Polarized Macrophages Show Diverse Pro-Angiogenic Characteristics Under Normo- and Hyperglycemic Conditions
Abstract
1. Introduction
2. Results
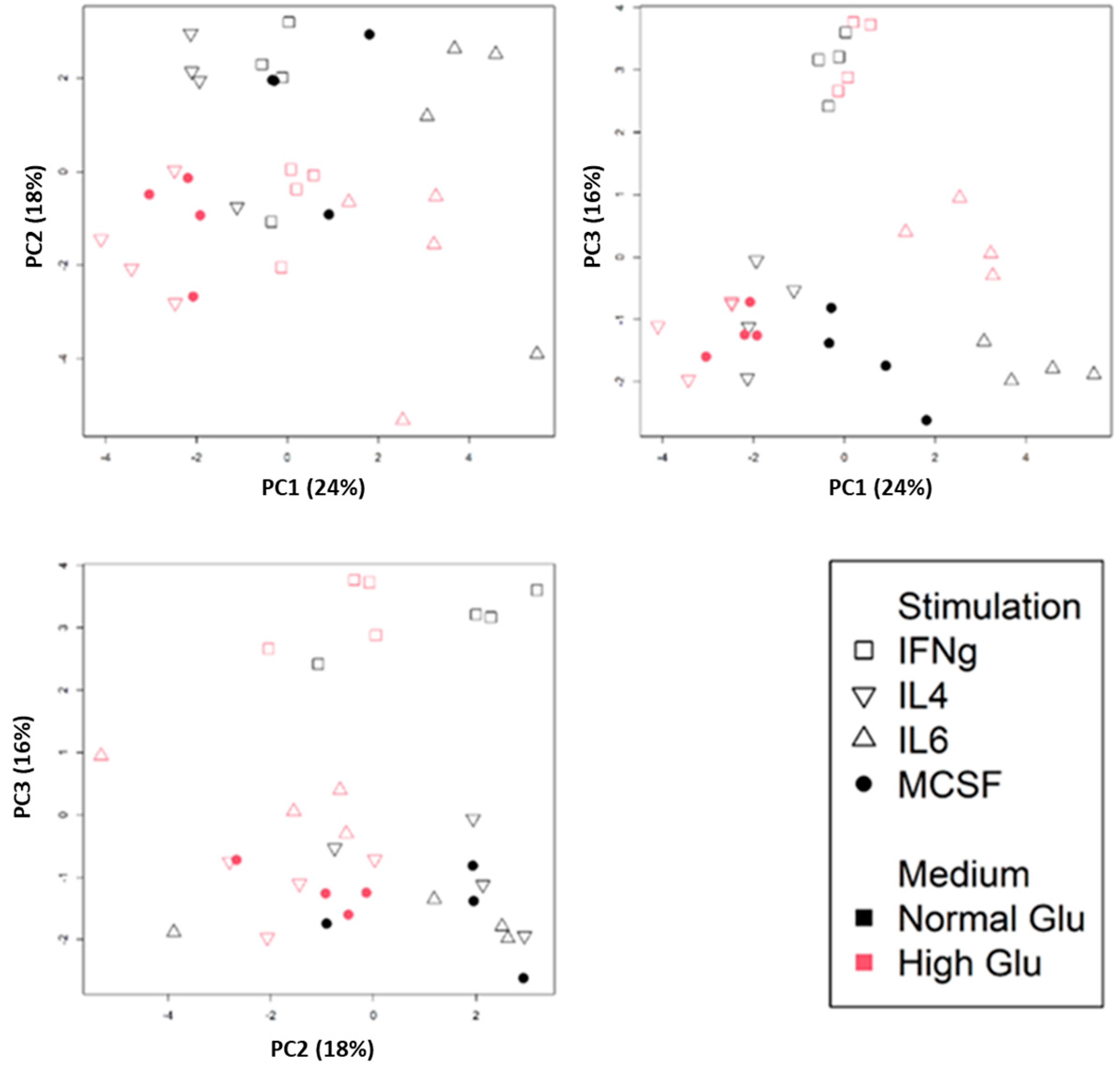
2.1. Basic Bioinformatic Analysis of Macrophages Polarized Under Normo- and Hyperglycemic Conditions
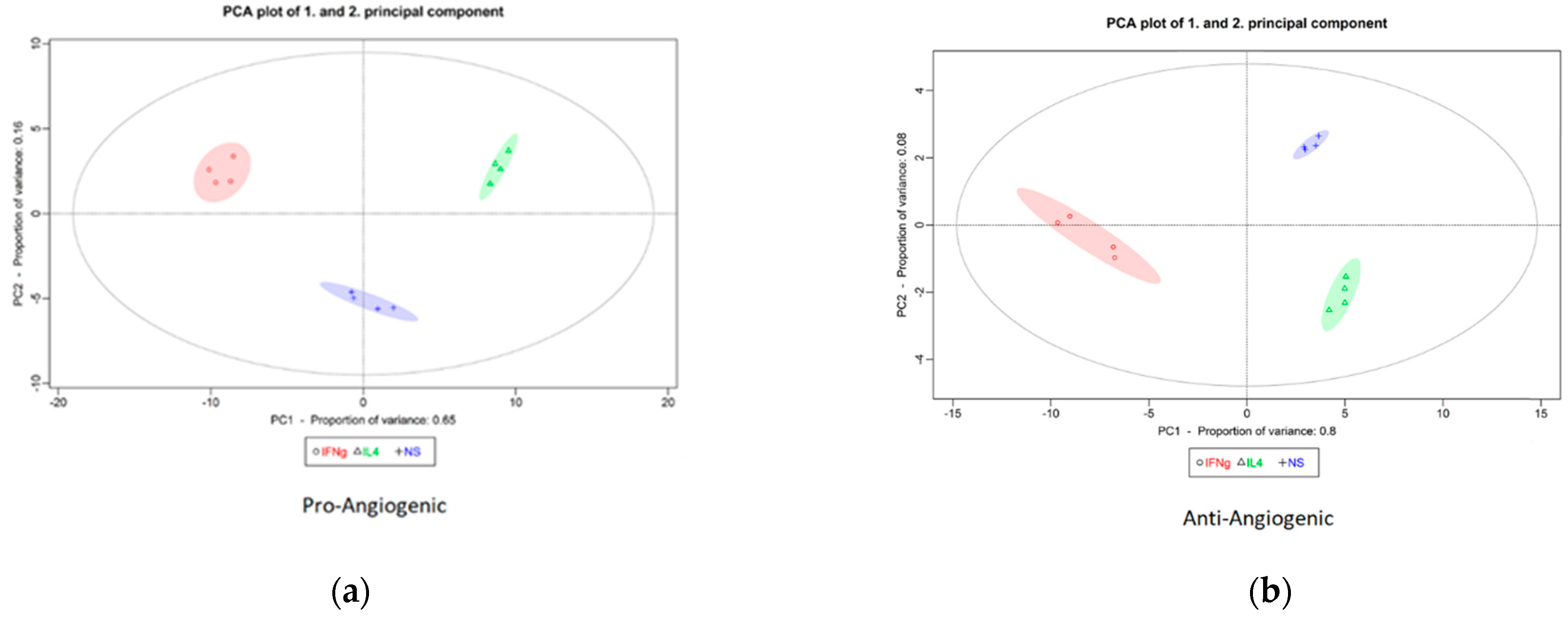
2.2. Identification of the Pro- and Anti-Angiogenic Genes Regulated upon M1 and M2 Macrophage Polarization
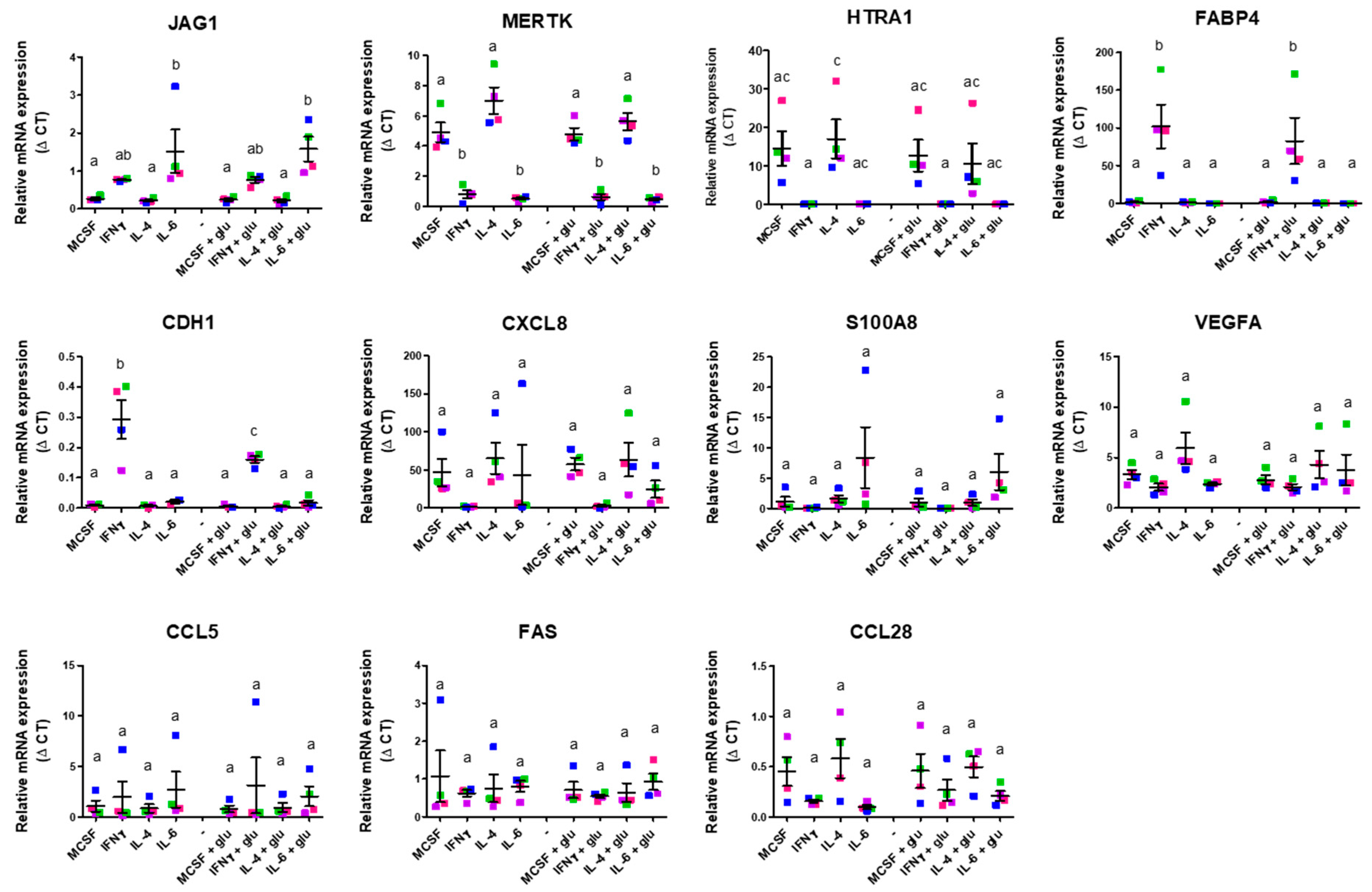
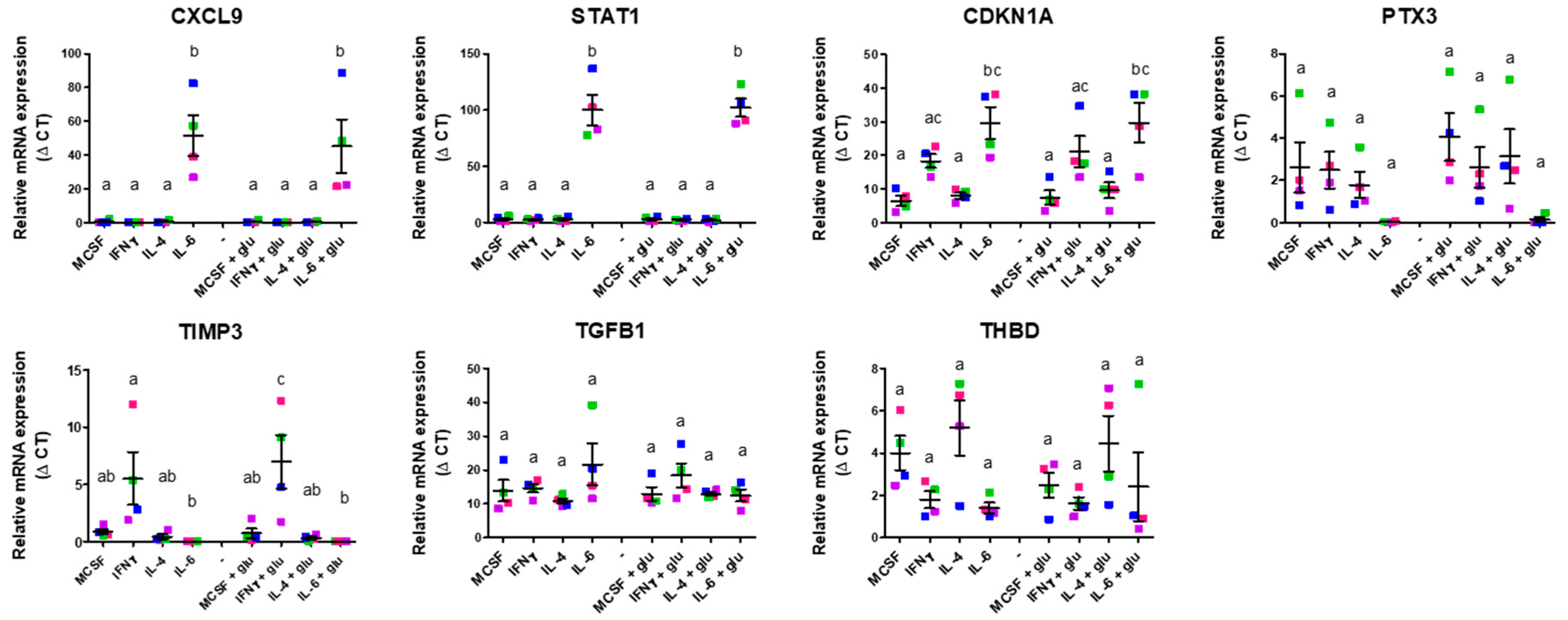
2.3. In Vitro Validation of Angiogenic Gene Expression of M1 and M2 Differentiation Under Standard and Hyperglycemic Conditions
3. Discussion
4. Materials and Methods
4.1. Recompilation and Analysis of Macrophage Datasets
4.2. Collection of Pro- and Anti-Angiogenic Signatures
4.3. Principal Component Analysis of Pro- and Anti-Angiogenic Genes in M0, M1, and M2 Macrophages
4.4. PBMC Isolation
4.5. Human Monocyte Isolation
4.6. Macrophage Polarization
4.7. Flow Cytometry
4.8. Real-Time Quantitative Polymerase Chain Reaction Analysis
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peluzzo, A.M.; Autieri, M.V. Challenging the paradigm: Anti-inflammatory interleukins and angiogenesis. Cells 2022, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Dudley, A.C.; Griffioen, A.W. Pathological angiogenesis: Mechanisms and therapeutic strategies. Angiogenesis 2023, 26, 313–347. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hong, H.; Tian, X.Y. The role of macrophages in vascular repair and regeneration after ischemic injury. Int. J. Mol. Sci. 2020, 21, 6328. [Google Scholar] [CrossRef]
- Larionova, I.; Kazakova, E.; Gerashchenko, T.; Kzhyshkowska, J. New Angiogenic regulators produced by TAMs: Perspective for targeting tumor angiogenesis. Cancers 2021, 13, 3253. [Google Scholar] [CrossRef]
- Shah, F.H.; Lee, H.W. Endothelial and macrophage interactions in the angiogenic niche. Cytokine Growth Factor. Rev. 2024, 78, 64–76. [Google Scholar] [CrossRef]
- Sanin, D.E.; Ge, Y.; Marinkovic, E.; Kabat, A.M.; Castoldi, A.; Caputa, G.; Grzes, K.M.; Curtis, J.D.; Thompson, E.A.; Willenborg, S.; et al. A common framework of monocyte-derived macrophage activation. Sci. Immunol. 2022, 7, eabl7482. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Katkar, G.; Ghosh, P. Macrophage states: There’s a method in the madness. Trends Immunol. 2023, 44, 954–964. [Google Scholar] [CrossRef]
- Yang, Z.; Tan, T.E.; Shao, Y.; Wong, T.Y.; Li, X. Classification of diabetic retinopathy: Past, present and future. Front. Endocrinol. 2022, 13, 1079217. [Google Scholar] [CrossRef]
- van der Pouw Kraan, T.C.T.M.; van der Laan, A.M.; Piek, J.J.; Horrevoets, A.J.G. Surfing the data tsunami, a bioinformatic dissection of the proangiogenic monocyte. Vasc. Pharmacol. 2012, 56, 297–305. [Google Scholar] [CrossRef]
- Raggi, F.; Pelassa, S.; Pierobon, D.; Penco, F.; Gattorno, M.; Novelli, F.; Eva, A.; Varesio, L.; Giovarelli, M.; Bosco, M.C. Regulation of human macrophage M1-M2 polarization balance by hypoxia and the triggering receptor expressed on myeloid cells-1. Front. Immunol. 2017, 8, 1097. [Google Scholar] [CrossRef] [PubMed]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 53, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.J.; O’Neill, C.L.; O’Doherty, T.M.; Knott, H.; Guduric-Fuchs, J.; Gardiner, T.A.; Stitt, A.W. Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol. Med. 2011, 17, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A.M.; Ahmad, A.; Allegaert, E.; Siddiquei, M.M.; Gikandi, P.W.; De Hertogh, G.; Opdenakker, G. Interleukin-11 overexpression and M2 macrophage density are associated with angiogenic activity in proliferative diabetic retinopathy. Ocul. Immunol. Inflamm. 2020, 28, 575–588. [Google Scholar] [CrossRef]
- Jeong, J.H.; Ojha, U.; Lee, Y.M. Pathological angiogenesis and inflammation in tissues. Arch. Pharm. Res. 2021, 44, 1–15. [Google Scholar] [CrossRef]
- Hagbi-Levi, S.; Grunin, M.; Jaouni, T.; Tiosano, L.; Rinsky, B.; Elbaz-Hayoun, S.; Peled, A.; Chowers, I. Proangiogenic characteristics of activated macrophages from patients with age-related macular degeneration. Neurobiol. Aging 2017, 51, 71–82. [Google Scholar] [CrossRef]
- Wang, W.; Lo, A.C.Y. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Li, H.; Meng, Y.; He, S.; Tan, X.; Zhang, Y.; Zhang, X.; Wang, L.; Zheng, W. Macrophages, chronic inflammation, and insulin resistance. Cells 2022, 11, 3001. [Google Scholar] [CrossRef]
- Moganti, K.; Li, F.; Schmuttermaier, C.; Riemann, S.; Kluter, H.; Gratchev, A.; Harmsen, M.C.; Kzhyshkowska, J. Hyperglycemia induces mixed M1/M2 cytokine profile in primary human monocyte-derived macrophages. Immunobiology 2017, 222, 952–959. [Google Scholar] [CrossRef]
- Kzhyshkowska, J.; Shen, J.; Larionova, I. Targeting of TAMs: Can we be more clever than cancer cells? Cell Mol. Immunol. 2024, 21, 1376–1409. [Google Scholar] [CrossRef]
- Mossel, D.M.; Moganti, K.; Riabov, V.; Weiss, C.; Kopf, S.; Cordero, J.; Dobreva, G.; Rots, M.G.; Kluter, H.; Harmsen, M.C.; et al. Epigenetic regulation of S100A9 and S100A12 expression in monocyte-macrophage system in hyperglycemic conditions. Front. Immunol. 2020, 11, 1071. [Google Scholar] [CrossRef] [PubMed]
- Badillo-Garcia, L.E.; Liu, Q.; Ziebner, K.; Balduff, M.; Sevastyanova, T.; Schmuttermaier, C.; Kluter, H.; Harmsen, M.; Kzhyshkowska, J. Hyperglycaemia amplifies TLR-mediated inflammatory response of M(IL4) macrophages to dyslipidemic ligands. J. Leukoc. Biol. 2024, 116, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Biselli-Chicote, P.M.; Oliveira, A.R.; Pavarino, E.C.; Goloni-Bertollo, E.M. VEGF gene alternative splicing: Pro- and anti-angiogenic isoforms in cancer. J. Cancer Res. Clin. Oncol. 2012, 138, 363–370. [Google Scholar] [CrossRef]
- Ahmad, A.; Nawaz, M.I. Molecular mechanism of VEGF and its role in pathological angiogenesis. J. Cell Biochem. 2022, 123, 1938–1965. [Google Scholar] [CrossRef] [PubMed]
- Ambarus, C.A.; Krausz, S.; van Eijk, M.; Hamann, J.; Radstake, T.R.; Reedquist, K.A.; Tak, P.P.; Baeten, D.L. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J. Immunol. Methods 2012, 375, 196–206. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Loke, P.; Lin, J.D. Redefining inflammatory macrophage phenotypes across stages and tissues by single-cell transcriptomics. Sci. Immunol. 2022, 7, eabo4652. [Google Scholar] [CrossRef]
- Blériot, C.; Dunsmore, G.; Alonso-Curbelo, D.; Ginhoux, F. A temporal perspective for tumor-associated macrophage identities and functions. Cancer Cell 2024, 42, 747–758. [Google Scholar] [CrossRef]
- Rahat, M.A.; Bitterman, H.; Lahat, N. Molecular mechanisms regulating macrophage response to hypoxia. Front. Immunol. 2011, 2, 45. [Google Scholar] [CrossRef]
- Wang, S.; Liu, G.; Li, Y.; Pan, Y. Metabolic reprogramming induces macrophage polarization in the tumor microenvironment. Front. Immunol. 2022, 13, 840029. [Google Scholar] [CrossRef]
- Geng, Y.; Fan, J.; Chen, L.; Zhang, C.; Qu, C.; Qian, L.; Chen, K.; Meng, Z.; Chen, Z.; Wang, P. A notch-dependent inflammatory feedback circuit between macrophages and cancer cells regulates pancreatic cancer metastasis. Cancer Res. 2021, 81, 64–76. [Google Scholar] [CrossRef]
- Allouch, A.; Voisin, L.; Zhang, Y.; Raza, S.Q.; Lecluse, Y.; Calvo, J.; Selimoglu-Buet, D.; de Botton, S.; Louache, F.; Pflumio, F.; et al. CDKN1A is a target for phagocytosis-mediated cellular immunotherapy in acute leukemia. Nat. Commun. 2022, 13, 6739. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T.; O’Brien, C.A.; Roberson, P.K.; Manolagas, S.C. Transcriptional activation of the p21(WAF1,CIP1,SDI1) gene by interleukin-6 type cytokines. A prerequisite for their pro-differentiating and anti-apoptotic effects on human osteoblastic cells. J. Biol. Chem. 1998, 273, 21137–21144. [Google Scholar] [CrossRef] [PubMed]
- Nasir, I.; McGuinness, C.; Poh, A.R.; Ernst, M.; Darcy, P.K.; Britt, K.L. Tumor macrophage functional heterogeneity can inform the development of novel cancer therapies. Trends Immunol. 2023, 44, 971–985. [Google Scholar] [CrossRef]
- Cohen, T.; Nahari, D.; Cerem, L.W.; Neufeld, G.; Levi, B.Z. Interleukin 6 induces the expression of vascular endothelial growth factor. J. Biol. Chem. 1996, 271, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Hojo, Y.; Ikeda, U.; Maeda, Y.; Takahashi, M.; Takizawa, T.; Okada, M.; Funayama, H.; Shimada, K. Interaction between human monocytes and vascular smooth muscle cells induces vascular endothelial growth factor expression. Atherosclerosis 2000, 150, 63–70. [Google Scholar] [CrossRef]
- Choi, B.R.; Johnson, K.R.; Maric, D.; McGavern, D.B. Monocyte-derived IL-6 programs microglia to rebuild damaged brain vasculature. Nat. Immunol. 2023, 24, 1110–1123. [Google Scholar] [CrossRef]
- Gopinathan, G.; Milagre, C.; Pearce, O.M.; Reynolds, L.E.; Hodivala-Dilke, K.; Leinster, D.A.; Zhong, H.; Hollingsworth, R.E.; Thompson, R.; Whiteford, J.R.; et al. Interleukin-6 stimulates defective angiogenesis. Cancer Res. 2015, 75, 3098–3107. [Google Scholar] [CrossRef]
- Kayakabe, K.; Kuroiwa, T.; Sakurai, N.; Ikeuchi, H.; Kadiombo, A.T.; Sakairi, T.; Matsumoto, T.; Maeshima, A.; Hiromura, K.; Nojima, Y. Interleukin-6 promotes destabilized angiogenesis by modulating angiopoietin expression in rheumatoid arthritis. Rheumatology 2012, 51, 1571–1579. [Google Scholar] [CrossRef]
- Roca, H.; Varsos, Z.S.; Sud, S.; Craig, M.J.; Ying, C.; Pienta, K.J. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J. Biol. Chem. 2009, 284, 34342–34354. [Google Scholar] [CrossRef] [PubMed]
- Braune, J.; Weyer, U.; Hobusch, C.; Mauer, J.; Bruning, J.C.; Bechmann, I.; Gericke, M. IL-6 regulates M2 polarization and local proliferation of adipose tissue macrophages in obesity. J. Immunol. 2017, 198, 2927–2934. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, P.; Wang, P.; Wang, W.; Liu, J. IL-6/ERK signaling pathway participates in type I IFN-programmed, unconventional M2-like macrophage polarization. Sci. Rep. 2023, 13, 1827. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, S.; Wang, Y.; Zhang, W.; Ma, K.; Hu, C.; Zhu, H.; Liang, S.; Liu, M.; Xu, N. IL-6 influences the polarization of macrophages and the formation and growth of colorectal tumor. Oncotarget 2018, 9, 17443–17454. [Google Scholar] [CrossRef]
- Ahmed, M.; de Winther, M.P.J.; Van den Bossche, J. Epigenetic mechanisms of macrophage activation in type 2 diabetes. Immunobiology 2017, 222, 937–943. [Google Scholar] [CrossRef]
- Marfella, R.; Sardu, C.; D’Onofrio, N.; Prattichizzo, F.; Scisciola, L.; Messina, V.; La Grotta, R.; Balestrieri, M.L.; Maggi, P.; Napoli, C.; et al. Glycaemic control is associated with SARS-CoV-2 breakthrough infections in vaccinated patients with type 2 diabetes. Nat. Commun. 2022, 13, 2318. [Google Scholar] [CrossRef]
- Prattichizzo, F.; de Candia, P.; Ceriello, A. Diabetes and kidney disease: Emphasis on treatment with SGLT-2 inhibitors and GLP-1 receptor agonists. Metabolism 2021, 120, 154799. [Google Scholar] [CrossRef]
- Wang, J.; Han, Y.; Huang, F.; Tang, L.; Mu, J.; Liang, Y. Diabetic macrophage small extracellular vesicles-associated miR-503/IGF1R axis regulates endothelial cell function and affects wound healing. Front. Immunol. 2023, 14, 1104890. [Google Scholar] [CrossRef]
- Wilson, C.L.; Miller, C.J. Simpleaffy: A bioConductor package for affymetrix quality control and data analysis. Bioinformatics 2005, 21, 3683–3685. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Gerds, T.A.; Nielsen, O.H.; Seidelin, J.B.; Troelsen, J.T.; Olsen, J. pcaGoPromoter--an R package for biological and regulatory interpretation of principal components in genome-wide gene expression data. PLoS ONE 2012, 7, e32394. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Aboud, S.; Karlen, K.; Hejdeman, B.; Urassa, W.; Biberfeld, G. Optimal blood mononuclear cell isolation procedures for gamma interferon enzyme-linked immunospot testing of healthy Swedish and Tanzanian subjects. Clin. Vaccine Immunol. 2008, 15, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.S.; Schiweck, C.; Arteaga-Henríquez, G.; Poletti, S.; Haarman, B.C.M.; Dik, W.A.; Schwarz, M.; Vrieze, E.; Mikova, O.; Joergens, S.; et al. Monocyte mitochondrial dysfunction, inflammaging, and inflammatory pyroptosis in major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110391. [Google Scholar] [CrossRef]
| DEG b | Up c | Down c | Common d | |
|---|---|---|---|---|
| M1-NG vs. M0-NG a | 1630 | 898 | 732 | |
| M1-HG vs. M0-NG | 1753 | 939 | 814 | 1190 |
| M2-NG vs. M0-NG | 1423 | 663 | 760 | |
| M2-HG vs. M0-NG | 1421 | 695 | 726 | 1188 |
| (A) | |||
| Order a | M1-NG vs. M0-NG | Count b | Benjamini c |
| 1 | GO:0006954~inflammatory response | 80 | 1.69 × 10−13 |
| 2 | GO:0007165~signal transduction | 133 | 0.0019 |
| 3 | GO:0050728~negative regulation of inflammatory response | 21 | 0.0016 |
| 4 | GO:0051056~regulation of small GTPase mediated signal transduction | 28 | 0.0024 |
| 5 | GO:0006915~apoptotic process | 75 | 0.0020 |
| 6 | GO:0006955~immune response | 60 | 0.0020 |
| 7 | GO:0006935~chemotaxis | 26 | 0.0023 |
| 8 | GO:0001525~angiogenesis | 38 | 0.0024 |
| 9 | GO:0000082~G1/S transition of mitotic cell cycle | 23 | 0.0028 |
| 10 | GO:0002250~adaptive immune response | 28 | 0.0065 |
| (B) | |||
| Order a | M1-HG vs. M0-NG | Count b | Benjamini c |
| 1 | GO:0006954~inflammatory response | 72 | 1.68 × 10−9 |
| 2 | GO:0001525~angiogenesis | 41 | 4.05 × 10−4 |
| 3 | GO:0006955~immune response | 61 | 0.0014 |
| 4 | GO:0032496~response to lipopolysaccharide | 31 | 0.0045 |
| 5 | GO:0006334~nucleosome assembly | 25 | 0.0058 |
| 6 | GO:0006935~chemotaxis | 25 | 0.0075 |
| 7 | GO:0050728~negative regulation of inflammatory response | 19 | 0.0102 |
| 8 | GO:0007067~mitotic nuclear division | 39 | 0.0099 |
| 9 | GO:0050729~positive regulation of inflammatory response | 18 | 0.0100 |
| 10 | GO:0006915~apoptotic process | 71 | 0.0094 |
| (A) | |||
| Order a | M2-NG vs. M0-NG | Count b | Benjamini c |
| 1 | GO:0006954~inflammatory response | 71 | 1.87 × 10−6 |
| 2 | GO:0051607~defense response to virus | 40 | 7.20 × 10−6 |
| 3 | GO:0045087~innate immune response | 72 | 6.22 × 10−5 |
| 4 | GO:0060337~type I interferon signaling pathway | 21 | 2.06 × 10−4 |
| 5 | GO:0060333~interferon-gamma-mediated signaling pathway | 21 | 0.0011 |
| 6 | GO:0071222~cellular response to lipopolysaccharide | 27 | 0.0017 |
| 7 | GO:0006955~immune response | 65 | 0.0023 |
| 8 | GO:0006919~activation of cysteine-type endopeptidase activity involved in apoptotic process | 22 | 0.0024 |
| 9 | GO:0002576~platelet degranulation | 25 | 0.0022 |
| 10 | GO:0002250~adaptive immune response | 31 | 0.0028 |
| 21 | GO:0001525~angiogenesis | 37 | 0.0316 |
| (B) | |||
| Order a | M2-HG vs. M0-NG | Count b | Benjamini c |
| 1 | GO:0051607~defense response to virus | 48 | 2.96 × 10−9 |
| 2 | GO:0006954~inflammatory response | 78 | 2.55 × 10−8 |
| 3 | GO:0045087~innate immune response | 79 | 3.50 × 10−6 |
| 4 | GO:0006955~immune response | 74 | 5.98 × 10−5 |
| 5 | GO:0006952~defense response | 22 | 2.48 × 10−4 |
| 6 | GO:0060337~type I interferon signaling pathway | 21 | 4.18 × 10−4 |
| 7 | GO:0060333~interferon-gamma-mediated signaling pathway | 22 | 5.23 × 10−4 |
| 8 | GO:0009615~response to virus | 27 | 0.0027 |
| 9 | GO:0045071~negative regulation of viral genome replication | 15 | 0.0032 |
| 10 | GO:0002250~adaptive immune response | 32 | 0.0040 |
| 24 | GO:0001525~angiogenesis | 38 | 0.0475 |
| M1(IFN-γ) vs. M0 | M2(IL-4) vs. M0 | |||
|---|---|---|---|---|
| up | down | up | down | |
| Pro-angiogenic genes | 22 (7%) a | 39 (13%) | 41 (14%) | 30 (10%) |
| Anti-angiogenic genes | 8 (7%) | 9 (8%) | 12 (11%) | 21 (18%) |
| M1(IFN-γ)-HG vs. M1(IFN-γ)-NG | M2(IL-4)-HG vs. M2(IL-4)-NG | |||
|---|---|---|---|---|
| up | down | up | down | |
| Pro-angiogenic genes | 48 (16%) a | 28 (9%) | 50 (17%) | 65 (22%) |
| Anti-angiogenic genes | 15 (13%) | 18 (16%) | 22 (19%) | 15 (13%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shariatzadeh, M.; Payán-Gómez, C.; Kzhyshkowska, J.; Dik, W.A.; Leenen, P.J.M. Polarized Macrophages Show Diverse Pro-Angiogenic Characteristics Under Normo- and Hyperglycemic Conditions. Int. J. Mol. Sci. 2025, 26, 4846. https://doi.org/10.3390/ijms26104846
Shariatzadeh M, Payán-Gómez C, Kzhyshkowska J, Dik WA, Leenen PJM. Polarized Macrophages Show Diverse Pro-Angiogenic Characteristics Under Normo- and Hyperglycemic Conditions. International Journal of Molecular Sciences. 2025; 26(10):4846. https://doi.org/10.3390/ijms26104846
Chicago/Turabian StyleShariatzadeh, Mahnaz, César Payán-Gómez, Julia Kzhyshkowska, Willem A. Dik, and Pieter J. M. Leenen. 2025. "Polarized Macrophages Show Diverse Pro-Angiogenic Characteristics Under Normo- and Hyperglycemic Conditions" International Journal of Molecular Sciences 26, no. 10: 4846. https://doi.org/10.3390/ijms26104846
APA StyleShariatzadeh, M., Payán-Gómez, C., Kzhyshkowska, J., Dik, W. A., & Leenen, P. J. M. (2025). Polarized Macrophages Show Diverse Pro-Angiogenic Characteristics Under Normo- and Hyperglycemic Conditions. International Journal of Molecular Sciences, 26(10), 4846. https://doi.org/10.3390/ijms26104846







