Sex-Associated Cerebellar and Hippocampal Volume Reduction in Alzheimer’s Disease: Insights from the Clinical ADNI Cohort and STZ Animal Model
Abstract
1. Introduction
2. Results
2.1. Animal AD Model Phase
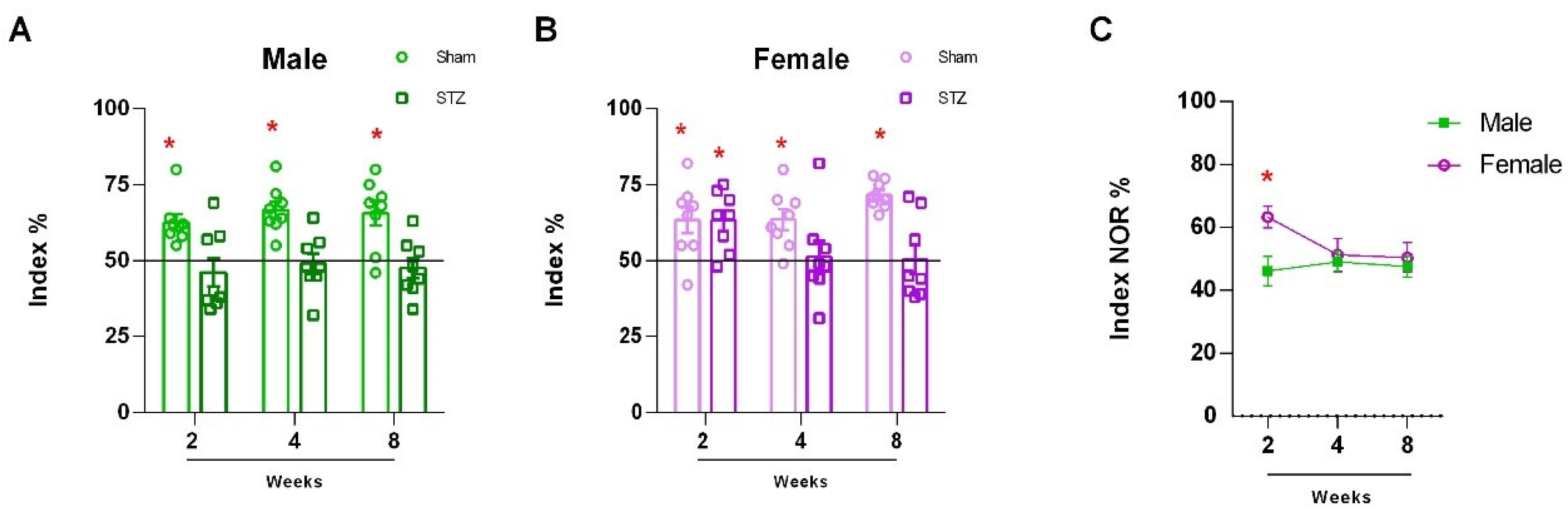
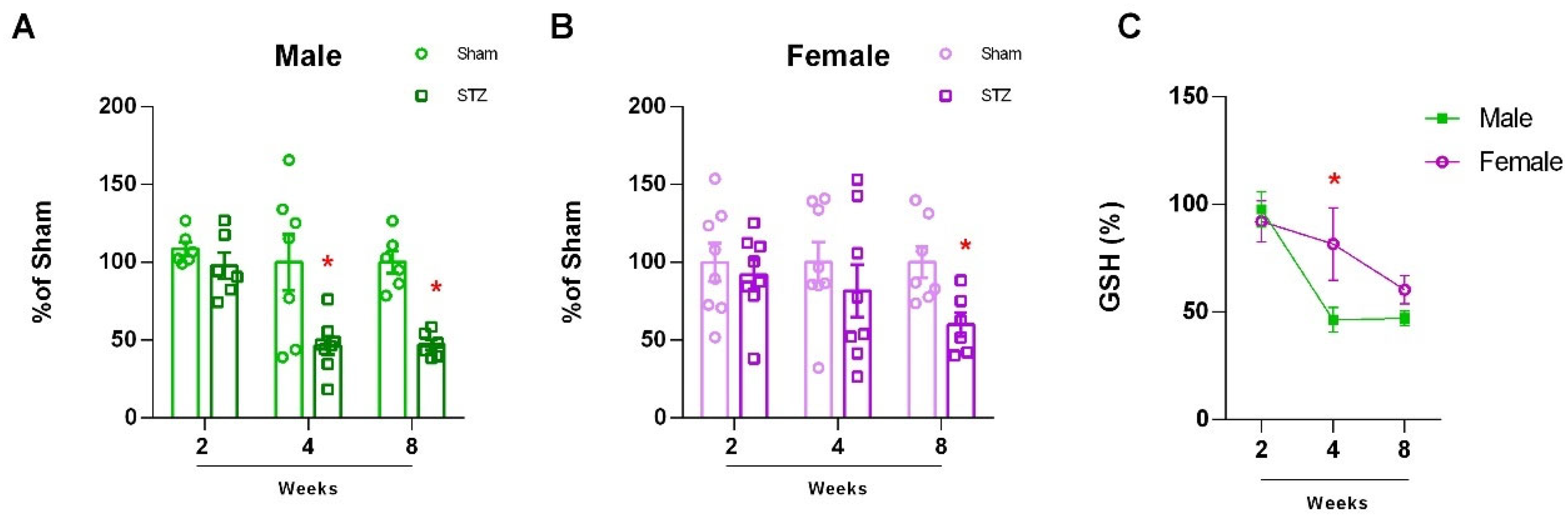
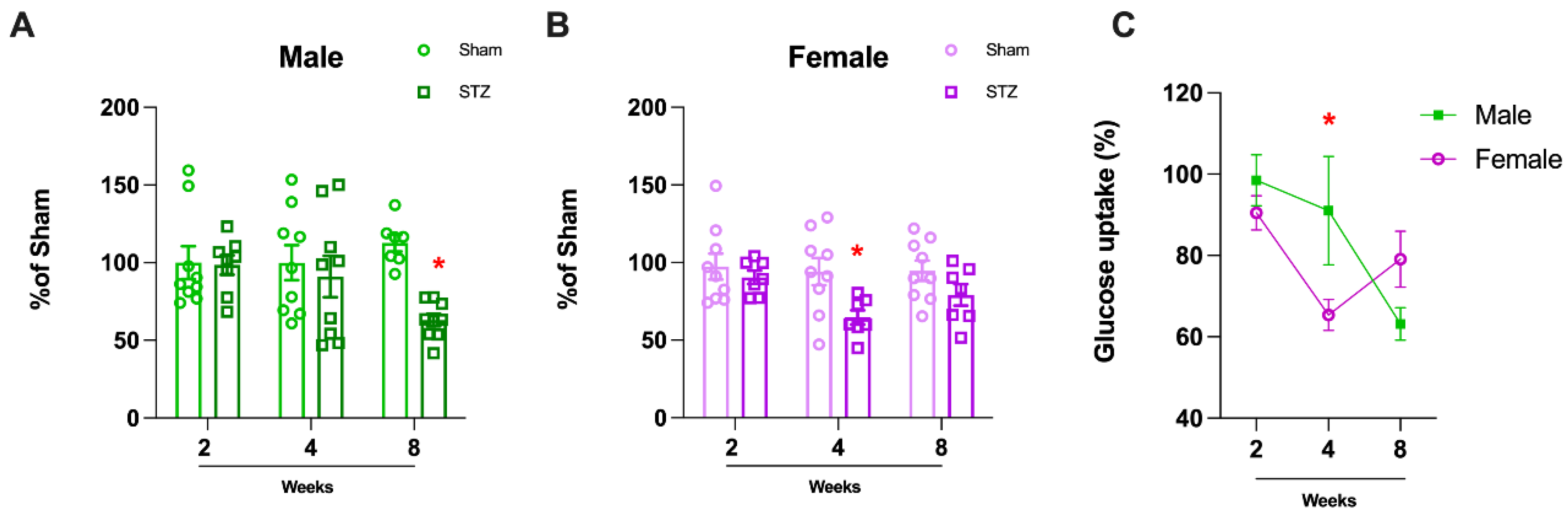
2.1.1. Sexually Dimorphic Cerebellar Response to STZ
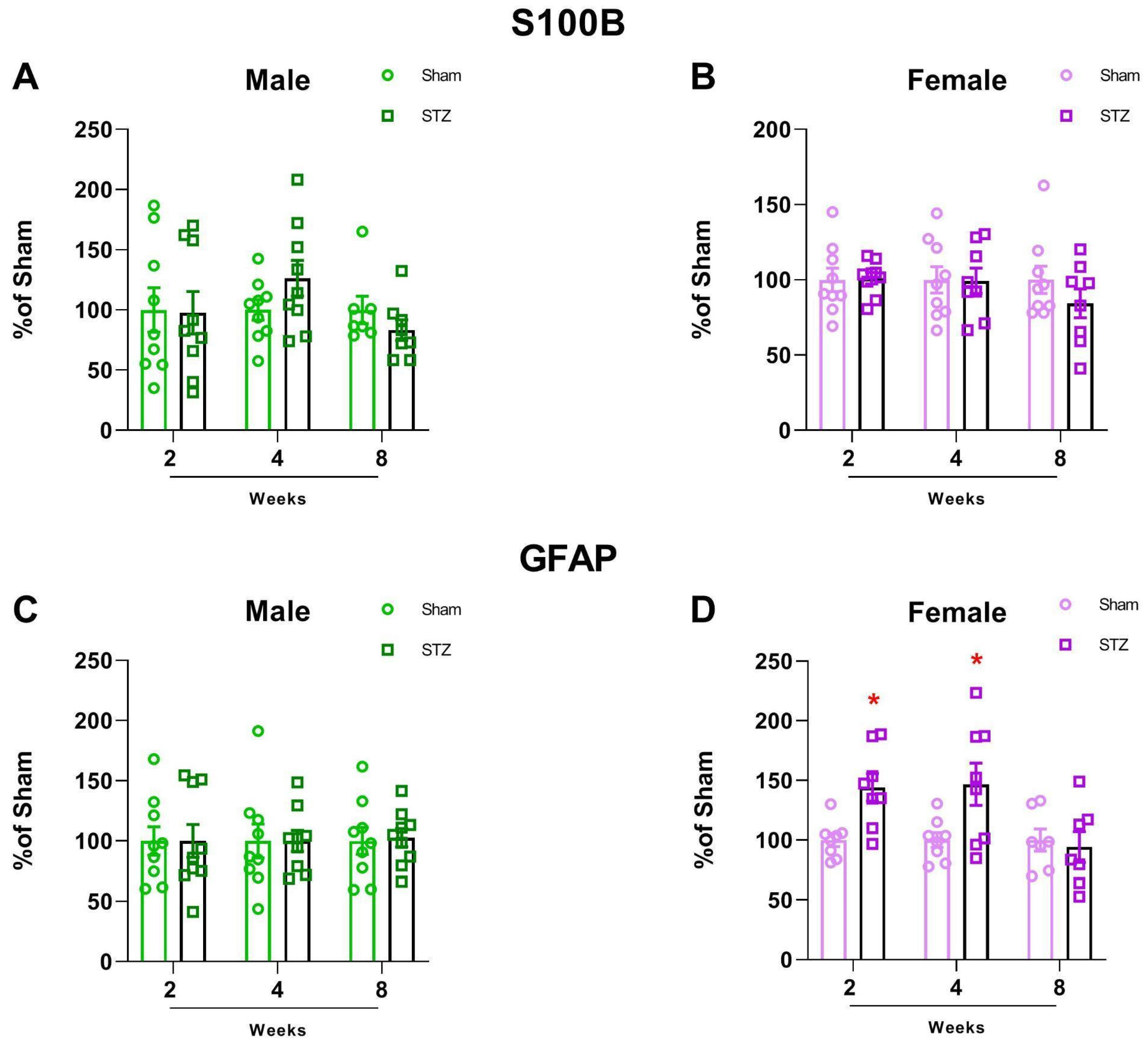
2.1.2. GFAP Is Altered in Female Rats After STZ Infusion
2.1.3. Cross-Sectional Analysis Comparing Cerebellum and Hippocampus Volume with GFAP Plasma Levels in the ADNI Cohort
2.1.4. Longitudinal Analysis of Cerebellum and Hippocampus Volume Changes
3. Discussion
4. Material and Methods
4.1. STZ-ICV Animal Model Experiments
4.1.1. Chemicals
4.1.2. Animals
4.1.3. Surgical Procedure
4.1.4. The Novel Object Recognition Test (NOR)
4.1.5. ELISA for S100B and GFAP
4.1.6. Quantification of Glutathione
4.1.7. Glucose Uptake
4.1.8. Western Blot Analysis for ChAT
4.1.9. Protein Determination
4.2. Statistical Analysis
4.2.1. Alzheimer’s Disease Neuroimaging Initiative (ADNI)
Florbetapir PET Amyloid Imaging
MRI—FreeSurfer Methods
Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2023, 19, 1598–1695. [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Parihar, M.S.; Hemnani, T. Alzheimer’s Disease Pathogenesis and Therapeutic Interventions. J. Clin. Neurosci. 2004, 11, 456–467. [Google Scholar] [CrossRef]
- Jeong, S. Molecular and Cellular Basis of Neurodegeneration in Alzheimer’s Disease. Mol. Cells 2017, 40, 613–620. [Google Scholar] [CrossRef]
- Laws, K.R.; Irvine, K.; Gale, T.M. Sex Differences in Alzheimer’s Disease. Curr. Opin. Psychiatry 2018, 31, 133–139. [Google Scholar] [CrossRef]
- Matsumoto, A.; Arai, Y. Sex Difference in Volume of the Ventromedial Nucleus of the Hypothalamus in the Rat. Endocrinol. Jpn. 1983, 30, 277–280. [Google Scholar] [CrossRef]
- McCarthy, M.M. Sexual Differentiation of the Brain in Man and Animals: Of Relevance to Klinefelter Syndrome? Am. J. Med. Genet C Semin Med. Genet. 2013, 163, 3–15. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The Cerebellum and Cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef]
- Alvarez, M.-I.; Rivas, L.; Lacruz, C.; Toledano, A. Astroglial Cell Subtypes in the Cerebella of Normal Adults, Elderly Adults, and Patients with Alzheimer’s Disease: A Histological and Immunohistochemical Comparison. Glia 2015, 63, 287–312. [Google Scholar] [CrossRef]
- Schmahmann, J.D. Cerebellum in Alzheimer’s Disease and Frontotemporal Dementia: Not a Silent Bystander. Brain 2016, 139, 1314–1318. [Google Scholar] [CrossRef]
- Dean, S.L.; McCarthy, M.M. Steroids, Sex and the Cerebellar Cortex: Implications for Human Disease. Cerebellum 2008, 7, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Sasaguri, H.; Hashimoto, S.; Watamura, N.; Sato, K.; Takamura, R.; Nagata, K.; Tsubuki, S.; Ohshima, T.; Yoshiki, A.; Sato, K.; et al. Recent Advances in the Modeling of Alzheimer’s Disease. Front. Neurosci. 2022, 16, 807473. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s Disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Armstrong, R.A. Risk Factors for Alzheimer’s Disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef]
- Evans, D.A.; Funkenstein, H.H.; Albert, M.S.; Scherr, P.A.; Cook, N.R.; Chown, M.J.; Hebert, L.E.; Hennekens, C.H.; Taylor, J.O. Prevalence of Alzheimer’s Disease in a Community Population of Older Persons. Higher than Previously Reported. JAMA 1989, 262, 2551–2556. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Khemka, V.K.; Banerjee, A.; Chatterjee, G.; Ganguly, A.; Biswas, A. Metabolic Risk Factors of Sporadic Alzheimer’s Disease: Implications in the Pathology, Pathogenesis and Treatment. Aging Dis. 2015, 6, 282–299. [Google Scholar] [CrossRef]
- Grieb, P. Intracerebroventricular Streptozotocin Injections as a Model of Alzheimer’s Disease: In Search of a Relevant Mechanism. Mol. Neurobiol. 2016, 53, 1741–1752. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Tota, S.K.; Kumar, A.; Ahmad, A.S. Streptozotocin Intracerebroventricular-Induced Neurotoxicity and Brain Insulin Resistance: A Therapeutic Intervention for Treatment of Sporadic Alzheimer’s Disease (sAD)-Like Pathology. Mol. Neurobiol. 2016, 53, 4548–4562. [Google Scholar] [CrossRef]
- Rodrigues, L.; Wartchow, K.M.; Suardi, L.Z.; Federhen, B.C.; Selistre, N.G.; Gonçalves, C.-A. Streptozotocin Causes Acute Responses on Hippocampal S100B and BDNF Proteins Linked to Glucose Metabolism Alterations. Neurochem. Int. 2019, 128, 85–93. [Google Scholar] [CrossRef]
- Craft, S.; Peskind, E.; Schwartz, M.W.; Schellenberg, G.D.; Raskind, M.; Porte, D. Cerebrospinal Fluid and Plasma Insulin Levels in Alzheimer’s Disease: Relationship to Severity of Dementia and Apolipoprotein E Genotype. Neurology 1998, 50, 164–168. [Google Scholar] [CrossRef]
- Hoyer, S. Glucose Metabolism and Insulin Receptor Signal Transduction in Alzheimer Disease. Eur. J. Pharmacol. 2004, 490, 115–125. [Google Scholar] [CrossRef]
- Craft, S.; Raman, R.; Chow, T.W.; Rafii, M.S.; Sun, C.-K.; Rissman, R.A.; Donohue, M.C.; Brewer, J.B.; Jenkins, C.; Harless, K.; et al. Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, M.; Wall, A.; Chiotis, K.; Saint-Aubert, L.; Wilking, H.; Sprycha, M.; Borg, B.; Thibblin, A.; Eriksson, J.; Sörensen, J.; et al. Tracer Kinetic Analysis of (S)-18F-THK5117 as a PET Tracer for Assessing Tau Pathology. J. Nucl. Med. 2016, 57, 574–581. [Google Scholar] [CrossRef]
- Lopresti, B.J.; Klunk, W.E.; Mathis, C.A.; Hoge, J.A.; Ziolko, S.K.; Lu, X.; Meltzer, C.C.; Schimmel, K.; Tsopelas, N.D.; DeKosky, S.T.; et al. Simplified Quantification of Pittsburgh Compound B Amyloid Imaging PET Studies: A Comparative Analysis. J. Nucl. Med. 2005, 46, 1959–1972. [Google Scholar]
- Frenzel, S.; Wittfeld, K.; Habes, M.; Klinger-König, J.; Bülow, R.; Völzke, H.; Grabe, H.J. A Biomarker for Alzheimer’s Disease Based on Patterns of Regional Brain Atrophy. Front. Psychiatry 2020, 10, 953. [Google Scholar] [CrossRef]
- Tremblay, C.; Rahayel, S.; Pastor-Bernier, A.; St-Onge, F.; Vo, A.; Rheault, F.; Daneault, V.; Morys, F.; Rajah, N.; Villeneuve, S.; et al. Pattern and Mechanisms of Atrophy Progression in Individuals with a Family History of Alzheimer’s Disease: A Comparative Study. arXiv 2024, arXiv:2024-03. [Google Scholar] [CrossRef]
- Sluimer, J.D.; van der Flier, W.M.; Karas, G.B.; van Schijndel, R.; Barnes, J.; Boyes, R.G.; Cover, K.S.; Olabarriaga, S.D.; Fox, N.C.; Scheltens, P.; et al. Accelerating Regional Atrophy Rates in the Progression from Normal Aging to Alzheimer’s Disease. Eur. Radiol. 2009, 19, 2826–2833. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Neuroenergetics: Calling upon Astrocytes to Satisfy Hungry Neurons. Neuroscientist 2004, 10, 53–62. [Google Scholar] [CrossRef]
- Kim, K.Y.; Shin, K.Y.; Chang, K.-A. GFAP as a Potential Biomarker for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Cells 2023, 12, 1309. [Google Scholar] [CrossRef]
- Pellerin, L. Brain Energetics (Thought Needs Food). Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 701–705. [Google Scholar] [CrossRef]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef] [PubMed]
- Biasibetti, R.; Almeida Dos Santos, J.P.; Rodrigues, L.; Wartchow, K.M.; Suardi, L.Z.; Nardin, P.; Selistre, N.G.; Vázquez, D.; Gonçalves, C.-A. Hippocampal Changes in STZ-Model of Alzheimer’s Disease Are Dependent on Sex. Behav. Brain Res. 2017, 316, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Samstag, C.L.; Chapman, N.H.; Gibbons, L.E.; Geller, J.; Loeb, N.; Dharap, S.; Yagi, M.; Cook, D.G.; Pagulayan, K.F.; Crane, P.K.; et al. Neuropathological Correlates of Vulnerability and Resilience in the Cerebellum in Alzheimer’s Disease. Alzheimers Dement 2024, 21, e14428. [Google Scholar] [CrossRef]
- Beard, E.; Lengacher, S.; Dias, S.; Magistretti, P.J.; Finsterwald, C. Astrocytes as Key Regulators of Brain Energy Metabolism: New Therapeutic Perspectives. Front. Physiol. 2021, 12, 825816. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Pellerin, L. Cellular Mechanisms of Brain Energy Metabolism. Relevance to Functional Brain Imaging and to Neurodegenerative Disorders. Ann. N Y Acad. Sci. 1996, 777, 380–387. [Google Scholar] [CrossRef]
- Balu, D.; Valencia-Olvera, A.C.; Islam, Z.; Mielczarek, C.; Hansen, A.; Perez Ramos, T.M.; York, J.; LaDu, M.J.; Tai, L.M. APOE Genotype and Sex Modulate Alzheimer’s Disease Pathology in Aged EFAD Transgenic Mice. Front. Aging Neurosci. 2023, 15, 1279343. [Google Scholar] [CrossRef]
- Dyer, A.H.; Lawlor, B.; Kennelly, S.P. NILVAD Study Group Sexual Dimorphism in the Association of APOE Ε4 Genotype with Cognitive Decline and Dementia Progression in Mild-to-Moderate Alzheimer Disease. Int. J. Geriatr. Psychiatry 2020, 35, 683–686. [Google Scholar] [CrossRef]
- Sauty, B.; Durrleman, S. Impact of Sex and APOE-Ε4 Genotype on Patterns of Regional Brain Atrophy in Alzheimer’s Disease and Healthy Aging. Front. Neurol. 2023, 14, 1161527. [Google Scholar] [CrossRef]
- Guo, K.; Li, G.; Quan, Z.; Wang, Y.; Wang, J.; Kang, F.; Wang, J. Extracerebral Normalization of 18F-FDG PET Imaging Combined with Behavioral CRS-R Scores Predict Recovery from Disorders of Consciousness. Neurocrit. Care, 2024; ahead of print. [Google Scholar] [CrossRef]
- Mosconi, L.; McHugh, P.F. FDG- and Amyloid-PET in Alzheimer’s Disease: Is the Whole Greater than the Sum of the Parts? Q J. Nucl. Med. Mol. Imaging 2011, 55, 250–264. [Google Scholar]
- Grünblatt, E.; Koutsilieri, E.; Hoyer, S.; Riederer, P. Gene Expression Alterations in Brain Areas of Intracerebroventricular Streptozotocin Treated Rat. J. Alzheimers Dis. 2006, 9, 261–271. [Google Scholar] [CrossRef]
- Hellweg, R.; Nitsch, R.; Hock, C.; Jaksch, M.; Hoyer, S. Nerve Growth Factor and Choline Acetyltransferase Activity Levels in the Rat Brain Following Experimental Impairment of Cerebral Glucose and Energy Metabolism. J. Neurosci. Res. 1992, 31, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Tahirovic, I.; Sofic, E.; Sapcanin, A.; Gavrankapetanovic, I.; Bach-Rojecky, L.; Salkovic-Petrisic, M.; Lackovic, Z.; Hoyer, S.; Riederer, P. Reduced Brain Antioxidant Capacity in Rat Models of Betacytotoxic-Induced Experimental Sporadic Alzheimer’s Disease and Diabetes Mellitus. Neurochem. Res. 2007, 32, 1709–1717. [Google Scholar] [CrossRef]
- Liang, K.J.; Carlson, E.S. Resistance, Vulnerability and Resilience: A Review of the Cognitive Cerebellum in Aging and Neurodegenerative Diseases. Neurobiol. Learn. Mem. 2020, 170, 106981. [Google Scholar] [CrossRef]
- Baldaçara, L.; Borgio, J.G.F.; Moraes, W.A.d.S.; Lacerda, A.L.T.; Montaño, M.B.M.M.; Tufik, S.; Bressan, R.A.; Ramos, L.R.; Jackowski, A.P. Cerebellar Volume in Patients with Dementia. Braz J. Psychiatry 2011, 33, 122–129. [Google Scholar] [CrossRef]
- Sugimura, T.; Saito, Y. Distinct Proportions of Cholinergic Neurons in the Rat Prepositus Hypoglossi Nucleus According to Their Cerebellar Projection Targets. J. Comp. Neurol. 2021, 529, 1541–1552. [Google Scholar] [CrossRef]
- Genrikhs, E.E.; Stelmashook, E.V.; Golyshev, S.A.; Aleksandrova, O.P.; Isaev, N.K. Streptozotocin Causes Neurotoxic Effect in Cultured Cerebellar Granule Neurons. Brain. Res. Bull. 2017, 130, 90–94. [Google Scholar] [CrossRef]
- Donato, R.; Sorci, G.; Riuzzi, F.; Arcuri, C.; Bianchi, R.; Brozzi, F.; Tubaro, C.; Giambanco, I. S100B’s Double Life: Intracellular Regulator and Extracellular Signal. Biochim. Biophys. Acta 2009, 1793, 1008–1022. [Google Scholar] [CrossRef]
- Sherif, R.N. Effect of Cerebrolysin on the Cerebellum of Diabetic Rats: An Imunohistochemical Study. Tissue Cell 2017, 49, 726–733. [Google Scholar] [CrossRef]
- Yu, T.-S.; Tensaouti, Y.; Stephanz, E.P.; Chintamen, S.; Rafikian, E.E.; Yang, M.; Kernie, S.G. Astrocytic ApoE Underlies Maturation of Hippocampal Neurons and Cognitive Recovery after Traumatic Brain Injury in Mice. Commun. Biol. 2021, 4, 1303. [Google Scholar] [CrossRef]
- McConnell, H.L.; Mishra, A. Cells of the Blood-Brain Barrier: An Overview of the Neurovascular Unit in Health and Disease. Methods Mol. Biol. 2022, 2492, 3–24. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, J.; Hou, Y.; Shi, X.; Liu, K. Prediction of Clinical Progression in Nervous System Diseases: Plasma Glial Fibrillary Acidic Protein (GFAP). Eur. J. Med. Res. 2024, 29, 51. [Google Scholar] [CrossRef] [PubMed]
- Merlo, S.; Spampinato, S.F.; Sortino, M.A. Early Compensatory Responses against Neuronal Injury: A New Therapeutic Window of Opportunity for Alzheimer’s Disease? CNS Neurosci. Ther. 2019, 25, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Torrealba, E.; Aguilar-Zerpa, N.; Garcia-Morales, P.; Díaz, M. Compensatory Mechanisms in Early Alzheimer’s Disease and Clinical Setting: The Need for Novel Neuropsychological Strategies. J. Alzheimers Dis. Rep. 2023, 7, 513–525. [Google Scholar] [CrossRef]
- Vicente-Acosta, A.; Herranz-Martín, S.; Pazos, M.R.; Galán-Cruz, J.; Amores, M.; Loria, F.; Díaz-Nido, J. Glial Cell Activation Precedes Neurodegeneration in the Cerebellar Cortex of the YG8–800 Murine Model of Friedreich Ataxia. Neurobiol. Dis. 2024, 200, 106631. [Google Scholar] [CrossRef]
- Guo, C.C.; Tan, R.; Hodges, J.R.; Hu, X.; Sami, S.; Hornberger, M. Network-Selective Vulnerability of the Human Cerebellum to Alzheimer’s Disease and Frontotemporal Dementia. Brain 2016, 139, 1527–1538. [Google Scholar] [CrossRef]
- Bellaver, B.; Ferrari-Souza, J.P.; Uglione da Ros, L.; Carter, S.F.; Rodriguez-Vieitez, E.; Nordberg, A.; Pellerin, L.; Rosa-Neto, P.; Leffa, D.T.; Zimmer, E.R. Astrocyte Biomarkers in Alzheimer Disease: A Systematic Review and Meta-Analysis. Neurology 2021, 96, E2944–E2955. [Google Scholar] [CrossRef]
- De Bastiani, M.A.; Bellaver, B.; Brum, W.S.; Souza, D.G.; Ferreira, P.C.L.; Rocha, A.S.; Povala, G.; Ferrari-Souza, J.P.; Benedet, A.L.; Ashton, N.J.; et al. Hippocampal GFAP-Positive Astrocyte Responses to Amyloid and Tau Pathologies. Brain. Behav. Immun. 2023, 110, 175–184. [Google Scholar] [CrossRef]
- Benedet, A.L.; Milà-Alomà, M.; Vrillon, A.; Ashton, N.J.; Pascoal, T.A.; Lussier, F.; Karikari, T.K.; Hourregue, C.; Cognat, E.; Dumurgier, J.; et al. Differences Between Plasma and Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels Across the Alzheimer Disease Continuum. JAMA Neurol. 2021, 78, 1471–1483. [Google Scholar] [CrossRef]
- Pereira, J.B.; Janelidze, S.; Smith, R.; Mattsson-Carlgren, N.; Palmqvist, S.; Teunissen, C.E.; Zetterberg, H.; Stomrud, E.; Ashton, N.J.; Blennow, K.; et al. Plasma GFAP Is an Early Marker of Amyloid-β but Not Tau Pathology in Alzheimer’s Disease. Brain 2021, 144, 3505–3516. [Google Scholar] [CrossRef]
- Nebel, R.A.; Aggarwal, N.T.; Barnes, L.L.; Gallagher, A.; Goldstein, J.M.; Kantarci, K.; Mallampalli, M.P.; Mormino, E.C.; Scott, L.; Yu, W.H.; et al. Understanding the Impact of Sex and Gender in Alzheimer’s Disease: A Call to Action. Alzheimers Dement 2018, 14, 1171–1183. [Google Scholar] [CrossRef]
- Moreira, A.P.; Vizuete, A.F.K.; Zin, L.E.F.; de Marques, C.O.; Pacheco, R.F.; Leal, M.B.; Gonçalves, C.-A. The Methylglyoxal/RAGE/NOX-2 Pathway Is Persistently Activated in the Hippocampus of Rats with STZ-Induced Sporadic Alzheimer’s Disease. Neurotox. Res. 2022, 40, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of A Beta-Deposition in the Human Brain and Its Relevance for the Development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Locke, T.M.; Soden, M.E.; Miller, S.M.; Hunker, A.; Knakal, C.; Licholai, J.A.; Dhillon, K.S.; Keene, C.D.; Zweifel, L.S.; Carlson, E.S. Dopamine D1 Receptor-Positive Neurons in the Lateral Nucleus of the Cerebellum Contribute to Cognitive Behavior. Biol. Psychiatry 2018, 84, 401–412. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. Coordinated Scaling of Cortical and Cerebellar Numbers of Neurons. Front. Neuroanat. 2010, 4, 12. [Google Scholar] [CrossRef]
- Fu, Y.; Rusznák, Z.; Herculano-Houzel, S.; Watson, C.; Paxinos, G. Cellular Composition Characterizing Postnatal Development and Maturation of the Mouse Brain and Spinal Cord. Brain. Struct. Funct. 2013, 218, 1337–1354. [Google Scholar] [CrossRef]
- Buckner, R.L.; Krienen, F.M. The Evolution of Distributed Association Networks in the Human Brain. Trends Cogn. Sci. 2013, 17, 648–665. [Google Scholar] [CrossRef]
- Barton, R.A.; Venditti, C. Rapid Evolution of the Cerebellum in Humans and Other Great Apes. Curr. Biol. 2014, 24, 2440–2444. [Google Scholar] [CrossRef]
- Biasibetti, R.; Tramontina, A.C.; Costa, A.P.; Dutra, M.F.; Quincozes-Santos, A.; Nardin, P.; Bernardi, C.L.; Wartchow, K.M.; Lunardi, P.S.; Gonçalves, C.-A. Green Tea (-)Epigallocatechin-3-Gallate Reverses Oxidative Stress and Reduces Acetylcholinesterase Activity in a Streptozotocin-Induced Model of Dementia. Behav. Brain Res. 2013, 236, 186–193. [Google Scholar] [CrossRef]
- Rodrigues, L.; Biasibetti, R.; Swarowsky, A.; Leite, M.C.; Quincozes-Santos, A.; Quilfeldt, J.A.; Achaval, M.; Gonçalves, C.-A. Hippocampal Alterations in Rats Submitted to Streptozotocin-Induced Dementia Model Are Prevented by Aminoguanidine. J. Alzheimers Dis. 2009, 17, 193–202. [Google Scholar] [CrossRef]
- Lissner, L.J.; Wartchow, K.M.; Toniazzo, A.P.; Gonçalves, C.-A.; Rodrigues, L. Object Recognition and Morris Water Maze to Detect Cognitive Impairment from Mild Hippocampal Damage in Rats: A Reflection Based on the Literature and Experience. Pharmacol. Biochem. Behav. 2021, 210, 173273. [Google Scholar] [CrossRef]
- Lissner, L.J.; Rodrigues, L.; Wartchow, K.M.; Borba, E.; Bobermin, L.D.; Fontella, F.U.; Hansen, F.; Quincozes-Santos, A.; Souza, D.O.G.; Gonçalves, C.-A. Short-Term Alterations in Behavior and Astroglial Function After Intracerebroventricular Infusion of Methylglyoxal in Rats. Neurochem. Res. 2020, 46, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Wartchow, K.M.; Rodrigues, L.; Lissner, L.J.; Federhen, B.C.; Selistre, N.G.; Moreira, A.; Gonçalves, C.-A.; Sesterheim, P. Insulin-Producing Cells from Mesenchymal Stromal Cells: Protection against Cognitive Impairment in Diabetic Rats Depends upon Implant Site. Life Sci. 2020, 251, 117587. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.C.; Galland, F.; Brolese, G.; Guerra, M.C.; Bortolotto, J.W.; Freitas, R.; de Almeida, L.M.V.; Gottfried, C.; Gonçalves, C.-A. A Simple, Sensitive and Widely Applicable ELISA for S100B: Methodological Features of the Measurement of This Glial Protein. J. Neurosci. Methods 2008, 169, 93–99. [Google Scholar] [CrossRef]
- Tramontina, F.; Leite, M.C.; Cereser, K.; de Souza, D.F.; Tramontina, A.C.; Nardin, P.; Andreazza, A.C.; Gottfried, C.; Kapczinski, F.; Gonçalves, C.-A. Immunoassay for Glial Fibrillary Acidic Protein: Antigen Recognition Is Affected by Its Phosphorylation State. J. Neurosci. Methods 2007, 162, 282–286. [Google Scholar] [CrossRef]
- Browne, R.W.; Armstrong, D. Reduced Glutathione and Glutathione Disulfide. Methods Mol. Biol. 1998, 108, 347–352. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate Uptake into Astrocytes Stimulates Aerobic Glycolysis: A Mechanism Coupling Neuronal Activity to Glucose Utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef]
- Wartchow, K.M.; Tramontina, A.C.; de Souza, D.F.; Biasibetti, R.; Bobermin, L.D.; Gonçalves, C.-A. Insulin Stimulates S100B Secretion and These Proteins Antagonistically Modulate Brain Glucose Metabolism. Neurochem. Res. 2016, 41, 1420–1429. [Google Scholar] [CrossRef]
- Peterson, G.L. A Simplification of the Protein Assay Method of Lowry et al. Which Is More Generally Applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]
- Reuter, M.; Rosas, H.D.; Fischl, B. Highly Accurate Inverse Consistent Registration: A Robust Approach. Neuroimage 2010, 53, 1181–1196. [Google Scholar] [CrossRef]
- Ségonne, F.; Dale, A.M.; Busa, E.; Glessner, M.; Salat, D.; Hahn, H.K.; Fischl, B. A Hybrid Approach to the Skull Stripping Problem in MRI. Neuroimage 2004, 22, 1060–1075. [Google Scholar] [CrossRef]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Sled, J.G.; Zijdenbos, A.P.; Evans, A.C. A Nonparametric Method for Automatic Correction of Intensity Nonuniformity in MRI Data. IEEE Trans. Med. Imaging 1998, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.M.; Sereno, M.I. Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. J. Cogn. Neurosci. 1993, 5, 162–176. [Google Scholar] [CrossRef]

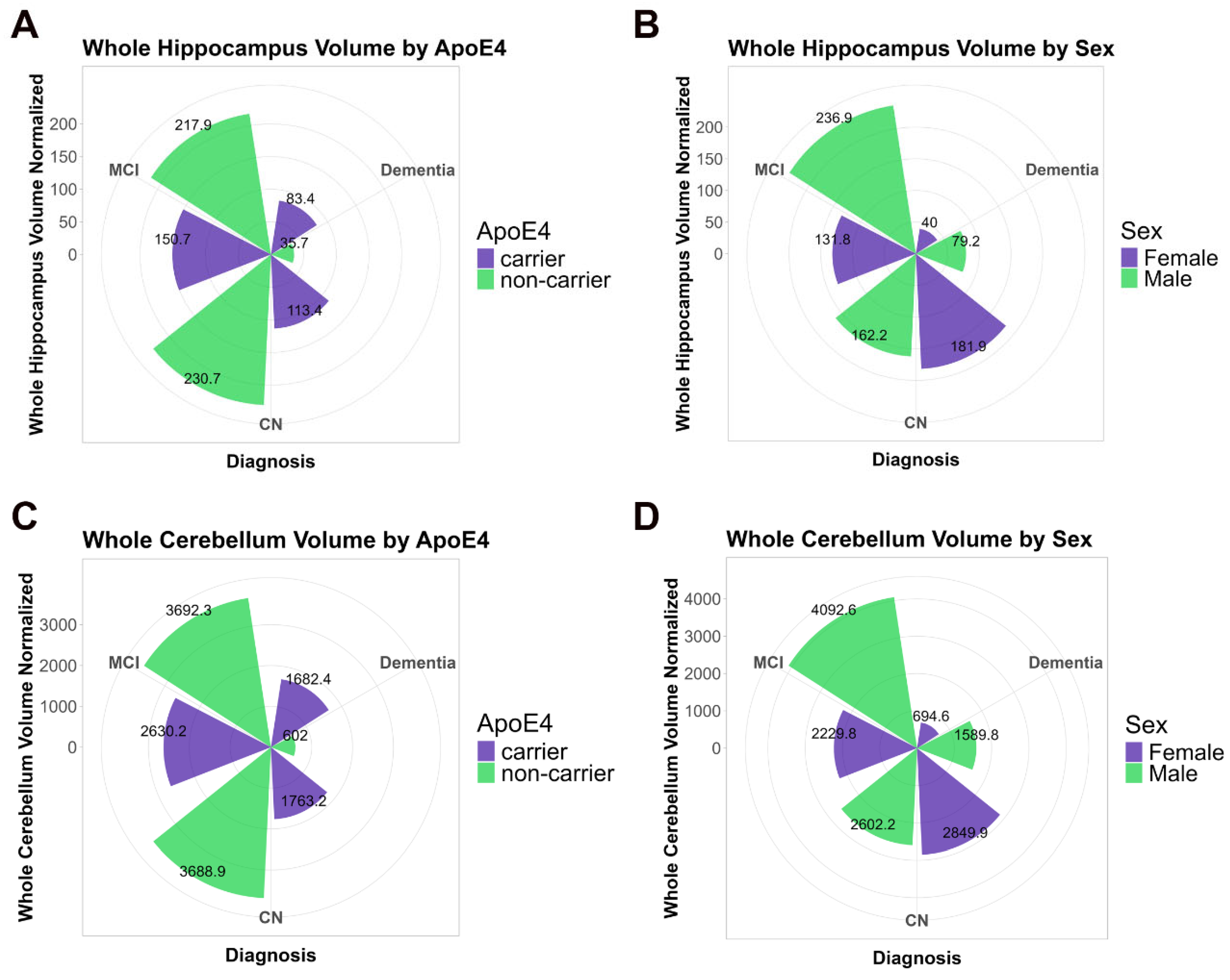
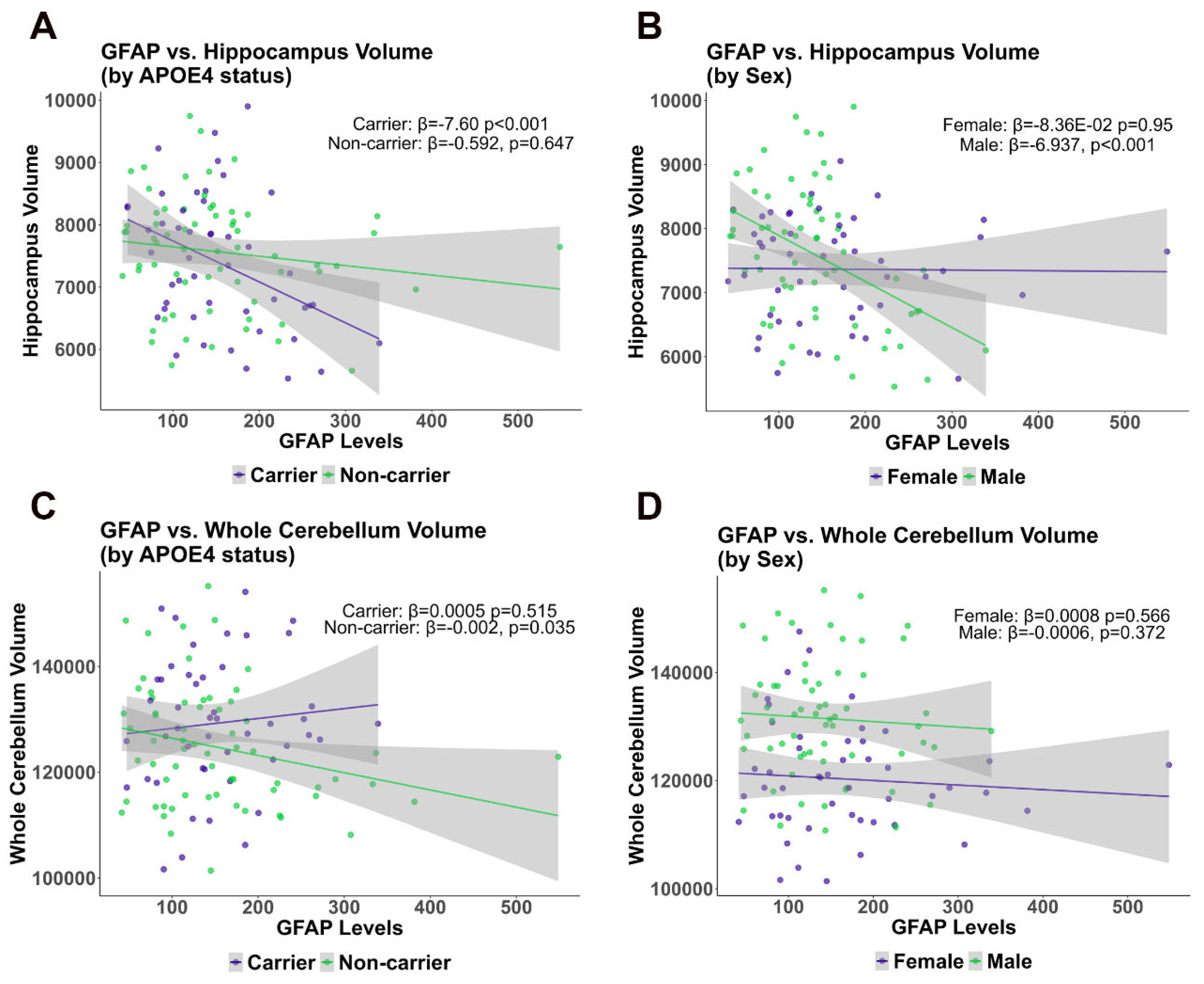

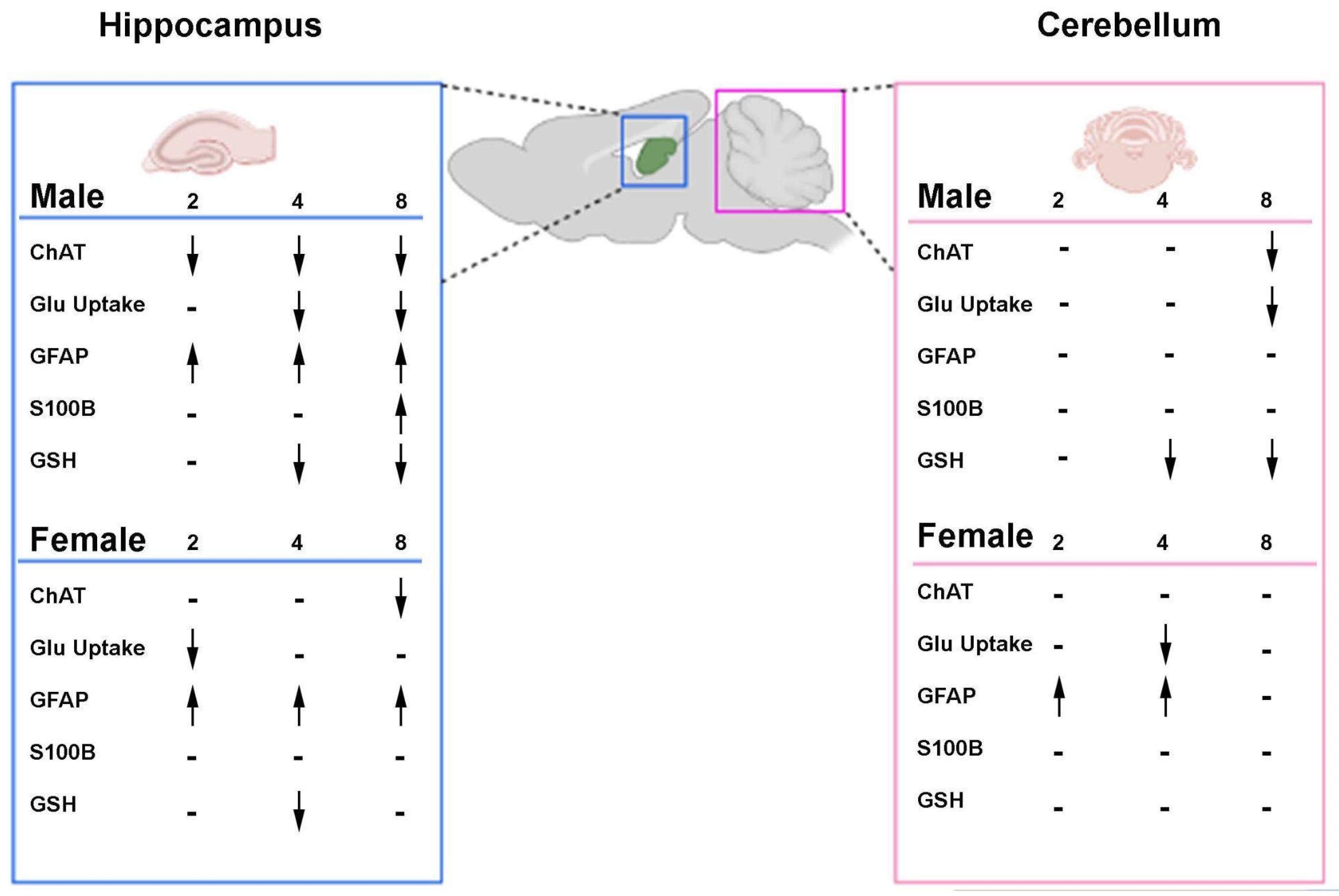
| Overall | ApoE4 Non-Carrier | ApoE4 Carrier | p-Value * | |
|---|---|---|---|---|
| N | 111 | 64 | 47 | |
| N (%) | N (%) | N (%) | ||
| Sex = Male | 63 (56.8) | 31 (48.4) | 32 (68.1) | 0.061 |
| Race | <0.0001 | |||
| Non-whites | 6 (5.4) | 5 (7.9) | 1 (2.1) | |
| Whites | 105 (94.6) | 59 (92.1) | 46 (97.9) | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Diagnosis | 0.013 | |||
| CN | 44 (39.6) | 30 (46.9) | 14 (29.8) | |
| MCI | 49 (44.1) | 29 (45.3) | 20 (42.6) | |
| Dementia | 18 (16.2) | 5 (7.8) | 13 (27.7) | |
| Age | 72.07 (6.36) | 73.02 (5.94) | 70.77 (6.75) | 0.067 |
| Education (years) | 16.31 (2.73) | 16.39 (2.55) | 16.19 (2.98) | 0.706 |
| GFAP | 152.15 (81.26) | 151.82 (91.74) | 152.62 (65.30) | 0.959 |
| Whole Cerebellum (SUVR) | 126,657.09 (12,057.85) | 124,737.36 (11,382.75) | 129,271.19 (12,574.43) | 0.05 |
| Hippocampus volume (mm3) | 7494.86 (984.53) | 7568.33 (903.69) | 7394.83 (1087.06) | 0.361 |
| ApoE4 Non-Carrier | ApoE4 Carrier | ||||
|---|---|---|---|---|---|
| Predictor | Outcome | β | p-Value | β | p-Value |
| Model 1 | |||||
| GFAP | Whole Cerebellum Vol. SUVR | −0.002124 | 0.0354 | 0.0005056 | 0.515 |
| Total Hippocampus vol (mm3) | −0.5919 | 0.64735 | −7.598 | 0.00097 | |
| Model 2 | |||||
| GFAP | Whole Cerebellum Vol. SUVR | −0.002046 | 0.04599 | 0.0003038 | 0.696 |
| Total Hippocampus vol (mm3) | −0.6497 | 0.61217 | −6.504 | 0.00465 | |
| Male | Female | ||||
|---|---|---|---|---|---|
| Predictor | Outcome | β | p-Value | β | p-Value |
| Model 1 | |||||
| GFAP | Whole Cerebellum Vol. SUVR | −0.0006723 | 0.3718 | −0.0008029 | 0.566 |
| Total Hippocampus vol (mm3) | −6.937 | 0.000904 | −8.36 × 10−2 | 0.949968 | |
| Model 2 | |||||
| GFAP | Whole Cerebellum Vol. SUVR | −0.0006808 | 0.3701 | −0.000828 | 0.563 |
| Total Hippocampus vol (mm3) | −6.721 | 0.000942 | 0.06878 | 0.958013 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wartchow, K.M.; Rodrigues, L.; Dartora, W.J.; Biasibetti, R.; Selistre, N.G.; Lazarian, A.; Barrios-Castellanos, C.; Bartelo, N.; Gonçalves, C.-A.; McIntire, L.B.J.; et al. Sex-Associated Cerebellar and Hippocampal Volume Reduction in Alzheimer’s Disease: Insights from the Clinical ADNI Cohort and STZ Animal Model. Int. J. Mol. Sci. 2025, 26, 4810. https://doi.org/10.3390/ijms26104810
Wartchow KM, Rodrigues L, Dartora WJ, Biasibetti R, Selistre NG, Lazarian A, Barrios-Castellanos C, Bartelo N, Gonçalves C-A, McIntire LBJ, et al. Sex-Associated Cerebellar and Hippocampal Volume Reduction in Alzheimer’s Disease: Insights from the Clinical ADNI Cohort and STZ Animal Model. International Journal of Molecular Sciences. 2025; 26(10):4810. https://doi.org/10.3390/ijms26104810
Chicago/Turabian StyleWartchow, Krista Mineia, Leticia Rodrigues, William Jones Dartora, Regina Biasibetti, Nicholas Guerini Selistre, Artur Lazarian, Carmen Barrios-Castellanos, Nicholas Bartelo, Carlos-Alberto Gonçalves, Laura Beth J. McIntire, and et al. 2025. "Sex-Associated Cerebellar and Hippocampal Volume Reduction in Alzheimer’s Disease: Insights from the Clinical ADNI Cohort and STZ Animal Model" International Journal of Molecular Sciences 26, no. 10: 4810. https://doi.org/10.3390/ijms26104810
APA StyleWartchow, K. M., Rodrigues, L., Dartora, W. J., Biasibetti, R., Selistre, N. G., Lazarian, A., Barrios-Castellanos, C., Bartelo, N., Gonçalves, C.-A., McIntire, L. B. J., & on behalf of Alzheimer’s Disease Neuroimaging Initiative (ADNI). (2025). Sex-Associated Cerebellar and Hippocampal Volume Reduction in Alzheimer’s Disease: Insights from the Clinical ADNI Cohort and STZ Animal Model. International Journal of Molecular Sciences, 26(10), 4810. https://doi.org/10.3390/ijms26104810








