Jasmonates in the Ethylene-Induced Resistance of Detached Citrus Fruits to Peel Damage
Abstract
1. Introduction
2. Results
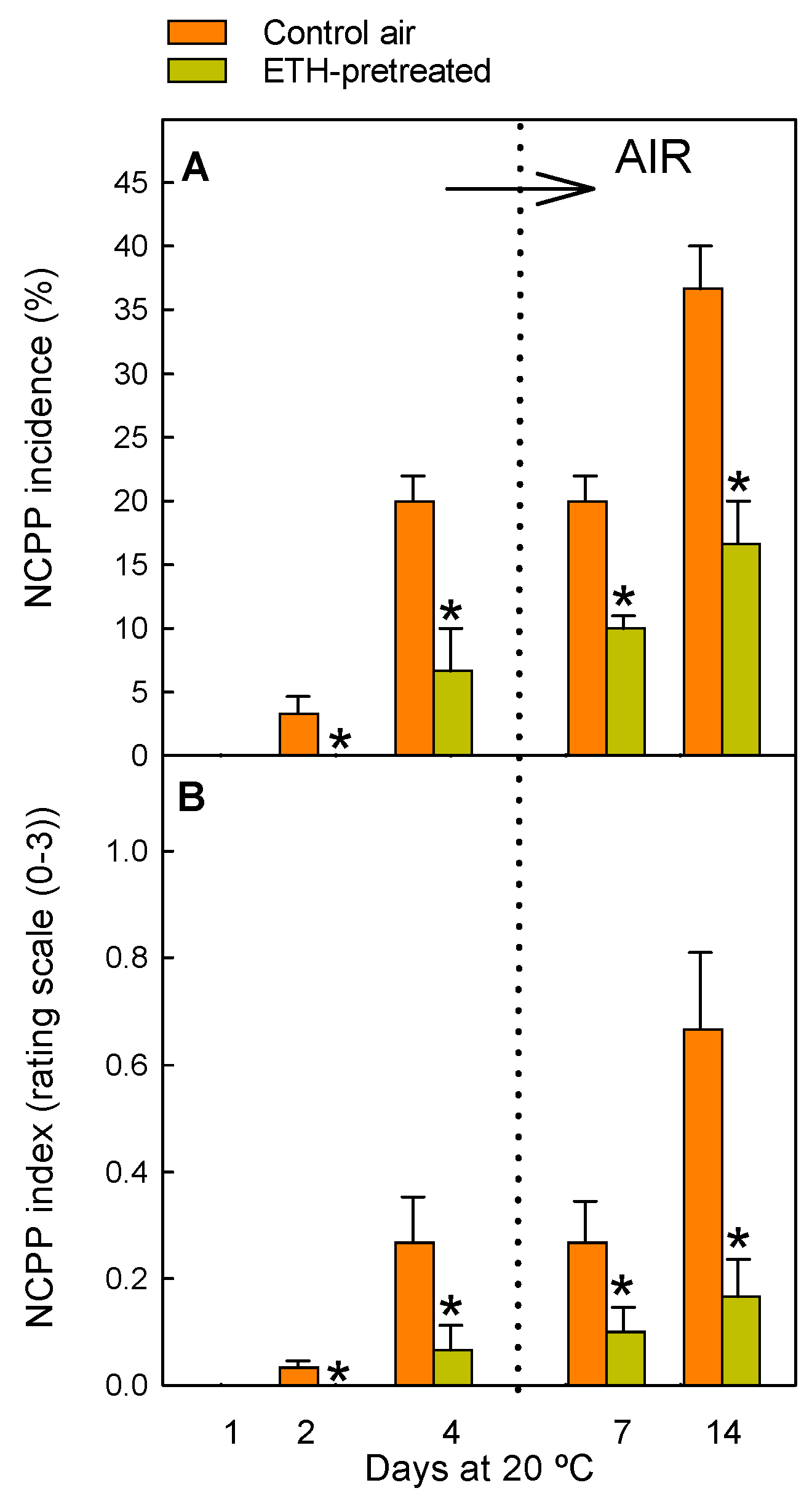
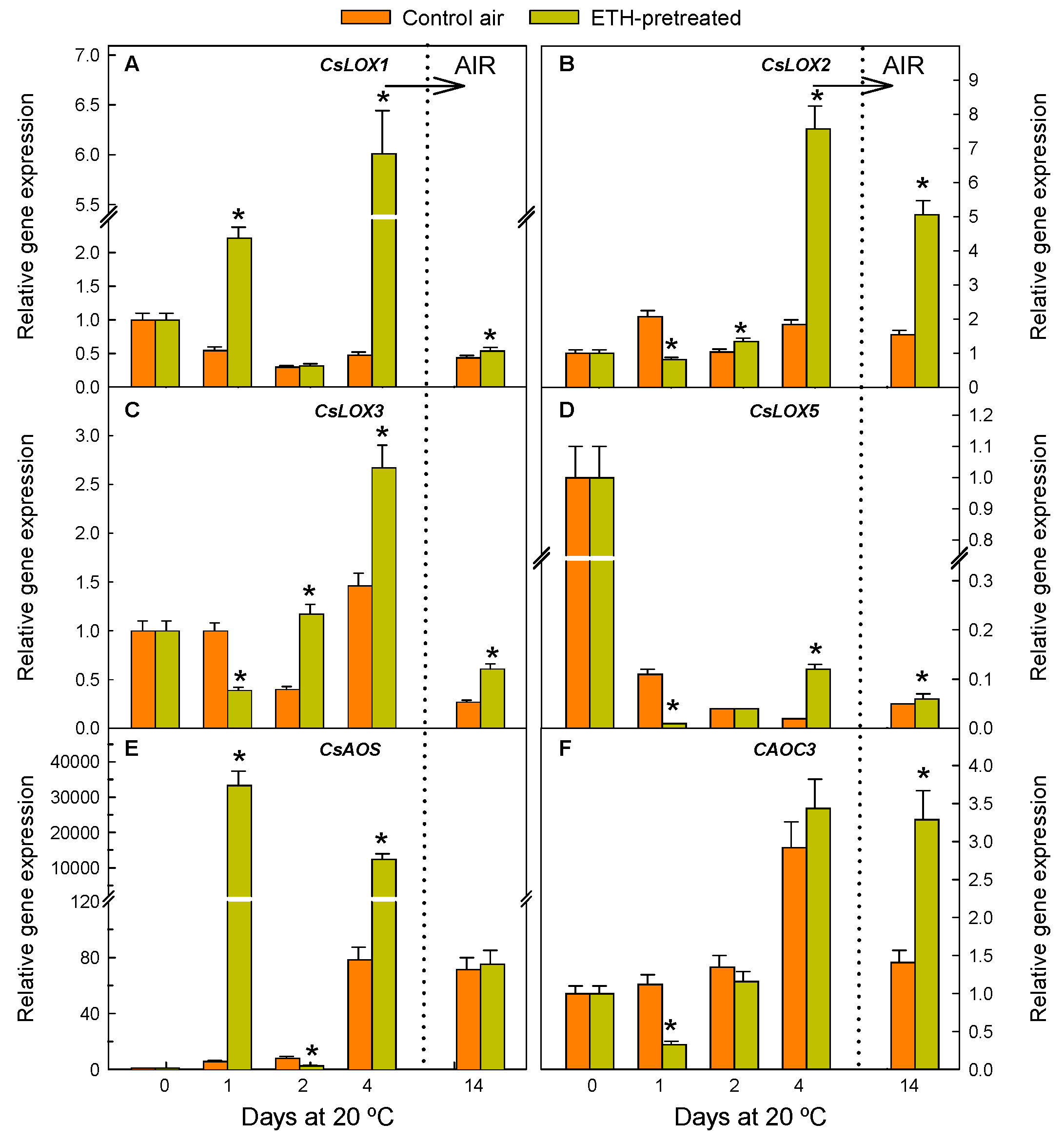
2.1. ETH Pretreatment of Detached Citrus Fruits and Postharvest Nutrient Deprivation Stress Modify the Regulation of Genes Involved in OPDA Synthesis
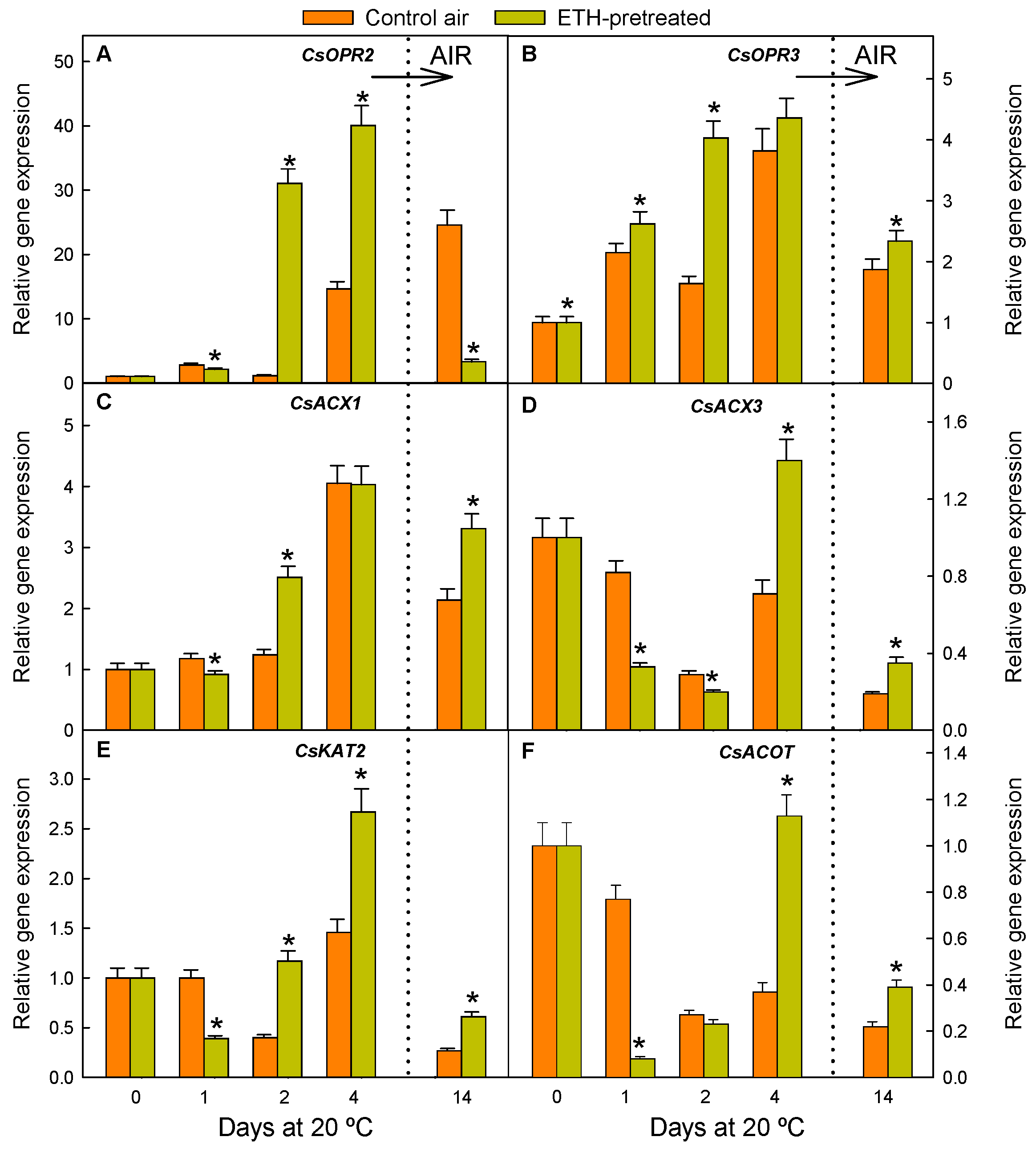
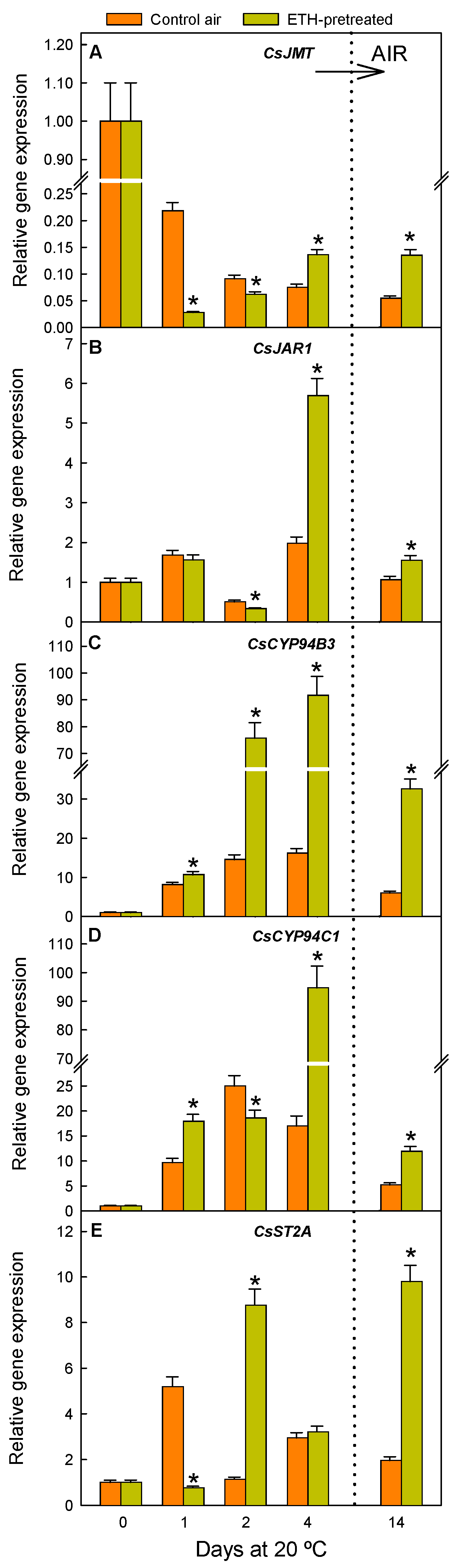
2.2. ETH Pretreatment and Nutrient Deprivation Modify the Postharvest Regulation of Genes Involved in JA, MeJA, and JAIle Accumulation
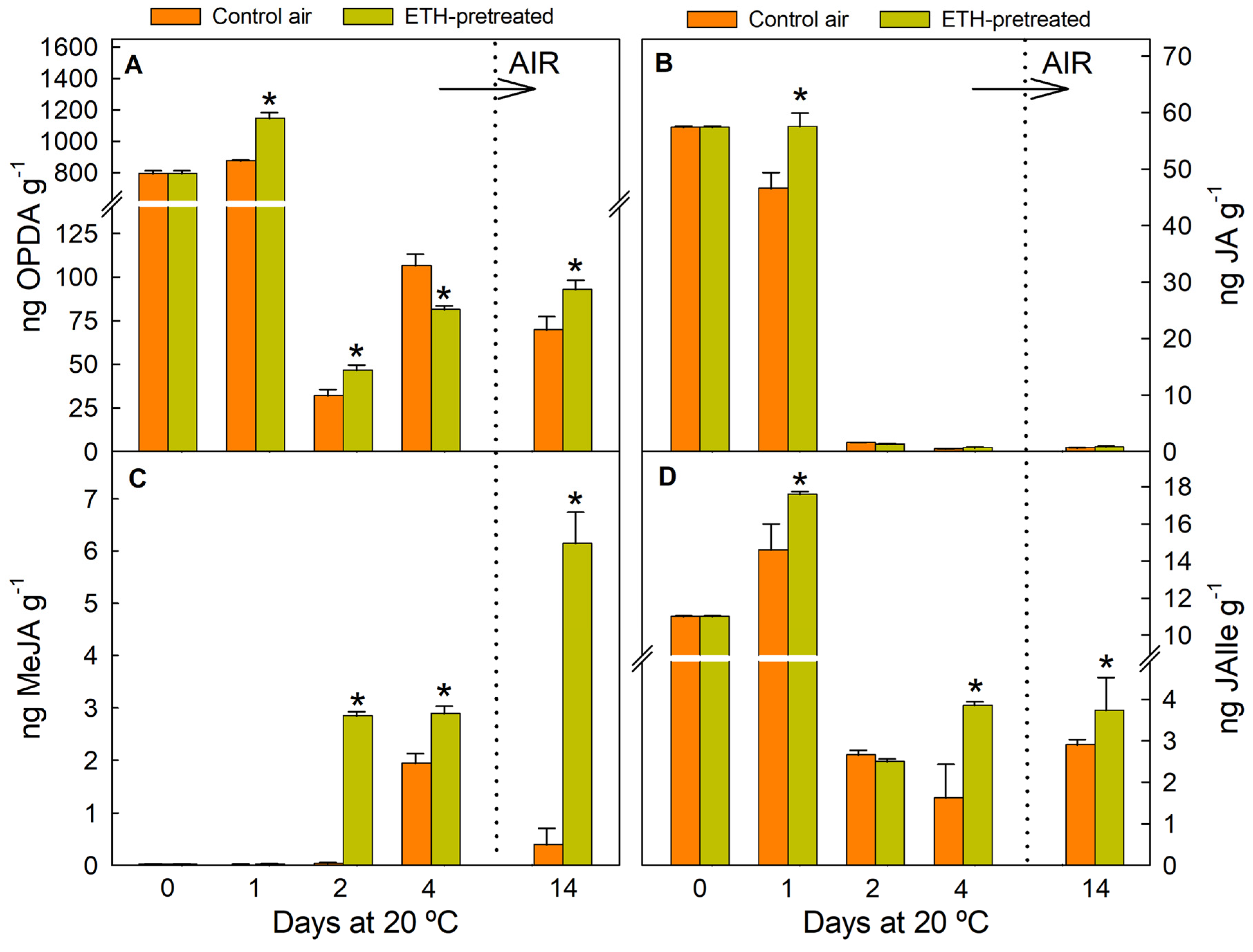
2.3. ETH Pretreatment and Nutrient Deprivation Modify the JA Levels in Detached Citrus Fruit
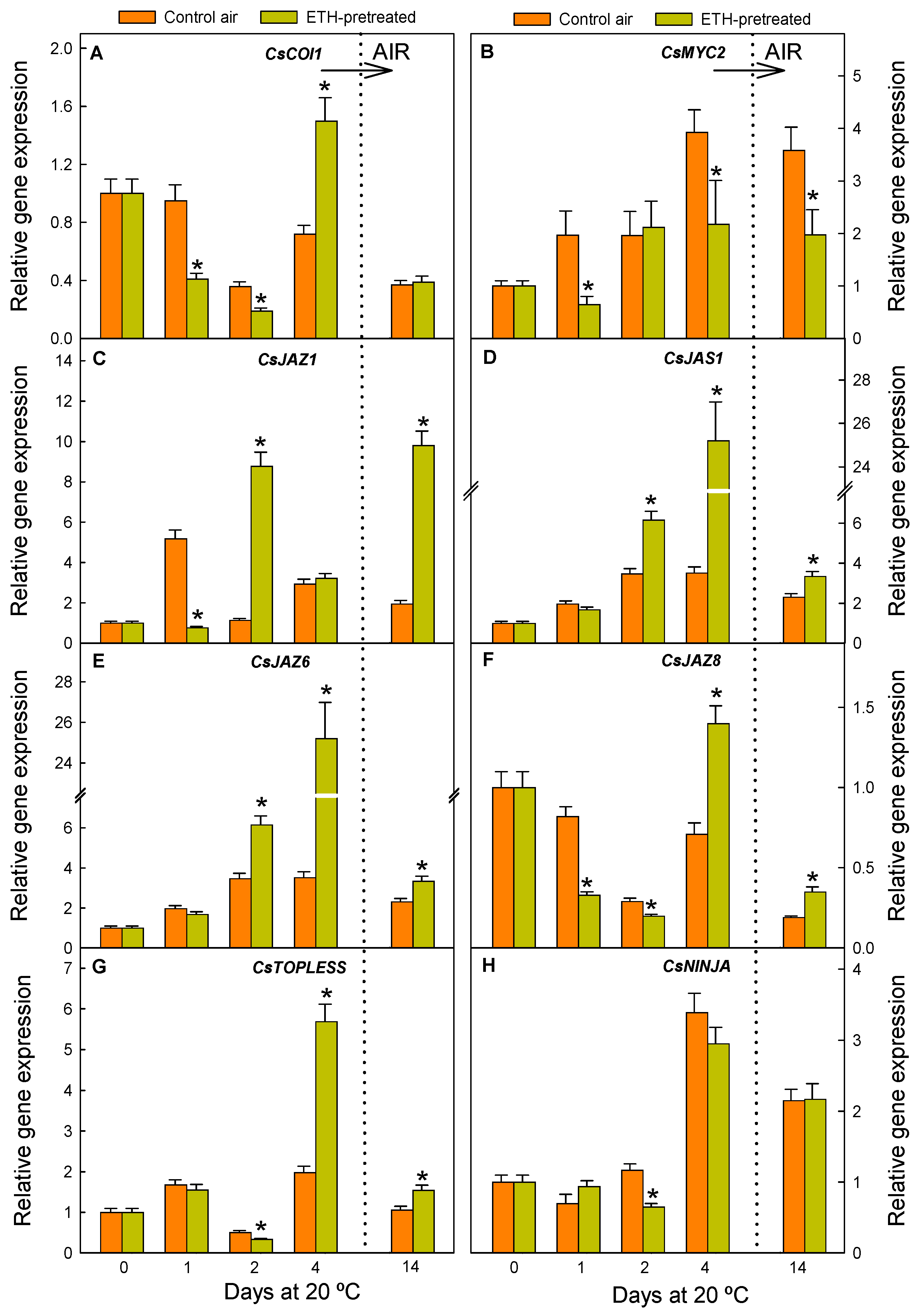
2.4. JA Perception and Signalling Is Affected by the ETH Pretreatment and Nutrient Deprivation in Citrus Fruit
3. Discussion
4. Materials and Methods
4.1. Fruit Material and Postharvest Treatments
4.2. Evaluation of NCPP Damage
4.3. Analysis of Jasmonate-Related Metabolites
4.4. Isolation of Total RNA and Synthesis of cDNA
4.5. Gene-Specific Primer Pairs and RT-qPCR Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACOT | Acyl-CoA thioesterase |
| ACX | Acyl-coenzyme A oxidase |
| AOC | Allene oxide cyclase |
| AOS | Allene oxide synthase |
| COI1 | Coronatine insensitive 1 |
| CYP94B3 | Cytochrome P450 94B3 |
| CYP94C1 | Cytochrome P450 94C1 |
| ETH | Ethylene |
| JAIle | Jasmonoyl–isoleucine |
| JA | Jasmonic acid |
| JAR1 | Jasmonoyl–isoleucine synthetase 1 |
| JAs | Jasmonates |
| JAZ | Jasmonate-zim domain |
| JMT | Jasmonic acid carboxyl methyltransferase |
| KAT | L-3-ketoacyl CoA thiolase |
| LOX | Lipoxygenase |
| MeJA | Methyl jasmonate |
| NCPP | Non-chilling peel pitting |
| NINJA | Novel interactor of JAZ |
| OPDA | cis-(+)-12-oxo-phytodienoic acid |
| OPR | 12-oxo-phytodienoic acid reductase |
| ST2A | Sulfotransferase 2A |
References
- Figueroa, C.R.; Jiang, C.Z.; Torres, C.A.; Fortes, A.M.; Alkan, N. Editorial: Regulation of fruit ripening and senescence. Front. Plant Sci. 2021, 12, 711458. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Shi, X.C.; Liu, F.Q.; Laborda, P. Effects of exogenous methyl jasmonate on quality and preservation of postharvest fruits: A review. Food Chem. 2021, 353, 129482. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.T.; Romero, P. Hormone profiling and heat-induced tolerance to cold stress in citrus fruit. Postharvest Biol. Technol. 2022, 194, 112088. [Google Scholar] [CrossRef]
- Alférez, F.; Agusti, M.; Zacarías, L. Postharvest rind staining in Navel oranges is aggravated by changes in storage relative humidity: Effect on respiration, ethylene production and water potential. Postharvest Biol. Technol. 2003, 28, 143–152. [Google Scholar] [CrossRef]
- Alquezar, B.; Mesejo, C.; Alferez, F.; Agustí, M.; Zacarias, L. Morphological and ultrastructural changes in peel of ‘Navelate’ oranges in relation to variations in relative humidity during postharvest storage and development of peel pitting. Postharvest Biol. Technol. 2010, 56, 163–170. [Google Scholar] [CrossRef]
- Romero, P.; Alférez, F.; Establés-Ortiz, B.; Lafuente, M.T. Insights into the regulation of molecular mechanisms involved in energy shortage in detached citrus fruit. Sci. Rep. 2020, 10, 1109. [Google Scholar] [CrossRef]
- Establés-Ortiz, B.; Romero, P.; Ballester, A.R.; González-Candelas, L.; Lafuente, M.T. Inhibiting ethylene perception with 1-methylcyclopropene triggers molecular responses aimed to cope with cell toxicity and increased respiration in citrus fruits. Plant Physiol. Biochem. 2016, 103, 154–166. [Google Scholar] [CrossRef]
- Riov, J.; Yang, S.F. Autoinhibition of ethylene production in citrus peel discs: Suppression of 1-aminocyclopropane-1-carboxylic acid synthesis. Plant Physiol. 1982, 69, 687–690. [Google Scholar] [CrossRef]
- Holland, N.; Nunes, F.L.S.; de Medeiros, I.U.D.; Lafuente, M.T. High-temperature conditioning induces chilling tolerance in mandarin fruit: A cell wall approach. J. Sci. Food Agric. 2012, 92, 3039–3045. [Google Scholar] [CrossRef]
- McCollum, G.; Maul, P. 1-Methylcyclopropene inhibits degreening but stimulates respiration and ethylene biosynthesis in grapefruit. HortScience 2007, 42, 120–124. [Google Scholar] [CrossRef]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Li, F.; Ali, M.; Zhang, X.; Liu, Y. Application of methyl jasmonate to control disease of postharvest fruit and vegetables: A Meta-analysis. Postharvest Biol. Technol. 2024, 208, 112667. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Fortiz, J.; Cruz, R.; Baez, R.; Wang, C.Y. Methyl jasmonate reduces chilling injury and maintains postharvest quality of mango fruit. J. Agric. Food Chem. 2000, 48, 515–519. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Tiznado-Hernández, M.E.; Zavaleta-Gatica, R.; Martínez-Téllez, M.A. Methyl jasmonate treatments reduce chilling injury and activate the defense response of guava fruits. Biochem. Biophys. Res. Commun. 2004, 313, 694–701. [Google Scholar] [CrossRef]
- Siboza, X.I.; Bertling, I.; Odindo, A.O. Salicylic acid and methyl jasmonate improve chilling tolerance in cold-stored lemon fruit (Citrus Limon). J. Plant Physiol. 2014, 171, 1722–1731. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Rahemi, M.; Eshghi, S.; Guillén, F.; Serrano, M.; Valero, D. Postharvest treatments with γ-aminobutyric acid, methyl jasmonate, or methyl salicylate enhance chilling tolerance of blood orange fruit at prolonged cold storage. J. Sci. Food Agric. 2019, 99, 6408–6417. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Valverde, J.M.; García-Pastor, M.E.; Valero, D.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M. Pre-harvest methyl jasmonate treatments increase antioxidant systems in lemon fruit without affecting yield or other fruit quality parameters. J. Sci. Food Agric. 2019, 99, 5035–5043. [Google Scholar] [CrossRef]
- Nham, N.T.; Macnish, A.J.; Zakharov, F.; Mitcham, E.J. ‘Bartlett’ pear fruit (Pyrus communis L.) ripening regulation by low temperatures involves genes associated with jasmonic acid, cold response, and transcription factors. Plant Sci. 2017, 260, 8–18. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Lindo-García, V.; Muñoz, P.; Larrigaudière, C.; Munné-Bosch, S.; Giné-Bordonaba, J. Interplay between hormones and assimilates during pear development and ripening and its relationship with the fruit postharvest behaviour. Plant Sci. 2020, 291, 110339. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Tomiyama, H.; Opio, P.; Komiya, R.; Saito, T.; Okawa, K.; Ohara, H.; Matsuura, H.; Lang, Y.; Igarashi, D. L-Isoleucine (Ile) promotes anthocyanin accumulation in apples. J. Plant Growth Regul. 2021, 40, 541–549. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, H.; Han, Z.; Wang, S.; Wang, T.; Li, Q.; Tian, J.; Wang, Y.; Zhang, X.; Xu, X.; et al. ERF4 affects fruit ripening by acting as a JAZ interactor between ethylene and jasmonic acid hormone signaling pathways. Hortic. Plant J. 2022, 8, 689–699. [Google Scholar] [CrossRef]
- Li, H.; Ai, Y.; Zeng, K.; Deng, L. The response of Midknight Valencia oranges to ethephon degreening varies in the turning and regreening stages. J. Sci. Food Agric. 2024, 104, 9569–9580. [Google Scholar] [CrossRef]
- Fujii, H.; Shimada, T.; Sugiyama, A.; Nishikawa, F.; Endo, T.; Nakano, M.; Ikoma, Y.; Shimizu, T.; Omura, M. Profiling ethylene-responsive genes in mature mandarin fruit using a citrus 22K oligoarray. Plant Sci. 2007, 173, 340–348. [Google Scholar] [CrossRef]
- Nardozza, S.; Cooney, J.; Boldingh, H.L.; Hewitt, K.G.; Trower, T.; Jones, D.; Thrimawithana, A.H.; Allan, A.C.; Richardson, A.C. Phytohormone and transcriptomic analysis reveals endogenous cytokinins affect kiwifruit growth under restricted carbon supply. Metabolites 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Contento, A.L.; Bassham, D.C. Increase in catalase-3 activity as a response to use of alternative catabolic substrates during sucrose starvation. Plant Phys. Biochem. 2010, 48, 232–238. [Google Scholar] [CrossRef]
- Guo, Q.; Yoshida, Y.; Major, I.T.; Wang, K.; Sugimoto, K.; Kapali, G.; Havko, N.E.; Benning, C.; Howe, G.A. JAZ repressors of metabolic defense promote growth and reproductive fitness in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E10768–E10777. [Google Scholar] [CrossRef]
- Kondo, S. Usage and action mechanism of oxylipins including jasmonic acid on physiological aspects of fruit production. Sci. Hortic. 2022, 295, 110893. [Google Scholar] [CrossRef]
- Taki, N.; Sasaki-Sekimoto, Y.; Obayashi, T.; Kikuta, A.; Kobayashi, K.; Ainai, T.; Yagi, K.; Sakurai, N.; Suzuki, H.; Masuda, T.; et al. 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 2005, 139, 1268–1283. [Google Scholar] [CrossRef]
- Hazman, M.; Hause, B.; Eiche, E.; Nick, P.; Riemann, M. Increased tolerance to salt stress in OPDA-deficient rice ALLENE OXIDE CYCLASE mutants is linked to an increased ROS-scavenging activity. J. Exp. Bot. 2015, 66, 3339–3352. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Strnad, M. Jasmonate signaling in plant stress responses and development—active and inactive compounds. N. Biotechnol. 2016, 33, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.J.K.; Howe, G.A. Catabolism and deactivation of the lipid-derived hormone jasmonoyl-isoleucine. Front. Plant Sci. 2012, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, K.; Bowen, J.; Allan, A.; Espley, R.; Karunairetnam, S.; Ferguson, I. Differential expression within the LOX gene family in ripening kiwifruit. J. Exp. Bot. 2006, 57, 3825–3836. [Google Scholar] [CrossRef]
- Torrigiani, P.; Fregola, F.; Ziosi, V.; Ruiz, K.B.; Kondo, S.; Costa, G. Differential expression of allene oxide synthase (AOS), and jasmonate relationship with ethylene biosynthesis in seed and mesocarp of developing peach fruit. Postharvest Biol. Technol. 2012, 63, 67–73. [Google Scholar] [CrossRef]
- Dourou, A.M.; Brizzolara, S.; Famiani, F.; Tonutti, P. Changes in volatile organic composition of olive oil extracted from cv. ‘Leccino’ fruit subjected to ethylene treatments at different ripening stages. J. Sci. Food Agric. 2021, 101, 3981–3986. [Google Scholar] [CrossRef]
- Yang, X.; Song, J.; Du, L.; Forney, C.; Campbell-Palmer, L.; Fillmore, S.; Wismer, P.; Zhang, Z. Ethylene and 1-MCP regulate major volatile biosynthetic pathways in apple fruit. Food Chem. 2016, 194, 325–336. [Google Scholar] [CrossRef]
- Lv, J.; Ge, Y.; Li, C.; Zhang, M.; Li, J. Identification and analysis of genes involved in the jasmonate pathway in response to ethephon and 1-methylcyclopropene during the ripening of apple fruit. J. Am. Soc. Hortic. Sci. 2017, 142, 184–191. [Google Scholar] [CrossRef]
- Yang, X.Y.; Jiang, W.J.; Yu, H.J. The expression profiling of the lipoxygenase (LOX) family genes during fruit development, abiotic stress and hormonal treatments in cucumber (Cucumis sativus L.). Int. J. Mol. Sci. 2012, 13, 2481–2500. [Google Scholar] [CrossRef]
- Lafuente, M.T.; Sampedro, R.; Romero, P. Transcriptional regulation of jasmonate metabolism and signaling by thermal stress and heat-induced chilling tolerance in ‘Fortune’ mandarin. Postharvest Biol. Technol. 2023, 203, 112399. [Google Scholar] [CrossRef]
- Fabi, J.P.; Mendes, L.R.B.C.; Lajolo, F.M.; do Nascimento, J.R.O. Transcript profiling of papaya fruit reveals differentially expressed genes associated with fruit ripening. Plant Sci. 2010, 179, 225–233. [Google Scholar] [CrossRef]
- Guang, Y.; Luo, S.; Ahammed, G.J.; Xiao, X.; Li, J.; Zhou, Y.; Yang, Y. The OPR gene family in watermelon: Genome-wide identification and expression profiling under hormone treatments and root-knot nematode infection. Plant Biol. 2021, 23, 80–88. [Google Scholar] [CrossRef]
- Fernández-Milmanda, G.L.; Crocco, C.D.; Reichelt, M.; Mazza, C.A.; Köllner, T.G.; Zhang, T.; Cargnel, M.D.; Lichy, M.Z.; Fiorucci, A.S.; Fankhauser, C.; et al. A light-dependent molecular link between competition cues and defence responses in plants. Nat. Plants 2020, 6, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Herrfurth, C.; Xin, M.; Savchenko, T.; Feussner, I.; Goossens, A.; De Smet, I. Warm temperature triggers JOX and ST2A-mediated jasmonate catabolism to promote plant growth. Nat. Commun. 2021, 12, 4804. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, F.; Krause, F.; Papenbrock, J. The multi-protein family of sulfotransferases in plants: Composition, occurrence, substrate specificity, and functions. Front. Plant Sci. 2014, 5, 556. [Google Scholar] [CrossRef]
- Kandel, S.; Sauveplane, V.; Compagnon, V.; Franke, R.; Millet, Y.; Schreiber, L.; Werck-Reichhart, D.; Pinot, F. Characterization of a methyl jasmonate and wounding-responsive cytochrome P450 of Arabidopsis thaliana catalyzing dicarboxylic fatty acid formation In Vitro. FEBS J. 2007, 274, 5116–5127. [Google Scholar] [CrossRef]
- Ziosi, V.; Bonghi, C.; Bregoli, A.M.; Trainotti, L.; Biondi, S.; Sutthiwal, S.; Kondo, S.; Costa, G.; Torrigiani, P. Jasmonate-induced transcriptional changes suggest a negative interference with the ripening syndrome in peach fruit. J. Exp. Bot. 2008, 59, 563–573. [Google Scholar] [CrossRef]
- Concha, C.M.; Figueroa, N.E.; Poblete, L.A.; Oñate, F.A.; Schwab, W.; Figueroa, C.R. Methyl jasmonate treatment induces changes in fruit ripening by modifying the expression of several ripening genes in Fragaria chiloensis fruit. Plant Physiol. Biochem. 2013, 70, 433–444. [Google Scholar] [CrossRef]
- Luo, D.; Huang, T.; Kou, X.; Zhang, Y.; Ba, L.; Wang, X.; Cao, S. MeJA enhances antioxidant activity and reduces membrane lipid degradation by maintaining energy charge levels in crystal grapes. Postharvest Biol. Technol. 2024, 216, 113078. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Trainotti, L.; Bonghi, C.; Ziosi, V.; Costa, G.; Torrigiani, P. Early methyl jasmonate application to peach delays fruit/seed development by altering the expression of multiple hormone-related genes. J. Plant Growth Regul. 2013, 32, 852–864. [Google Scholar] [CrossRef]
- Yu, W.; Yu, M.; Zhao, R.; Sheng, J.; Li, Y.; Shen, L. Ethylene perception is associated with methyl-jasmonate-mediated immune response against Botrytis cinerea in tomato fruit. J. Agric. Food Chem. 2019, 67, 6725–6735. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.; Li, Z.; Min, D.; Zhang, X.; Ai, W.; Li, J.; Zhou, J.; Li, Z.; Li, F.; Li, X. CRISPR/Cas9-mediated SlMYC2 mutagenesis adverse to tomato plant growth and MeJA-induced fruit resistance to Botrytis cinerea. J. Agric. Food Chem. 2020, 68, 5529–5538. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Yang, X.; Wang, L.; Kebbeh, M.; Wang, Y.; Dai, B.; Shen, S.; Zheng, X.; Zhou, H. Methyl jasmonate treatment regulates α-linolenic acid metabolism and jasmonate acid signaling pathway to improve chilling tolerance in both stony hard and melting flesh peaches. Postharvest Biol. Technol. 2022, 190, 111960. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, H.; Xu, X.; Yu, Z. Postharvest application of methyl jasmonate inhibited ethylene biosynthesis and signaling in peach through activating the negative feedback of JA-signaling pathway. Postharvest Biol. Technol. 2024, 213, 112965. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Li, G.; Zhou, X.; Fu, W.; Jiang, Y.; Liu, T.; Ji, S. Exogenous ATP alleviated aroma fading by regulating LOX pathway and fatty acids synthesis in ‘Nanguo’ pears after refrigeration. Sci. Hortic. 2018, 240, 522–529. [Google Scholar] [CrossRef]
- Siboza, X.I.; Bertling, I.; Odindo, A.O. Enzymatic antioxidants in response to methyl jasmonate and salicylic acid and their effect on chilling tolerance in lemon fruit [Citrus limon (L.) Burm. F.]. Sci. Hortic. 2017, 225, 659–667. [Google Scholar] [CrossRef]
- Flors, V.; Ton, J.; Van Doorn, R.; Jakab, G.; García-Agustín, P.; Mauch-Mani, B. Interplay between JA, SA and ABA signalling during basal and induced resistance against Pseudomonas syringae and Alternaria brassicicola. Plant J. 2008, 54, 81–92. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lafuente, M.T.; Sampedro, R.; Romero, P. Jasmonates in the Ethylene-Induced Resistance of Detached Citrus Fruits to Peel Damage. Int. J. Mol. Sci. 2025, 26, 4805. https://doi.org/10.3390/ijms26104805
Lafuente MT, Sampedro R, Romero P. Jasmonates in the Ethylene-Induced Resistance of Detached Citrus Fruits to Peel Damage. International Journal of Molecular Sciences. 2025; 26(10):4805. https://doi.org/10.3390/ijms26104805
Chicago/Turabian StyleLafuente, María T., Raúl Sampedro, and Paco Romero. 2025. "Jasmonates in the Ethylene-Induced Resistance of Detached Citrus Fruits to Peel Damage" International Journal of Molecular Sciences 26, no. 10: 4805. https://doi.org/10.3390/ijms26104805
APA StyleLafuente, M. T., Sampedro, R., & Romero, P. (2025). Jasmonates in the Ethylene-Induced Resistance of Detached Citrus Fruits to Peel Damage. International Journal of Molecular Sciences, 26(10), 4805. https://doi.org/10.3390/ijms26104805





