Mechanism of Curcumol Targeting the OTUB1/TGFBI Ubiquitination Pathway in the Inhibition of Angiogenesis in Colon Cancer
Abstract
1. Introduction
2. Results
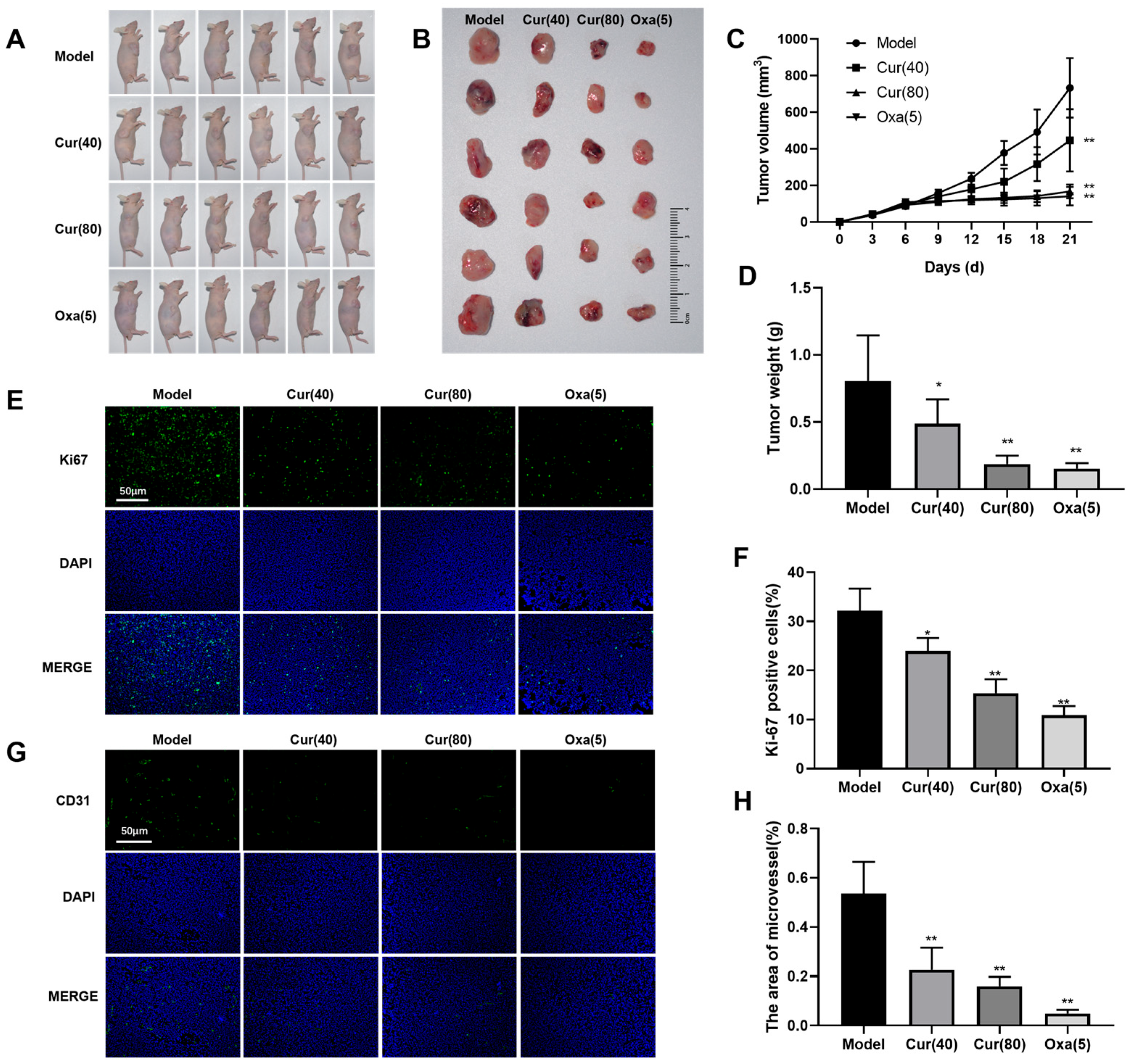
2.1. Curcumol Inhibits Tumor Formation in Nude Mice with Subcutaneous Colon Cancer
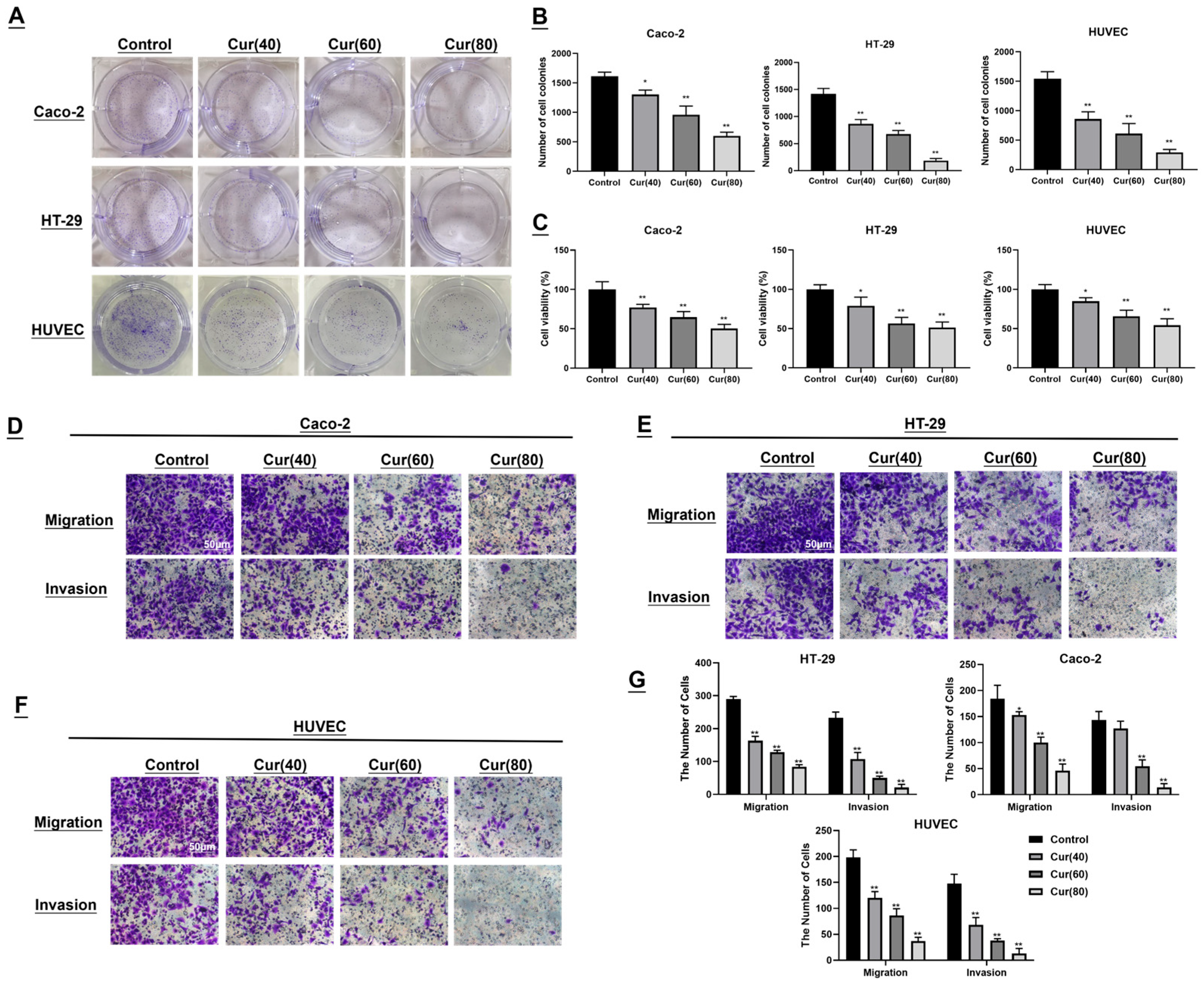
2.2. The Impact of Curcumol on the Colon Cancer Cell Lines Caco-2/HT-29 and Human Umbilical Vein Endothelial Cells (HUVECs) Was Investigated
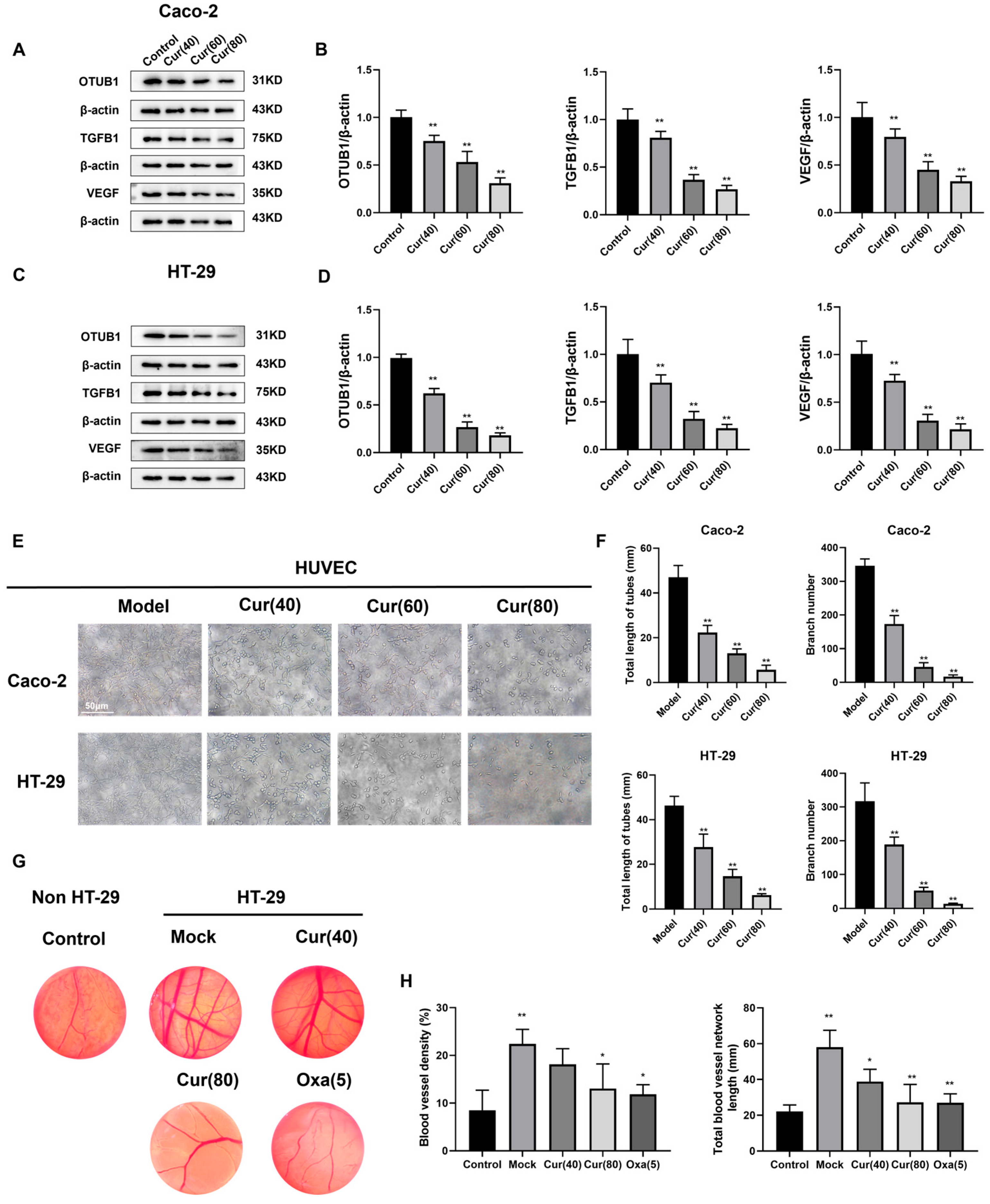
2.3. Curcumol Reduces Angiogenesis in Subcutaneous HT-29 Cell Tumors in Nude Mice
2.4. Curcumol Reduces the Angiogenesis of Caco-2/HT-29 Colorectal Cancer Cells
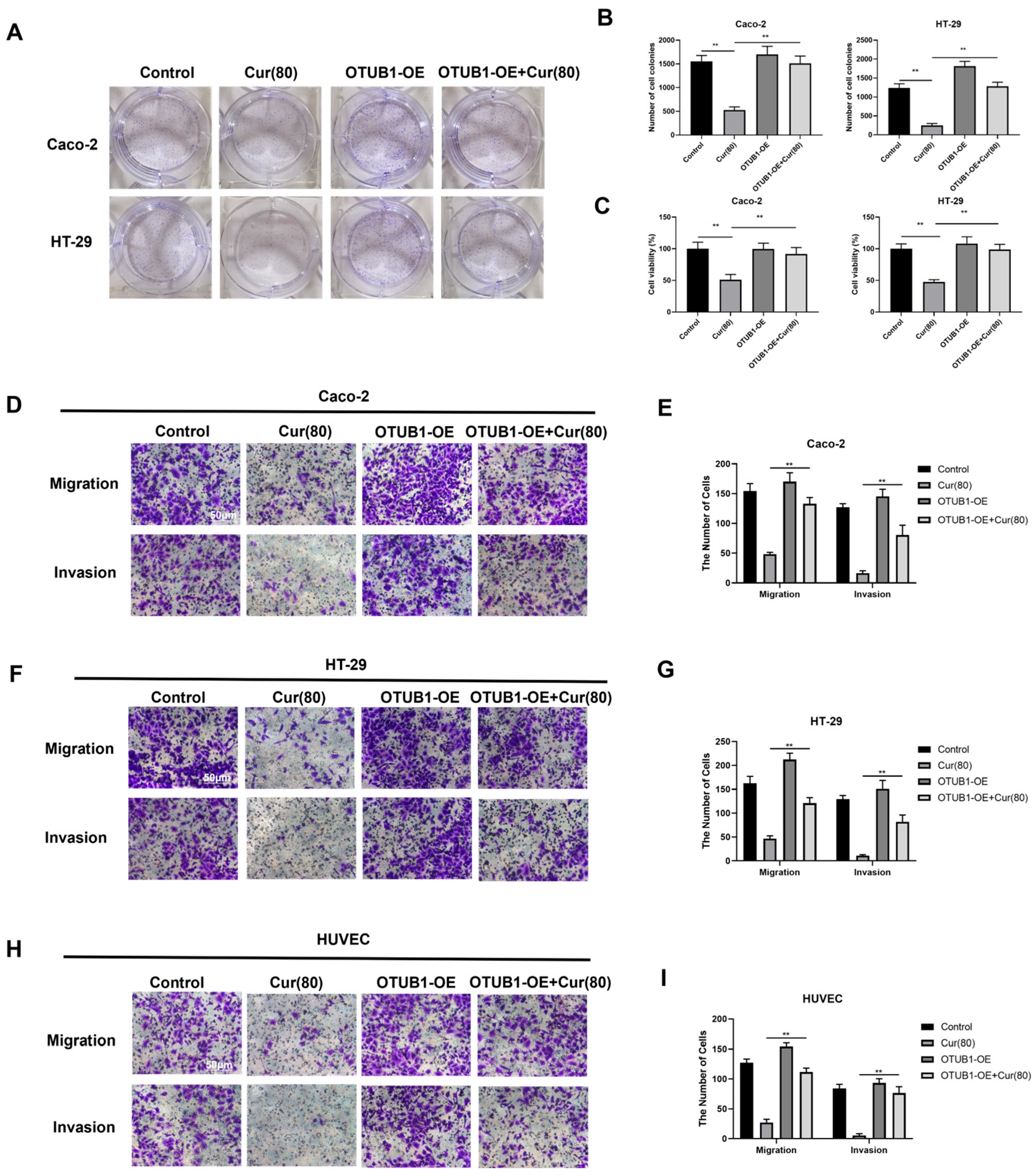
2.5. Curcumol Inhibits the Biological Activity of Human Colon Cancer Cell Lines Caco-2 and HT-29, Which Exhibit an Overexpression of OTUB1
2.6. Curcumol Inhibits the Angiogenic Ability of Caco-2/HT-29 Colorectal Cancer Cells by Downregulating OTUB1 Expression
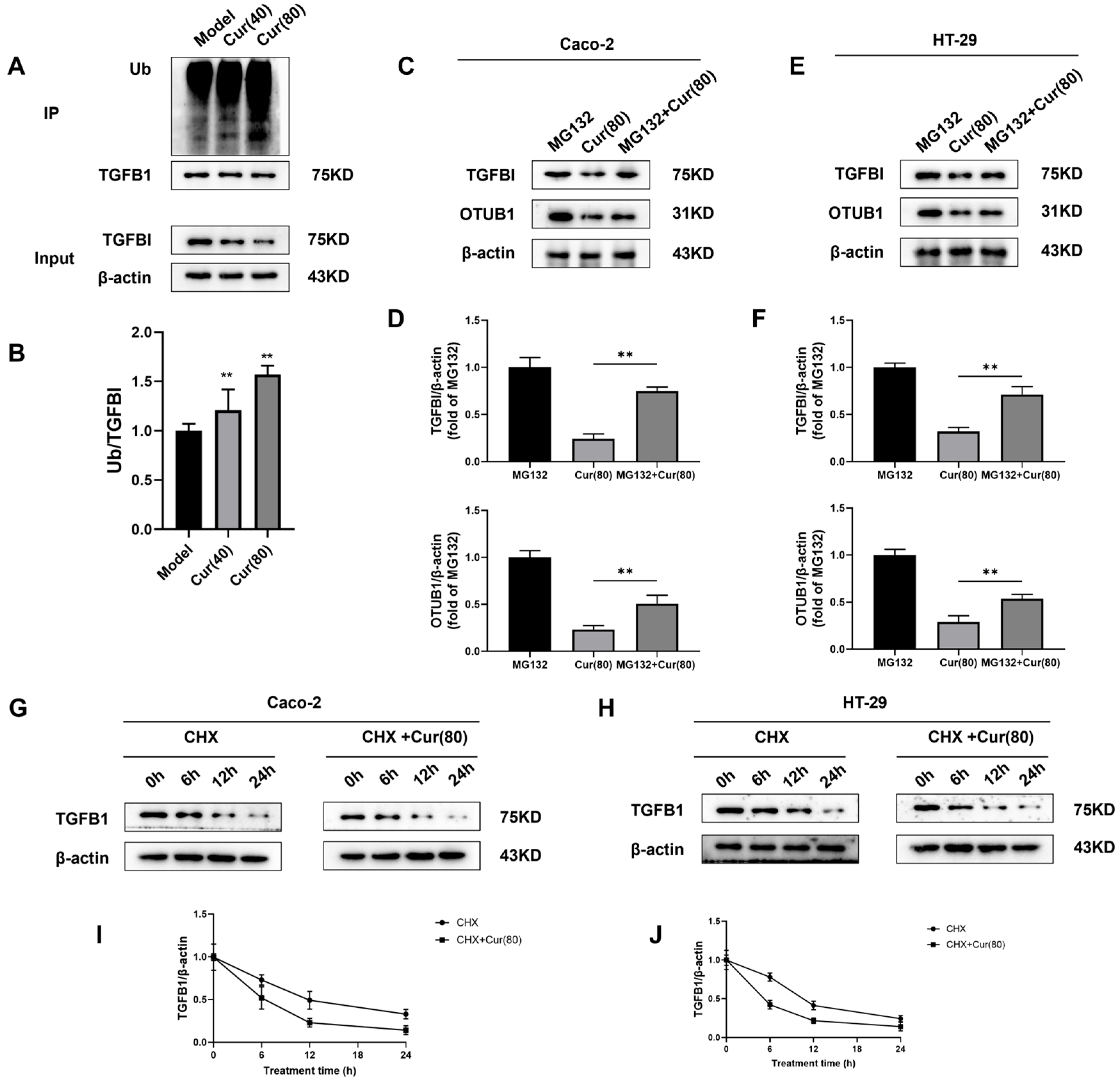
2.7. Curcumol Regulates the Deubiquitination of TGFBI by OTUB1 to Inhibit Angiogenesis Induced by Colorectal Cancer
3. Discussion
4. Materials and Methods
4.1. Chemical Substances and Reagents
4.2. Cell Line Cultivation and OTUB1 Overexpression
4.3. Animal Model and Categorization
4.4. Immunofluorescence Staining of Ki67
4.5. Immunofluorescent Analysis of CD31
4.6. Experiment on Colony Formation
4.7. Methyl Thiazolyl Tetrazolium (MTT) Assay for Cell Viability
4.8. Transwell Assay for Cellular Migration and Invasion
4.9. The Immunofluorescence of Tumor Tissue
4.10. Tubule Formation Assay
4.11. Chick Embryo Allantoic Membrane (CAM) Experiment
4.12. Western Blot
4.13. Quantitative qRT-PCR
4.14. Immunoprecipitation (IP)
4.15. Determination of the Half-Life of TGFBI Protein
4.16. Ubiquitination Experiments in Cells
4.17. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAM | chick embryo allantoic membrane |
| CHX | cycloheximide |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | dimethyl sulfoxide |
| DR | Drug Resistance |
| DTX | docetaxel |
| DUB | deubiquitinating |
| EMT | epithelial-mesenchymal transition |
| FBS | fetal bovine serum |
| PFA | formaldehyde |
| HUVEC | human umbilical vein endothelial cells |
| mCRC | metastatic colorectal cancer |
| MMP | matrix metalloproteinase |
| MTT | Methyl thiazolyl tetrazolium |
| PBS | phosphate-buffered saline |
| SD | standard deviation |
| SDF-1 | stromal cell-derived factor |
| WHO | the World Health Organization |
| VEGF | vascular endothelial growth factor |
References
- Abedizadeh, R.; Majidi, F.; Khorasani, H.R.; Abedi, H.; Sabour, D. Colorectal cancer: A comprehensive review of carcinogenesis, diagnosis, and novel strategies for classified treatments. Cancer Metastasis Rev. 2024, 43, 729–753. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Dwivedi, S.K.D.; Bhattacharya, R.; Mukherjee, P.; Rao, G. VEGF signaling: Role in angiogenesis and beyond. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189079. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, H.X.; Wu, C.T.; Wang, W.Q.; Jin, W.; Gao, H.L.; Li, H.; Zhang, S.R.; Xu, J.Z.; Qi, Z.H.; et al. Angiogenesis in pancreatic cancer: Current research status and clinical implications. Angiogenesis 2019, 22, 15–36. [Google Scholar] [CrossRef]
- Griffioen, A.W.; Dudley, A.C. The rising impact of angiogenesis research. Angiogenesis 2022, 25, 435–437. [Google Scholar] [CrossRef]
- Giantonio, B.J.; Catalano, P.J.; Meropol, N.J.; O’Dwyer, P.J.; Mitchell, E.P.; Alberts, S.R.; Schwartz, M.A.; Benson, A.B., 3rd. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 1539–1544. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Zheng, X.; Lin, Z.; Zhuang, Q.; Xie, H.; Liang, Y.; Xu, Y.; Cui, L.; Liu, X.; et al. Normalization of Tumor Vessels by Lenvatinib-Based Metallo-Nanodrugs Alleviates Hypoxia and Enhances Calreticulin-Mediated Immune Responses in Orthotopic HCC and Organoids. Small 2023, 19, e2207786. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis. Annu. Rev. Med. 2006, 57, 1–18. [Google Scholar] [CrossRef]
- Dudics, S.; Langan, D.; Meka, R.R.; Venkatesha, S.H.; Berman, B.M.; Che, C.T.; Moudgil, K.D. Natural Products for the Treatment of Autoimmune Arthritis: Their Mechanisms of Action, Targeted Delivery, and Interplay with the Host Microbiome. Int. J. Mol. Sci. 2018, 19, 2508. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Wei, W.; Rasul, A.; Sadiqa, A.; Sarfraz, I.; Hussain, G.; Nageen, B.; Liu, X.; Watanabe, N.; Selamoglu, Z.; Ali, M.; et al. Curcumol: From Plant Roots to Cancer Roots. Int. J. Biol. Sci. 2019, 15, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.B.; Trinh, T.A.; Lee, T.K.; Yamabe, N.; Kang, K.S.; Song, J.H.; Choi, S.; Lee, S.; Jang, T.S.; Kim, K.H.; et al. Curcuzedoalide contributes to the cytotoxicity of Curcuma zedoaria rhizomes against human gastric cancer AGS cells through induction of apoptosis. J. Ethnopharmacol. 2018, 213, 48–55. [Google Scholar] [CrossRef]
- Lee, T.K.; Lee, D.; Lee, S.R.; Ko, Y.J.; Sung Kang, K.; Chung, S.J.; Kim, K.H. Sesquiterpenes from Curcuma zedoaria rhizomes and their cytotoxicity against human gastric cancer AGS cells. Bioorg. Chem. 2019, 87, 117–122. [Google Scholar] [CrossRef]
- Wei, Z.L.; Juan, W.; Tong, D.; Juan, L.X.; Sa, L.Y.; Jie, H.F.M.; Xiao, G.; Xiang, L.G.; Jie, H.M.; Xu, C. Curcumol inhibits breast cancer growth via NCL/ERα36 and the PI3K/AKT pathway. Food Funct. 2023, 14, 874–885. [Google Scholar] [CrossRef]
- Xu, W.; Ding, J.; Kuang, S.; Li, B.; Sun, T.; Zhu, C.; Liu, J.; Zhu, L.; Li, Y.; Sheng, W. Icariin-Curcumol promotes docetaxel sensitivity in prostate cancer through modulation of the PI3K-Akt signaling pathway and the Warburg effect. Cancer Cell Int. 2023, 23, 190. [Google Scholar] [CrossRef]
- Guan, X.; Yu, D.; HuangFu, M.; Huang, Z.; Dou, T.; Liu, Y.; Zhou, L.; Li, X.; Wang, L.; Liu, H.; et al. Curcumol inhibits EBV-positive Nasopharyngeal carcinoma migration and invasion by targeting nucleolin. Biochem. Pharmacol. 2021, 192, 114742. [Google Scholar] [CrossRef]
- Liu, C. Curcumol Targeting PAX8 Inhibits Ovarian Cancer Cell Migration and Invasion and Increases Chemotherapy Sensitivity of Niraparib. J. Oncol. 2022, 2022, 3941630. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, K.; Wang, M.; Wang, Q.; Chang, H.; Liang, Y.; Wang, Q.; Zhao, J.; Tang, T.; Yang, S. Ubiquitination Flow Repressors: Enhancing Wound Healing of Infectious Diabetic Ulcers through Stabilization of Polyubiquitinated Hypoxia-Inducible Factor-1α by Theranostic Nitric Oxide Nanogenerators. Adv. Mater. 2021, 33, e2103593. [Google Scholar] [CrossRef]
- Wu, L.; Xiao, J.; Yi, D.; Ding, H.; Wang, R.; Duan, Z.; Liu, Z.; Shi, X.; Shen, M.; Sang, J. Cytosolic Cadherin 4 promotes angiogenesis and metastasis in papillary thyroid cancer by suppressing the ubiquitination/degradation of β-catenin. J. Transl. Med. 2024, 22, 201. [Google Scholar] [CrossRef]
- Nakada, S.; Tai, I.; Panier, S.; Al-Hakim, A.; Iemura, S.; Juang, Y.C.; O’Donnell, L.; Kumakubo, A.; Munro, M.; Sicheri, F.; et al. Non-canonical inhibition of DNA damage-dependent ubiquitination by OTUB1. Nature 2010, 466, 941–946. [Google Scholar] [CrossRef]
- Juang, Y.C.; Landry, M.C.; Sanches, M.; Vittal, V.; Leung, C.C.; Ceccarelli, D.F.; Mateo, A.R.; Pruneda, J.N.; Mao, D.Y.; Szilard, R.K.; et al. OTUB1 co-opts Lys48-linked ubiquitin recognition to suppress E2 enzyme function. Mol. Cell 2012, 45, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Sun, L.; Song, T. OTUB1-mediated inhibition of ubiquitination: A growing list of effectors, multiplex mechanisms, and versatile functions. Front. Mol. Biosci. 2023, 10, 1261273. [Google Scholar] [CrossRef] [PubMed]
- Saldana, M.; VanderVorst, K.; Berg, A.L.; Lee, H.; Carraway, K.L. Otubain 1: A non-canonical deubiquitinase with an emerging role in cancer. Endocr. Relat. Cancer 2019, 26, R1–R14. [Google Scholar] [CrossRef]
- Zhou, K.; Mai, H.; Zheng, S.; Cai, W.; Yang, X.; Chen, Z.; Zhan, B. OTUB1-mediated deubiquitination of FOXM1 up-regulates ECT-2 to promote tumor progression in renal cell carcinoma. Cell Biosci. 2020, 10, 50. [Google Scholar] [CrossRef]
- Seo, S.U.; Woo, S.M.; Kim, M.W.; Lee, E.W.; Min, K.J.; Kwon, T.K. Phosphorylation of OTUB1 at Tyr 26 stabilizes the mTORC1 component, Raptor. Cell Death Differ. 2023, 30, 82–93. [Google Scholar] [CrossRef]
- Zhao, Y.; Ruan, J.; Li, Z.; Su, X.; Chen, K.; Lin, Y.; Cai, Y.; Wang, P.; Liu, B.; Schlüter, D.; et al. OTUB1 inhibits breast cancer by non-canonically stabilizing CCN6. Clin. Transl. Med. 2023, 13, e1385. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Wu, D.; Tian, C.; Gao, J.; Yang, Q.; Hong, X.; Gu, F.; Zhang, K.; Hu, Y.; et al. OTUB1 Targets CHK1 for Deubiquitination and Stabilization to Facilitate Lung Cancer Progression and Radioresistance. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 1222–1233. [Google Scholar] [CrossRef]
- Liu, X.; Deng, H.; Tang, J.; Wang, Z.; Zhu, C.; Cai, X.; Rong, F.; Chen, X.; Sun, X.; Jia, S.; et al. OTUB1 augments hypoxia signaling via its non-canonical ubiquitination inhibition of HIF-1α during hypoxia adaptation. Cell Death Dis. 2022, 13, 560. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, J.; Fu, X.; Du, W.; Zhou, L.; Meng, X.; Yu, H.; Lin, J.; Ye, W.; Liu, J.; et al. OTUB1 promotes metastasis and serves as a marker of poor prognosis in colorectal cancer. Mol. Cancer 2014, 13, 258. [Google Scholar] [CrossRef]
- Huang, H.; Tang, Q.; Li, S.; Qin, Y.; Zhu, G. TGFBI: A novel therapeutic target for cancer. Int. Immunopharmacol. 2024, 134, 112180. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, J.S.; Kim, H.J.; Schlaepfer, D.D.; Kim, I.S.; Nam, J.O. Endothelial angiogenic activity and adipose angiogenesis is controlled by extracellular matrix protein TGFBI. Sci. Rep. 2021, 11, 9644. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, X.; Yang, E.; Li, Y.; Geng, Y.; Chen, Z.; Huang, S.; Zhang, D.; Wu, H. OTUB1 Catalytic-Independently Deubiquitinates TGFBI and Mediates the Angiogenesis in Infantile Hemangioma by Regulating Glycolysis. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 654–673. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef]
- Hoseinkhani, Z.; Norooznezhad, F.; Rastegari-Pouyani, M.; Mansouri, K. Medicinal Plants Extracts with Antiangiogenic Activity: Where Is the Link? Adv. Pharm. Bull. 2020, 10, 370–378. [Google Scholar] [CrossRef]
- Gupta, M.K.; Qin, R.Y. Mechanism and its regulation of tumor-induced angiogenesis. World J. Gastroenterol. 2003, 9, 1144–1155. [Google Scholar] [CrossRef]
- van de Schootbrugge, C.; Bussink, J.; Span, P.N.; Sweep, F.C.; Grénman, R.; Stegeman, H.; Pruijn, G.J.; Kaanders, J.H.; Boelens, W.C. αB-crystallin stimulates VEGF secretion and tumor cell migration and correlates with enhanced distant metastasis in head and neck squamous cell carcinoma. BMC Cancer 2013, 13, 128. [Google Scholar] [CrossRef]
- Li, R.; Qi, Y.; Jiang, M.; Zhang, T.; Wang, H.; Wang, L.; Han, M. Primary tumor-secreted VEGF induces vascular hyperpermeability in premetastatic lung via the occludin phosphorylation/ubiquitination pathway. Mol. Carcinog. 2019, 58, 2316–2326. [Google Scholar] [CrossRef]
- Su, C.H.; Wu, Y.J.; Chang, C.Y.; Tien, T.Y.; Tseng, S.W.; Tsai, C.H.; Bettinger, T.; Tsai, C.H.; Yeh, H.I. The increase of VEGF secretion from endothelial progenitor cells post ultrasonic VEGF gene delivery enhances the proliferation and migration of endothelial cells. Ultrasound Med. Biol. 2013, 39, 134–145. [Google Scholar] [CrossRef]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. = Rev. Roum. De Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Edelmann, M.J.; Iphöfer, A.; Akutsu, M.; Altun, M.; di Gleria, K.; Kramer, H.B.; Fiebiger, E.; Dhe-Paganon, S.; Kessler, B.M. Structural basis and specificity of human otubain 1-mediated deubiquitination. Biochem. J. 2009, 418, 379–390. [Google Scholar] [CrossRef]
- Karunarathna, U.; Kongsema, M.; Zona, S.; Gong, C.; Cabrera, E.; Gomes, A.R.; Man, E.P.; Khongkow, P.; Tsang, J.W.; Khoo, U.S.; et al. OTUB1 inhibits the ubiquitination and degradation of FOXM1 in breast cancer and epirubicin resistance. Oncogene 2016, 35, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.G.; Li, Y.; Sun, X.X.; Dai, M.S. Otub1 stabilizes MDMX and promotes its proapoptotic function at the mitochondria. Oncotarget 2017, 8, 11053–11062. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Xu, R.; Huang, X.; Tang, Z.; Tian, Y.; Zhang, J.; Zheng, X. Deubiquitinating enzyme OTUB1 promotes cancer cell immunosuppression via preventing ER-associated degradation of immune checkpoint protein PD-L1. Cell Death Differ. 2021, 28, 1773–1789. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.P.; Vasaikar, S.V.; Tomczak, K.; Tripathi, S.; den Hollander, P.; Arslan, E.; Chakraborty, P.; Soundararajan, R.; Jolly, M.K.; Rai, K.; et al. Identification of EMT signaling cross-talk and gene regulatory networks by single-cell RNA sequencing. Proc. Natl. Acad. Sci. USA 2021, 118, e2102050118. [Google Scholar] [CrossRef]
- Zhan, S.; Bai, X.; Zhao, Y.; Tuoheti, K.; Yisha, Z.; Zuo, Y.; Lu, P.; Liu, T. TGFBI promotes proliferation and epithelial-mesenchymal transition in renal cell carcinoma through PI3K/AKT/mTOR/HIF-1α pathway. Cancer Cell Int. 2024, 24, 265. [Google Scholar] [CrossRef]
- Ma, C.; Rong, Y.; Radiloff, D.R.; Datto, M.B.; Centeno, B.; Bao, S.; Cheng, A.W.; Lin, F.; Jiang, S.; Yeatman, T.J.; et al. Extracellular matrix protein betaig-h3/TGFBI promotes metastasis of colon cancer by enhancing cell extravasation. Genes Dev. 2008, 22, 308–321. [Google Scholar] [CrossRef]
- Chiavarina, B.; Costanza, B.; Ronca, R.; Blomme, A.; Rezzola, S.; Chiodelli, P.; Giguelay, A.; Belthier, G.; Doumont, G.; Van Simaeys, G.; et al. Metastatic colorectal cancer cells maintain the TGFβ program and use TGFBI to fuel angiogenesis. Theranostics 2021, 11, 1626–1640. [Google Scholar] [CrossRef]
- Suzuki, M.; Yokobori, T.; Gombodorj, N.; Yashiro, M.; Turtoi, A.; Handa, T.; Ogata, K.; Oyama, T.; Shirabe, K.; Kuwano, H. High stromal transforming growth factor β-induced expression is a novel marker of progression and poor prognosis in gastric cancer. J. Surg. Oncol. 2018, 118, 966–974. [Google Scholar] [CrossRef]
- Costanza, B.; Rademaker, G.; Tiamiou, A.; De Tullio, P.; Leenders, J.; Blomme, A.; Bellier, J.; Bianchi, E.; Turtoi, A.; Delvenne, P.; et al. Transforming growth factor beta-induced, an extracellular matrix interacting protein, enhances glycolysis and promotes pancreatic cancer cell migration. Int. J. Cancer 2019, 145, 1570–1584. [Google Scholar] [CrossRef]
- Avraamides, C.J.; Garmy-Susini, B.; Varner, J.A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604–617. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Tao, Y.; Li, X.; Qin, J.; Bai, Z.; Chi, B.; Yan, W.; Chen, X. Curcumol inhibits colorectal cancer proliferation by targeting miR-21 and modulated PTEN/PI3K/Akt pathways. Life Sci. 2019, 221, 354–361. [Google Scholar] [CrossRef]



| Gene Name | Primer Sequences (5′–3′) |
|---|---|
| TGFBI-F | TCAAAGATGGTGTCCCTCGC |
| TGFBI-R | ACATCCGGTCCATGGTGAAC |
| OTUB1-F | GCTGTGCAGAATCCTCTGGT |
| OTUB1-R | AAGCCAAACGCTCGGTAGAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Wu, W.; Hou, D.; Zhao, Y.; Ye, J.; Shen, L.; Zhao, T.; Wu, X. Mechanism of Curcumol Targeting the OTUB1/TGFBI Ubiquitination Pathway in the Inhibition of Angiogenesis in Colon Cancer. Int. J. Mol. Sci. 2025, 26, 4899. https://doi.org/10.3390/ijms26104899
Zhu Y, Wu W, Hou D, Zhao Y, Ye J, Shen L, Zhao T, Wu X. Mechanism of Curcumol Targeting the OTUB1/TGFBI Ubiquitination Pathway in the Inhibition of Angiogenesis in Colon Cancer. International Journal of Molecular Sciences. 2025; 26(10):4899. https://doi.org/10.3390/ijms26104899
Chicago/Turabian StyleZhu, Yimiao, Wenya Wu, Dahai Hou, Yu Zhao, Jinshu Ye, Lizong Shen, Tong Zhao, and Xiaoyu Wu. 2025. "Mechanism of Curcumol Targeting the OTUB1/TGFBI Ubiquitination Pathway in the Inhibition of Angiogenesis in Colon Cancer" International Journal of Molecular Sciences 26, no. 10: 4899. https://doi.org/10.3390/ijms26104899
APA StyleZhu, Y., Wu, W., Hou, D., Zhao, Y., Ye, J., Shen, L., Zhao, T., & Wu, X. (2025). Mechanism of Curcumol Targeting the OTUB1/TGFBI Ubiquitination Pathway in the Inhibition of Angiogenesis in Colon Cancer. International Journal of Molecular Sciences, 26(10), 4899. https://doi.org/10.3390/ijms26104899





