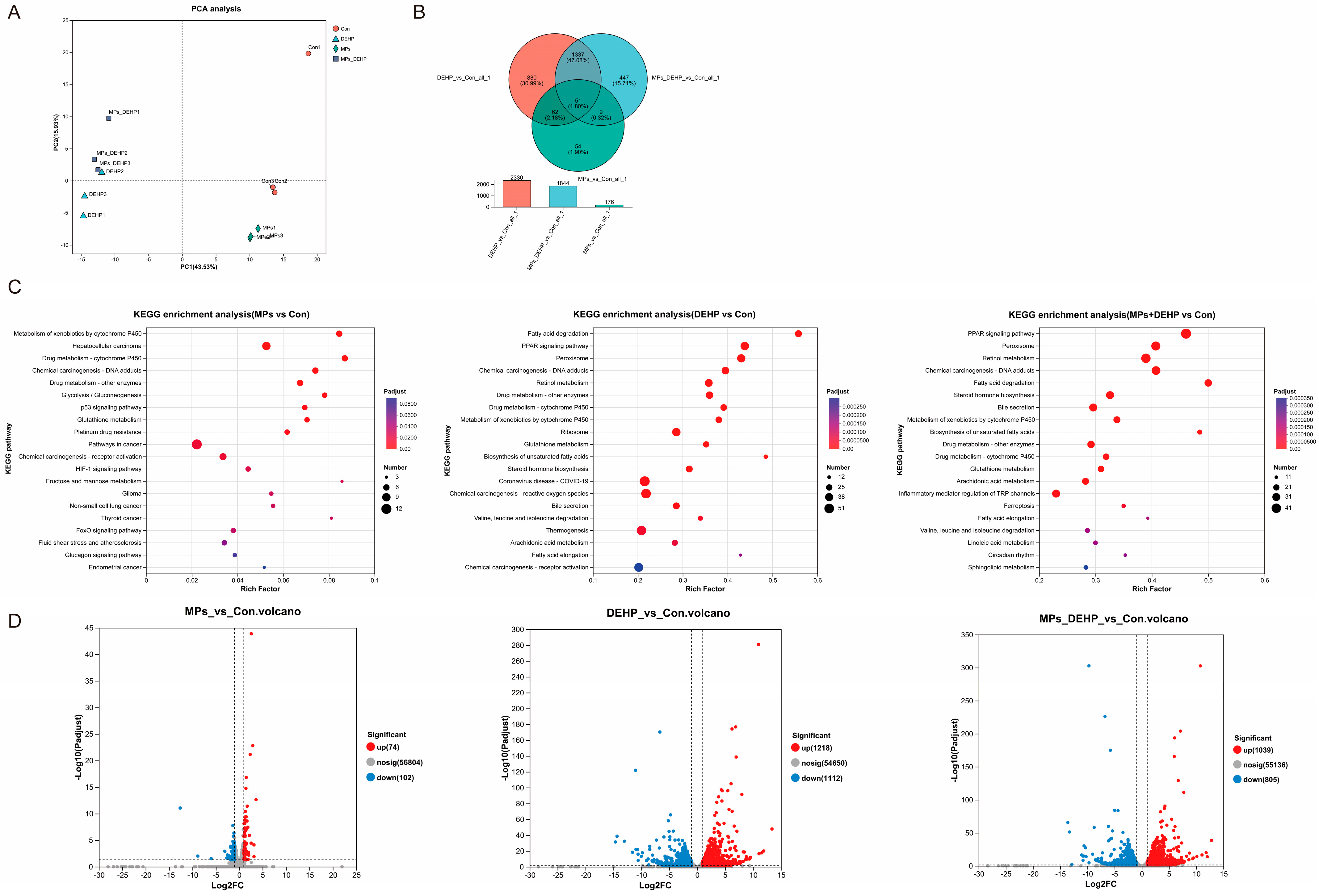
2.3. Effects of MPs, DEHP, and MPs + DEHP on Transcriptomic Profiles in Mice
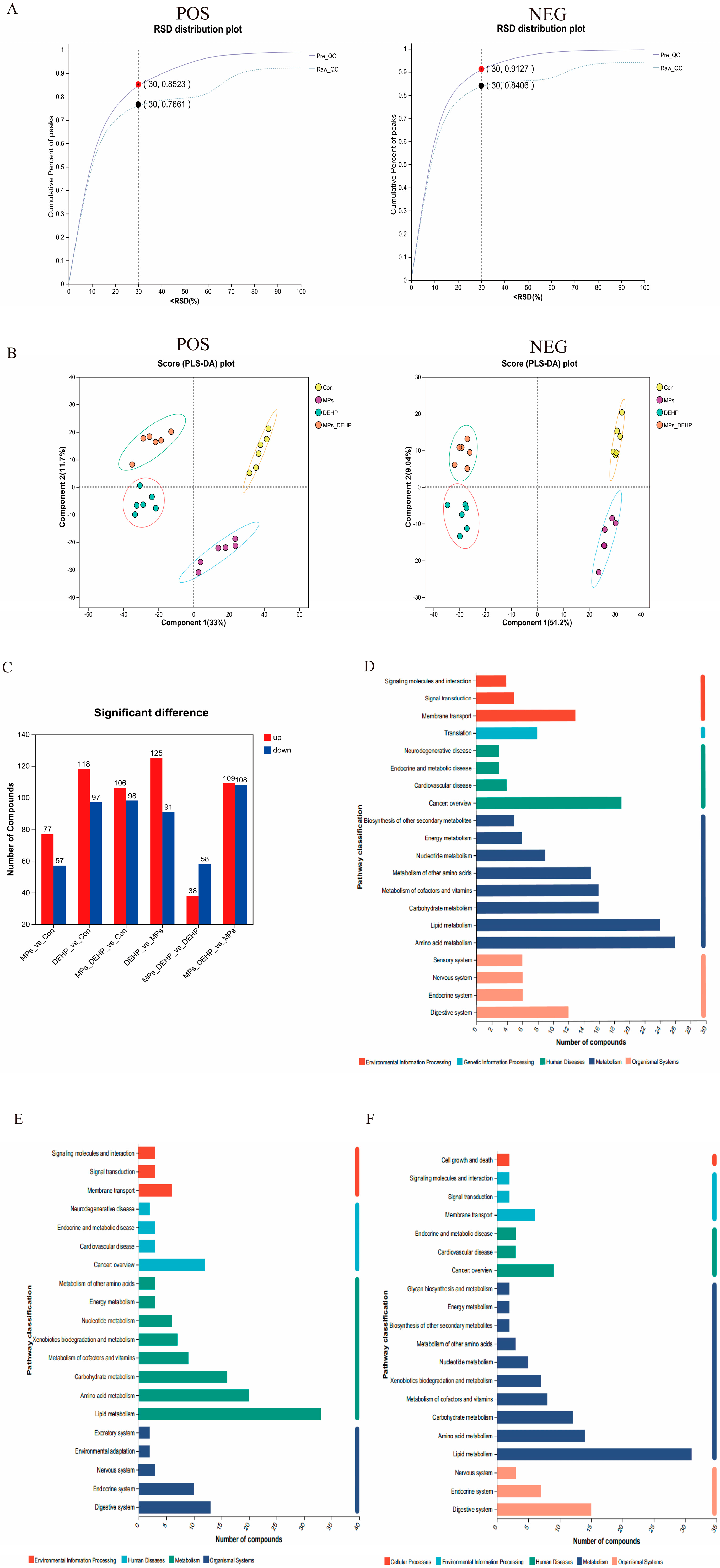
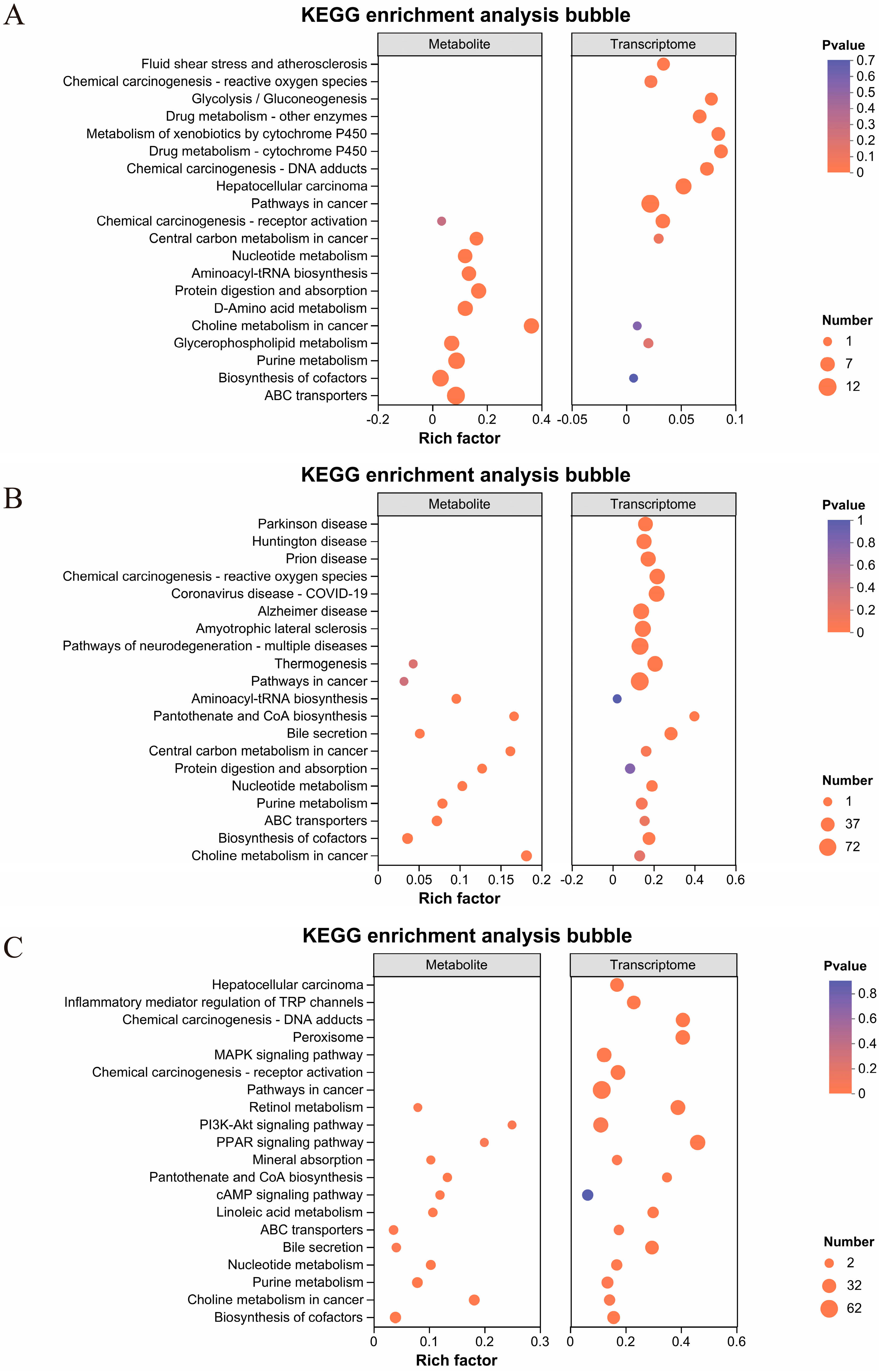
2.4. Effects of MPs, DEHP, and MPs + DEHP on Metabolomics Profiles in Mice
2.5. Integrated Analysis of Transcriptomic and Metabolic Profiles in Mouse Liver Tissue
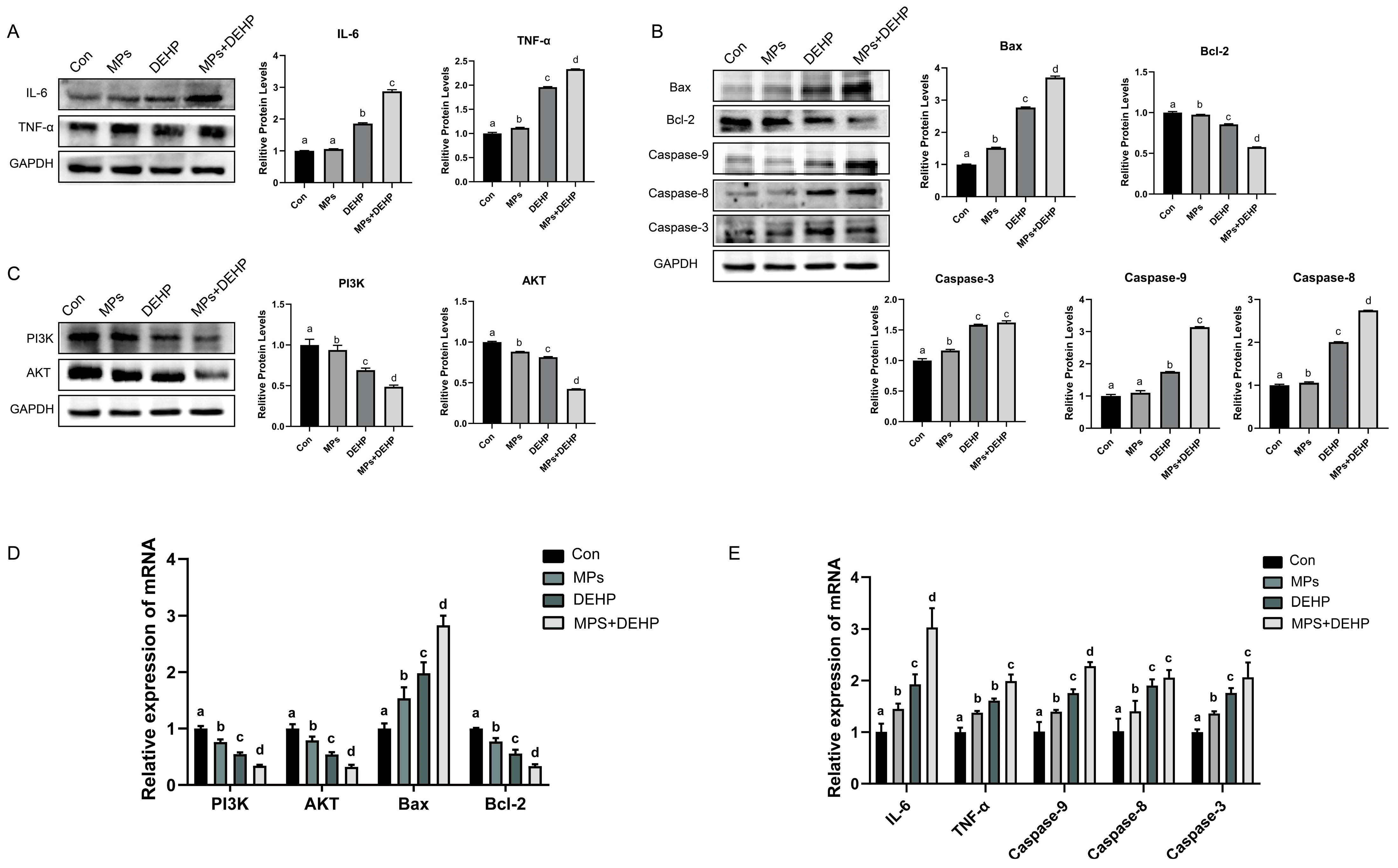
2.6. Effects of MPs, DEHP, and MPs + DEHP on the PI3K/AKT Pathway in Mouse Liver Cells
3. Discussion
3.1. Effects of MP and DEHP Exposure on the Mouse Liver
3.2. Effects of MP and DEHP Exposure on Oxidative Stress and Inflammatory Responses in Mice
3.3. Effects of MP and DEHP Exposure on Disruption of Amino Acid Metabolism of Mice
3.4. Effects of MP and DEHP Exposure on Lipid Metabolism Disorders and Membrane Integrity of Mice
3.5. Effects of MP and DEHP Exposure on the PI3K/AKT Signaling Pathway and Liver Apoptosis
3.6. Effects of MP and DEHP Exposure on Systemic Implications
4. Materials and Methods
4.1. Chemicals and Animals
4.2. Experimental Design and Exposure
4.3. Measurement of Oxidative Stress Markers
4.4. Determination of Biochemical Parameters
4.5. H&E Staining
4.6. RNA Sequencing and Transcriptomic Analysis
4.7. UHPLC-MS/MS Metabolomic Analysis
4.8. Integrated Analysis of Transcriptomics and Metabolomics
4.9. Western Blot Analysis
4.10. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banerjee, A.; Shelver, W.L. Micro- and nanoplastic induced cellular toxicity in mammals: A review. Sci. Total Environ. 2021, 755 Pt 2, 142518. [Google Scholar] [CrossRef]
- Basto, M.N.; Nicastro, K.R.; Tavares, A.I.; McQuaid, C.D.; Casero, M.; Azevedo, F.; Zardi, G.I. Plastic ingestion in aquatic birds in Portugal. Mar. Pollut. Bull. 2019, 138, 19–24. [Google Scholar] [CrossRef]
- Stubbins, A.; Law, K.L.; Muñoz, S.E.; Bianchi, T.S.; Zhu, L. Plastics in the Earth system. Science 2021, 373, 51–55. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, X.; Qi, X.; Liu, X.; Zhang, Y.; Qiao, S.; Lin, H. Di-(2-Ethylhexyl) Phthalate and Microplastics Induced Neuronal Apoptosis through the PI3K/AKT Pathway and Mitochondrial Dysfunction. J. Agric. Food Chem. 2022, 70, 10771–10781. [Google Scholar] [CrossRef]
- Okoffo, E.D.; O’Brien, S.; Ribeiro, F.; Burrows, S.D.; Toapanta, T.; Rauert, C.; Obrien, J.W.; Tscharke, B.J.; Wang, X.; Thomas, K.V. Plastic particles in soil: State of the knowledge on sources, occurrence and distribution, analytical methods and ecological impacts. Environ. Sci. Process. Impacts 2021, 23, 240–274. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Basu, S.; Shetti, N.P.; Nadagouda, M.N.; Aminabhavi, T.M. Microplastics in the environment: Occurrence, perils, and eradication. Chem. Eng. J. 2021, 408, 127317. [Google Scholar] [CrossRef]
- Qin, Z.H.; Mou, J.H.; Chao, C.Y.H.; Chopra, S.S.; Daoud, W.; Leu, S.Y.; Ning, Z.; Tso, C.Y.; Chan, C.K.; Tang, S.; et al. Biotechnology of Plastic Waste Degradation, Recycling, and Valorization: Current Advances and Future Perspectives. ChemSusChem 2021, 14, 4103–4114. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Wang, S.; Li, G.; Du, L.; Dong, X. Microplastics in the soil: A review of distribution, anthropogenic impact, and interaction with soil microorganisms based on meta-analysis. Sci. Total Environ. 2022, 832, 154975. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, Z.; Shen, R.; Wang, M.; Huang, Y.; Ren, H.; Zhang, Y.; Lemos, B. Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environ. Int. 2020, 143, 105916. [Google Scholar] [CrossRef] [PubMed]
- Shengchen, W.; Jing, L.; Yujie, Y.; Yue, W.; Shiwen, X. Polystyrene microplastics-induced ROS overproduction disrupts the skeletal muscle regeneration by converting myoblasts into adipocytes. J. Hazard. Mater. 2021, 417, 125962. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuan, Q.; Zhang, L.; Meng, L.; Fu, X.; Hou, Y. Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mater. 2022, 424 Pt C, 127629. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Zhang, X.Q.; Zhang, Z.M.; Wang, N.B.; Liu, H.; Zhang, R.R.; Sun, A.L.; Chen, J.; Shi, X.Z. Transcriptome sequencing and metabolite analysis reveal the single and combined effects of microplastics and di-(2-ethylhexyl) phthalate on Peneaus vannamei. Sci. Total Environ. 2023, 867, 161549. [Google Scholar] [CrossRef]
- Liang, B.; Huang, Y.; Zhong, Y.; Li, Z.; Ye, R.; Wang, B.; Zhang, B.; Meng, H.; Lin, X.; Du, J.; et al. Brain single-nucleus transcriptomics highlights that polystyrene nanoplastics potentially induce Parkinson’s disease-like neurodegeneration by causing energy metabolism disorders in mice. J. Hazard. Mater. 2022, 430, 128459. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Wu, P.; Lin, S.; Cao, G.; Wu, J.; Jin, H.; Wang, C.; Wong, M.H.; Yang, Z.; Cai, Z. Absorption, distribution, metabolism, excretion and toxicity of microplastics in the human body and health implications. J. Hazard. Mater. 2022, 437, 129361. [Google Scholar] [CrossRef]
- Pironti, C.; Notarstefano, V.; Ricciardi, M.; Motta, O.; Giorgini, E.; Montano, L. First Evidence of Microplastics in Human Urine, a Preliminary Study of Intake in the Human Body. Toxics 2022, 11, 40. [Google Scholar] [CrossRef]
- Roslan, N.S.; Lee, Y.Y.; Ibrahim, Y.S.; Tuan Anuar, S.; Yusof, K.; Lai, L.A.; Brentnall, T. Detection of microplastics in human tissues and organs: A scoping review. J. Glob. Health 2024, 14, 04179. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Weng, J.; Jin, Z.; Cao, Y.; Jiang, H.; Zhang, Z. Detection and characterization of microplastics in the human testis and semen. Sci. Total Environ. 2023, 877, 162713. [Google Scholar] [CrossRef]
- Montano, L.; Raimondo, S.; Piscopo, M.; Ricciardi, M.; Guglielmino, A.; Chamayou, S.; Gentile, R.; Gentile, M.; Rapisarda, P.; Oliveri Conti, G.; et al. First evidence of microplastics in human ovarian follicular fluid: An emerging threat to female fertility. Ecotoxicol. Environ. Saf. 2025, 291, 117868. [Google Scholar] [CrossRef]
- Chen, C.C.; Zhu, X.; Xu, H.; Chen, F.; Ma, J.; Pan, K. Copper Adsorption to Microplastics and Natural Particles in Seawater: A Comparison of Kinetics, Isotherms, and Bioavailability. Environ. Sci. Technol. 2021, 55, 13923–13931. [Google Scholar] [CrossRef]
- Li, R.; Zhu, L.; Yang, K.; Li, H.; Zhu, Y.G.; Cui, L. Impact of Urbanization on Antibiotic Resistome in Different Microplastics: Evidence from a Large-Scale Whole River Analysis. Environ. Sci. Technol. 2021, 55, 8760–8770. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Tao, Y.; Guo, X.; Cui, Y.; Li, Z. Phthalates (PAEs) and reproductive toxicity: Hypothalamic-pituitary-gonadal (HPG) axis aspects. J. Hazard. Mater. 2023, 459, 132182. [Google Scholar] [CrossRef]
- Radke, E.G.; Galizia, A.; Thayer, K.A.; Cooper, G.S. Phthalate exposure and metabolic effects: A systematic review of the human epidemiological evidence. Environ. Int. 2019, 132, 104768. [Google Scholar] [CrossRef]
- Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006, 29, 134–139; discussion 181–185. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef]
- Thayer, K.A.; Heindel, J.J.; Bucher, J.R.; Gallo, M.A. Role of environmental chemicals in diabetes and obesity: A National Toxicology Program workshop review. Environ. Health Perspect. 2012, 120, 779–789. [Google Scholar] [CrossRef]
- Kay, V.R.; Bloom, M.S.; Foster, W.G. Reproductive and developmental effects of phthalate diesters in males. Crit. Rev. Toxicol. 2014, 44, 467–498. [Google Scholar] [CrossRef] [PubMed]
- Erythropel, H.C.; Maric, M.; Nicell, J.A.; Leask, R.L.; Yargeau, V. Leaching of the plasticizer di(2-ethylhexyl)phthalate (DEHP) from plastic containers and the question of human exposure. Appl. Microbiol. Biotechnol. 2014, 98, 9967–9981. [Google Scholar] [CrossRef]
- Wowkonowicz, P.; Kijeńska, M.; Koda, E. Potential environmental risk assessment of di-2-ethylhexyl phthalate emissions from a municipal solid waste landfill leachate. PeerJ 2021, 9, e12163. [Google Scholar] [CrossRef]
- Jiang, F.W.; Yang, Z.Y.; Bian, Y.F.; Cui, J.G.; Zhang, H.; Zhao, Y.; Li, J.L. The novel role of the aquaporin water channel in lycopene preventing DEHP-induced renal ionic homeostasis disturbance in mice. Ecotoxicol. Environ. Saf. 2021, 226, 112836. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Ye, S.; Tang, S.; Liang, N.; Liang, Y.; Xiao, F. Polystyrene microplastics trigger hepatocyte apoptosis and abnormal glycolytic flux via ROS-driven calcium overload. J. Hazard. Mater. 2021, 417, 126025. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, L.; Shin, N.; Lascarez-Lagunas, L.I.; Martinez-Garcia, M.; Nadarajan, S.; Karthikraj, R.; Kannan, K.; Colaiácovo, M.P. Environmentally-relevant exposure to diethylhexyl phthalate (DEHP) alters regulation of double-strand break formation and crossover designation leading to germline dysfunction in Caenorhabditis elegans. PLoS Genet. 2020, 16, e1008529. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Q.; Yu, H.; Yang, L.; Sun, Y.; Xu, N.; Wang, N.; Lei, Z.; Hou, J.; Jin, Y.; et al. Polystyrene microplastics induce blood-testis barrier disruption regulated by the MAPK-Nrf2 signaling pathway in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 47921–47931. [Google Scholar] [CrossRef]
- Coffin, S.; Lee, I.; Gan, J.; Schlenk, D. Simulated digestion of polystyrene foam enhances desorption of diethylhexyl phthalate (DEHP) and In vitro estrogenic activity in a size-dependent manner. Environ. Pollut. 2019, 246, 452–462. [Google Scholar] [CrossRef]
- Huang, W.; Xiao, J.; Shi, X.; Zheng, S.; Li, H.; Liu, C.; Wu, K. Effects of di-(2-ethylhexyl) phthalate (DEHP) on behavior and dopamine signaling in zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2022, 93, 103885. [Google Scholar] [CrossRef] [PubMed]
- Capela, D.; Mhaouty-Kodja, S. Effects of pubertal exposure to low doses of di-(2-ethylexyl)phthalate on reproductive behaviors in male mice. Chemosphere 2021, 263, 128191. [Google Scholar] [CrossRef]
- Adamovsky, O.; Buerger, A.N.; Vespalcova, H.; Sohag, S.R.; Hanlon, A.T.; Ginn, P.E.; Craft, S.L.; Smatana, S.; Budinska, E.; Persico, M.; et al. Evaluation of Microbiome-Host Relationships in the Zebrafish Gastrointestinal System Reveals Adaptive Immunity Is a Target of Bis(2-ethylhexyl) Phthalate (DEHP) Exposure. Environ. Sci. Technol. 2020, 54, 5719–5728. [Google Scholar] [CrossRef]
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and diet: A review of the food monitoring and epidemiology data. Environ. Health A Glob. Access Sci. Source 2014, 13, 43. [Google Scholar]
- Pakalin, S.; Aschberger, K.; Cosgrove, O.; Lund, B.-O.; Paya-Perez, A.; Vergro, S. European Union Risk Assessment Report—Bis (2-Ethylhexyl) Phthalate (DEHP); OPOCE: Luxembourg, 2008. [Google Scholar]
- Conkle, J.L.; Báez Del Valle, C.D.; Turner, J.W. Are We Underestimating Microplastic Contamination in Aquatic Environments? Environ. Manag. 2018, 61, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef] [PubMed]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Wang, S.; Lai, X.; Deng, Y.; Song, Y. Correlation between mouse age and human age in anti-tumor research: Significance and method establishment. Life Sci. 2020, 242, 117242. [Google Scholar] [CrossRef]
- Agrawal, S.; Dhiman, R.K.; Limdi, J.K. Evaluation of abnormal liver function tests. Postgrad. Med. J. 2016, 92, 223–234. [Google Scholar] [CrossRef]
- Sakka, S.G. Assessing liver function. Curr. Opin. Crit. Care 2007, 13, 207–214. [Google Scholar] [CrossRef]
- Wang, H.; Ran, J.; Jiang, T. Urea. Sub-Cell. Biochem. 2014, 73, 7–29. [Google Scholar]
- Kim, J.H.; Yu, Y.B.; Choi, J.H. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef]
- Wang, W.N.; Zhou, J.; Wang, P.; Tian, T.T.; Zheng, Y.; Liu, Y.; Mai, W.J.; Wang, A.L. Oxidative stress, DNA damage and antioxidant enzyme gene expression in the Pacific white shrimp, Litopenaeus vannamei when exposed to acute pH stress. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2009, 150, 428–435. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Guo, M.; Shao, Y.; Liu, J.; Jiang, G.; Xing, M. Arsenite renal apoptotic effects in chickens co-aggravated by oxidative stress and inflammatory response. Met. Integr. Biometal Sci. 2018, 10, 1805–1813. [Google Scholar] [CrossRef]
- Cui, J.; Shan, K.; Yang, Q.; Qi, Y.; Qu, H.; Li, J.; Wang, R.; Jia, L.; Chen, W.; Feng, N.; et al. Prostaglandin E(3) attenuates macrophage-associated inflammation and prostate tumour growth by modulating polarization. J. Cell. Mol. Med. 2021, 25, 5586–5601. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef]
- Schenker, S.; Hu, Z.Q.; Johnson, R.F.; Yang, Y.; Frosto, T.; Elliott, B.D.; Henderson, G.I.; Mock, D.M. Human placental biotin transport: Normal characteristics and effect of ethanol. Alcohol. Clin. Exp. Res. 1993, 17, 566–575. [Google Scholar] [CrossRef]
- Aghaei, Z.; Mercer, G.V.; Schneider, C.M.; Sled, J.G.; Macgowan, C.K.; Baschat, A.A.; Kingdom, J.C.; Helm, P.A.; Simpson, A.J.; Simpson, M.J.; et al. Maternal exposure to polystyrene microplastics alters placental metabolism in mice. Metabolomics Off. J. Metabolomic Soc. 2022, 19, 1. [Google Scholar] [CrossRef]
- Mühlhausen, C.; Burckhardt, B.C.; Hagos, Y.; Burckhardt, G.; Keyser, B.; Lukacs, Z.; Ullrich, K.; Braulke, T. Membrane translocation of glutaric acid and its derivatives. J. Inherit. Metab. Dis. 2008, 31, 188–193. [Google Scholar] [CrossRef]
- Balazs, L.; Okolicany, J.; Ferrebee, M.; Tolley, B.; Tigyi, G. Topical application of the phospholipid growth factor lysophosphatidic acid promotes wound healing in vivo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R466–R472. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Qiao, R.; Bonilla, M.M.; Yang, X.; Ren, H.; Lemos, B. Evidence that microplastics aggravate the toxicity of organophosphorus flame retardants in mice (Mus musculus). J. Hazard. Mater. 2018, 357, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Perrotti, F.; Rosa, C.; Cicalini, I.; Sacchetta, P.; Del Boccio, P.; Genovesi, D.; Pieragostino, D. Advances in Lipidomics for Cancer Biomarkers Discovery. Int. J. Mol. Sci. 2016, 17, 1992. [Google Scholar] [CrossRef]
- Su, H.; Yuan, P.; Lei, H.; Zhang, L.; Deng, D.; Zhang, L.; Chen, X. Long-term chronic exposure to di-(2-ethylhexyl)-phthalate induces obesity via disruption of host lipid metabolism and gut microbiota in mice. Chemosphere 2022, 287 Pt 4, 132414. [Google Scholar] [CrossRef]
- Mårtensson, C.U.; Doan, K.N.; Becker, T. Effects of lipids on mitochondrial functions. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2017, 1862, 102–113. [Google Scholar] [CrossRef]
- Shimizu, S. [Pantothenic acid]. Nihon rinsho. Jpn. J. Clin. Med. 1999, 57, 2218–2222. [Google Scholar]
- Czumaj, A.; Szrok-Jurga, S.; Hebanowska, A.; Turyn, J.; Swierczynski, J.; Sledzinski, T.; Stelmanska, E. The Pathophysiological Role of CoA. Int. J. Mol. Sci. 2020, 21, 9057. [Google Scholar] [CrossRef]
- Teperino, R.; Schoonjans, K.; Auwerx, J. Histone methyl transferases and demethylases; can they link metabolism and transcription? Cell Metab. 2010, 12, 321–327. [Google Scholar] [CrossRef]
- Hao, C.; Cheng, X.; Xia, H.; Ma, X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci. Rep. 2012, 32, 619–629. [Google Scholar] [CrossRef]
- Barouki, R.; Samson, M.; Blanc, E.B.; Colombo, M.; Zucman-Rossi, J.; Lazaridis, K.N.; Miller, G.W.; Coumoul, X. The exposome and liver disease-how environmental factors affect liver health. J. Hepatol. 2023, 79, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Hu, J.C.; Li, P.Y.; Huang, G.J.; Kuo, Y.H.; Chao, C.Y. Torenia concolor Lindley var. formosana Yamazaki extracts improve inflammatory response and lipid accumulation via PPARs activation. BioMedicine 2017, 7, 18. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Tang, Y.X.; Qiu, B.H.; Talukder, M.; Li, X.N.; Li, J.L. Di-2-ethylhexyl phthalate (DEHP) induced lipid metabolism disorder in liver via activating the LXR/SREBP-1c/PPARα/γ and NF-κB signaling pathway. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2022, 165, 113119. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liang, J.; Zhang, S.; Yan, H.; Luan, T. Di-2-ethylhexyl phthalate disrupts hepatic lipid metabolism in obese mice by activating the LXR/SREBP-1c and PPAR-α signaling pathways. Sci. Total Environ. 2024, 914, 169919. [Google Scholar] [CrossRef] [PubMed]
- Feige, J.N.; Gerber, A.; Casals-Casas, C.; Yang, Q.; Winkler, C.; Bedu, E.; Bueno, M.; Gelman, L.; Auwerx, J.; Gonzalez, F.J.; et al. The pollutant diethylhexyl phthalate regulates hepatic energy metabolism via species-specific PPARalpha-dependent mechanisms. Environ. Health Perspect. 2010, 118, 234–241. [Google Scholar] [CrossRef]
- Osaki, M.; Oshimura, M.; Ito, H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis Int. J. Program. Cell Death 2004, 9, 667–676. [Google Scholar] [CrossRef]
- Khezri, M.R.; Jafari, R.; Yousefi, K.; Zolbanin, N.M. The PI3K/AKT signaling pathway in cancer: Molecular mechanisms and possible therapeutic interventions. Exp. Mol. Pathol. 2022, 127, 104787. [Google Scholar] [CrossRef]
- Mao, Y.; Xi, L.; Li, Q.; Cai, Z.; Lai, Y.; Zhang, X.; Yu, C. Regulation of cell apoptosis and proliferation in pancreatic cancer through PI3K/Akt pathway via Polo-like kinase 1. Oncol. Rep. 2016, 36, 49–56. [Google Scholar] [CrossRef]
- Lee, C.Y.; Suk, F.M.; Twu, Y.C.; Liao, Y.J. Long-Term Exposure to Low-Dose Di-(2-ethylhexyl) Phthalate Impairs Cholesterol Metabolism in Hepatic Stellate Cells and Exacerbates Liver Librosis. Int. J. Environ. Res. Public Health 2020, 17, 3802. [Google Scholar] [CrossRef]
- Li, L.; Wang, F.; Zhang, J.; Wang, K.; De, X.; Li, L.; Zhang, Y. Typical phthalic acid esters induce apoptosis by regulating the PI3K/Akt/Bcl-2 signaling pathway in rat insulinoma cells. Ecotoxicol. Environ. Saf. 2021, 208, 111461. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, W.; Yang, F.; Feng, T.; Zhou, M.; Yu, Y.; Yu, X.; Zhao, W.; Yi, F.; Tang, W.; et al. Interleukin-6-stimulated progranulin expression contributes to the malignancy of hepatocellular carcinoma cells by activating mTOR signaling. Sci. Rep. 2016, 6, 21260. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sun, X.; Guo, T.; Su, H.; Chen, Q.; Gong, Z.; Qi, J.; Zhao, X. Interleukin-1 receptor antagonist inhibits angiogenesis via blockage IL-1α/PI3K/NF-κβ pathway in human colon cancer cell. Cancer Manag. Res. 2017, 9, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Lv, Y.; Wang, Z.; Ma, J.; Mi, C.; Li, X.; Xu, G.H.; Piao, L.X.; Zheng, S.Z.; Jin, X. Imperatorin efficiently blocks TNF-α-mediated activation of ROS/PI3K/Akt/NF-κB pathway. Oncol. Rep. 2017, 37, 3397–3404. [Google Scholar] [CrossRef]
- Erkekoglu, P.; Kocer-Gumusel, B. Genotoxicity of phthalates. Toxicol. Mech. Methods 2014, 24, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, C.; Scarciello, G.; Bianchi, A.R.; Berman, B.; Chianese, T.; Scudiero, R.; Rosati, L.; De Maio, A.; Lettieri, G.; Piscopo, M. Toxicological effects and potential reproductive risk of microplastic-induced molecular changes in protamine-like proteins and their DNA binding. Chem.-Biol. Interact. 2025, 405, 111309. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Z.; Zhu, R.; Gou, D.; Jia, P.P.; Pei, D.S. An insight into sex-specific neurotoxicity and molecular mechanisms of DEHP: A critical review. Environ. Pollut. 2023, 316 Pt 2, 120673. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, Y.; Wang, Y.; Li, Z.; Li, X.; Bao, H.; Li, J.; Zhou, D. Transcriptome Sequencing and Metabolite Analysis Revealed the Single and Combined Effects of Microplastics and Di-(2-ethylhexyl) Phthalate on Mouse Liver. Int. J. Mol. Sci. 2025, 26, 4943. https://doi.org/10.3390/ijms26104943
Zhang J, Li Y, Wang Y, Li Z, Li X, Bao H, Li J, Zhou D. Transcriptome Sequencing and Metabolite Analysis Revealed the Single and Combined Effects of Microplastics and Di-(2-ethylhexyl) Phthalate on Mouse Liver. International Journal of Molecular Sciences. 2025; 26(10):4943. https://doi.org/10.3390/ijms26104943
Chicago/Turabian StyleZhang, Jiabin, Yangcheng Li, Yihan Wang, Zeyu Li, Xiaolei Li, Hongxia Bao, Jiakui Li, and Donghai Zhou. 2025. "Transcriptome Sequencing and Metabolite Analysis Revealed the Single and Combined Effects of Microplastics and Di-(2-ethylhexyl) Phthalate on Mouse Liver" International Journal of Molecular Sciences 26, no. 10: 4943. https://doi.org/10.3390/ijms26104943
APA StyleZhang, J., Li, Y., Wang, Y., Li, Z., Li, X., Bao, H., Li, J., & Zhou, D. (2025). Transcriptome Sequencing and Metabolite Analysis Revealed the Single and Combined Effects of Microplastics and Di-(2-ethylhexyl) Phthalate on Mouse Liver. International Journal of Molecular Sciences, 26(10), 4943. https://doi.org/10.3390/ijms26104943






