Impact of Chronic Fluoxetine Exposure on Oocyte Development and Reproductive Outcomes in a Mouse Model
Abstract
1. Introduction
2. Results
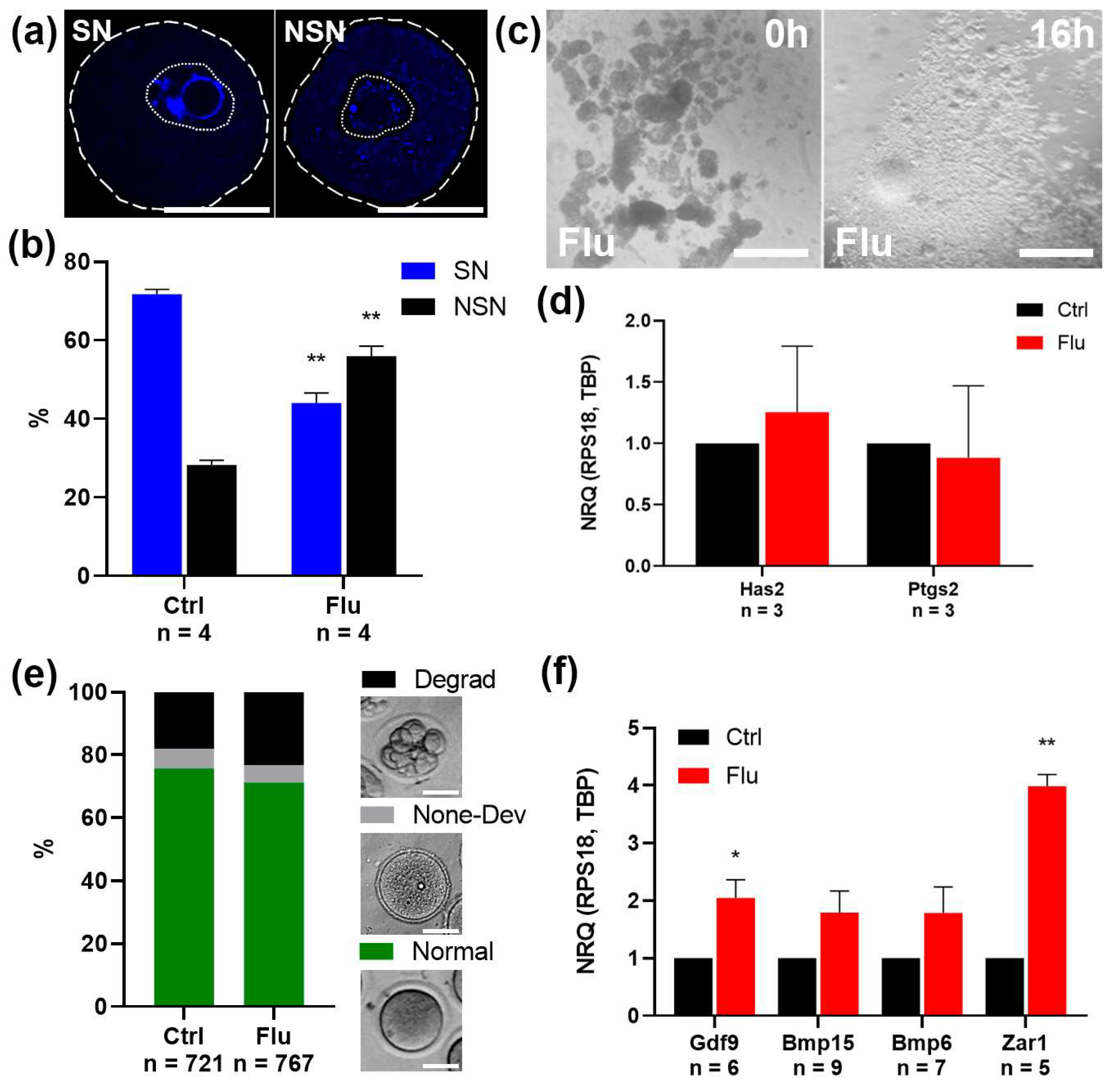
2.1. Effects of Fluoxetine on the Quantity and Quality of Ovulated MII Oocytes
2.2. Effects of Fluoxetine on GV Oocyte Maturation and Cumulus Expansion In Vitro (IVM)
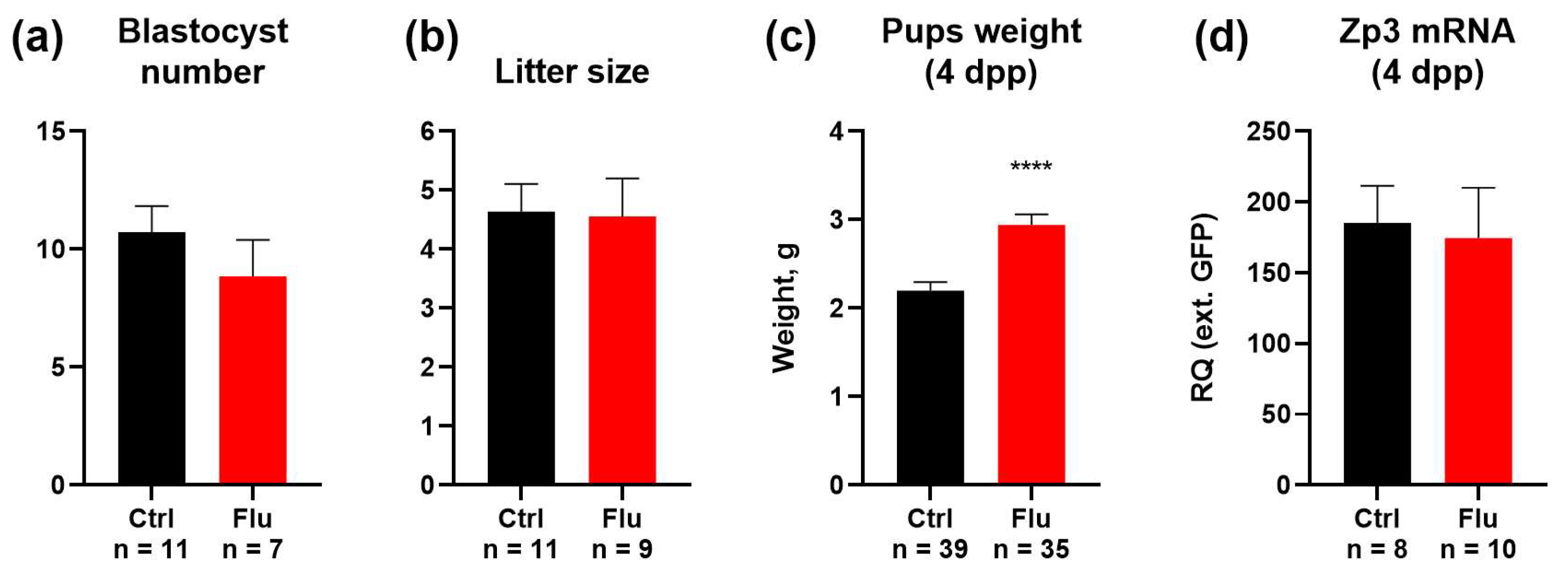
2.3. Effects of Fluoxetine on Reproduction and Developmental Potential
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Chemicals
4.2. Superovulation Protocol and Oocyte and Embryo Retrieval
4.3. In Vitro Oocyte Maturation and Cumulus–Oocyte Complex Expansion
4.4. Immunohistochemistry
4.5. Image Analysis
4.6. Western Blotting
4.7. Quantitative Polymerase Chain Reaction (qPCR)
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARTs | Assisted reproductive technologies |
| COC | Cumulus–oocyte complex |
| GV | Germinal vesicle (meiotic maturation stage) |
| GVBD | Germinal vesicle breakdown |
| IVM | In vitro maturation |
| MII | Metaphase II (meiotic maturation stage) |
| NSN | Non-surrounded nucleolus (chromatin configuration) |
| PCR | Polymerase chain reaction |
| PMSG | Pregnant mare serum gonadotropin |
| RAS | Russian Academy of Sciences |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| SN | Surrounded nucleolus (chromatin configuration) |
| SSRI | Selective serotonin reuptake inhibitor |
References
- Tukur, H.A.; Aljumaah, R.S.; Swelum, A.A.-A.; Alowaimer, A.N.; Saadeldin, I.M. The Making of a Competent Oocyte—A Review of Oocyte Development and Its Regulation. J. Anim. Reprod. Biotechnol. 2020, 35, 2–11. [Google Scholar] [CrossRef]
- Ruiz-Santiago, C.; Rodríguez-Pinacho, C.; Pérez-sánchez, G.; Acosta-cruz, E. Effects of Selective Serotonin Reuptake Inhibitors on Endocrine System (Review). Biomed. Rep. 2024, 21, 128. [Google Scholar] [CrossRef] [PubMed]
- Cesta, C.E.; Viktorin, A.; Olsson, H.; Johansson, V.; Sjölander, A.; Bergh, C.; Skalkidou, A.; Nygren, K.G.; Cnattingius, S.; Iliadou, A.N. Depression, Anxiety, and Antidepressant Treatment in Women: Association with in Vitro Fertilization Outcome. Fertil. Steril. 2016, 105, 1594–1602.e3. [Google Scholar] [CrossRef] [PubMed]
- Berardelli, R.; Margarito, E.; Ghiggia, F.; Picu, A.; Balbo, M.; Bonelli, L.; Giordano, R.; Karamouzis, I.; Bo, M.; Ghigo, E.; et al. Neuroendocrine Effects of Citalopram, a Selective Serotonin Re-Uptake Inhibitor, during Lifespan in Humans. J. Endocrinol. Invest. 2010, 33, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Dubé, F.; Amireault, P. Local Serotonergic Signaling in Mammalian Follicles, Oocytes and Early Embryos. Life Sci. 2007, 81, 1627–1637. [Google Scholar] [CrossRef]
- Frolova, V.S.; Ivanova, A.D.; Konorova, M.S.; Shmukler, Y.B.; Nikishin, D.A. Spatial Organization of the Components of the Serotonergic System in the Early Mouse Development. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2023, 17, S59–S64. [Google Scholar] [CrossRef]
- Kaihola, H.; Yaldir, F.G.; Hreinsson, J.; Hörnaeus, K.; Bergquist, J.; Olivier, J.D.A.; Åkerud, H.; Sundström-Poromaa, I. Effects of Fluoxetine on Human Embryo Development. Front. Cell Neurosci. 2016, 10, 160. [Google Scholar] [CrossRef]
- Domar, A.D.; Moragianni, V.A.; Ryley, D.A.; Urato, A.C. The Risks of Selective Serotonin Reuptake Inhibitor Use in Infertile Women: A Review of the Impact on Fertility, Pregnancy, Neonatal Health and Beyond. Hum. Reprod. 2013, 28, 160–171. [Google Scholar] [CrossRef]
- Alyoshina, N.M.; Tkachenko, M.D.; Nikishina, Y.O.; Nikishin, D.A.; Koltzov, N.K. Serotonin Transporter Activity in Mouse Oocytes Is a Positive Indicator of Follicular Growth and Oocyte Maturity. Int. J. Mol. Sci. 2023, 24, 11247. [Google Scholar] [CrossRef]
- Alyoshina, N.M.; Rousanova, V.R.; Malchenko, L.A.; Khramova, Y.V.; Nikishina, Y.O.; Konduktorova, V.V.; Evstifeeva, A.Y.; Nikishin, D.A. Analysis of the Ovarian Marker Genes Expression Revealed the Antagonistic Effects of Serotonin and Androstenedione on the Functional State of Mouse Granulosa Cells in Primary Culture. Russ. J. Dev. Biol. 2023, 54, 165–176. [Google Scholar] [CrossRef]
- Domingues, R.R.; Wiltbank, M.C.; Hernandez, L.L. The Antidepressant Fluoxetine (Prozac®) Modulates Estrogen Signaling in the Uterus and Alters Estrous Cycles in Mice. Mol. Cell Endocrinol. 2023, 559, 111783. [Google Scholar] [CrossRef] [PubMed]
- Mansoriyan, M.; Torabzadeh Khorasani, P.; Ramezani, M. Effect of Fluoxetine on Ovarian and Oviduct Tissue in Female Mature Balb/C Mice. J. Adv. Med. Biomed. Res. 2018, 26, 100–110. [Google Scholar]
- Romero-Reyes, J.; Cárdenas, M.; Damián-Matsumura, P.; Domínguez, R.; Ayala, M.E. Inhibition of Serotonin Reuptake in the Prepubertal Rat Ovary by Fluoxetine and Effects on Ovarian Functions. Reprod. Toxicol. 2016, 59, 80–88. [Google Scholar] [CrossRef]
- Achary, B. Electron Micrograph Studies on the Effects of Fluoxetine in Depression-Induced Adult Female Rat Ovaries. Indian. J. Sci. Technol. 2021, 14, 406–414. [Google Scholar] [CrossRef]
- Nikishin, D.A.; Alyoshina, N.M.; Semenova, M.L.; Shmukler, Y.B. Analysis of Expression and Functional Activity of Aromatic L-Amino Acid Decarboxylase (DDC) and Serotonin Transporter (SERT) as Potential Sources of Serotonin in Mouse Ovary. Int. J. Mol. Sci. 2019, 20, 3070. [Google Scholar] [CrossRef]
- González-Menció, F.; Manns, J.; Murphy, B.D. FSH and LH Activity of PMSG from Mares at Different Stages of Gestation. Anim. Reprod. Sci. 1978, 1, 137–144. [Google Scholar] [CrossRef]
- Conti, M.; Franciosi, F. Acquisition of Oocyte Competence to Develop as an Embryo: Integrated Nuclear and Cytoplasmic Events. Hum. Reprod. Update 2018, 24, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Bogolyubova, I.; Salimov, D.; Bogolyubov, D. Chromatin Configuration in Diplotene Mouse and Human Oocytes during the Period of Transcriptional Activity Extinction. Int. J. Mol. Sci. 2023, 24, 11517. [Google Scholar] [CrossRef]
- Fountas, S.; Petinaki, E.; Bolaris, S.; Kargakou, M.; Dafopoulos, S.; Zikopoulos, A.; Moustakli, E.; Sotiriou, S.; Dafopoulos, K. The Roles of GDF-9, BMP-15, BMP-4 and EMMPRIN in Folliculogenesis and In Vitro Fertilization. J. Clin. Med. 2024, 13, 3775. [Google Scholar] [CrossRef]
- Rong, Y.; Ji, S.-Y.; Zhu, Y.-Z.; Wu, Y.-W.; Shen, L.; Fan, H.-Y. ZAR1 and ZAR2 Are Required for Oocyte Meiotic Maturation by Regulating the Maternal Transcriptome and MRNA Translational Activation. Nucleic Acids Res. 2019, 47, 11387–11402. [Google Scholar] [CrossRef]
- Farrelly, L.A.; Thompson, R.E.; Zhao, S.; Lepack, A.E.; Lyu, Y.; Bhanu, N.V.; Zhang, B.; Loh, Y.-H.E.; Ramakrishnan, A.; Vadodaria, K.C.; et al. Histone Serotonylation Is a Permissive Modification That Enhances TFIID Binding to H3K4me3. Nature 2019, 567, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Dvoran, M.; Nemcova, L.; Kalous, J. An Interplay between Epigenetics and Translation in Oocyte Maturation and Embryo Development: Assisted Reproduction Perspective. Biomedicines 2022, 10, 1689. [Google Scholar] [CrossRef] [PubMed]
- Fanibunda, S.E.; Deb, S.; Maniyadath, B.; Tiwari, P.; Ghai, U.; Gupta, S.; Figueiredo, D.; Weisstaub, N.; Gingrich, J.A.; Vaidya, A.D.B.; et al. Serotonin Regulates Mitochondrial Biogenesis and Function in Rodent Cortical Neurons via the 5-HT2A Receptor and SIRT1-PGC-1α Axis. Proc. Natl. Acad. Sci. USA 2019, 116, 11028–11037. [Google Scholar] [CrossRef]
- Basu, B.; Desai, R.; Balaji, J.; Chaerkady, R.; Sriram, V.; Maiti, S.; Panicker, M.M. Serotonin in Pre-Implantation Mouse Embryos Is Localized to the Mitochondria and Can Modulate Mitochondrial Potential. Reproduction 2008, 135, 657–669. [Google Scholar] [CrossRef]
- Domingues, R.R.; Fricke, H.P.; Sheftel, C.M.; Bell, A.M.; Sartori, L.C.; Manuel, R.S.J.; Krajco, C.J.; Wiltbank, M.C.; Hernandez, L.L. Effect of Low and High Doses of Two Selective Serotonin Reuptake Inhibitors on Pregnancy Outcomes and Neonatal Mortality. Toxics 2022, 10, 11. [Google Scholar] [CrossRef]
- Oberlander, T.F.; Warburton, W.; Misri, S.; Aghajanian, J.; Hertzman, C. Neonatal Outcomes after Prenatal Exposure to Selective Serotonin Reuptake Inhibitor Antidepressants and Maternal Depression Using Population-Based Linked Health Data. Arch. Gen. Psychiatry 2006, 63, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.; Ho, H.T.B.; Hu, T.; Hebert, M.F.; Wang, J. Serotonin Transporter Deficiency Drives Estrogen-Dependent Obesity and Glucose Intolerance. Sci. Rep. 2017, 7, 1137. [Google Scholar] [CrossRef]
- Domingues, R.R.; Wiltbank, M.C.; Hernandez, L.L. Maternal Serotonin: Implications for the Use of Selective Serotonin Reuptake Inhibitors during Gestation. Biol. Reprod. 2023, 109, 17–28. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Mo, M.; Muyiduli, X.; Wang, S.; Li, M.; Jiang, S.; Wu, Y.; Shao, B.; Shen, Y.; et al. The Association of Gestational Diabetes Mellitus with Fetal Birth Weight. J. Diabetes Complicat. 2018, 32, 635–642. [Google Scholar] [CrossRef]
- Lopatina, M.V.; Petritskaya, E.N.; Ivlieva, A.L. Dependence of Fluid Consumption of Laboratory Mice on Diet. Lab. Anim. Sci. Res. 2018, 3, 96–99. [Google Scholar]
- Zha, W.; Hu, T.; Hebert, M.F.; Wang, J. Effect of Pregnancy on Paroxetine-Induced Adiposity and Glucose Intolerance in Mice. J. Pharmacol. Exp. Ther. 2019, 371, 113–120. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | NCBI Gene ID | Forward Primer | Reverse Primer |
|---|---|---|---|
| Bmp15 | 12155 | GAATCTGATGCCTCTTGTCCTT | ATGGCATGGTTGGGTGAAT |
| Bmp6 | 12161 | ACCGTACTTTGTGGCAGAGC | GAAAAGGCAAAGAGCAGAGTTAG |
| Gdf9 | 14566 | GCCTCCCCGACCTTTAGA | TGCCTCAGACTCCACATTTTC |
| GFP | - | CATGGCCGACAAGCAGAAGAAC | GGCGGCGGTCACGAACTC |
| Has2 | 15117 | GCGGAAGAAGGGACAACA | TGCGGTGCCACAATACTG |
| Ptgs2 | 19225 | CCCTCCGGTGTTTGTCCTT | CCTGCAGCATTTTTCATCTTGTA |
| Rps18 | 20084 | AAGAAAATTCGAGCCCATAGAGG | TAACAGCAAAGGCCCAGAGACT |
| Tbp | 21374 | GTAGCGGTGGCGGGTATCT | CGTCTTCAATGTTCTGGGTTATCT |
| Zar1 | 317755 | GTGATTCGGATGCCCCTCG | GGGCAGGCACATCTAGTTCTT |
| Zp3 | 22788 | TGCCAGACCCGAACTCCT | TAGCTGGCGCGACTTTGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkachenko, M.D.; Alyoshina, N.M.; Nikishina, Y.O.; Frolova, V.S.; Nikishin, D.A. Impact of Chronic Fluoxetine Exposure on Oocyte Development and Reproductive Outcomes in a Mouse Model. Int. J. Mol. Sci. 2025, 26, 4858. https://doi.org/10.3390/ijms26104858
Tkachenko MD, Alyoshina NM, Nikishina YO, Frolova VS, Nikishin DA. Impact of Chronic Fluoxetine Exposure on Oocyte Development and Reproductive Outcomes in a Mouse Model. International Journal of Molecular Sciences. 2025; 26(10):4858. https://doi.org/10.3390/ijms26104858
Chicago/Turabian StyleTkachenko, Maria D., Nina M. Alyoshina, Yulia O. Nikishina, Veronika S. Frolova, and Denis A. Nikishin. 2025. "Impact of Chronic Fluoxetine Exposure on Oocyte Development and Reproductive Outcomes in a Mouse Model" International Journal of Molecular Sciences 26, no. 10: 4858. https://doi.org/10.3390/ijms26104858
APA StyleTkachenko, M. D., Alyoshina, N. M., Nikishina, Y. O., Frolova, V. S., & Nikishin, D. A. (2025). Impact of Chronic Fluoxetine Exposure on Oocyte Development and Reproductive Outcomes in a Mouse Model. International Journal of Molecular Sciences, 26(10), 4858. https://doi.org/10.3390/ijms26104858





