Additive Value of Rheumatoid Factor Isotypes in Sjögren’s Syndrome Patients with Joint Complaints of Different Etiologies—Can Rheumatoid Factor IgA Serve as an Early, Poor Prognostic Biomarker Candidate?
Abstract
:1. Introduction
2. Results
2.1. Demographics
2.2. Laboratory Parameters
2.2.1. Basic Laboratory Parameters
2.2.2. Rheumatoid Factor Isotypes
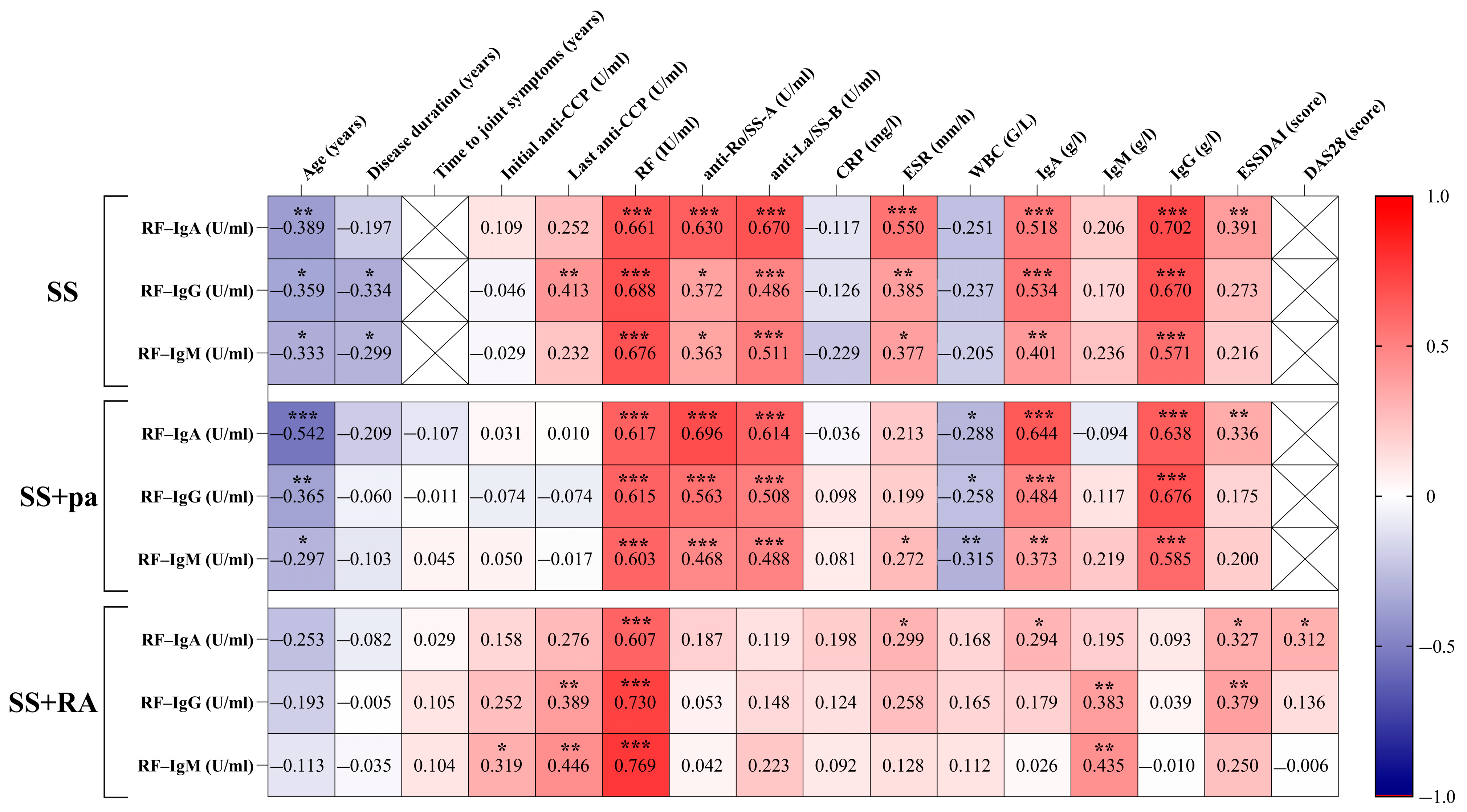
2.2.3. Correlations Between Patient Groups, Rheumatoid Factor Isotypes, and Disease-Specific Parameters
2.2.4. Differences Among Patient Groups According to the Negative or Positive RF Isotype Results
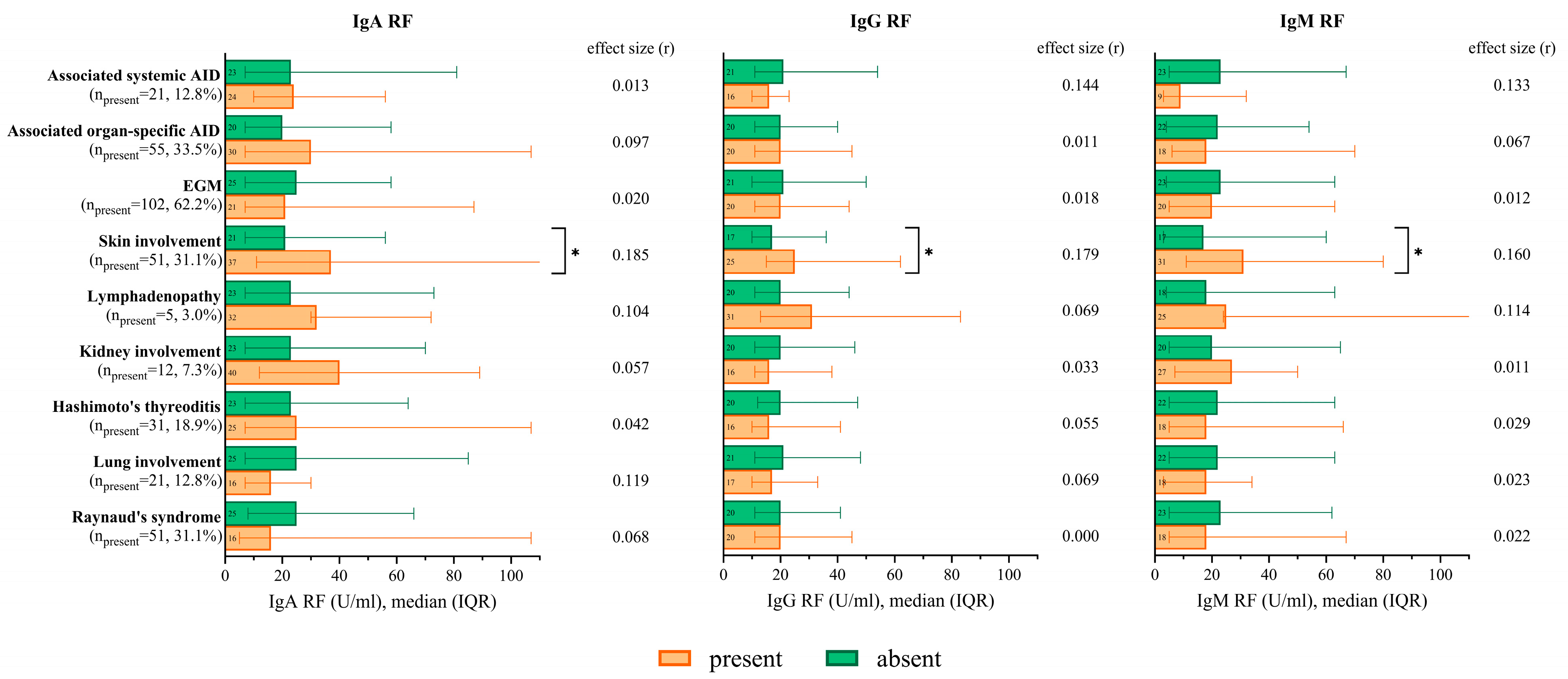
2.2.5. RF Isotype Levels According to the Occurrence of Extraglandular Manifestations and Associated Autoimmune Diseases
2.2.6. RF Isotypes in the Case of Low/Normal or Elevated Immunoglobulin Levels
2.2.7. Combination of Immunoglobulin and RF Isotype Qualitative Results and Their Occurrence in Patient Groups
3. Discussion
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AID | Autoimmune disease |
| ANA | Antinuclear antibody |
| CCP | Cyclic citrullinated peptide |
| DAS28 | Disease activity score |
| EGM | Extraglandular manifestation |
| ELISA | Enzyme-linked immunosorbent assay |
| ESR | Erythrocyte sedimentation rate |
| ESSDAI | EULAR Sjögren’s syndrome disease activity index |
| EULAR | European Alliance of Associations for Rheumatology |
| HLA | Human leukocyte antigen |
| Ig | Immunoglobulin |
| IQR | Interquartile range |
| MRI | Magnetic resonance imaging |
| pa | Polyarthritis |
| RA | Rheumatoid arthritis |
| RF | Rheumatoid factor |
| SD | Standard deviation |
| SS | Sjögren’s syndrome |
References
- Negrini, S.; Emmi, G.; Greco, M.; Borro, M.; Sardanelli, F.; Murdaca, G.; Indiveri, F.; Puppo, M. Sjögren’s syndrome: A systemic autoimmune disease. Clin. Exp. Med. 2022, 22, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Fauchais, A.L.; Ouattara, B.; Gondran, G.; Lalloué, F.; Petit, D.; Ly, K.; Lambert, M.; Launay, D.; Loustaud-Ratti, V.; Bezanahari, H.; et al. Articular manifestations in primary Sjögren’s syndrome: Clinical signifcance and prognosis of 188 patients. Rheumatology 2010, 49, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Aradi, Z.; Nagy, G.; Horváth, I.F.; Antal-Szalmás, P.; Szántó, A. Polyarthritis in Sjögren’s Syndrome: Difficulties in Distinguishing Extraglandular Manifestation and Associated Rheumatoid Arthritis. Diagnostics 2024, 14, 1494. [Google Scholar] [CrossRef]
- Tiwari, V.; Jandu, J.S.; Bergman, M.J. Rheumatoid Factor. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ingegnoli, F.; Castelli, R.; Gualtierotti, R. Rheumatoid Factors: Clinical Applications. Dis. Markers 2013, 35, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Fiona, A.; Barbara, C.B. Sjögren’s syndrome. J. Dtsch. Dermatol. Gesellschaft 2022, 20, 980–1002. [Google Scholar] [CrossRef]
- Murdaca, G.; Tagliafico, L.; Page, E.; Paladin, F.; Gangemi, S. Gender Differences in the Interplay between Vitamin D and Microbiota in Allergic and Autoimmune Diseases. Biomedicines 2024, 12, 1023. [Google Scholar] [CrossRef]
- Murdaca, G.; Allegra, A.; Tonacci, A.; Musolino, C.; Ricciardi, L.; Gangemi, S. Mast Cells and Vitamin D Status: A Clinical and Bilological Link in the Onset of Allergy and Bone Diseases. Biomedicines 2022, 10, 1877. [Google Scholar] [CrossRef]
- Contini, P.; Murdaca, G.; Puppo, F.; Negrini, S. HLA-G Expressing Immune Cells in Immune Mediated Diseases. Front. Immunol. 2020, 11, 1613. [Google Scholar] [CrossRef]
- Nocturne, G.; Mariette, X. B cells in the pathogenesis of primary Sjögren syndrome. Nat. Rev. Rheumatol. 2018, 14, 133–145. [Google Scholar] [CrossRef]
- Meek, B.; Kelder, J.C.; Claessen, A.M.E.; Jan van Houte, A.; Borg, E.J.T. Rheumatoid factor isotype and Ro epitope distribution in primary Sjögren syndrome and rheumatoid arthritis with keratoconjunctivitis sicca. Rheum. Int. 2018, 38, 1487–1493. [Google Scholar] [CrossRef]
- Lee, K.A.; Kim, K.W.; Kim, B.M.; Won, J.Y.; Kim, H.A.; Moon, H.W.; Kim, H.R.; Lee, S.H. Clinical and diagnostic significance of serum immunoglobulin A rheumatoid factor in primary Sjogren’s syndrome. Clin. Oral. Investig. 2019, 23, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Maślińska, M.; Mańczak, M.; Kwiatkowska, B.; Ramsperger, V.; Shen, L.; Suresh, L. IgA immunoglobulin isotype of rheumatoid factor in primary Sjögren’s syndrome. Rheum. Int. 2021, 41, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Hermosillo-Villafranca, J.A.; Guillén-Lozoya, A.H.; Vega-Morales, D.; Pérez-Onofrea, I.; Gracia-Aréchiga, T.S.; Garza-Elizondo, M.A.; Garza-Alpirez, A.; Rodriguez-Rodriguez, D.R. Role of rheumatoid factor isotypes and anti-citrullinated peptide antibodies in the differential diagnosis of non-selected patients with inflammatory arthralgia. Reumatol. Clin. 2021, 17, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Jónsson, T.; Steinsson, K.; Jónsson, H.; Geirsson, Á.J.; Thorsteinsson, J.; Valdimarsson, H. Combined elevation of IgM and IgA rheumatoid factor has high diagnostic specificity for rheumatoid arthritis. Rheum. Int. 1998, 18, 119–122. [Google Scholar] [CrossRef]
- Van Hoovels, L.; Cruyssen, B.V.; Sieghart, D.; Bonroy, C.; Nagy, E.; Pullerits, R.; Čučnik, S.; Dahle, C.; Heijnen, I.; Bernasconi, L.; et al. IgA rheumatoid factor in rheumatoid arthritis. Clin. Chem. Lab. Med. 2022, 60, 1617–1626. [Google Scholar] [CrossRef]
- Steiner, G.; Toes, R.E.M. Autoantibodies in rheumatoid arthritis—Rheumatoid factor, anticitrullinated protein antibodies and beyond. Curr. Opin. Rheumatol. 2024, 36, 217–224. [Google Scholar] [CrossRef]
- Seror, R.; Bowman, S.; Brito-Zerón, P. EULAR Sjögren’s syndrome disease activity index (ESSDAI): A user guide. RMD Open 2015, 1, e000022. [Google Scholar] [CrossRef]
- van Riel, P.M.; Renskers, L. The Disease Activity Score (DAS) and the Disease Activity Score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin. Exp. Rheumatol. 2016, 34 (Suppl. 101), S40–S44. [Google Scholar]
- Alghamdi, M.A.; Bahlas, S.M.; Alamry, S.A.; Mattar, E.H.; Redwan, E.M. Exploring Anticitrullinated Antibodies (ACPAs) and Serum-Derived Exosomes Cargoes. Antibodies 2025, 14, 10. [Google Scholar] [CrossRef]
- Bodakci, E. Clinical Characteristics of Distinct Supbgroups of Patients with Primary Sjögren’s Syndrome Classified by Serological Profiles: A Comparison Study. J. Pers. Med. 2024, 14, 967. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]


| SS | SS+pa | SS+RA | p-Value | Effect Size | |
|---|---|---|---|---|---|
| Patients (n = 164) | 45 (27.4%) [20.8–34.9%] | 73 (44.5%) [36.8–52.5%] | 46 (28%) [21.3–35.6%] | ||
| Women | 42 (93.3%) | 71 (97.3%) | 45 (97.8%) | 0.445 | V = 0.099 |
| Age (years) | 58.9 ± 14.1 | 60 ± 10.1 | 63.3 ± 10.8 | 0.156 | η2 = 0.023 |
| Disease duration (years) | 11 (9–15) | 11 (8–19) | 13 (10–20) | 0.094 | η2 = 0.017 |
| IgA (g/L) | 2.1 (1.6–3) | 2.4 (1.7–3.2) | 2.2 (1.7–3.2) | 0.874 | η2 = 0.011 |
| IgG (g/L) | 13.3 (10.3–19.2) 3 | 11.9 (9.4–17.3) 3 | 10.5 (8.5–11.6) 1,2 | 0.004 | η2 = 0.055 |
| IgM (g/L) | 0.9 (0.7–1.6) | 1.1 (0.8–1.9) | 1 (0.7–1.3) | 0.156 | η2 = 0.011 |
| CRP (mg/L) | 2 (0.9–5.3) | 2 (1.1–3.9) | 2.4 (1.2–5.6) | 0.430 | η2 = 0.002 |
| RF (IU/mL) | 26 (14–47) | 23 (13–40) | 28 (12–89) | 0.437 | η2 = 0.002 |
| IgA RF | IgG RF | IgM RF | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive (n = 89) | Negative (n = 75) | p | Effect Size | Positive (n = 83) | Negative (n = 81) | p | Effect Size | Positive (n = 83) | Negative (n = 81) | p | Effect Size | |
| Patient groups | ||||||||||||
| SS | 29 (32.6) | 16 (21.3) | 0.108 | 0.126 a | 25 (30.1) | 20 (24.7) | 0.436 | 0.061 a | 25 (30.1) | 20 (24.7) | 0.436 | 0.061 a |
| SS+pa | 35 (39.3) | 38 (50.7) | 0.145 | 0.114 a | 33 (39.8) | 40 (49.4) | 0.215 | 0.097 a | 33 (39.8) | 40 (49.4) | 0.215 | 0.097 a |
| SS+RA | 25 (28.1) | 21 (28) | 0.990 | 0.001 a | 25 (30.1) | 21 (25.9) | 0.550 | 0.047 a | 25 (30.1) | 21 (25.9) | 0.550 | 0.047 a |
| Demographics | ||||||||||||
| Age (years) | 56.8 ± 11.9 | 65.2 ± 9.3 | <0.001 | 0.775 b | 57.4 ± 12.7 | 63.9 ± 9.3 | <0.001 | 0.580 b | 58 ± 12.4 | 63.2 ± 10.1 | 0.004 | 0.457 b |
| Disease duration (years) | 11 (8–15) | 13 (10–21) | 0.013 | 0.193 c | 13 (9–20) | 13 (9–20) | 0.217 | 0.096 c | 11 (8–18) | 12 (9–19) | 0.349 | 0.073 c |
| Disease-specific data | ||||||||||||
| Time to joint symptoms (years) | 0 (−1–2) | 0 (0–2) | 0.465 | 0.067 c | 0 (−0.5–2) | 0 (−0.5–2) | 0.738 | 0.031 c | 0 (−1–2) | 0 (−1–2) | 0.952 | 0.006 c |
| DAS28 (score) | 3 (2–3) | 2 (2–3) | 0.051 | 0.288 c | 2 (2–3) | 3 (2–3) | 0.843 | 0.029 c | 2 (2–3) | 3 (2–3) | 0.869 | 0.024 c |
| ESSDAI (score) | 2 (1–4) | 1 (0–2) | <0.001 | 0.272 c | 2 (1–4) | 1 (0–2) | 0.005 | 0.222 c | 2 (1–4) | 1 (0–2) | 0.028 | 0.171 c |
| Laboratory parameters | ||||||||||||
| Initial anti-CCP (+) | 23 (26.1) | 8 (10.7) | 0.012 | 0.196 a | 21 (25.6) | 10 (12.3) | 0.031 | 0.169 a | 22 (26.8) | 9 (11.1) | 0.011 | 0.200 a |
| Last anti-CCP (+) | 21 (23.6) | 11 (14.7) | 0.151 | 0.112 a | 22 (26.5) | 10 (12.3) | 0.022 | 0.179 a | 22 (26.5) | 10 (12.3) | 0.022 | 0.179 a |
| RF (+) | 82 (92.1) | 38 (50.7) | <0.001 | 0.466 a | 75 (90.4) | 45 (55.6) | <0.001 | 0.393 a | 78 (94) | 42 (51.9) | <0.001 | 0.475 a |
| anti-Ro/SS-A (+) | 70 (78.7) | 22 (29.3) | <0.001 | 0.495 a | 54 (65.1) | 38 (46.9) | 0.019 | 0.183 a | 55 (66.3) | 37 (45.7) | 0.008 | 0.207 a |
| anti-La/SS-B (+) | 55 (61.8) | 11 (14.7) | <0.001 | 0.479 a | 45 (54.2) | 21 (25.9) | <0.001 | 0.288 a | 49 (59) | 17 (21) | <0.001 | 0.388 a |
| ANA (+) | 80 (89.9) | 37 (49.3) | <0.001 | 0.447 a | 66 (79.5) | 51 (63) | 0.019 | 0.183 a | 69 (83.1) | 48 (59.3) | 0.001 | 0.264 a |
| CRP (high) | 26 (29.2) | 16 (21.3) | 0.249 | 0.090 a | 23 (27.7) | 19 (23.5) | 0.533 | 0.049 a | 23 (27.7) | 19 (23.5) | 0.533 | 0.049 a |
| ESR (high) | 44 (49.4) | 20 (26.7) | 0.003 | 0.233 a | 45 (54.2) | 19 (23.5) | <0.001 | 0.315 a | 44 (53) | 20 (24.7) | <0.001 | 0.290 a |
| WBC (normal) | 54 (60.7) | 54 (72) | 0.128 | 0.119 a | 50 (60.2) | 58 (71.6) | 0.125 | 0.120 a | 51 (61.4) | 57 (70.4) | 0.228 | 0.094 a |
| IgA (g/L) | 2.8 (2.0–3.6) | 1.9 (1.4–2.5) | <0.001 | 0.345 c | 2.8 (2.0–3.5) | 1.9 (1.4–2.5) | <0.001 | 0.316 c | 2.7 (1.9–3.5) | 1.9 (1.4–2.6) | <0.001 | 0.276 c |
| IgG (g/L) | 14.7 (11.1–19.5) | 10.3 (8.7–11.8) | <0.001 | 0.426 c | 16.2 (10.9–19.7) | 10.3 (8.5–11.8) | <0.001 | 0.454 c | 14.4 (10.7–19.7) | 10.4 (8.5–12) | <0.001 | 0.394 c |
| IgM (g/L) | 1.09 (0.7–1.54) | 0.97 (0.65–1.82) | 0.784 | 0.021 c | 1.14 (0.9–1.63) | 0.87 (0.63–1.54) | 0.008 | 0.207 c | 1.14 (0.88–1.84) | 0.87 (0.58–1.47) | 0.003 | 0.232 c |
| IgA RF | IgG RF | IgM RF | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive (n = 89) | Negative (n = 75) | p | Effect Size (V) | Positive (n = 83) | Negative (n = 81) | p | Effect Size (V) | Positive (n = 83) | Negative (n = 81) | p | Effect Size (V) | |
| Methylprednisolone | 48 (53.9) | 31 (41.3) | 0.108 | 0.126 | 42 (50.6) | 37 (45.7) | 0.528 | 0.049 | 42 (50.6) | 37 (45.7) | 0.528 | 0.049 |
| Methotrexate | 24 (27) | 15 (20) | 0.297 | 0.082 | 21 (25.3) | 18 (22.2) | 0.643 | 0.036 | 20 (24.1) | 19 (23.5) | 0.923 | 0.008 |
| Leflunomide | 11 (12.4) | 7 (9.3) | 0.537 | 0.048 | 11 (13.3) | 7 (8.6) | 0.345 | 0.074 | 10 (12) | 8 (9.9) | 0.656 | 0.035 |
| Sulfasalazine | 2 (2.2) | 7 (9.3) | 0.081 | 0.155 | 1 (1.2) | 8 (9.9) | 0.017 | 0.190 | 2 (2.4) | 7 (8.6) | 0.097 | 0.137 |
| Azathioprine | 4 (4.5) | 4 (5.3) | 1.000 | 0.019 | 5 (6) | 3 (3.7) | 0.720 | 0.054 | 5 (6) | 3 (3.7) | 0.720 | 0.054 |
| Chloroquine | 30 (33.7) | 22 (29.3) | 0.549 | 0.047 | 25 (30.1) | 27 (33.3) | 0.658 | 0.034 | 27 (32.5) | 25 (30.9) | 0.819 | 0.018 |
| Certolizumab | 3 (3.4) | 3 (4) | 1.000 | 0.017 | 5 (6) | 1 (1.2) | 0.210 | 0.128 | 5 (6) | 1 (1.2) | 0.210 | 0.128 |
| Tocilizumab | 2 (2.2) | 1 (1.3) | 1.000 | 0.034 | 2 (2.4) | 1 (1.2) | 1.000 | 0.044 | 3 (3.6) | 0 (0) | 0.246 | 0.135 |
| SS (n = 45) | SS+pa (n = 73) | SS+RA (n = 46) | p-Value | Effect Size (V) | |
|---|---|---|---|---|---|
| High total IgG + high IgA RF | 18 (40%) 2,3 | 19 (26%) 1 | 8 (17.4%) 1 | 0.050 | 0.191 |
| Specificity (95% CI) | 77.3 (69–83.9) | 71.4 (61.4–79.7) | 68.6 (59.8–76.3) | ||
| Sensitivity (95% CI) | 40 (27–54.6) | 26 (17.3–37.1) | 17.4 (9.1–30.7) | ||
| Normal total IgG + high IgM RF | 7 (15.6%) 3 | 15 (20.5%) 3 | 17 (37%) 1,2 | 0.039 | 0.199 |
| Specificity (95% CI) | 73.1 (64.5–80.3) | 73.6 (63.8–81.6) | 81.4 (73.4–87.4) | ||
| Sensitivity (95% CI) | 15.6 (7.7–28.8) | 20.6 (12.9–31.2) | 37 (24.5–51.4) | ||
| High total IgG + normal IgM RF | 4 (8.9%) | 9 (12.3%) | 1 (2.2%) | 0.128 | 0.162 |
| Specificity (95% CI) | 91.6 (85.2–95.4) | 94.5 (87.8–97.6) | 89 (82.1–93.5) | ||
| Sensitivity (95% CI) | 8.9 (3.5–20.7) | 12.3 (6.6–21.8) | 2.2 (0.1–11.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aradi, Z.; Bói, B.; Nagy, G.; Antal-Szalmás, P.; Mezei, K.; Horváth, I.F.; Szántó, A. Additive Value of Rheumatoid Factor Isotypes in Sjögren’s Syndrome Patients with Joint Complaints of Different Etiologies—Can Rheumatoid Factor IgA Serve as an Early, Poor Prognostic Biomarker Candidate? Int. J. Mol. Sci. 2025, 26, 4797. https://doi.org/10.3390/ijms26104797
Aradi Z, Bói B, Nagy G, Antal-Szalmás P, Mezei K, Horváth IF, Szántó A. Additive Value of Rheumatoid Factor Isotypes in Sjögren’s Syndrome Patients with Joint Complaints of Different Etiologies—Can Rheumatoid Factor IgA Serve as an Early, Poor Prognostic Biomarker Candidate? International Journal of Molecular Sciences. 2025; 26(10):4797. https://doi.org/10.3390/ijms26104797
Chicago/Turabian StyleAradi, Zsófia, Bernadett Bói, Gábor Nagy, Péter Antal-Szalmás, Kincső Mezei, Ildikó Fanny Horváth, and Antónia Szántó. 2025. "Additive Value of Rheumatoid Factor Isotypes in Sjögren’s Syndrome Patients with Joint Complaints of Different Etiologies—Can Rheumatoid Factor IgA Serve as an Early, Poor Prognostic Biomarker Candidate?" International Journal of Molecular Sciences 26, no. 10: 4797. https://doi.org/10.3390/ijms26104797
APA StyleAradi, Z., Bói, B., Nagy, G., Antal-Szalmás, P., Mezei, K., Horváth, I. F., & Szántó, A. (2025). Additive Value of Rheumatoid Factor Isotypes in Sjögren’s Syndrome Patients with Joint Complaints of Different Etiologies—Can Rheumatoid Factor IgA Serve as an Early, Poor Prognostic Biomarker Candidate? International Journal of Molecular Sciences, 26(10), 4797. https://doi.org/10.3390/ijms26104797





