Cation and Spin Interactions in Cadmium Ferrite: A Quantum Mechanical Study
Abstract
1. Introduction
2. Result and Discussion
2.1. Energy, Geometric Structure, Charge and Spin Distribution, and Electronic Structure
2.2. Lattice Dynamics
2.3. Spectroscopic Characterization
3. Materials and Methods
4. Conclusions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Rahman, A.; Zulfiqar, S.; Haq, A.U.; Alsafari, I.A.; Qazi, U.Y.; Warsi, M.F.; Shahid, M. Cd-Gd-Doped Nickel Spinel Ferrite Nanoparticles and Their Nanocomposites with Reduced Graphene Oxide for Catalysis and Antibacterial Activity Studies. Ceram. Int. 2021, 47, 9513–9521. [Google Scholar] [CrossRef]
- Senapati, K.K.; Borgohain, C.; Phukan, P. Synthesis of Highly Stable CoFe2O4 Nanoparticles and Their Use as Magnetically Separable Catalyst for Knoevenagel Reaction in Aqueous Medium. J. Mol. Catal. A Chem. 2011, 339, 24–31. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled Preparation, Oxygen Reduction/Evolution Reaction Application, and Beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef]
- Wu, K.; Li, J.; Zhang, C. Zinc Ferrite Based Gas Sensors: A Review. Ceram. Int. 2019, 45, 11143–11157. [Google Scholar] [CrossRef]
- Silva, O.; Morais, P. Investigation of Anisotropy in Cadmium Ferrite-Based Ionic Magnetic Fluid Using Magnetic Resonance. J. Magn. Magn. Mater. 2005, 289, 136–138. [Google Scholar] [CrossRef]
- Nayak, P. Synthesis and Characterization of Cadmium Ferrite. Mater. Chem. Phys. 2008, 112, 24–26. [Google Scholar] [CrossRef]
- Su, M.; Liao, C.; Chan, T.; Shih, K.; Xiao, T.; Chen, D.; Kong, L.; Song, G. Incorporation of Cadmium and Nickel into Ferrite Spinel Solid Solution: X-ray Diffraction and X-Ray Absorption Fine Structure Analyses. Environ. Sci. Technol. 2018, 52, 775–782. [Google Scholar] [CrossRef]
- Yokoyama, M.; Sato, T.; Ohta, E.; Sato, T. Magnetization of Cadmium Ferrite Prepared by Coprecipitation. J. Appl. Phys. 1996, 80, 1015–1019. [Google Scholar] [CrossRef]
- Sinha, M.; Pradhan, S. Synthesis of Nanocrystalline Cd--Zn Ferrite by Ball Milling and Its Stability at Elevated Temperatures. J. Alloys Compd. 2010, 489, 91–98. [Google Scholar] [CrossRef]
- Naseri, M. Optical and Magnetic Properties of Monophasic Cadmium Ferrite (CdFe2O4) Nanostructure Prepared by Thermal Treatment Method. J. Magn. Magn. Mater. 2015, 392, 107–113. [Google Scholar] [CrossRef]
- Vasanthi, V.; Shanmugavani, A.; Sanjeeviraja, C.; Selvan, R.K. Microwave Assisted Combustion Synthesis of CdFe2O4: Magnetic and Electrical Properties. J. Magn. Magn. Mater. 2012, 324, 2100–2107. [Google Scholar] [CrossRef]
- Lou, X.; Liu, S.; Shi, D.; Chu, W. Ethanol-Sensing Characteristics of CdFe2O4 Sensor Prepared by Sol--Gel Method. Mater. Chem. Phys. 2007, 105, 67–70. [Google Scholar] [CrossRef]
- Deraz, N.; Hessien, M.M. Structural and Magnetic Properties of Pure and Doped Nanocrystalline Cadmium Ferrite. J. Alloys Compd. 2009, 475, 832–839. [Google Scholar] [CrossRef]
- Bakuzis, A.; Skeff Neto, K.; Gravina, P.; Figueiredo, L.; Morais, P.; Silva, L.; Azevedo, R.; Silva, O. Magneto-Optical Properties of a Highly Transparent Cadmium Ferrite-Based Magnetic Fluid. Appl. Phys. Lett. 2004, 84, 2355–2357. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, J.; Randhawa, B. Essence of Superparamagnetism in Cadmium Ferrite Induced by Various Organic Fuels via Novel Solution Combustion Method. Ceram. Int. 2014, 40, 12235–12243. [Google Scholar] [CrossRef]
- Shi, W.; Liu, X.; Zhang, T.; Wang, Q.; Zhang, L. Magnetic Nano-Sized Cadmium Ferrite as an Efficient Catalyst for the Degradation of Congo Red in the Presence of Microwave Irradiation. RSC Adv. 2015, 5, 51027–51034. [Google Scholar] [CrossRef]
- Chen, N.-S.; Yang, X.-J.; Liu, E.-S.; Huang, J.-L. Reducing Gas-Sensing Properties of Ferrite Compounds MFe2O4 (M = Cu, Zn, Cd and Mg. Sens. Actuators B Chem. 2000, 66, 178–180. [Google Scholar] [CrossRef]
- Nekvapil, F.; Bunge, A.; Radu, T.; Cinta Pinzaru, S.; Turcu, R. Raman Spectra Tell Us so Much More: Raman Features and Saturation Magnetization for Efficient Analysis of Manganese Zinc Ferrite Nanoparticles. J. Raman Spectrosc. 2020, 51, 959–968. [Google Scholar] [CrossRef]
- Andhare, D.D.; Patade, S.R.; Jadhav, S.A.; Somvanshi, S.B.; Jadhav, K.M. Rietveld Refined Structural, Morphological, Raman and Magnetic Investigations of Superparamagnetic Zn–Co Nanospinel Ferrites Prepared by Cost-Effective Co-Precipitation Route. Appl. Phys. A Mater. Sci. Process 2021, 127, 480. [Google Scholar] [CrossRef]
- Murugesan, C.; Chandrasekaran, G. Impact of Gd3+ Substitution on the Structural, Magnetic and Electrical Properties of Cobalt Ferrite Nanoparticles. RSC Adv. 2015, 5, 73714–73725. [Google Scholar] [CrossRef]
- Chandramohan, P.; Srinivasan, M.P.; Velmurugan, S.; Narasimhan, S.V. Cation Distribution and Particle Size Effect on Raman Spectrum of CoFe2O4. J. Solid. State Chem. 2011, 184, 89–96. [Google Scholar] [CrossRef]
- Graves, P.; Johnston, C.; Campaniello, J. Raman Scattering in Spinel Structure Ferrites. Mater. Res. Bull. 1988, 23, 1651–1660. [Google Scholar] [CrossRef]
- Patil, S.; Bhojya Naik, H.; Nagaraju, G.; Shiralgi, Y. Sugarcane Juice Facilitated Eco-Friendly Synthesis of Solar Light Active CdFe2O4 Nanoparticles and Its Photocatalytic Application. Eur. Phys. J. Plus 2018, 133, 229. [Google Scholar] [CrossRef]
- Reitz, C.; Suchomski, C.; Chakravadhanula, V.S.K.; Djerdj, I.; Jagličić, Z.; Brezesinski, T. Morphology, Microstructure, and Magnetic Properties of Ordered Large-Pore Mesoporous Cadmium Ferrite Thin Film Spin Glasses. Inorg. Chem. 2013, 52, 3744–3754. [Google Scholar] [CrossRef]
- Kardile, H.; Somvanshi, S.B.; Chavan, A.R.; Pandit, A.; Jadhav, K. Effect of Cd2+ Doping on Structural, Morphological, Optical, Magnetic and Wettability Properties of Nickel Ferrite Thin Films. Optik 2020, 207, 164462. [Google Scholar] [CrossRef]
- Yokoyama, M.; Ohta, E.; Sato, T. Magnetic Properties of Ultrafine Particles and Bulk Materials of Cadmium Ferrite. J. Magn. Magn. Mater. 1998, 183, 173–180. [Google Scholar] [CrossRef]
- Almutairi, T.S. Unraveling the Complex Interplay of Phase Transitions in Spinel Ferrites: A Comprehensive Quantum Mechanical Vibrational Study of ZnFe2O4. ACS Omega 2023, 8, 36999–37010. [Google Scholar] [CrossRef]
- Harris, V.G.; Andalib, P. Goodenough–Kanamori–Anderson Rules of Super Exchange Applied to Ferrite Systems. In Modern Ferrites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022. [Google Scholar]
- White, W.; DeAngelis, B. Interpretation of the Vibrational Spectra of Spinels. Spectrochim. Acta A 1967, 23, 985–995. [Google Scholar] [CrossRef]
- Gillot, B.; Thiebaut, D.; Laarj, M. Synthesis of Stoichiometric Cadmium Substituted Magnetites and Formation by Oxidation of Solid Solutions of Cadmium Ferrite and Gamma-Iron Oxide. Thermochim. Acta 1999, 342, 167–174. [Google Scholar] [CrossRef]
- Saravanan, M.; Girisun, T.S. Nonlinear Optical Absorption and Optical Limiting Properties of Cadmium Ferrite. Mater. Chem. Phys. 2015, 160, 413–419. [Google Scholar] [CrossRef]
- Chethan, B.; Ravikiran, Y.; Vijayakumari, S.; Rajprakash, H.; Thomas, S. Nickel Substituted Cadmium Ferrite as Room Temperature Operable Humidity Sensor. Sens. Actuators A Phys. 2018, 280, 466–474. [Google Scholar] [CrossRef]
- Almutairi, T.S. Phase Transitions and Spectral Shifts: A Quantum Mechanical Exploration of Vibrational Frequency in Magnesium Ferrite. RSC Adv. 2024, 14, 2727–2740. [Google Scholar] [CrossRef]
- Almutairi, T.S. Unveiling the Impact of Spin and Cation Dynamics on Raman Spectroscopy in Co-Ferrite. ACS Phys. Chem. Au 2025, 5, 171–182. [Google Scholar] [CrossRef]
- Almutairi, T.S. Probing the Influence of Cation Distribution on the Vibrational Dynamics and Phase Transitions in Mg-Doped Zinc Ferrites: Quantum Mechanical Study. AIP Adv. 2024, 14, 055025. [Google Scholar] [CrossRef]
- Hu, T.; Chu, X.; Gao, F.; Dong, Y.; Sun, W.; Bai, L. Acetone Sensing Properties of Reduced Graphene Oxide-CdFe2O4 Composites Prepared by Hydrothermal Method. Mater. Sci. Semicond. Process 2015, 34, 146–153. [Google Scholar] [CrossRef]
- Dovesi, R.; Orlando, R.; Erba, A.; Zicovich-wilson, C.M.; Civalleri, B.; Casassa, S.; Maschio, L.; Ferrabone, M.; Pierre, M.D.L.; Arco, P.D.; et al. C RYSTAL 14: A Program for the Ab Initio Investigation of Crystalline Solids. Int. J. Quantum Chem. 2014, 114, 1287–1317. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. I. The Effect of the Exchange-only Gradient Correction. J. Chem. Phys. 1992, 3, 2155–2160. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Chemical Accuracy in the Computation of NMR Shieldings: The PBE0 Model. Chem. Phys. Lett. 1998, 298, 113–119. [Google Scholar] [CrossRef]
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the Exchange Screening Parameter on the Performance of Screened Hybrid Functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef]
- Fritsch, D. Electronic and Optical Properties of Spinel Zinc Ferrite: Ab Initio Hybrid Functional Calculations. J. Phys. Condens. Matter 2018, 30, 095502. [Google Scholar] [CrossRef]
- Catti, M.; Valerio, G.; Dovesi, R. Theoretical Study of Electronic, Magnetic, and Structural Properties of -Fe2O3 (Hematite). Phys. Rev. B 1995, 51, 7441–7450. [Google Scholar] [CrossRef]
- Towler, M.; Allan, N.; Harrison, N.M.; Saunders, V.; Mackrodt, W.; Apra, E. Ab Initio Study of Mno and Nio. Phys. Rev. B 1994, 50, 5041. [Google Scholar] [CrossRef]
- Dou, Y.; Egdell, R.; Law, D.; Harrison, N.; Searle, B. An Experimental and Theoretical Investigation of the Electronic Structure of CdO. J. Phys. Condens. Matter 1998, 10, 8447. [Google Scholar] [CrossRef][Green Version]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Pisani, C.; Dovesi, R.; Roetti, C. Hartree-Fock Ab Initio Treatment of Crystalline Systems; Lecture Notes in Chemistry; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9783642933851. [Google Scholar]
- Zicovich-Wilson, C.M.; Pascale, F.; Roetti, C.; Saunders, V.R.; Orlando, R.; Dovesi, R. Calculation of the Vibration Frequencies of α-Quartz: The Effect of Hamiltonian and Basis Set. J. Comput. Chem. 2004, 25, 1873–1881. [Google Scholar] [CrossRef]
- Pascale, F.; Zicovich-Wilson, C.M.; López Gejo, F.; Civalleri, B.; Orlando, R.; Dovesi, R. The Calculation of the Vibrational Frequencies of Crystalline Compounds and Its Implementation in the CRYSTAL Code; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Maschio, L.; Kirtman, B.; Rérat, M.; Orlando, R.; Dovesi, R. Ab Initio Analytical Raman Intensities for Periodic Systems through a Coupled Perturbed Hartree-Fock/Kohn-Sham Method in an Atomic Orbital Basis. I. Theory. J. Chem. Phys. 2013, 139, 164101. [Google Scholar] [CrossRef]
- Maschio, L.; Kirtman, B.; Rerat, M.; Orlando, R.; Dovesi, R. Comment on “Ab Initio Analytical Infrared Intensities for Periodic Systems through a Coupled Perturbed Hartree-Fock/Kohn-Sham Method” [J. Chem. Phys. 137, 204113 (2012)]. J. Chem. Phys. 2013, 139, 167101. [Google Scholar] [CrossRef]
- Hess, B.A., Jr.; Schaad, L.J.; Carsky, P.; Zahradnik, R. Ab Initio Calculations of Vibrational Spectra and Their Use in the Identification of Unusual Molecules. Chem. Rev. 1986, 86, 709–730. [Google Scholar] [CrossRef]
- Ferrero, M.; Ŕrat, M.; Orlando, R.; Dovesi, R. Coupled Perturbed Hartree-Fock for Periodic Systems: The Role of Symmetry and Related Computational Aspects. J. Chem. Phys. 2008, 128, 014110. [Google Scholar] [CrossRef]
- Ferrero, M.; Rérat, M.; Orlando, R.; Dovesi, R.; Bush, I.J. Coupled Perturbed Kohn-Sham Calculation of Static Polarizabilities of Periodic Compounds. In Proceedings of the Journal of Physics: Conference Series; Institute of Physics Publishing: Bristol, UK, 2008; Volume 117. [Google Scholar]
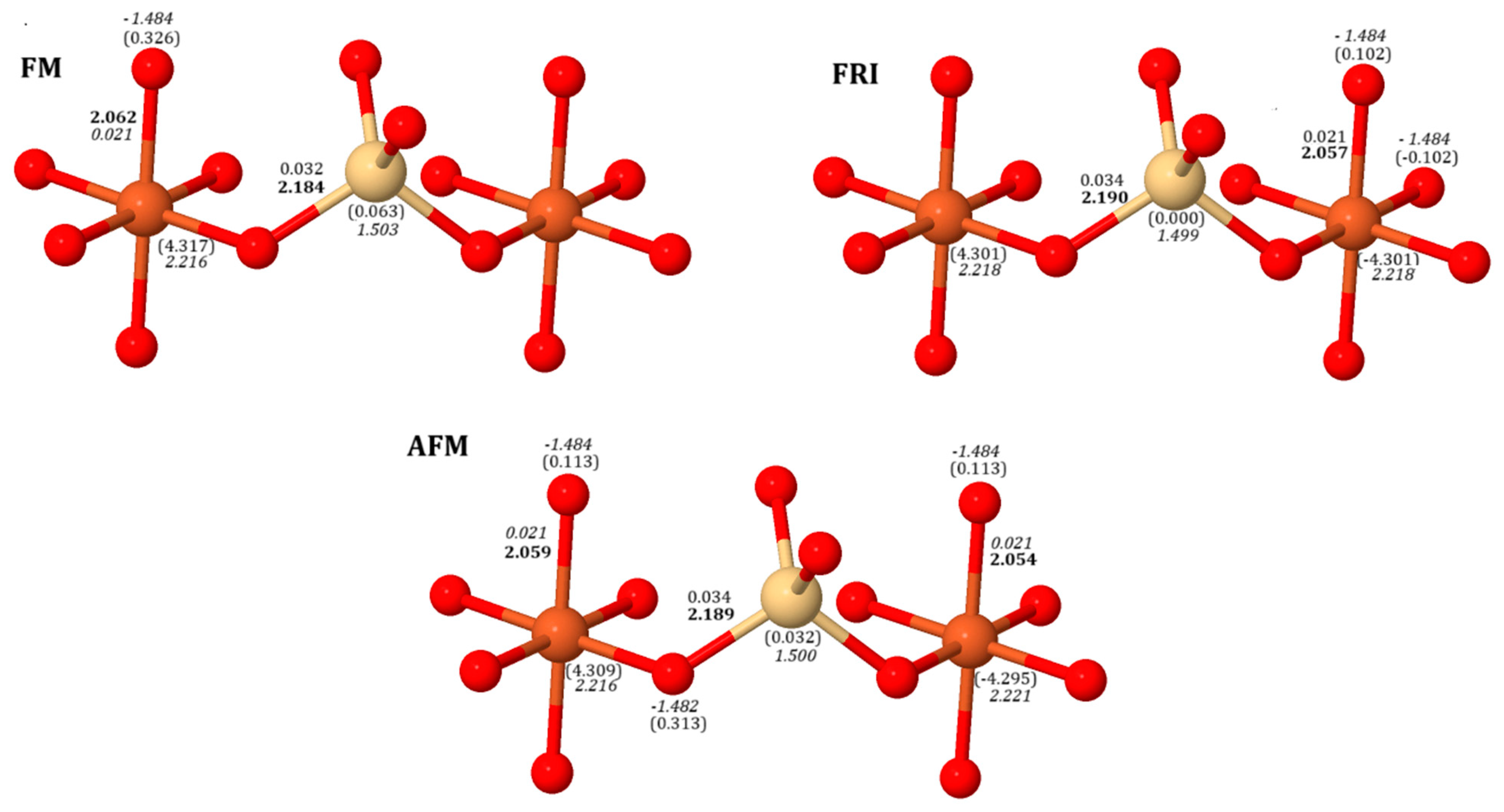
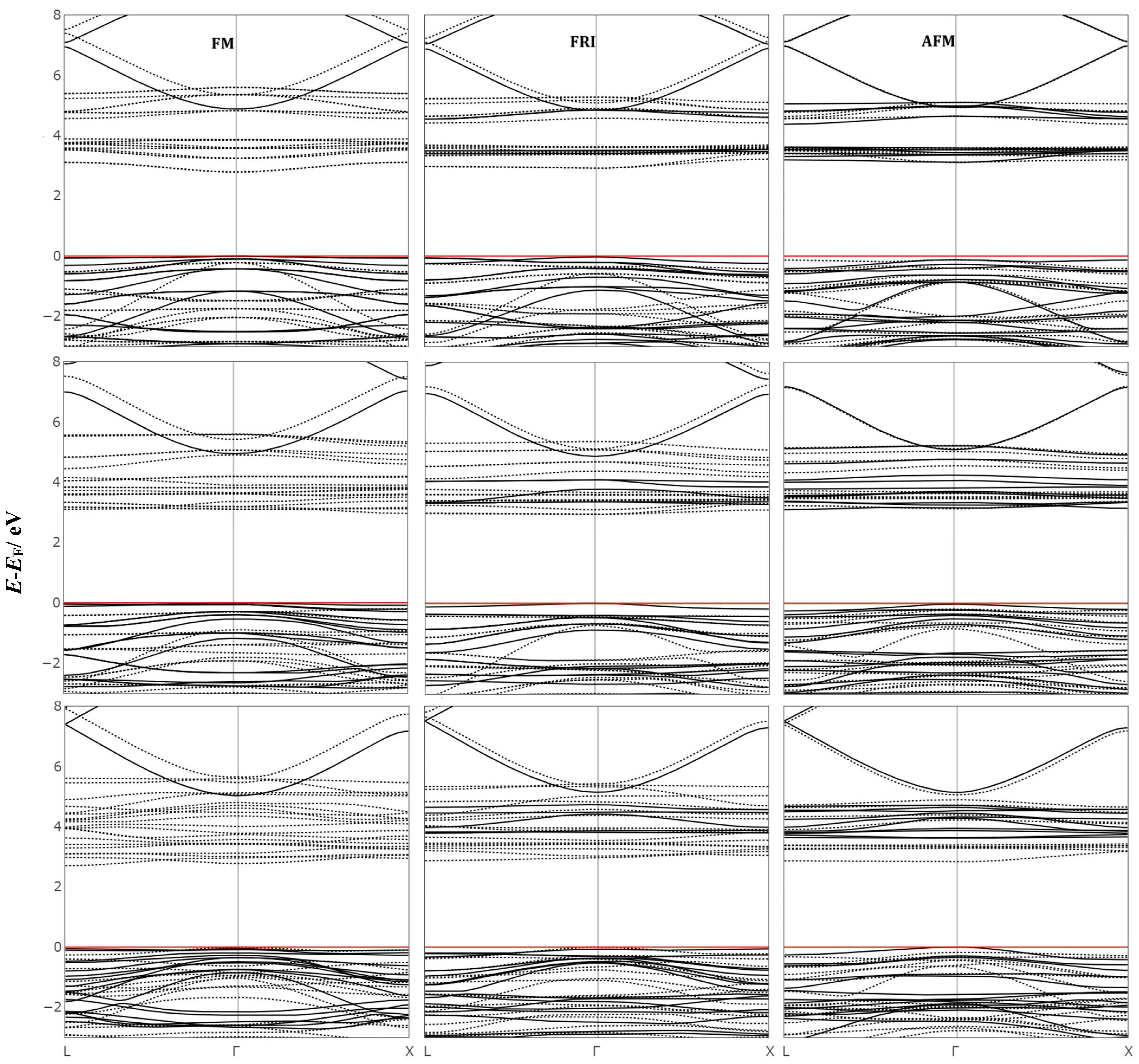
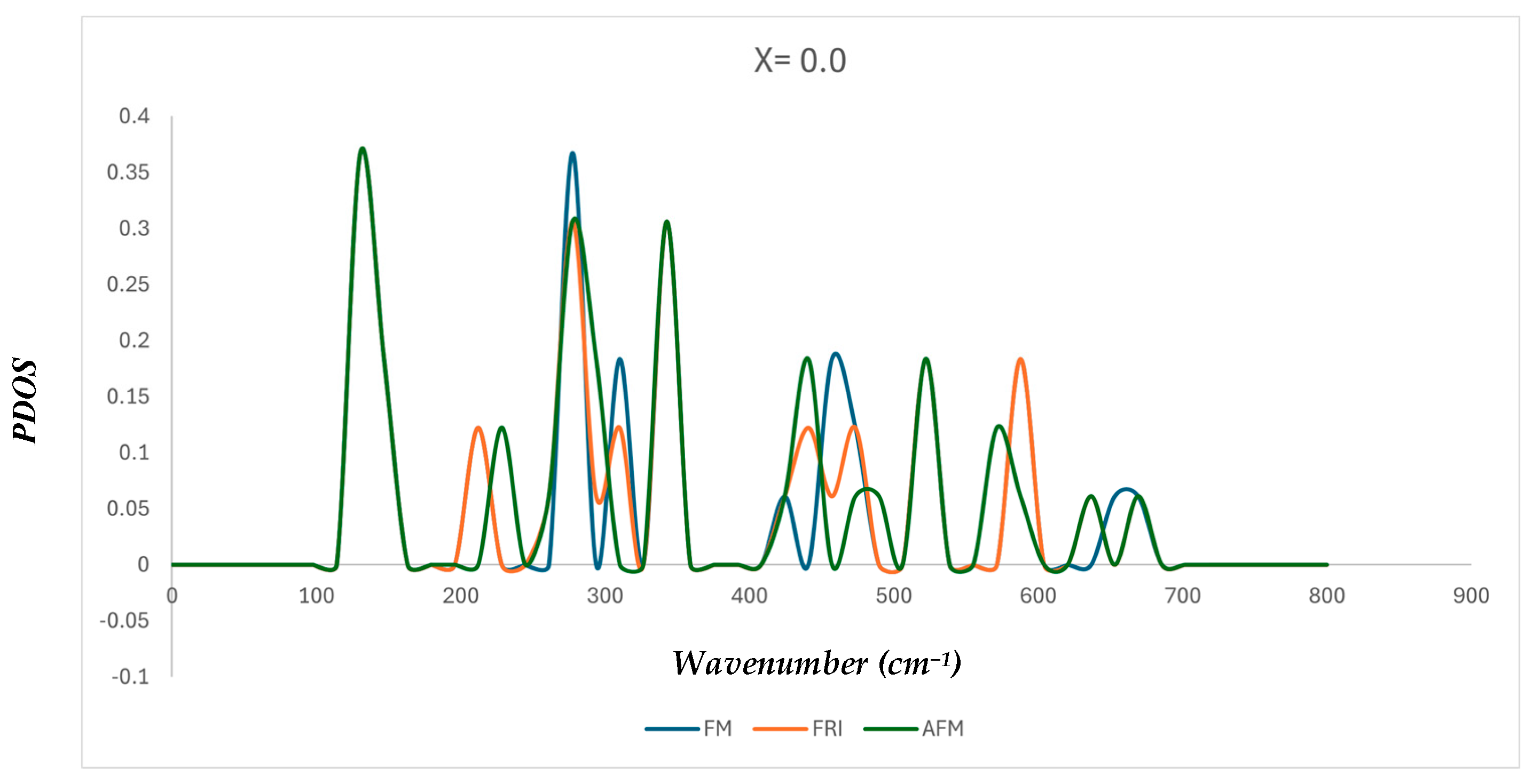
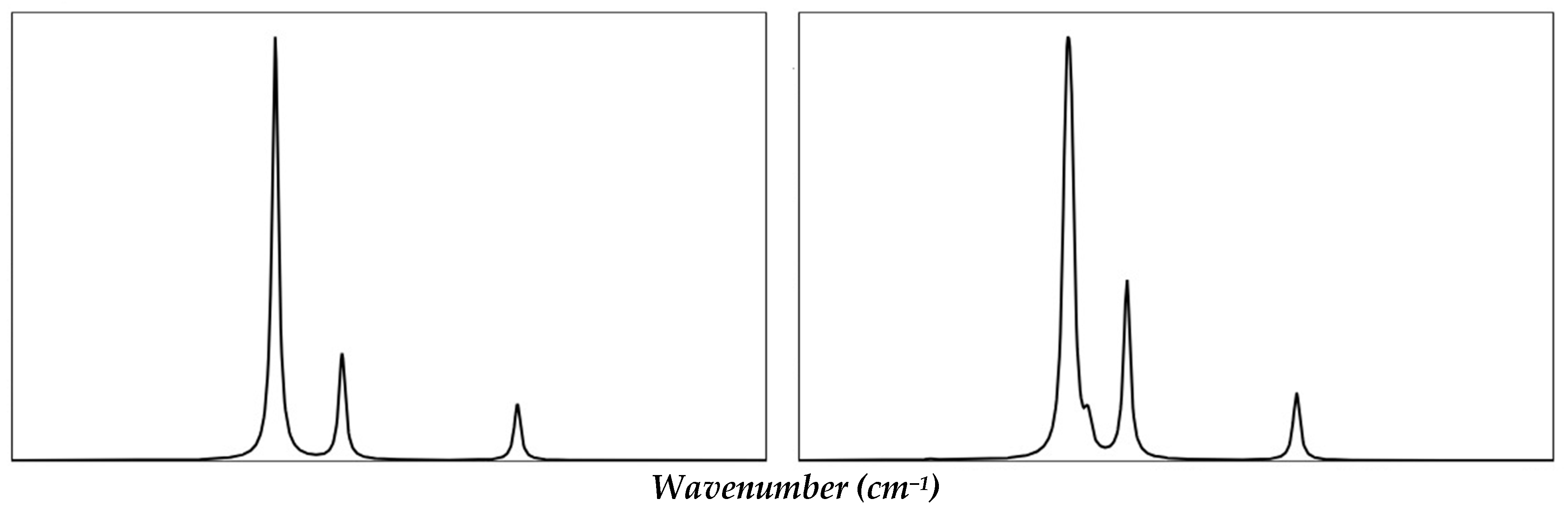


| Functionals | (X = 0) | (X = 0.5) | (X = 1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FM | FRI | AFM | FM | FRI | AFM | FM | FRI | AFM | ||
| B3LYP | E | −16,591.3505 | −16,591.3506 | −16,591.3508 | −16,591.3068 | −16,591.3192 | −16,591.3150 | −16,591.2923 | −16,591.3055 | −16,591.3173 |
| ER | (0.008) | (0.005) | (0.000) | (1.197) | (0.859) | (0.974) | (1.591) | (1.232) | (0.911) | |
| HSE06 | E | −16,590.0006 | −16,590.0008 | −16,590.0009 | −16,589.95282 | −16,589.9644 | −16,589.9602 | −16,589.9379 | −16,589.9502 | −16,589.9613 |
| ER | (0.008) | (0.003) | (0.000) | (1.308) | (0.993) | (1.107) | (1.714) | (1.379) | (1.077) | |
| PBE0 | E | −16,590.0070 | −16,590.0071 | −16,590.0072 | −16,589.9591 | −16,589.9702 | −16,589.9660 | −16,589.9438 | −16,589.9558 | −16,589.9665 |
| ER | (0.005) | (0.003) | (0.000) | (1.308) | (1.006) | (1.121) | (1.725) | (1.398) | (1.107) | |
| Energy | X = 0 | X = 0.5 | X = 1 | |||
|---|---|---|---|---|---|---|
| FM | AFM | FM | AFM | FM | AFM | |
| EL | −451,473.6017 | −451,473.6077 | −451,472.4116 | −451,472.6339 | −451,472.0165 | −451,472.6978 |
| (0.006) | (0.000) | (1.196) | (0.973) | (1.591) | (0.909) | |
| E0 | 0.848033 | 0.837199 | 0.838940 | 0.832946 | 0.829726 | 0.835760 |
| ET | 0.419251 | 0.424238 | 0.424223 | 0.4273647 | 0.424812 | 0.423246 |
| S (meV/(cell.K)) | 2.413010 | 2.451891 | 2.474677 | 2.5096526 | 2.458753 | 2.469079 |
| PV | 0.000108 | 0.000108 | 0.000109 | 0.0001084 | 0.000108 | 0.000106 |
| TS | 0.719439 | 0.7310314 | 0.737824 | 0.7482529 | 0.733077 | 0.736156 |
| ET + PV-TS | −0.300078 | −0.3066847 | −0.313492 | −0.3207797 | −0.308156 | −0.312802 |
| EL + E0 + ET + PV-TS | −451,473.0537 | −451,473.0772 | −451,471.8861 | −451,472.1217 | −451,471.4950 | −451,472.1748 |
| (0.023) | (0.000) | (1.191) | (0.955) | (1.582) | (0.902) | |
| Functionals | CdFO | Fe (Oh) μB | Fe (Td) μB | Cd (Oh) μB | Cd (Td) μB | O μB | ||
|---|---|---|---|---|---|---|---|---|
| B3LYP | X = 0 | FM | 4.317 (4) | 0.063 (2) | 0.326 (8) | |||
| FRI | 4.309 (3) | _ | _ | 0.032 (2) | 0.313 (2) | |||
| −4.295 | 0.113 (6) | |||||||
| AFM | 4.301 (2) | _ | _ | 0.000 (2) | −0.102 (4) | |||
| −4.301 (2) | −0.102 (4) | |||||||
| X = 0.5 | 4.304 (3) | 4.275 | 0.039 | 0.074 | 0.357 | 0.368 | ||
| FM | 0.341(3) | 0.318(3) | ||||||
| 4.278(3) | −4.214 | −0.008 | 0.074 | 0.111 | 0.364 | |||
| FRI | −0.028 (3) | 0.308 (3) | ||||||
| AFM | 4.274 (2) −4.287 | −4.234 | −0.019 | 0.023 | −0.041 | 0.120 | ||
| −0.164 (2) | −0.010 (2) | |||||||
| −0.055 | 0.291 | |||||||
| X = 1 | FM | 4.341 (2) | 4.261 (2) | 0.045 (2) | ̶ | 0.350 (4) | 0.326 (4) | |
| FRI | 4.304 (2) | −4.194 | −0.002 (2) | _ | 0.054 (2) | 0.341 (2) | ||
| 4.256 | −0.042 (2) | 0.315 (2) | ||||||
| AFM | 4.270 (2) | −4.195 (2) | −0.053 (2) | ̶ | 0.047(4) | −0.057(4) | ||
| HSE06 | X = 0 | FM | 4.372 (4) | ̶ | ̶ | 0.064 (2) | 0.298 (8) | |
| FRI | 4.365 (3) | _ | _ | 0.033 (2) | 0.287 (2) | |||
| −4.354 | 0.104 (6) | |||||||
| AFM | 4.359 (2) | _ | _ | 0.000 (2) | −0.093 (4) | |||
| −4.360 (2) | 0.094 (4) | |||||||
| X = 0.5 | FM | 4.361 (3) | 4.337 | 0.035 | 0.075 | 0.324 | 0.334 | |
| 0.314 (3) | 0.290 (3) | |||||||
| FRI | 4.337 (3) | −4.279 | −0.009 | 0.075 | 0.099 | 0.333 | ||
| −0.025 (3) | 0.282 (3) | |||||||
| AFM | 4.334 (2)−4.347 | −4.298 | −0.018 | 0.024 | −0.038 | 0.110 | ||
| −0.154 (2) | −0.006 (2) | |||||||
| −0.045 | 0.267 | |||||||
| X = 1 | FM | 4.400 (2) | 4.318 (2) | 0.043 (2) | - | 0.319 (4) | ||
| 0.319 (4) | ||||||||
| FRI | 4.366 (2) | −4.257 | −0.005 (2) | - | 0.051 (2) | 0.312 (2) | ||
| 4.312 | −0.044 (2) | 0.292 (2) | ||||||
| AFM | 4.334 (2) | −4.257 (2) | −0.055 (2) | - | 0.044 (4) | |||
| −0.055 (4) | ||||||||
| PBE0 | X = 0 | FM | 4.380 (4) | ̶ | ̶ | 0.064 (2) | 0.294 (6) | |
| FRI | 4.374 (3) | _ | _ | 0.033 (2) | 0.283 (2) | |||
| −4.364 | 0.102 (6) | |||||||
| AFM | 4.369 (2) | _ | _ | −0.000 (2) | −0.093 (4) | |||
| −4.369 (2) | 0.093 (4) | |||||||
| X = 0.5 | FM | 4.369 (3) | 4.346 | 0.035 | 0.075 | 0.319 | 0.331 | |
| 0.310 (3) | 0.286 (3) | |||||||
| FRI | 4.346 (3) | −4.289 | 0.075 | −0.010 | 0.097 | 0.329 | ||
| −0.026 (3) | 0.279 (3) | |||||||
| AFM | 4.344 (2)−4.356 | −4.308 | −0.018 | 0.024 | −0.038 | 0.109 | ||
| −0.153 (2) | −0.007 (2) | |||||||
| −0.046 | 0.266 | |||||||
| X = 1 | FM | 4.409 (2) | 4.327 (2) | 0.043 (2) | - | 0.315 (4) | ||
| 0.296 (4) | ||||||||
| FRI | 4.376 (2) | −4.268 | −0.005 (2) | - | 0.050 (2) | 0.308 (2) | ||
| 4.321 | −0.045 (2) | 0.288 (2) | ||||||
| AFM | 4.346 (2) | −4.268 (2) | −0.055 (2) | - | 0.043 (4) | |||
| −0.055 (4) | ||||||||
| Primitive Formula | Inversion Degree | Spin Arrangement | Band Gap | |||||
|---|---|---|---|---|---|---|---|---|
| B3LYP | HSE06 | PBE0 | ||||||
| (Cd2)[Fe4]O8 | X = 0.0 | FM | 4.884 | 3.012 | 4.719 | 3.120 | 5.436 | 3.790 |
| FRI | 3.430 | 2.916 | 3.542 | 3.043 | 4.311 | 3.763 | ||
| AFM | 3.112 | 3.112 | 3.251 | 3.246 | 3.977 | 3.977 | ||
| (CdFe)[CdFe3]O8 | X = 0.5 | FM | 4.951 | 3.018 | 4.740 | 2.950 | 5.455 | 3.633 |
| FRI | 3.221 | 3.120 | 3.228 | 3.161 | 3.988 | 3.876 | ||
| AFM | 3.111 | 3.145 | 3.144 | 3.260 | 3.870 | 4.001 | ||
| (Fe2)[ Cd2Fe2]O8 | X = 1.0 | FM | 5.078 | 2.677 | 4.858 | 2.700 | 5.561 | 3.392 |
| FRI | 3.430 | 2.916 | 3.750 | 2.922 | 4.502 | 3.643 | ||
| AFM | 3.523 | 3.005 | 3.565 | 3.079 | 4.305 | 3.808 | ||
| Vibrational Modes (cm−1) | Ref. [31] | Ref. [32] | Ref. [23] | Current Work (X = 0.0) | |
|---|---|---|---|---|---|
| FM | AFM | ||||
| v1 | 549.61–553.47 | 553–578 | 548–549 | 536.36 | 527.47 |
| v2 | 424.26–410.75 | 438–403 | 374–380 | 350.10 | 348.04 |
| v3 | 279.02 | 285.00 Sh-305.12 | |||
| v4 | 153.38 | 137.49 | |||
| X= 0.0 | X =1.0 | ||
|---|---|---|---|
| FM | FM | AFM | |
| T2g (1) | 138.26 | I | I |
| 199.10 | 199.57 | ||
| 206.96 | 209.02 | ||
| 227.00 | 230.68 | ||
| 262.98 | 255.89 | ||
| 302.33 | |||
| I | 306.95 | ||
| 317.03 | |||
| 312.22 | |||
| 324.24 | 332.23 | ||
| 328.27 | |||
| Eg (1) | 349.37 | I | 337.12 |
| T2g (2) | 462.57 | 390.50 | 413.13 |
| 464.58 | |||
| 451.39 | 479.93 | ||
| 483.01 | I | ||
| T2g (3) | 591.82 | 535.77 | 541.58 |
| 552.35 | 556.23 | ||
| 580.72 | 583.89 | ||
| A1g | 686.15 | 697.72 | 685.11 |
| Raman Symmetry Assignment | Ref. [36] | Ref. [23] | Ref. [24] | Ref. [25] | Current Work (X = 0) | |
|---|---|---|---|---|---|---|
| FM | AFM | |||||
| T2g (1) | 138 | 137 | ||||
| Eg | 325–329 | 314 | 349 | 347 | ||
| T2g (2) | 438–445 | 331 | 463 | 452 | ||
| T2g (3) | 446 | 472 | 592 | 588 | ||
| A1g | 644 | 634–636 | 652 | 674 | 686 | 682 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairi, T.S. Cation and Spin Interactions in Cadmium Ferrite: A Quantum Mechanical Study. Int. J. Mol. Sci. 2025, 26, 4912. https://doi.org/10.3390/ijms26104912
Almutairi TS. Cation and Spin Interactions in Cadmium Ferrite: A Quantum Mechanical Study. International Journal of Molecular Sciences. 2025; 26(10):4912. https://doi.org/10.3390/ijms26104912
Chicago/Turabian StyleAlmutairi, Tahani Saad. 2025. "Cation and Spin Interactions in Cadmium Ferrite: A Quantum Mechanical Study" International Journal of Molecular Sciences 26, no. 10: 4912. https://doi.org/10.3390/ijms26104912
APA StyleAlmutairi, T. S. (2025). Cation and Spin Interactions in Cadmium Ferrite: A Quantum Mechanical Study. International Journal of Molecular Sciences, 26(10), 4912. https://doi.org/10.3390/ijms26104912






