Significance of Midkine Signaling in Women’s Cancers: Novel Biomarker and Therapeutic Target
Abstract
1. Introduction
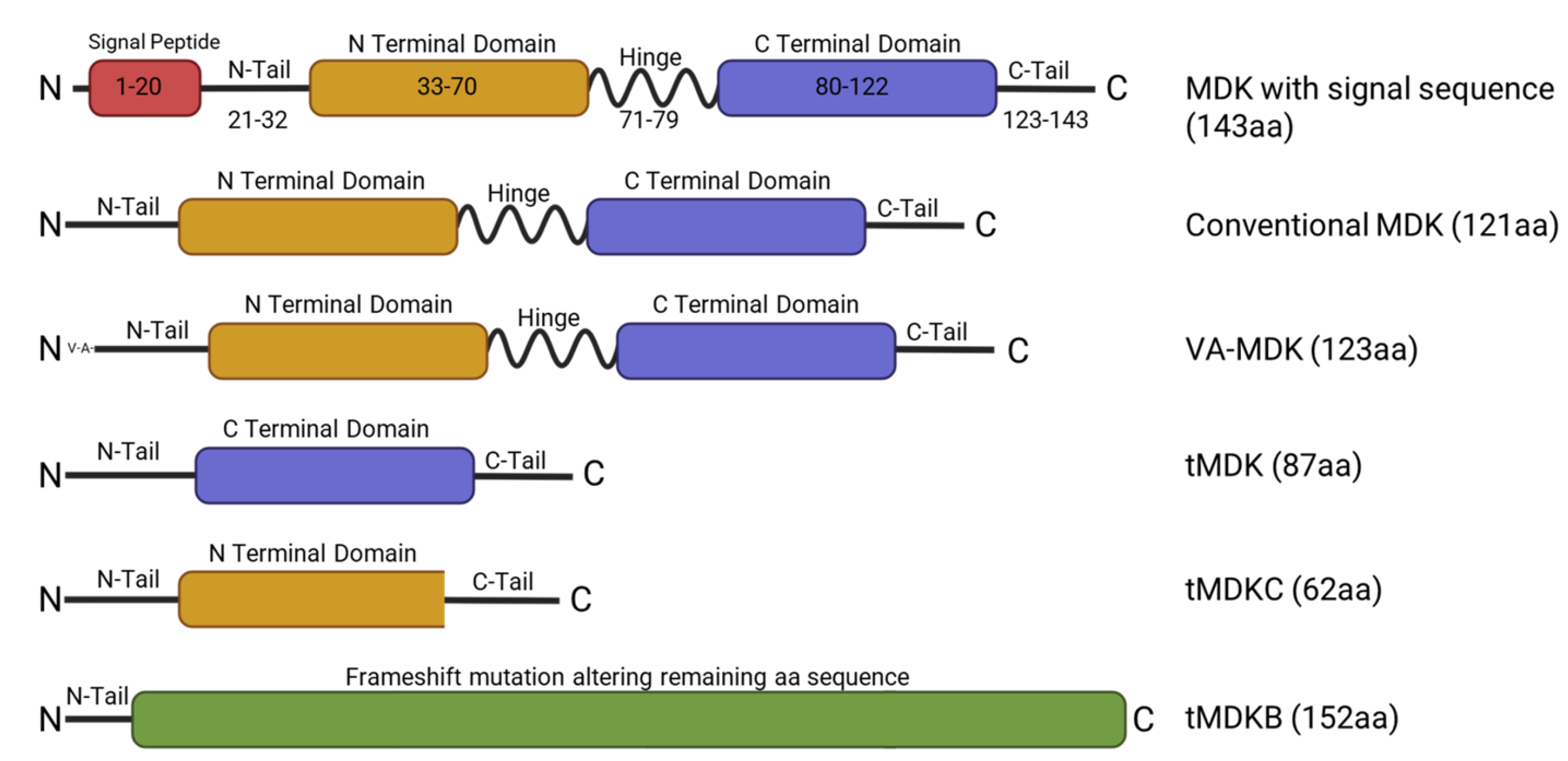
2. Midkine Structure and Regulation
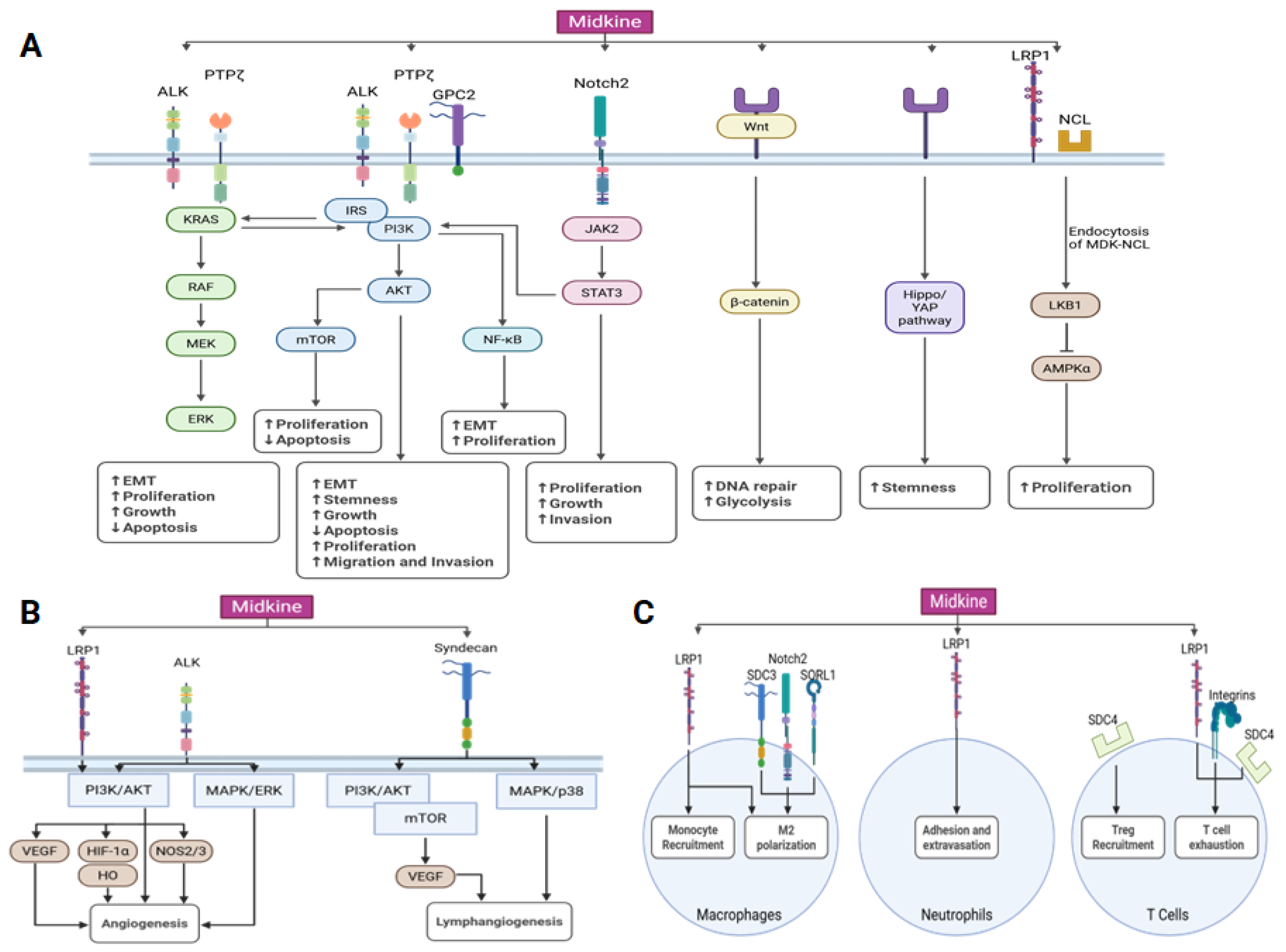
3. Midkine Signaling Pathways
3.1. Midkine Receptors
| MDK Receptor | Known Downstream Pathways | Function | References |
|---|---|---|---|
| ALK | PI3k/AKT, MAPK/ERK, KRAS/RAF | Promotes cancer cell growth and proliferation; suppresses apoptosis; promotes angiogenesis | [41,54] |
| Glypicans | PI3K/AKT | Promotes proliferation, adhesion, migration and invasion | [39,40] |
| Integrins | LRP6 and PTPζ | Facilitates cancer cell migration; promotes T-cell exhaustion | [44] |
| LRP1 | LKB1/AMPKα, PI3K/AKT | Enhances proliferation of cancer cells; promotes recruitment and M2 polarization of tumor-associated macrophages; promotes adhesion and extravasation of PMNs; promotes T-cell exhaustion. Facilitates angiogenesis and anchorage independent growth | [37,42,58,59], |
| Notch2 | JAK2/STAT3 | Promotes EMT, proliferation, and growth of cancer cells; promotes M2 polarization of tumor-associated macrophages | [43] |
| NCL | LKB1/AMPKα | Promotes cancer cell proliferation; important in crosstalk between cancer cells and the tumor microenvironment, promotes a malignant phenotype in endothelial cells and and helps to promote an immunosuppressed tumor microenvironment. | [47,48,49] |
| PTPζ | KRAS/RAF; PI3K/AKT/mTOR, NFκB | Promotes cancer cell proliferation and suppresses apoptosis | [36,37,57] |
| SORL1 | Promotes M2 polarization of tumor-associated macrophages | [59] | |
| Syndecans | PI3K/AKT, MAPK/p38 | Promotes Treg recruitment, T-cell exhaustion, M2 polarization of tumor-associated macrophages, and lymphangiogenesis | [38,60,61] |
3.2. Midkine in Cell Proliferation
3.3. Midkine in Estrogen Signaling
3.4. Midkine in Cancer Stemness
3.5. Midkine in Angiogeneiss
3.6. Midkine Signaling in Hypoxia
3.7. Midkine Signaling in Metastasis
3.8. Midkine Signaling in the Tumor Microenvironment
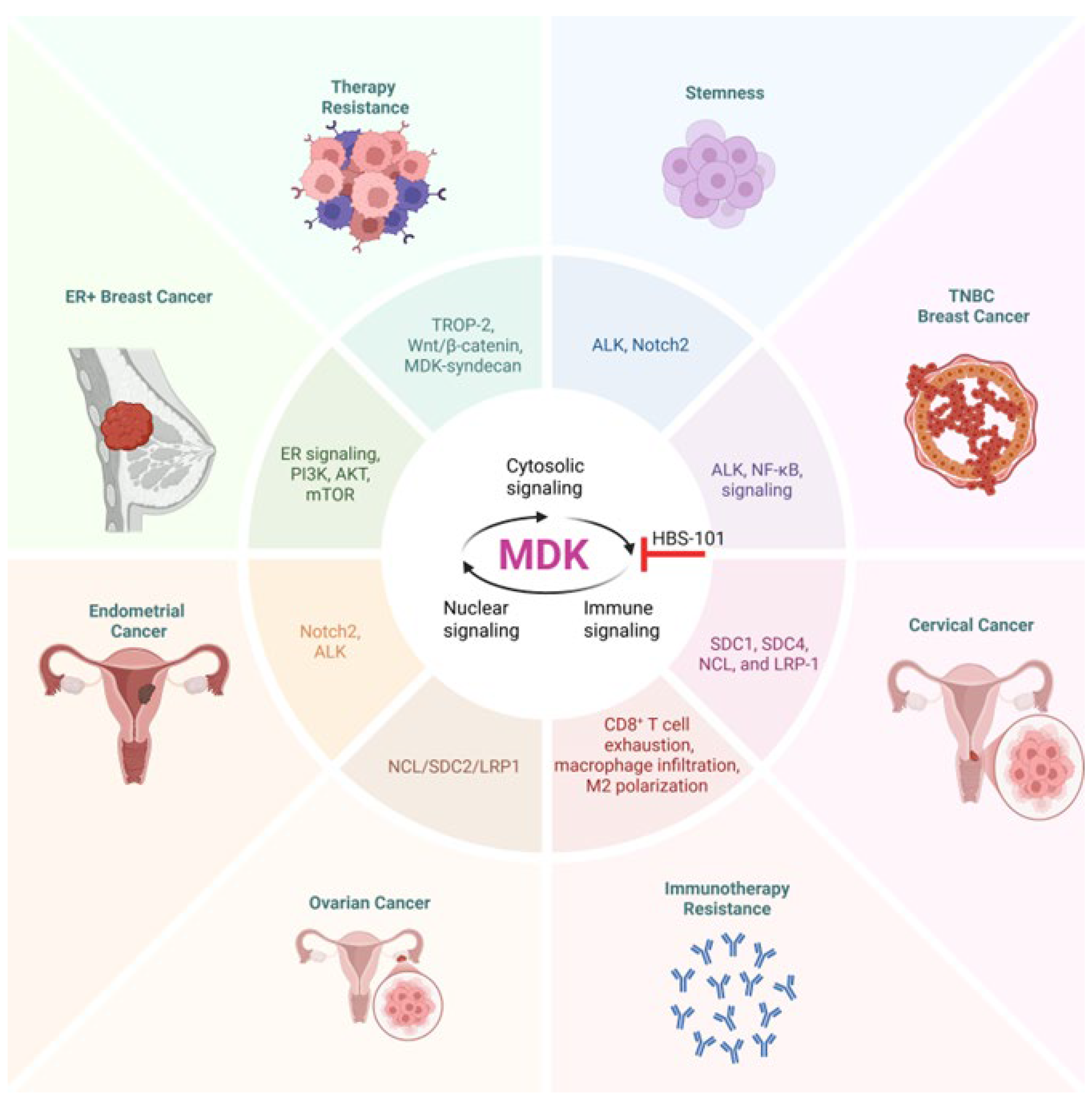
4. Midkine Signaling in Women’s Cancers
4.1. Midkine Signaling in Breast Cancer
4.2. Midkine as a Biomarker in Breast Cancer
4.3. Midkine in Ovarian Cancer
4.4. Midkine as a Biomarker in Ovarian Cancer
4.5. Midkine in Endometrial Cancer
4.6. Midkine as a Biomarker in Endometrial Cancer
4.7. Midkine in Cervical Cancer
4.8. Midkine as a Biomarker in Cervical Cancer
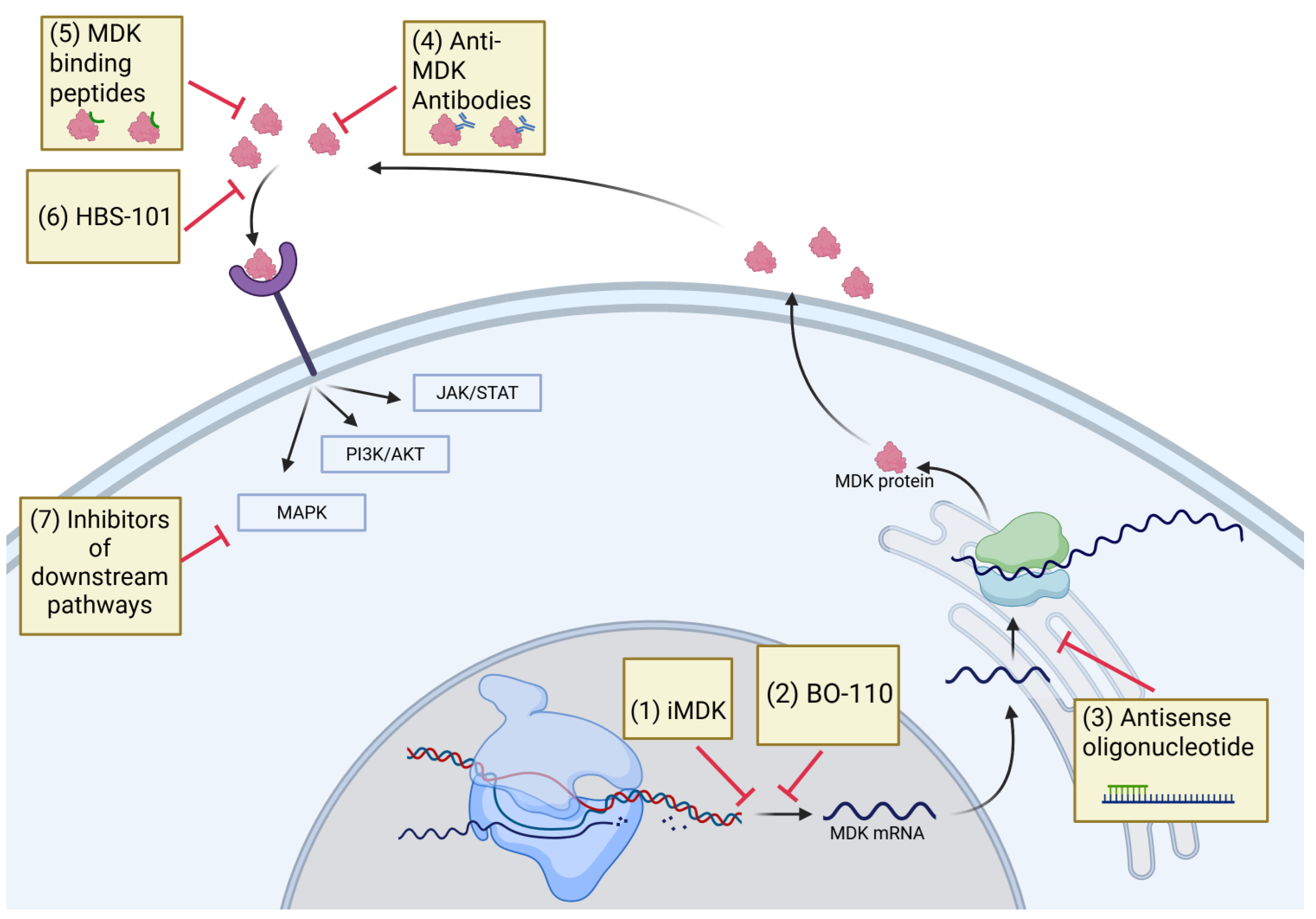
5. Therapeutic Targeting of MDK
6. Conclusions and Future Directions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-flurouracil |
| AGR2 | anterior gradient protein 2 homolog |
| AKT | protein kinase B |
| AMPKα | AMP-activated protein kinase |
| ALK | anaplastic lymphoma kinase |
| Arg1 | arginase 1 |
| AUC | area under the curve |
| BCL2 | B-cell lymphoma 2 protein |
| BMI | body mass index |
| CA125 | cancer antigen 125 |
| CA 15-3 | cancer antigen 15-3 |
| CAM | chick chorioallantoic membrane |
| CAK | CDK-activating kinase |
| CCL4 | chemokine (C-C motif) ligand 4 |
| CDK4/6 | cyclin-dependent kinase 4/6 |
| CEA | carcinoembryonic antigen |
| CSF | cerebrospinal fluid |
| CTLA4 | cytotoxic T-lymphocyte-associated protein 4 |
| EMT | epithelial to mesenchymal transition |
| ER | estrogen receptor |
| ERK | MAP-kinase/extracellular signal-regulated kinase |
| GPC2 | glypican 2 |
| HE4 | human epididymis protein 4 |
| HER2 | human epidermal growth factor receptor 2 |
| HUVECs | human umbilical vein endothelial cells |
| IFN-α | interferon alpha |
| IFN-β | interferon beta |
| IFN-γ | interferon gamma |
| JAK/STAT | Janus kinase/signal transducers and activators of transcription |
| KRAS | Kirsten rat sarcoma virus |
| lncRNA | long non-coding RNA |
| LKB1 | serine/threonine kinase 11 |
| LRP1 | low-density lipoprotein receptor-related protein 1 |
| MAPK | mitogen-activated protein kinase |
| MDK | midkine |
| MDSC | myeloid-derived suppressor cell |
| MHC | MHC class 1 polypeptide-related sequence A and B |
| miR | microRNAs |
| MICA/B | MHC class 1 polypeptide-related sequence A and B |
| mTOR | mammalian target of rapamycin |
| NCL | nucleolin |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK cells | natural killer cells |
| NOS2 | inducible nitric oxide synthase |
| Notch2 | neurogenic locus notch homolog protein 2 |
| PD-1 | programmed cell-death protein 1 |
| PD-L1 | programmed death ligand 1 |
| PI3K | phosphatidylinositol 3-kinase |
| PMNs | polymorphonuclear cells |
| PTPζ | protein tyrosine phosphatase ζ |
| RAF | rapidly accelerated fibrosarcoma |
| SCCA | squamous cell carcinoma antigen |
| SDC1 | syndecan1 |
| SDC2 | syndecan 2 |
| SDC3 | syndecan 3 |
| SDC4 | syndecan 4 |
| siRNA | small interfering RNA |
| SORL1 | sortilin-related receptor 1 |
| SREBF1 | sterol regulatory element-binding transcription factor 1 |
| STAT3 | signal transducer and activator of transcription 3 |
| STRAD | STE20-related adaptor alpha |
| TAZ | transcriptional coactivator with PDZ-binding motif |
| TGF-β | transforming growth factor beta |
| tMDK | truncated MDK |
| TNBC | triple-negative breast cancer |
| Treg | regulatory T cell |
| TROP-2 | tumor-associated calcium signal transducer 2 |
| USP12 | ubiquitin-specific protease 12 |
| VA-MDK | valine-alanine MDK |
| VEGF | vascular endothelial growth factor |
| VEGFR3 | VEGF receptor 3 |
| Wnt | wingless and integrated |
| YAP | yes-associated protein |
References
- Kadomatsu, K.; Tomomura, M.; Muramatsu, T. cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem. Biophys. Res. Commun. 1988, 151, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Kadomatsu, K.; Huang, R.P.; Suganuma, T.; Murata, F.; Muramatsu, T. A retinoic acid responsive gene MK found in the teratocarcinoma system is expressed in spatially and temporally controlled manner during mouse embryogenesis. J. Cell Biol. 1990, 110, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.; Yao, S. The midkine family of growth factors: Diverse roles in nervous system formation and maintenance. Br. J. Pharmacol. 2014, 171, 905–912. [Google Scholar] [CrossRef]
- Filippou, P.S.; Karagiannis, G.S.; Constantinidou, A. Midkine (MDK) growth factor: A key player in cancer progression and a promising therapeutic target. Oncogene 2020, 39, 2040–2054. [Google Scholar] [CrossRef]
- Hirota, Y.; Osuga, Y.; Nose, E.; Koga, K.; Yoshino, O.; Hirata, T.; Yano, T.; Tsutsumi, O.; Sakuma, S.; Muramatsu, T.; et al. The presence of midkine and its possible implication in human ovarian follicles. Am. J. Reprod. Immunol. 2007, 58, 367–373. [Google Scholar] [CrossRef]
- Cadenas, J.; Pors, S.E.; Hansen, C.P.; Olufsen, S.M.; Subiran, C.; Bøtkjær, J.A.; La Cour Poulsen, L.; Fedder, J.; Dueholm, M.; Colmorn, L.B.; et al. Midkine characterization in human ovaries: Potential new variants in follicles. FS Sci. 2023, 4, 294–301. [Google Scholar] [CrossRef]
- Cernkovich, E.R.; Deng, J.; Hua, K.; Harp, J.B. Midkine is an autocrine activator of signal transducer and activator of transcription 3 in 3T3-L1 cells. Endocrinology 2007, 148, 1598–1604. [Google Scholar] [CrossRef]
- Wu, B.; Li, Y.; Nie, N.; Shen, X.; Jiang, W.; Liu, Y.; Gong, L.; An, C.; Zhao, K.; Yao, X.; et al. SFRP4+ stromal cell subpopulation with IGF1 signaling in human endometrial regeneration. Cell Discov. 2022, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Muramatsu, H.; Ishiguro, N.; Muramatsu, T. Midkine, a Heparin-Binding Growth Factor, Is Fundamentally Involved in the Pathogenesis of Rheumatoid Arthritis. Arthritis Rheum. 2004, 50, 1420–1429. [Google Scholar] [CrossRef]
- Aynacıoğlu, A.; Bilir, A.; Tuna, M.Y. Involvement of midkine in autoimmune and autoinflammatory diseases. Mod. Rheumatol. 2019, 29, 567–571. [Google Scholar] [CrossRef]
- Wang, J.; Takeuchi, H.; Sonobe, Y.; Jin, S.; Mizuno, T.; Miyakawa, S.; Fujiwara, M.; Nakamura, Y.; Kato, T.; Muramatsu, H.; et al. Inhibition of midkine alleviates experimental autoimmune encephalomyelitis through the expansion of regulatory T cell population. Proc. Natl. Acad. Sci. USA 2008, 105, 3915–3920. [Google Scholar] [CrossRef]
- Salman, I.N.; Mohammed, N.U.G.; Shaban, A.; Abed, B.A.; Ali Mutar, S.; Omran, H.H. Clinical relevance of midkine as a biomarker predicting atherosclerotic risk factors in individuals with type-2 diabetes mellitus: A cross-sectional study. J. Diabetes Metab. Disord. 2025, 24, 20. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Wang, G.; Yin, S.H.; Yu, X.H. Midkine: A multifaceted driver of atherosclerosis. Clin. Chim. Acta 2021, 521, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Sun, H.; Wang, Y.; Zhang, L.; Xia, Z.; Peng, L.; Hou, Y.; Shen, W.; Liu, R.; Peng, Y. Midkine, a potential link between obesity and insulin resistance. PLoS ONE 2014, 9, e88299. [Google Scholar] [CrossRef]
- Jones, D.R. Measuring midkine: The utility of midkine as a biomarker in cancer and other diseases. Br. J. Pharmacol. 2014, 171, 2925–2939. [Google Scholar] [CrossRef] [PubMed]
- Kerzerho, J.; Adotevi, O.; Castelli, F.A.; Dosset, M.; Bernardeau, K.; Szely, N.; Lang, F.; Tartour, E.; Maillere, B. The angiogenic growth factor and biomarker midkine is a tumor-shared antigen. J. Immunol. 2010, 185, 418–423. [Google Scholar] [CrossRef]
- Zhang, D.; Ding, L.; Li, Y.; Ren, J.; Shi, G.; Wang, Y.; Zhao, S.; Ni, Y.; Hou, Y. Midkine derived from cancer-associated fibroblasts promotes cisplatin-resistance via up-regulation of the expression of lncRNA ANRIL in tumour cells. Sci. Rep. 2017, 7, 16231. [Google Scholar] [CrossRef] [PubMed]
- Said, E.A.; Al-Dughaishi, S.; Al-Hatmi, W.; Al-Reesi, I.; Al-Riyami, M.; Al-Balushi, M.S.; Al-Bimani, A.; Al-Busaidi, J.Z.; Al-Khabori, M.; Al-Kindi, S.; et al. Human macrophages and monocyte-derived dendritic cells stimulate the proliferation of endothelial cells through midkine production. PLoS ONE 2022, 17, e0267662. [Google Scholar] [CrossRef]
- Uehara, K.; Matsubara, S.; Kadomatsu, K.; Tsutsui, J.-i.; Muramatsu, T. Genomic Structure of Human Midkine (MK), a Retinoi1c Acid-Responsive Growth/Differentiation Factor1. J. Biochem. 1992, 111, 563–567. [Google Scholar] [CrossRef]
- Kaname, T.; Kuwano, A.; Murano, I.; Uehara, K.; Muramatsu, T.; Kajii, T. Midkine gene (MDK), a gene for prenatal differentiation and neuroregulation, maps to band 11p11.2 by fluorescence in situ hybridization. Genomics 1993, 17, 514–515. [Google Scholar] [CrossRef]
- Tao, P.; Xu, D.; Lin, S.; Ouyang, G.L.; Chang, Y.; Chen, Q.; Yuan, Y.; Zhuo, X.; Luo, Q.; Li, J.; et al. Abnormal expression, highly efficient detection and novel truncations of midkine in human tumors, cancers and cell lines. Cancer Lett. 2007, 253, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, T. Structure and function of midkine as the basis of its pharmacological effects. Br. J. Pharmacol. 2014, 171, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, T. Midkine and pleiotrophin: Two related proteins involved in development, survival, inflammation and tumorigenesis. J. Biochem. 2002, 132, 359–371. [Google Scholar] [CrossRef]
- Fabri, L.; Maruta, H.; Muramatsu, H.; Muramatsu, T.; Simpson, R.J.; Burgess, A.W.; Nice, E.C. Structural characterisation of native and recombinant forms of the neurotrophic cytokine MK. J. Chromatogr. 1993, 646, 213–225. [Google Scholar] [CrossRef]
- Pedraza, R.C.; Matsubara, S.; Muramatsu, T. A Retinoic Acid-Responsive Element in Human Midkine Gene1. J. Biochem. 1995, 117, 845–849. [Google Scholar] [CrossRef]
- Zhao, G.; Nie, Y.; Lv, M.; He, L.; Wang, T.; Hou, Y. ERβ-mediated estradiol enhances epithelial mesenchymal transition of lung adenocarcinoma through increasing transcription of midkine. Mol. Endocrinol. 2012, 26, 1304–1315. [Google Scholar] [CrossRef]
- Reynolds, P.R.; Mucenski, M.L.; Le Cras, T.D.; Nichols, W.C.; Whitsett, J.A. Midkine is regulated by hypoxia and causes pulmonary vascular remodeling. J. Biol. Chem. 2004, 279, 37124–37132. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Matsubara, S.; Pedraza, C.; Ozawa, M.; Tsutsui, J.; Takamatsu, H.; Noguchi, H.; Akiyama, T.; Muramatsu, T. Midkine as a novel target gene for the Wilms’ tumor suppressor gene (WT1). Oncogene 1996, 13, 2197–2203. [Google Scholar]
- Luo, J.; Wang, X.; Xia, Z.; Yang, L.; Ding, Z.; Chen, S.; Lai, B.; Zhang, N. Transcriptional factor specificity protein 1 (SP1) promotes the proliferation of glioma cells by up-regulating midkine (MDK). Mol. Biol. Cell 2015, 26, 430–439. [Google Scholar] [CrossRef]
- Meng, X.; Duan, C.; Pang, H.; Chen, Q.; Han, B.; Zha, C.; Dinislam, M.; Wu, P.; Li, Z.; Zhao, S.; et al. DNA damage repair alterations modulate M2 polarization of microglia to remodel the tumor microenvironment via the p53-mediated MDK expression in glioma. EBioMedicine 2019, 41, 185–199. [Google Scholar] [CrossRef]
- Lu, J.; Liu, Q.H.; Wang, F.; Tan, J.J.; Deng, Y.Q.; Peng, X.H.; Liu, X.; Zhang, B.; Xu, X.; Li, X.P. Exosomal miR-9 inhibits angiogenesis by targeting MDK and regulating PDK/AKT pathway in nasopharyngeal carcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Pan, Y.; Li, X.; Zhang, X.; Xue, Y.; Wang, T.; Zhao, S.; Hou, Y. Dihydroartemisinin and Curcumin Synergistically Induce Apoptosis in SKOV3 Cells Via Upregulation of MiR-124 Targeting Midkine. Cell Physiol. Biochem. 2017, 43, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Li, C.; Wang, F.; Han, F.; Zhu, L. The Long Noncoding RNA ZFAS1 Potentiates the Development of Hepatocellular Carcinoma via the microRNA-624/MDK/ERK/JNK/P38 Signaling Pathway. Onco Targets Ther. 2020, 13, 4431–4444. [Google Scholar] [CrossRef]
- Han, X.; Li, M.; Xu, J.; Fu, J.; Wang, X.; Wang, J.; Xia, T.; Wang, S.; Ma, G. miR-1275 targets MDK/AKT signaling to inhibit breast cancer chemoresistance by lessening the properties of cancer stem cells. Int. J. Biol. Sci. 2023, 19, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, B.; Chen, W.; Man, X. MicroRNA-188 inhibits biological activity of lung cancer stem cells through targeting MDK and mediating the Hippo pathway. Exp. Physiol. 2020, 105, 1360–1372. [Google Scholar] [CrossRef]
- Maeda, N.; Ichihara-Tanaka, K.; Kimura, T.; Kadomatsu, K.; Muramatsu, T.; Noda, M. A receptor-like protein-tyrosine phosphatase PTPzeta/RPTPbeta binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPzeta. J. Biol. Chem. 1999, 274, 12474–12479. [Google Scholar] [CrossRef]
- Sakaguchi, N.; Muramatsu, H.; Ichihara-Tanaka, K.; Maeda, N.; Noda, M.; Yamamoto, T.; Michikawa, M.; Ikematsu, S.; Sakuma, S.; Muramatsu, T. Receptor-type protein tyrosine phosphatase zeta as a component of the signaling receptor complex for midkine-dependent survival of embryonic neurons. Neurosci. Res. 2003, 45, 219–224. [Google Scholar] [CrossRef]
- Deepa, S.S.; Yamada, S.; Zako, M.; Goldberger, O.; Sugahara, K. Chondroitin Sulfate Chains on Syndecan-1 and Syndecan-4 from Normal Murine Mammary Gland Epithelial Cells Are Structurally and Functionally Distinct and Cooperate with Heparan Sulfate Chains to Bind Growth Factors: A novel function to control binding of midkine, pleiotrophin, and basic fibroblast growth factor. J. Biol. Chem. 2004, 279, 37368–37376. [Google Scholar] [CrossRef]
- Chen, S.; Liao, J.; Li, J.; Wang, S. GPC2 promotes prostate cancer progression via MDK-mediated activation of PI3K/AKT signaling pathway. Funct. Integr. Genom. 2024, 24, 127. [Google Scholar] [CrossRef]
- Kurosawa, N.; Chen, G.Y.; Kadomatsu, K.; Ikematsu, S.; Sakuma, S.; Muramatsu, T. Glypican-2 binds to midkine: The role of glypican-2 in neuronal cell adhesion and neurite outgrowth. Glycoconj. J. 2001, 18, 499–507. [Google Scholar] [CrossRef]
- Stoica, G.E.; Kuo, A.; Powers, C.; Bowden, E.T.; Sale, E.B.; Riegel, A.T.; Wellstein, A. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J. Biol. Chem. 2002, 277, 35990–35998. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, H.; Zou, K.; Sakaguchi, N.; Ikematsu, S.; Sakuma, S.; Muramatsu, T. LDL receptor-related protein as a component of the midkine receptor. Biochem. Biophys. Res. Commun. 2000, 270, 936–941. [Google Scholar] [CrossRef]
- Huang, Y.; Hoque, M.O.; Wu, F.; Trink, B.; Sidransky, D.; Ratovitski, E.A. Midkine induces epithelial-mesenchymal transition through Notch2/Jak2-Stat3 signaling in human keratinocytes. Cell Cycle 2008, 7, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, H.; Zou, P.; Suzuki, H.; Oda, Y.; Chen, G.Y.; Sakaguchi, N.; Sakuma, S.; Maeda, N.; Noda, M.; Takada, Y.; et al. alpha4beta1- and alpha6beta1-integrins are functional receptors for midkine, a heparin-binding growth factor. J. Cell Sci. 2004, 117, 5405–5415. [Google Scholar] [CrossRef]
- Pan, X.W.; Chen, W.J.; Xu, D.; Guan, W.B.; Li, L.; Chen, J.X.; Chen, W.J.; Dong, K.Q.; Ye, J.Q.; Gan, S.S.; et al. Molecular subtyping and characterization of clear cell renal cell carcinoma by tumor differentiation trajectories. iScience 2023, 26, 108370. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, N.; Kumar, P.; Wang, C.; Leu, J.S.; Flynn, W.F.; Gao, R.; Baskin, D.S.; Pichumani, K.; Ijare, O.B.; Wood, S.L.; et al. Single-cell analysis of human glioma and immune cells identifies S100A4 as an immunotherapy target. Nat. Commun. 2022, 13, 767. [Google Scholar] [CrossRef]
- Shibata, Y.; Muramatsu, T.; Hirai, M.; Inui, T.; Kimura, T.; Saito, H.; McCormick, L.M.; Bu, G.; Kadomatsu, K. Nuclear targeting by the growth factor midkine. Mol. Cell Biol. 2002, 22, 6788–6796. [Google Scholar] [CrossRef]
- Yu, X.; Xie, L.; Ge, J.; Li, H.; Zhong, S.; Liu, X. Integrating single-cell RNA-seq and spatial transcriptomics reveals MDK-NCL dependent immunosuppressive environment in endometrial carcinoma. Front. Immunol. 2023, 14, 1145300. [Google Scholar] [CrossRef]
- Hovanessian, A.G. Midkine, a cytokine that inhibits HIV infection by binding to the cell surface expressed nucleolin. Cell Res. 2006, 16, 174–181. [Google Scholar] [CrossRef]
- Xia, T.; Chen, D.; Liu, X.; Qi, H.; Wang, W.; Chen, H.; Ling, T.; Otkur, W.; Zhang, C.-S.; Kim, J.; et al. Midkine noncanonically suppresses AMPK activation through disrupting the LKB1-STRAD-Mo25 complex. Cell Death Dis. 2022, 13, 414. [Google Scholar] [CrossRef]
- Thorpe, L.M.; Yuzugullu, H.; Zhao, J.J. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer 2015, 15, 7–24. [Google Scholar] [CrossRef]
- Olmeda, D.; Cerezo-Wallis, D.; Riveiro-Falkenbach, E.; Pennacchi, P.C.; Contreras-Alcalde, M.; Ibarz, N.; Cifdaloz, M.; Catena, X.; Calvo, T.G.; Cañón, E.; et al. Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine. Nature 2017, 546, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qu, X.; Zhang, X.; Luo, Y.; Zhang, Y.; Luo, Y.; Hou, K.; Liu, Y. Midkine positively regulates the proliferation of human gastric cancer cells. Cancer Lett. 2009, 279, 137–144. [Google Scholar] [CrossRef]
- Kuo, A.H.; Stoica, G.E.; Riegel, A.T.; Wellstein, A. Recruitment of insulin receptor substrate-1 and activation of NF-kappaB essential for midkine growth signaling through anaplastic lymphoma kinase. Oncogene 2007, 26, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Bie, C.; Chen, Y.; Tang, H.; Li, Q.; Zhong, L.; Peng, X.; Shi, Y.; Lin, J.; Lai, J.; Wu, S.; et al. Insulin-Like Growth Factor 1 Receptor Drives Hepatocellular Carcinoma Growth and Invasion by Activating Stat3-Midkine-Stat3 Loop. Dig. Dis. Sci. 2022, 67, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, K.; Qiu, L.; Yue, J.; Jiang, H.; Deng, Q.; Zhou, R.; Yin, Z.; Ma, S.; Ke, Y. Cancer-associated fibroblasts facilitate DNA damage repair by promoting the glycolysis in non-small cell lung cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166670. [Google Scholar] [CrossRef]
- Liedert, A.; Mattausch, L.; Röntgen, V.; Blakytny, R.; Vogele, D.; Pahl, M.; Bindl, R.; Neunaber, C.; Schinke, T.; Harroch, S.; et al. Midkine-deficiency increases the anabolic response of cortical bone to mechanical loading. Bone 2011, 48, 945–951. [Google Scholar] [CrossRef]
- Chen, S.; Bu, G.; Takei, Y.; Sakamoto, K.; Ikematsu, S.; Muramatsu, T.; Kadomatsu, K. Midkine and LDL-receptor-related protein 1 contribute to the anchorage-independent cell growth of cancer cells. J. Cell Sci. 2007, 120, 4009–4015. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, C.; Liu, L.; Hu, Y.; Yang, B.; Qiu, S.; Li, Y.; Cao, D.; Ju, Z.; Ge, J.; et al. Single-cell RNA-sequencing atlas reveals an MDK-dependent immunosuppressive environment in ErbB pathway-mutated gallbladder cancer. J. Hepatol. 2021, 75, 1128–1141. [Google Scholar] [CrossRef]
- Mitsiadis, T.A.; Salmivirta, M.; Muramatsu, T.; Muramatsu, H.; Rauvala, H.; Lehtonen, E.; Jalkanen, M.; Thesleff, I. Expression of the heparin-binding cytokines, midkine (MK) and HB-GAM (pleiotrophin) is associated with epithelial-mesenchymal interactions during fetal development and organogenesis. Development 1995, 121, 37–51. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kojima, Y.; Sakamoto, T.; Ozato, Y.; Nakano, Y.; Abe, T.; Hosoda, K.; Saito, H.; Higuchi, S.; Hisamatsu, Y.; et al. Spatial and single-cell colocalisation analysis reveals MDK-mediated immunosuppressive environment with regulatory T cells in colorectal carcinogenesis. EBioMedicine 2024, 103, 105102. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhu, S.; Wu, M.; Han, W.; Yu, Y. Functional Receptors and Intracellular Signal Pathways of Midkine (MK) and Pleiotrophin (PTN). Biol. Pharm. Bull. 2014, 37, 511–520. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Osuga, Y.; Koga, K.; Yoshino, O.; Hirata, T.; Harada, M.; Morimoto, C.; Yano, T.; Tsutsumi, O.; Sakuma, S.; et al. Possible implication of midkine in the development of endometriosis. Hum. Reprod. 2005, 20, 1084–1089. [Google Scholar] [CrossRef]
- Liu, X.Y.; Liu, Y.B.; Xu, J.C.; Zhang, Y.F.; Ruan, Y.Y.; Zhao, Y.; Wu, L.F.; Hu, J.W.; Zhang, Z.; He, M.J.; et al. Single-cell transcriptomic analysis deciphers key transitional signatures associated with oncogenic evolution in human intramucosal oesophageal squamous cell carcinoma. Clin. Transl. Med. 2023, 13, e1203. [Google Scholar] [CrossRef]
- Qi, M.; Ikematsu, S.; Ichihara-Tanaka, K.; Sakuma, S.; Muramatsu, T.; Kadomatsu, K. Midkine rescues Wilms’ tumor cells from cisplatin-induced apoptosis: Regulation of Bcl-2 expression by Midkine. J. Biochem. 2000, 127, 269–277. [Google Scholar] [CrossRef]
- Mirkin, B.L.; Clark, S.; Zheng, X.; Chu, F.; White, B.D.; Greene, M.; Rebbaa, A. Identification of midkine as a mediator for intercellular transfer of drug resistance. Oncogene 2005, 24, 4965–4974. [Google Scholar] [CrossRef] [PubMed]
- Clusan, L.; Ferrière, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 6834. [Google Scholar] [CrossRef]
- Yu, K.; Huang, Z.Y.; Xu, X.L.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Receptor Function: Impact on the Human Endometrium. Front Endocrinol 2022, 13, 827724. [Google Scholar] [CrossRef]
- Kozieł, M.J.; Piastowska-Ciesielska, A.W. Estrogens, Estrogen Receptors and Tumor Microenvironment in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 14673. [Google Scholar] [CrossRef]
- Liang, J.; Shang, Y. Estrogen and cancer. Annu. Rev. Physiol. 2013, 75, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rees, M.C.P.; Bicknell, R. The isolation and long-term culture of normal human endometrial epithelium and stroma: Expression of mRNAs for angiogenic polypeptides basally and on oestrogen and progesterone challenges. J. Cell Sci. 1995, 108, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Hu, C.; Xu, J.; Yu, P.; Yuan, L.; Li, Z.; Liang, H.; Zhang, Y.; Chen, J.; Wei, Q.; et al. The estrogen response in fibroblasts promotes ovarian metastases of gastric cancer. Nat. Commun. 2024, 15, 8447. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.E. Dienogest in long-term treatment of endometriosis. Int. J. Women’s Health 2011, 3, 175–184. [Google Scholar] [CrossRef]
- Nirgianakis, K.; Grandi, G.; McKinnon, B.; Bersinger, N.; Cagnacci, A.; Mueller, M. Dienogest mediates midkine suppression in endometriosis. Hum. Reprod. 2016, 31, 1981–1986. [Google Scholar] [CrossRef]
- Tamm-Rosenstein, K.; Simm, J.; Suhorutshenko, M.; Salumets, A.; Metsis, M. Changes in the transcriptome of the human endometrial Ishikawa cancer cell line induced by estrogen, progesterone, tamoxifen, and mifepristone (RU486) as detected by RNA-sequencing. PLoS ONE 2013, 8, e68907. [Google Scholar] [CrossRef]
- Cleary, M.P.; Grossmann, M.E. Minireview: Obesity and breast cancer: The estrogen connection. Endocrinology 2009, 150, 2537–2542. [Google Scholar] [CrossRef]
- Wichmann, I.A.; Cuello, M.A. Obesity and gynecological cancers: A toxic relationship. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. 1), 123–134. [Google Scholar] [CrossRef]
- Wang, H.; Du, Y.; Huang, S.; Sun, X.; Ye, Y.; Sun, H.; Chu, X.; Shan, X.; Yuan, Y.; Shen, L.; et al. Single-cell analysis reveals a subpopulation of adipose progenitor cells that impairs glucose homeostasis. Nat. Commun. 2024, 15, 4827. [Google Scholar] [CrossRef]
- Çelik, H.H.A.; Korkmaz, S. Evaluation of serum midkine levels and metabolic parameters in patients with hidradenitis suppurativa. Arch. Dermatol. Res. 2023, 315, 1909–1914. [Google Scholar] [CrossRef]
- Jee, Y.H.; Lee, K.S.; Yue, S.; Leschek, E.W.; Boden, M.G.; Jadra, A.; Klibanski, A.; Vaidyanathan, P.; Misra, M.; Chang, Y.P.; et al. Plasma midkine concentrations in healthy children, children with increased and decreased adiposity, and children with short stature. PLoS ONE 2019, 14, e0224103. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Iyengar, N.M.; Zahid, H.; Carter, K.M.; Byun, D.J.; Choi, M.H.; Sun, Q.; Savenkov, O.; Louka, C.; Liu, C.; et al. Obesity promotes breast epithelium DNA damage in women carrying a germline mutation in BRCA1 or BRCA2. Sci. Transl. Med. 2023, 15, eade1857. [Google Scholar] [CrossRef]
- López-Valero, I.; Dávila, D.; González-Martínez, J.; Salvador-Tormo, N.; Lorente, M.; Saiz-Ladera, C.; Torres, S.; Gabicagogeascoa, E.; Hernández-Tiedra, S.; García-Taboada, E.; et al. Midkine signaling maintains the self-renewal and tumorigenic capacity of glioma initiating cells. Theranostics 2020, 10, 5120–5136. [Google Scholar] [CrossRef]
- Lee, S.H.; Suh, H.N.; Lee, Y.J.; Seo, B.N.; Ha, J.W.; Han, H.J. Midkine prevented hypoxic injury of mouse embryonic stem cells through activation of Akt and HIF-1α via low-density lipoprotein receptor-related protein-1. J. Cell. Physiol. 2012, 227, 1731–1739. [Google Scholar] [CrossRef]
- Zhao, S.L.; Zhang, Y.J.; Li, M.H.; Zhang, X.L.; Chen, S.L. Mesenchymal stem cells with overexpression of midkine enhance cell survival and attenuate cardiac dysfunction in a rat model of myocardial infarction. Stem Cell Res. Ther. 2014, 5, 37. [Google Scholar] [CrossRef]
- Yao, X.; Tan, Z.; Gu, B.; Wu, R.R.; Liu, Y.K.; Dai, L.C.; Zhang, M. Promotion of self-renewal of embryonic stem cells by midkine. Acta Pharmacol. Sin. 2010, 31, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Doganlar, Z.B.; Doganlar, O.; Turkekul, K.; Serttas, R. Inhibition of Midkine Suppresses Prostate Cancer CD133(+) Stem Cell Growth and Migration. Am. J. Med. Sci. 2017, 354, 299–309. [Google Scholar] [CrossRef]
- Weckbach, L.T.; Preissner, K.T.; Deindl, E. The Role of Midkine in Arteriogenesis, Involving Mechanosensing, Endothelial Cell Proliferation, and Vasodilation. Int. J. Mol. Sci. 2018, 19, 2559. [Google Scholar] [CrossRef] [PubMed]
- Weckbach, L.T.; Groesser, L.; Borgolte, J.; Pagel, J.I.; Pogoda, F.; Schymeinsky, J.; Müller-Höcker, J.; Shakibaei, M.; Muramatsu, T.; Deindl, E.; et al. Midkine acts as proangiogenic cytokine in hypoxia-induced angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H429–H438. [Google Scholar] [CrossRef]
- Lautz, T.; Lasch, M.; Borgolte, J.; Troidl, K.; Pagel, J.I.; Caballero-Martinez, A.; Kleinert, E.C.; Walzog, B.; Deindl, E. Midkine Controls Arteriogenesis by Regulating the Bioavailability of Vascular Endothelial Growth Factor A and the Expression of Nitric Oxide Synthase 1 and 3. EBioMedicine 2018, 27, 237–246. [Google Scholar] [CrossRef]
- Choudhuri, R.; Zhang, H.T.; Donnini, S.; Ziche, M.; Bicknell, R. An angiogenic role for the neurokines midkine and pleiotrophin in tumorigenesis. Cancer Res. 1997, 57, 1814–1819. [Google Scholar] [PubMed]
- Dai, L.C.; Wang, X.; Yao, X.; Lu, Y.L.; Ping, J.L.; He, J.F. Antisense oligonucleotide targeting midkine suppresses in vivo angiogenesis. World J. Gastroenterol. 2007, 13, 1208–1213. [Google Scholar] [CrossRef]
- Shin, D.H.; Jo, J.Y.; Kim, S.H.; Choi, M.; Han, C.; Choi, B.K.; Kim, S.S. Midkine Is a Potential Therapeutic Target of Tumorigenesis, Angiogenesis, and Metastasis in Non-Small Cell Lung Cancer. Cancers 2020, 12, 2402. [Google Scholar] [CrossRef]
- Huang, H.L.; Shen, J.F.; Min, L.S.; Ping, J.L.; Lu, Y.L.; Dai, L.C. Inhibitory effect of midkine-binding peptide on tumor proliferation and migration. Int. J. Clin. Exp. Pathol. 2015, 8, 5387–5394. [Google Scholar] [PubMed]
- Masui, M.; Okui, T.; Shimo, T.; Takabatake, K.; Fukazawa, T.; Matsumoto, K.; Kurio, N.; Ibaragi, S.; Naomoto, Y.; Nagatsuka, H.; et al. Novel Midkine Inhibitor iMDK Inhibits Tumor Growth and Angiogenesis in Oral Squamous Cell Carcinoma. Anticancer Res. 2016, 36, 2775–2781. [Google Scholar]
- Sheng, B.; Wei, Z.; Wu, X.; Li, Y.; Liu, Z. USP12 promotes breast cancer angiogenesis by maintaining midkine stability. Cell Death Dis. 2021, 12, 1074. [Google Scholar] [CrossRef]
- Wu, L.; Chen, H.; Fu, C.; Xing, M.; Fang, H.; Yang, F.; Yang, Q.; Zhang, Y.; Li, W.; Chen, Z. Midkine mediates dysfunction of liver sinusoidal endothelial cells through integrin α4 and α6. Vasc. Pharmacol. 2022, 147, 107113. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Shen, L.; Ren, Q.; Zeng, Q.; He, X. Prognostic and Clinicopathological Significance of Hypoxia-Inducible Factor-1α in Endometrial Cancer: A Meta-Analysis. Front. Oncol. 2020, 10, 587420. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, M.; Schmid, T.; Xin, Z.; Kozhuharova, L.; Yu, W.K.; Huang, Y.; Cai, F.; Biskup, E. Hypoxia in Breast Cancer-Scientific Translation to Therapeutic and Diagnostic Clinical Applications. Front. Oncol. 2021, 11, 652266. [Google Scholar] [CrossRef]
- Osada, R.; Horiuchi, A.; Kikuchi, N.; Yoshida, J.; Hayashi, A.; Ota, M.; Katsuyama, Y.; Mellilo, G.; Konishi, I. Expression of hypoxia-inducible factor 1α, hypoxia-inducible factor 2α, and von Hippel–Lindau protein in epithelial ovarian neoplasms and allelic loss of von Hippel-Lindau gene: Nuclear expression of hypoxia-inducible factor 1α is an independent prognostic factor in ovarian carcinoma. Hum. Pathol. 2007, 38, 1310–1320. [Google Scholar] [CrossRef]
- Waller, J.; Onderdonk, B.; Flood, A.; Swartz, H.; Shah, J.; Shah, A.; Aydogan, B.; Halpern, H.; Hasan, Y. The clinical utility of imaging methods used to measure hypoxia in cervical cancer. Br. J. Radiol. 2020, 93, 20190640. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Tong, S.; Gao, L. Identification of MDK as a Hypoxia- and Epithelial-Mesenchymal Transition-Related Gene Biomarker of Glioblastoma Based on a Novel Risk Model and In Vitro Experiments. Biomedicines 2024, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, N.; Wang, Q.; Fan, X.; Xin, Y.; Wang, S. Midkine inhibition enhances anti-PD-1 immunotherapy in sorafenib-treated hepatocellular carcinoma via preventing immunosuppressive MDSCs infiltration. Cell Death Discov. 2023, 9, 92. [Google Scholar] [CrossRef]
- Li, X.; Zhao, S.; Bian, X.; Zhang, L.; Lu, L.; Pei, S.; Dong, L.; Shi, W.; Huang, L.; Zhang, X.; et al. Signatures of EMT, immunosuppression, and inflammation in primary and recurrent human cutaneous squamous cell carcinoma at single-cell resolution. Theranostics 2022, 12, 7532–7549. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, Z.; Lu, N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adhes. Migr. 2015, 9, 317–324. [Google Scholar] [CrossRef]
- Yang, K.D.; Wang, Y.; Zhang, F.; Li, Q.L.; Luo, B.H.; Feng, D.Y.; Zeng, Z.J. CAF-derived midkine promotes EMT and cisplatin resistance by upregulating lncRNA ST7-AS1 in gastric cancer. Mol. Cell Biochem. 2022, 477, 2493–2505. [Google Scholar] [CrossRef]
- Hu, B.; Qin, C.; Li, L.; Wei, L.; Mo, X.; Fan, H.; Lei, Y.; Wei, F.; Zou, D. Midkine promotes glioblastoma progression via PI3K-Akt signaling. Cancer Cell Int. 2021, 21, 509. [Google Scholar] [CrossRef]
- Güngör, C.; Zander, H.; Effenberger, K.E.; Vashist, Y.K.; Kalinina, T.; Izbicki, J.R.; Yekebas, E.; Bockhorn, M. Notch Signaling Activated by Replication Stress–Induced Expression of Midkine Drives Epithelial–Mesenchymal Transition and Chemoresistance in Pancreatic Cancer. Cancer Res. 2011, 71, 5009–5019. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, Q.; Li, R.; Chen, S.; Tan, J.; Li, L.; Dong, X.; Huang, C.; Wen, T.; Liu, J. Targeting MDK Abrogates IFN-γ-Elicited Metastasis inCancers of Various Origins. Front. Oncol. 2022, 12, 885656. [Google Scholar] [CrossRef]
- Cohen, S.; Shoshana, O.Y.; Zelman-Toister, E.; Maharshak, N.; Binsky-Ehrenreich, I.; Gordin, M.; Hazan-Halevy, I.; Herishanu, Y.; Shvidel, L.; Haran, M.; et al. The cytokine midkine and its receptor RPTPζ regulate B cell survival in a pathway induced by CD74. J. Immunol. 2012, 188, 259–269. [Google Scholar] [CrossRef]
- Hayward, S.; Gachehiladze, M.; Badr, N.; Andrijes, R.; Molostvov, G.; Paniushkina, L.; Sopikova, B.; Slobodová, Z.; Mgebrishvili, G.; Sharma, N.; et al. The CD151-midkine pathway regulates the immune microenvironment in inflammatory breast cancer. J. Pathol. 2020, 251, 63–73. [Google Scholar] [CrossRef]
- Cerezo-Wallis, D.; Contreras-Alcalde, M.; Troulé, K.; Catena, X.; Mucientes, C.; Calvo, T.G.; Cañón, E.; Tejedo, C.; Pennacchi, P.C.; Hogan, S.; et al. Midkine rewires the melanoma microenvironment toward a tolerogenic and immune-resistant state. Nat. Med. 2020, 26, 1865–1877. [Google Scholar] [CrossRef]
- Weckbach, L.T.; Muramatsu, T.; Walzog, B. Midkine in Inflammation. Sci. World J. 2011, 11, 517152. [Google Scholar] [CrossRef] [PubMed]
- Sato, W.; Kadomatsu, K.; Yuzawa, Y.; Muramatsu, H.; Hotta, N.; Matsuo, S.; Muramatsu, T. Midkine Is Involved in Neutrophil Infiltration into the Tubulointerstitium in Ischemic Renal Injury1. J. Immunol. 2001, 167, 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Inoh, K.; Muramatsu, H.; Ochiai, K.; Torii, S.; Muramatsu, T. Midkine, a heparin-binding cytokine, plays key roles in intraperitoneal adhesions. Biochem. Biophys. Res. Commun. 2004, 317, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Sonobe, Y.; Li, H.; Jin, S.; Kishida, S.; Kadomatsu, K.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Midkine inhibits inducible regulatory T cell differentiation by suppressing the development of tolerogenic dendritic cells. J. Immunol. 2012, 188, 2602–2611. [Google Scholar] [CrossRef]
- Shi, N.; Chen, S.; Wang, D.; Wu, T.; Zhang, N.; Chen, M.; Ding, X. MDK promotes M2 macrophage polarization to remodel the tumour microenvironment in clear cell renal cell carcinoma. Sci. Rep. 2024, 14, 18254. [Google Scholar] [CrossRef]
- Li, Y.S.; Lai, W.P.; Yin, K.; Zheng, M.M.; Tu, H.Y.; Guo, W.B.; Li, L.; Lin, S.H.; Wang, Z.; Zeng, L.; et al. Lipid-associated macrophages for osimertinib resistance and leptomeningeal metastases in NSCLC. Cell Rep. 2024, 43, 114613. [Google Scholar] [CrossRef]
- Weckbach, L.T.; Gola, A.; Winkelmann, M.; Jakob, S.M.; Groesser, L.; Borgolte, J.; Pogoda, F.; Pick, R.; Pruenster, M.; Müller-Höcker, J.; et al. The cytokine midkine supports neutrophil trafficking during acute inflammation by promoting adhesion via β2 integrins (CD11/CD18). Blood 2014, 123, 1887–1896. [Google Scholar] [CrossRef]
- Yan, P.; Jimenez, E.R.; Li, Z.; Bui, T.; Seehawer, M.; Nishida, J.; Foidart, P.; Stevens, L.E.; Xie, Y.; Gomez, M.M.; et al. Midkine as a driver of age-related changes and increase in mammary tumorigenesis. Cancer Cell 2024, 42, 1936–1954.e1939. [Google Scholar] [CrossRef]
- Said, E.A.; Al-Dughaishi, S.; Al-Hatmi, W.; Al-Reesi, I.; Al-Balushi, M.S.; Al-Bimani, A.; Al-Busaidi, J.Z.; Al-Riyami, M.; Al-Khabori, M.; Al-Kindi, S.; et al. Differential Production of Midkine and Pleiotrophin by Innate APCs upon Stimulation through Nucleic Acid-Sensing TLRs. J. Immunol. Res. 2023, 2023, 7944102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, H.; Nie, Y.; Mi, Q.; Chen, X.; Hou, Y. Midkine upregulates MICA/B expression in human gastric cancer cells and decreases natural killer cell cytotoxicity. Cancer Immunol. Immunother. 2012, 61, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Pan, Y.; Xiong, M.; Sanapala, S.; Anastasaki, C.; Cobb, O.; Dahiya, S.; Gutmann, D.H. Midkine activation of CD8(+) T cells establishes a neuron-immune-cancer axis responsible for low-grade glioma growth. Nat. Commun. 2020, 11, 2177. [Google Scholar] [CrossRef] [PubMed]
- Quah, H.S.; Cao, E.Y.; Suteja, L.; Li, C.H.; Leong, H.S.; Chong, F.T.; Gupta, S.; Arcinas, C.; Ouyang, J.F.; Ang, V.; et al. Single cell analysis in head and neck cancer reveals potential immune evasion mechanisms during early metastasis. Nat. Commun. 2023, 14, 1680. [Google Scholar] [CrossRef]
- Luo, L.; Yang, P.; Mastoraki, S.; Rao, X.; Wang, Y.; Kettner, N.M.; Raghavendra, A.S.; Tripathy, D.; Damodaran, S.; Hunt, K.K.; et al. Single-cell RNA sequencing identifies molecular biomarkers predicting late progression to CDK4/6 inhibition in patients with HR+/HER2- metastatic breast cancer. Mol. Cancer 2025, 24, 48. [Google Scholar] [CrossRef]
- Tian, Z.; Tang, J.; Liao, X.; Yang, Q.; Wu, Y.; Wu, G. An immune-related prognostic signature for predicting breast cancer recurrence. Cancer Med. 2020, 9, 7672–7685. [Google Scholar] [CrossRef]
- Li, F.; Tian, P.; Zhang, J.; Kou, C. The clinical and prognostic significance of midkine in breast cancer patients. Tumour Biol. 2015, 36, 9789–9794. [Google Scholar] [CrossRef]
- Miyashiro, I.; Kaname, T.; Shin, E.; Wakasugi, E.; Monden, T.; Takatsuka, Y.; Kikkawa, N.; Muramatsu, T.; Monden, M.; Akiyama, T. Midkine expression in human breast cancers: Expression of truncated form. Breast Cancer Res. Treat. 1997, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li Qin, L.; LIAN HUANG, H.; LIANG PING, J.; XU, W.; LI, J.; CHENG DAI, L. Expression of midkine and endoglin in breast carcinomas with different immunohistochemical profiles. APMIS 2011, 119, 103–110. [Google Scholar] [CrossRef]
- Zhang, L.; Song, L.; Xu, Y.; Xu, Y.; Zheng, M.; Zhang, P.; Wang, Q. Midkine promotes breast cancer cell proliferation and migration by upregulating NR3C1 expression and activating the NF-κB pathway. Mol. Biol. Rep. 2022, 49, 2953–2961. [Google Scholar] [CrossRef]
- Choi, J.; Gyamfi, J.; Jang, H.; Koo, J.S. The role of tumor-associated macrophage in breast cancer biology. Histol. Histopathol. 2018, 33, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Wang, Z.; Sun, Z.; Wei, J.; Zhang, L.; Ju, H.; Wang, T.; Zhang, C.; Guan, M.; Pan, S. Single-cell RNA sequencing reveals the characteristics of cerebrospinal fluid tumour environment in breast cancer and lung cancer leptomeningeal metastases. Clin. Transl. Med. 2022, 12, e885. [Google Scholar] [CrossRef]
- Ibusuki, M.; Fujimori, H.; Yamamoto, Y.; Ota, K.; Ueda, M.; Shinriki, S.; Taketomi, M.; Sakuma, S.; Shinohara, M.; Iwase, H.; et al. Midkine in plasma as a novel breast cancer marker. Cancer Sci. 2009, 100, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Çetin Sorkun, H.; Akbulut, M.; Enli, Y.; Tepeli, E.; Özkan, S.; Erdem, E. Quantitative comparison of immunohistochemical and PCR analysis of midkine expression in breast cancer types and serum midkine level. Turk. J. Med. Sci. 2016, 46, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Giamanco, N.M.; Jee, Y.H.; Wellstein, A.; Shriver, C.D.; Summers, T.A.; Baron, J. Midkine and pleiotrophin concentrations in needle biopsies of breast and lung masses. Cancer Biomark. 2017, 20, 299–307. [Google Scholar] [CrossRef]
- Wu, X.; Zhi, X.; Ji, M.; Wang, Q.; Li, Y.; Xie, J.; Zhao, S. Midkine as a potential diagnostic marker in epithelial ovarian cancer for cisplatin/paclitaxel combination clinical therapy. Am. J. Cancer Res. 2015, 5, 629–638. [Google Scholar]
- Nakanishi, T.; Kadomatsu, K.; Okamoto, T.; Tomoda, Y.; Muramatsu, T. Expression of midkine and pleiotropin in ovarian tumors. Obstet. Gynecol. 1997, 90, 285–290. [Google Scholar] [CrossRef]
- Matsumoto, T.; Oda, Y.; Hasegawa, Y.; Hashimura, M.; Oguri, Y.; Inoue, H.; Yokoi, A.; Tochimoto, M.; Nakagawa, M.; Jiang, Z.; et al. Anaplastic Lymphoma Kinase Overexpression Is Associated with Aggressive Phenotypic Characteristics of Ovarian High-Grade Serous Carcinoma. Am. J. Pathol. 2021, 191, 1837–1850. [Google Scholar] [CrossRef]
- Zhou, W.; Ma, J.; Zhao, H.; Wang, Q.; Guo, X.; Chen, L.; Cao, Z.; Xu, J.; Zhang, B.; Zhou, X. Serum Exosomes From Epithelial Ovarian Cancer Patients Contain LRP1, Which Promotes the Migration of Epithelial Ovarian Cancer Cell. Mol. Cell Proteom. 2023, 22, 100520. [Google Scholar] [CrossRef]
- Wolde, T.; Bhardwaj, V.; Reyad-Ul-Ferdous, M.; Qin, P.; Pandey, V. The Integrated Bioinformatic Approach Reveals the Prognostic Significance of LRP1 Expression in Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 7996. [Google Scholar] [CrossRef]
- Liu, Q.; Tan, J.; Zhao, Z.; Li, R.; Zheng, L.; Chen, X.; Li, L.; Dong, X.; Wen, T.; Liu, J. Combined Usage of MDK Inhibitor Augments Interferon-γ Anti-Tumor Activity in the SKOV3 Human Ovarian Cancer Cell Line. Biomedicines 2022, 11, 8. [Google Scholar] [CrossRef]
- Carvalho, R.F.; do Canto, L.M.; Abildgaard, C.; Aagaard, M.M.; Tronhjem, M.S.; Waldstrøm, M.; Jensen, L.H.; Steffensen, K.D.; Rogatto, S.R. Single-cell and bulk RNA sequencing reveal ligands and receptors associated with worse overall survival in serous ovarian cancer. Cell Commun. Signal. 2022, 20, 176. [Google Scholar] [CrossRef]
- Zeller, C.; Dai, W.; Steele, N.L.; Siddiq, A.; Walley, A.J.; Wilhelm-Benartzi, C.S.M.; Rizzo, S.; van der Zee, A.; Plumb, J.A.; Brown, R. Candidate DNA methylation drivers of acquired cisplatin resistance in ovarian cancer identified by methylome and expression profiling. Oncogene 2012, 31, 4567–4576. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Xiong, C.; Huang, F.; Zhang, M.; Mo, Y.; Bai, H. Big data-based identification of methylated genes associated with drug resistance and prognosis in ovarian cancer. Medicine 2020, 99, e20802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y.; Song, X.; Wang, X.; Quan, L.; Xu, P.; Zhao, L.; Song, W.; Liu, Q.; Zhou, X. Immune escape between endoplasmic reticulum stress-related cancer cells and exhausted CD8+T cells leads to neoadjuvant chemotherapy resistance in ovarian cancer. Biochem. Biophys. Res. Commun. 2024, 733, 150686. [Google Scholar] [CrossRef] [PubMed]
- Enroth, S.; Berggrund, M.; Lycke, M.; Lundberg, M.; Assarsson, E.; Olovsson, M.; Stålberg, K.; Sundfeldt, K.; Gyllensten, U. A two-step strategy for identification of plasma protein biomarkers for endometrial and ovarian cancer. Clin. Proteom. 2018, 15, 38. [Google Scholar] [CrossRef]
- Leandersson, P.; Åkesson, A.; Hedenfalk, I.; Malander, S.; Borgfeldt, C. A multiplex biomarker assay improves the diagnostic performance of HE4 and CA125 in ovarian tumor patients. PLoS ONE 2020, 15, e0240418. [Google Scholar] [CrossRef]
- Rice, G.E.; Edgell, T.A.; Autelitano, D.J. Evaluation of midkine and anterior gradient 2 in a multimarker panel for the detection of ovarian cancer. J. Exp. Clin. Cancer Res. 2010, 29, 62. [Google Scholar] [CrossRef]
- Boylan, K.L.M.; Geschwind, K.; Koopmeiners, J.S.; Geller, M.A.; Starr, T.K.; Skubitz, A.P.N. A multiplex platform for the identification of ovarian cancer biomarkers. Clin. Proteom. 2017, 14, 34. [Google Scholar] [CrossRef]
- Bartha, Á.; Győrffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef]
- Tanabe, K.; Matsumoto, M.; Ikematsu, S.; Nagase, S.; Hatakeyama, A.; Takano, T.; Niikura, H.; Ito, K.; Kadomatsu, K.; Hayashi, S.; et al. Midkine and its clinical significance in endometrial carcinoma. Cancer Sci. 2008, 99, 1125–1130. [Google Scholar] [CrossRef]
- Yokoi, A.; Nakamura, Y.; Hashimura, M.; Oguri, Y.; Matsumoto, T.; Nakagawa, M.; Ishibashi, Y.; Ito, T.; Ohhigata, K.; Harada, Y.; et al. Anaplastic lymphoma kinase overexpression enhances aggressive phenotypic characteristics of endometrial carcinoma. BMC Cancer 2023, 23, 765. [Google Scholar] [CrossRef] [PubMed]
- Lachej, N.; Dabkeviciene, D.; Simiene, J.; Sabaliauskaite, R.; Jonusiene, V.; Brasiunas, V.; Sasnauskiene, A.; Vaicekauskaite, I.; Brasiuniene, B.; Kanopiene, D.; et al. Components of NOTCH Signaling for Uterine Cancer Patients’ Prognosis. J. Oncol. 2022, 2022, 8199306. [Google Scholar] [CrossRef]
- Inoue, H.; Hashimura, M.; Akiya, M.; Chiba, R.; Saegusa, M. Functional role of ALK-related signal cascades on modulation of epithelial-mesenchymal transition and apoptosis in uterine carcinosarcoma. Mol. Cancer 2017, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Bilir, A.; Erguven, M.; Ermis, E.; Sencan, M.; Yazihan, N. Combination of imatinib mesylate with lithium chloride and medroxyprogesterone acetate is highly active in Ishikawa endometrial carcinoma in vitro. J. Gynecol. Oncol. 2011, 22, 225–232. [Google Scholar] [CrossRef]
- Torres, A.; Pac-Sosińska, M.; Wiktor, K.; Paszkowski, T.; Maciejewski, R.; Torres, K. CD44, TGM2 and EpCAM as novel plasma markers in endometrial cancer diagnosis. BMC Cancer 2019, 19, 401. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef]
- Fei, H.; Chen, T.; Jiang, H. Autocrine and paracrine effects of MDK promote lymph node metastasis of cervical squamous cell carcinoma. iScience 2024, 27, 110077. [Google Scholar] [CrossRef]
- Sin, S.T.K.; Li, Y.; Liu, M.; Ma, S.; Guan, X.Y. TROP-2 exhibits tumor suppressive functions in cervical cancer by dual inhibition of IGF-1R and ALK signaling. Gynecol. Oncol. 2019, 152, 185–193. [Google Scholar] [CrossRef]
- Kang, H.C.; Kim, I.-J.; Park, H.-W.; Jang, S.-G.; Ahn, S.-A.; Yoon, S.N.; Chang, H.-J.; Yoo, B.C.; Park, J.-G. Regulation of MDK expression in human cancer cells modulates sensitivities to various anticancer drugs: MDK overexpression confers to a multi-drug resistance. Cancer Lett. 2007, 247, 40–47. [Google Scholar] [CrossRef]
- Hu, G.; Xiao, Y.; Ma, C.; Wang, J.; Qian, X.; Wu, X.; Zhu, F.; Sun, S.; Qian, J. Lumican is a potential predictor on the efficacy of concurrent chemoradiotherapy in cervical squamous cell carcinoma. Heliyon 2023, 9, e18011. [Google Scholar] [CrossRef]
- Zhang, T.; Zhuang, L.; Muaibati, M.; Wang, D.; Abasi, A.; Tong, Q.; Ma, D.; Jin, L.; Huang, X. Identification of cervical cancer stem cells using single-cell transcriptomes of normal cervix, cervical premalignant lesions, and cervical cancer. eBioMedicine 2023, 92, 104612. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.S.; Park, W.I.; Sung, S.H.; Choi, E.A.; Chung, H.W.; Woo, B.H. Immunohistochemical and quantitative competitive PCR analyses of midkine and pleiotrophin expression in cervical cancer. Gynecol. Oncol. 2003, 88, 289–297. [Google Scholar] [CrossRef]
- Nakamura, E.; Kadomatsu, K.; Yuasa, S.; Muramatsu, H.; Mamiya, T.; Nabeshima, T.; Fan, Q.W.; Ishiguro, K.; Igakura, T.; Matsubara, S.; et al. Disruption of the midkine gene (Mdk) resulted in altered expression of a calcium binding protein in the hippocampus of infant mice and their abnormal behaviour. Genes Cells 1998, 3, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Maeda, Y.; Fukazawa, T.; Yamatsuji, T.; Takaoka, M.; Bao, X.H.; Matsuoka, J.; Okui, T.; Shimo, T.; Takigawa, N.; et al. Inhibition of the growth factor MDK/midkine by a novel small molecule compound to treat non-small cell lung cancer. PLoS ONE 2013, 8, e71093. [Google Scholar] [CrossRef]
- Ishida, N.; Fukazawa, T.; Maeda, Y.; Yamatsuji, T.; Kato, K.; Matsumoto, K.; Shimo, T.; Takigawa, N.; Whitsett, J.A.; Naomoto, Y. A novel PI3K inhibitor iMDK suppresses non-small cell lung Cancer cooperatively with A MEK inhibitor. Exp. Cell Res. 2015, 335, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Kariya, R.; Gunya, S.; Cheevapruk, K.; Okada, S. Midkine inhibitor (iMDK) induces apoptosis of primary effusion lymphoma via G2/M cell cycle arrest. Leuk. Res. 2022, 116, 106826. [Google Scholar] [CrossRef]
- Maehara, H.; Kaname, T.; Yanagi, K.; Hanzawa, H.; Owan, I.; Kinjou, T.; Kadomatsu, K.; Ikematsu, S.; Iwamasa, T.; Kanaya, F.; et al. Midkine as a novel target for antibody therapy in osteosarcoma. Biochem. Biophys. Res. Commun. 2007, 358, 757–762. [Google Scholar] [CrossRef]
- Mahajan, M.; Rodriguez Sanchez, A.L.; Jayamohan, S.; Vijayan, D.K.; Johnson, J.D.; Xie, H.; Wang, Y.; Liang, D.; Sanchez, J.R.; Subbarayalu, P.; et al. The discovery and characterization of HBS-101, a novel inhibitor of midkine, as a therapeutic agent for the treatment of triple negative breast cancer. Mol. Cancer Ther. 2025. [Google Scholar] [CrossRef]
- Olmeda, D.; Cerezo-Wallis, D.; Mucientes, C.; Calvo, T.G.; Cañón, E.; Alonso-Curbelo, D.; Ibarz, N.; Muñoz, J.; Rodriguez-Peralto, J.L.; Ortiz-Romero, P.; et al. Live imaging of neolymphangiogenesis identifies acute antimetastatic roles of dsRNA mimics. EMBO Mol. Med. 2021, 13, e12924. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aller, E.J.; Nair, H.B.; Vadlamudi, R.K.; Viswanadhapalli, S. Significance of Midkine Signaling in Women’s Cancers: Novel Biomarker and Therapeutic Target. Int. J. Mol. Sci. 2025, 26, 4809. https://doi.org/10.3390/ijms26104809
Aller EJ, Nair HB, Vadlamudi RK, Viswanadhapalli S. Significance of Midkine Signaling in Women’s Cancers: Novel Biomarker and Therapeutic Target. International Journal of Molecular Sciences. 2025; 26(10):4809. https://doi.org/10.3390/ijms26104809
Chicago/Turabian StyleAller, Emily J., Hareesh B. Nair, Ratna K. Vadlamudi, and Suryavathi Viswanadhapalli. 2025. "Significance of Midkine Signaling in Women’s Cancers: Novel Biomarker and Therapeutic Target" International Journal of Molecular Sciences 26, no. 10: 4809. https://doi.org/10.3390/ijms26104809
APA StyleAller, E. J., Nair, H. B., Vadlamudi, R. K., & Viswanadhapalli, S. (2025). Significance of Midkine Signaling in Women’s Cancers: Novel Biomarker and Therapeutic Target. International Journal of Molecular Sciences, 26(10), 4809. https://doi.org/10.3390/ijms26104809






