Abstract
A library of neoisopulegol-based amino and thiol adducts was developed from (+)-neoisopulegol, derived from commercially available (−)-isopulegol. Michael addition of different nucleophiles towards its highly active α,β-unsaturated γ-lactone motif was accomplished, resulting in diverse amino and thiol analogs in stereoselective reactions. Then, the lactone ring was opened, with NH3 and benzylamine furnishing primary amide and N-benzyl-substituted amide derivatives, respectively. The in vitro antimicrobial effect of prepared compounds was also explored. The results revealed that naphthylmethyl-substituted β-aminolactone, the most promising compound, displayed selective inhibition for the Gram-positive bacteria S. aureus with an MIC (minimum inhibitory concentration) value of 12.5 μM. A docking study was performed to interpret the obtained results.
1. Introduction
The druglike chemical space contains an immense number of small molecules (>1060 possibilities); therefore, finding bioactive compounds among these small molecules is challenging. A possible solution to this problem is biology-oriented synthesis (BIOS). BIOS is based on the realization that 3D structures of protein domains as well as scaffolds of natural products (which evolved to interact with these proteins) are highly conserved in biology. Hence, it is reasonable to assume that analogs of bioactive natural products are more likely to inhabit a biologically relevant chemical space than other compounds. Indeed, compound libraries created according to BIOS principles show increased hit rates in biochemical and biological screens compared to traditional compound libraries [1,2].
Monoterpenes and monoterpenoids (C10 compounds containing two isoprene units) are abundant in nature. They show great structural diversity (including various extents of functionalization), often contain a number of chiral centers, and many of them are available at affordable prices (importantly, in a number of cases, both enantiomers are accessible) [3,4]. Therefore, they are often employed as enantiopure starting compounds for the synthesis of various complex molecules, such as natural products [3,4,5], β-amino acids [6], etc. Some synthetically useful members of this compound family are depicted in Figure 1. It is also worth mentioning that monoterpenes and monoterpenoids often show biological activity [7], making them promising agents from the viewpoint of BIOS.
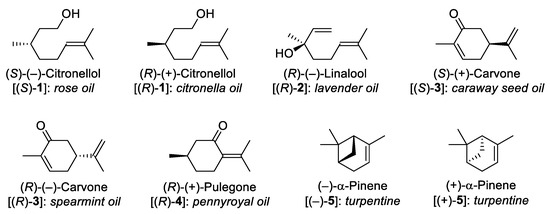
Figure 1.
Examples of monoterpenes and monoterpenoids which are commonly used in chiral pool syntheses.
Sesquiterpene lactones are a large and diverse subclass of C15 terpenoids (Figure 2). Their characteristic structural element is a γ-lactone ring (in most cases, it is an α-methylene-γ-lactone motif). The main sources of sesquiterpene lactones are plants of the Asteraceae family, but they are also found in numerous other plants and in some fungi. Sesquiterpene lactones usually show cytotoxic, antitumor, or antibacterial activities [8,9,10].
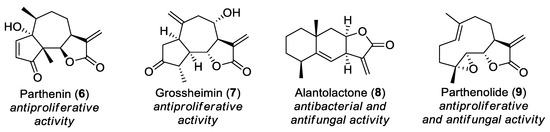
Figure 2.
Examples of sesquiterpene lactones.
Many sesquiterpene lactones are reasonably accessible, and their α-methylene-γ-lactone motif can be transformed by a variety of ways. Therefore, from the viewpoint of BIOS, these terpenoids can serve as valuable starting compounds in the synthesis of compound libraries. Indeed, Michael adducts of sesquiterpene lactones have been widely studied, and diverse biological activities were reported (Figure 3) [11,12,13,14,15,16,17,18,19,20].
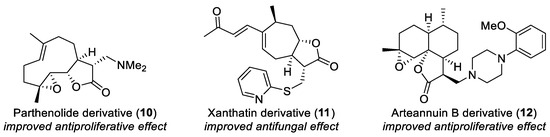
Figure 3.
Bioactive Michael adducts of sesquiterpene lactones.
In contrast with sesquiterpene lactones, monoterpene lactones are rare in nature (Figure 4). Pleurolactone (13) was isolated from two edible Pleurotus mushrooms (P. eryngii and P. cornucopiae) [21,22], and shows moderate inhibitory activity against nitric oxide production in lipopolysaccaride-activated macrophages [22]. cis,cis-p-Menthenolide (14) is the second major component of the essential oil of Mentha suaveolens ssp. insularis, and it inhibits quorum sensing of Chromobacterium violaceum [23]. Paeonilactone B (15) was isolated from the roots of the plant Paeonia albiflora (also known as P. lactiflora), together with paeonilactone C (16). The latter compound can be considered the O-benzoylated oxa-Michael adduct of paeonilactone B and water, and both directly and indirectly suppresses stimulated muscle twitchings of frog sciatic nerve–sartorius muscle preparations [24].
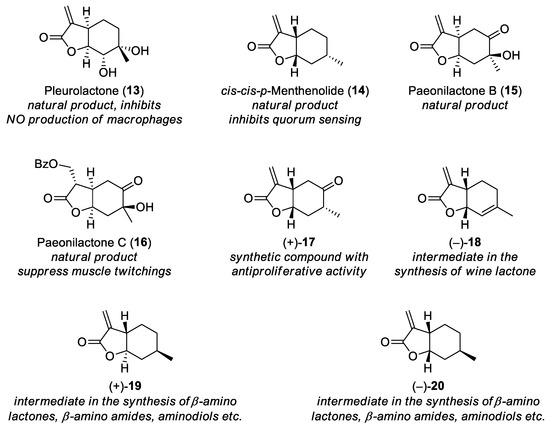
Figure 4.
Monoterpene lactones and their relevant properties.
Synthetic exomethylene lactones with a monoterpene skeleton are also known (Figure 4). Compound (+)-17 possesses antiproliferative activity [25], while other compounds are useful intermediates in organic synthesis, e.g., lactone (−)-18 can be transformed into the natural compound wine lactone [26,27] or its epimer [27]. Lactones (+)-19 [5,28,29,30,31,32] and (−)-20 [5,29,33] are especially versatile. These can be obtained in some steps [5,34,35,36,37] from readily available (−)-isopulegol (an intermediate in the synthesis of menthol [5]), and our research group utilized them extensively to prepare β-amino lactones [29,33], β-amino amides [29], aminodiols [29,33], and other products [5,29,30,31,32,33].
Based on our previous experiences with (+)-neoisopulegol-derived α-methylene-γ-lactone (−)-20 and the importance of bioactive Michael adducts of sesquiterpene lactones, our aim was the synthesis of Michael adducts of compound (−)-20 and investigation of the antimicrobial effect of the obtained products. A docking model was also developed for the most potent analogs.
2. Results and Discussion
2.1. Synthesis of (−)-α-Methylene-γ-Butyrolactone (−)-20
(+)-Neoisopulegol 22 was synthesized in two steps from commercially available (−)-isopulegol 21 according to modified literature methods. First, oxidation of the hydroxy group of (−)-21 with pyridinium chlorochromate (PCC) provided (−)-isopulegone [5,38], which, in turn, was diastereoselectively reduced with a stoichiometric amount of L-Selectride into the desired (+)-neoisopulegol (+)-22 (Scheme 1) [5,36].
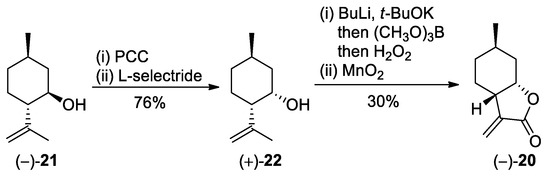
Scheme 1.
Preparation of (−)-α-methylene-γ-butyrolactone (−)-20.
From (+)-neoisopulegol 22, the key intermediate (−)-α-methylene-γ-butyrolactone (−)-20 was prepared by modified literature methods. First, allylic borylation and subsequent treatment with H2O2 resulted in regioselective hydroxylation [5,37]. Then, treatment of the obtained diol with MnO2 led to the oxidation of the primary alcohol motif to the carboxylic acid group, followed by spontaneous ring closure to form lactone (−)-20 (Scheme 1) [5,36].
2.2. Synthesis of (+)-Neoisopulegol-Based Amino Adducts
Nucleophilic addition of benzylamines to (−)-α-methylene-γ-butyrolactone (−)-20 provided (+)-neoisopulegol-based β-aminolactone 23 [29] and its analogs 24–30 with high diastereoselectivity (Scheme 1, Table 1). Previously, we observed that transformation of β-aminolactones into β-amino amides sometimes improves biological activity [29]. As a consequence, the ring opening of aminolactone 23 was performed with NH3/MeOH and benzylamine. The products were primary amide 31 and benzyl-substituted β-aminoamide 32 [29], respectively (Scheme 2).

Table 1.
Nucleophilic addition of (−)-20 with benzylamines.
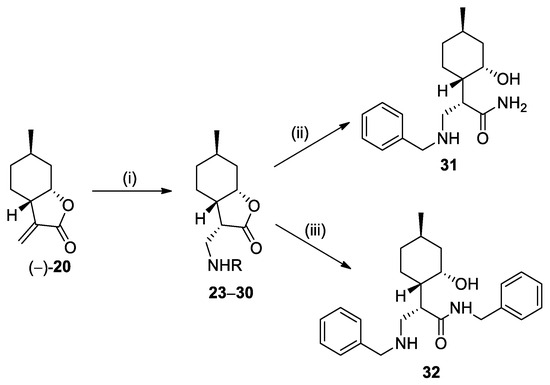
Scheme 2.
Preparation of (+)-neoisopulegol-based β-aminolactones and amides. Conditions: (i) RNH2 (1.5 equiv.), dry EtOH, 25 °C, 24 h, 42–90%; (ii) 25% NH3 in MeOH, 25 °C, 24 h, 94%; (iii) BnNH2 (2 equiv.), dry THF, 70 °C, 48 h, 70%.
2.3. Synthesis of (+)-Neoisopulegol-Based Thiol Adducts
Besides amines, Michael addition of (−)-α-methylene-γ-butyrolactone (−)-20 was also performed with various thiols. Unlike the conjugate addition of amines, formation of thiol adducts required Et3N as base [14,31]. Utilizing substituted benzyl mercaptans, the corresponding thiol adducts 33–35 were obtained (Scheme 3, Table 2). Nucleophilic addition was also successfully performed with cysteine derivatives as sulfur nucleophiles [39], yielding thiol adducts 38–43 (Scheme 3, Table 3). Similar to our previous work [31], ring opening of lactone 33 with NH3 and benzylamine was also performed, providing primary amide 36 and benzyl-substituted amide 37 (Scheme 3).
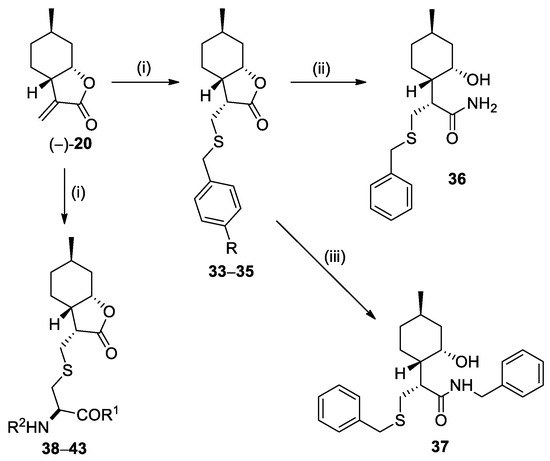
Scheme 3.
Preparation of (+)-neoisopulegol-based thiol adducts. Conditions: (i) RSH or cysteine derivatives (1.5 equiv.), Et3N (2.8 equiv.), EtOH, 25 °C, 24 h, 41–93%; (ii) 25% NH3 in MeOH, 25 °C, 24 h, 95%; (iii) BnNH2 (2 equiv.), dry THF, 70 °C, 48 h, 54%.

Table 2.
Nucleophilic addition of (–)-20 with benzyl mercaptans.

Table 3.
Nucleophilic addition of (−)-20 with cysteine derivatives.
2.4. Synthesis of (+)-Neoisopulegol-Based Azole Adducts
A simple method to obtain the azole adducts of an α-methylene-γ-butyrolactone in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) as a base was previously described in the literature [32,40]. When this procedure was applied to (−)-α-methylene-γ-butyrolactone (−)-20, the desired products were obtained in moderate yields (Scheme 4, Table 4). In contrast with the reactions depicted in Scheme 2 and Scheme 3, these reactions were not completely diastereoselective.
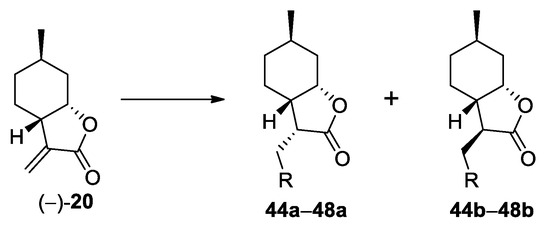
Scheme 4.
Preparation of (+)-neoisopulegol-based azole adducts. Conditions: azole (2 equiv.), DBU (1 equiv.), dry acetonitrile, 70 °C, 24 h, 12–57%.

Table 4.
Nucleophilic addition of (–)-20 with azoles.
Similar to our previous work [32], the ring opening of lactones 47a and 47b was successfully performed to obtain primary amides 49a and 49b and benzyl-substituted amides 50a and 50b, respectively (Scheme 5).
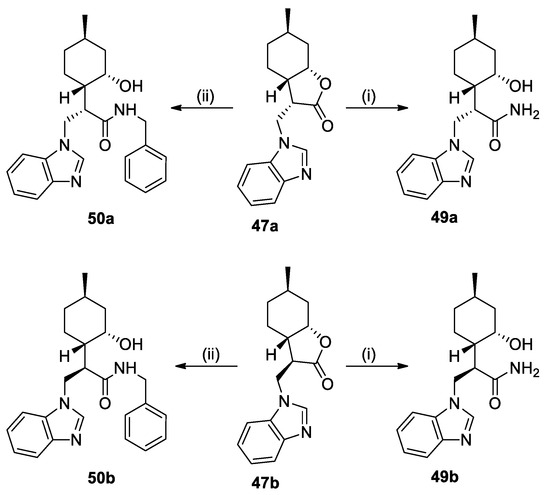
Scheme 5.
Lactone ring opening of azole adducts 47a and 47b. Conditions: (i) 25% NH3 in MeOH, 25 °C, 24 h, 59% (49a) or 69% (49b); (ii) BnNH2 (2 equiv.), dry THF, 70 °C, 72 h, 39% (50a) or 60% (50b).
2.5. Determination of Relative Configuration of Synthesized (+)-Neoisopulegol-Based Amino and Thiol Adducts
In order to determine the relative configuration of the synthesized (+)-neoisopulegol-based Michael adducts, from each compound family, some selected compounds were subjected to NOESY experiments. Once their stereochemistry was determined using NOE signals (or the lack of NOE signals) between H-3, H-3a, and H-7a, the configurations of related compounds were assigned by analogy. The results are summarized in Figure 5. Amino adducts, thiol adducts, and the major product azole adducts had the same stereochemistry at C-3, while the minor product azole adducts are C-3 epimers of the major product azole adducts.
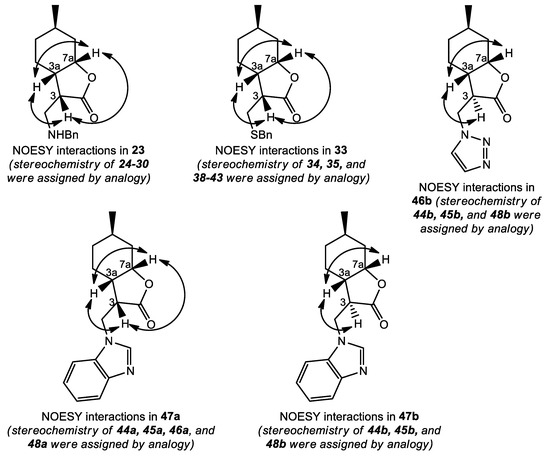
Figure 5.
Determination of the relative configurations of (+)-neoisopulegol-based Michael adducts.
2.6. Antimicrobial Assay
Since several sesquiterpene lactone-derived amino and thiol analogs exerted a significant antimicrobial effect [16,20], in vitro antimicrobial effects of the prepared amino and thiol analogs were tested [41] on three bacterial strains (Staphylococcus aureus ATCC 25923, S. aureus MRSA ATCC 43300, and Escherichia coli ATCC 25922). The results indicated that β-aminolactones 23–30 showed moderate activity on the S. aureus ATCC 25923 strain (the most active compound, naphthylmethyl-substituted β-aminolactone 30, exhibited an MIC value of 12.5 μM on this Gram-positive bacterium), but they were not effective on the other two strains (Table 5). All other compounds lacked antibacterial activity (see Table S1 in Supplementary Materials for biological screening data of all compounds).

Table 5.
Antibacterial activity of β-aminolactones on S. aureus ATCC 25923. Results of the antimicrobial assay of other compounds are provided in Table S1 in Supplementary Materials.
2.7. Docking Study
Molecular docking was conducted to predict the possible protein targets of the most promising compounds 28 and 30 (see Table 5). To explore potential binding sites, a variety of S. aureus proteins with key roles in bacterial survival and resistance mechanisms were selected to be used as templates, including penicillin-binding protein 2a (PBP2a) (PDB code: 4CJN, resolution: 1.95 Å) [42], dihydrofolate reductase (DHFR) (PDB code: 2W9S, resolution: 1.80 Å) [43], DNA gyrase (GyrB ATPase domain) (PDB code: 3U2D, resolution: 1.85 Å) [44], and the C(30) carotenoid dehydrosqualene synthase (PDB code: 2ZCQ, resolution: 2.38 Å) [45]. The CDOCKER method [46] allowed us to investigate the ligand–receptor interactions and to predict binding energies (Table S2 in Supplementary Materials). Both compounds demonstrated favorable binding interactions with DNA gyrase (PDB code: 3U2D) and dehydrosqualene synthase (PDB code: 2ZCQ) (Figure 6 and Figure 7). Compound 28 showed robust binding to 2ZCQ through hydrogen bonding with ASP A:48 and ASN A:168, and a π–cation bonding with ARG A:45, achieving a CDOCKER interaction energy range of −47.5769 to −56.8457 kcal/mol. Similarly, compound 30 exhibited strong hydrogen bonding with ASN A:54, THR A:173, and ASP A:81 in 3U2D, accompanied by notable van der Waals interactions, resulting in a CDOCKER interaction energy range of −40.2136 to −47.49286 kcal/mol. These results suggest that the amino group plays a major role in forming strong interactions, such as hydrogen bonds and ionic interactions, with the residues within the binding site. Additionally, it should be noted that the naphthyl group contributes to forming further van der Waals bonds, which may explain why compound 30 has the highest activity among the other compounds. However, the role of the remaining functional groups is limited to minor interactions, which may explain the in vitro assay results. Furthermore, the in silico ADMET analysis [47] proposes promising pharmacokinetic and safety profiles for the tested compounds (Table S3 in Supplementary Materials).
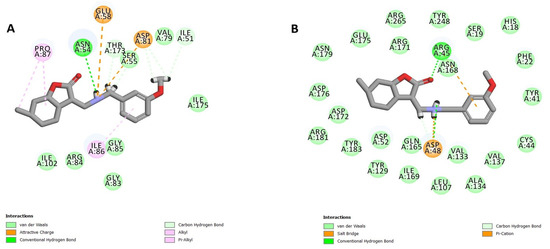
Figure 6.
A 2D diagram of the interactions between compound 28 and the binding site of the protein templates ((A): 3U2D, (B): 2ZCQ).
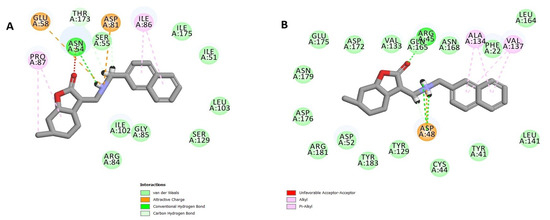
Figure 7.
A 2D diagram of the interactions between compound 30 and the binding site of the protein templates ((A): 3U2D, (B): 2ZCQ).
3. Materials and Methods
3.1. General Methods
Commercially available solvents were used as obtained from suppliers (Molar Chemicals Ltd., Halásztelek, Hungary; Merck Ltd., Budapest, Hungary and VWR International Ltd., Debrecen, Hungary), while applied solvents were dried according to standard procedures. Optical rotations were measured in MeOH at 20 °C with a Perkin-Elmer 341 polarimeter (PerkinElmer Inc., Shelton, CT, USA). Chromatographic separations and monitoring of reactions were carried out on a Merck Kieselgel 60 (Merck Ltd., Budapest, Hungary). HRMS flow injection analysis was performed with a Thermo Scientific Orbitrap Exploris 240 hybrid quadrupole-Orbitrap (Thermo Fischer Scientific, Waltham, MA, USA) mass spectrometer coupled to a Waters Acquity I-Class UPLC TM (Waters, Manchester, UK). GC measurements for direct separation of commercially available enantiomers of isopulegol, to determine the enantiomeric purity of the starting material (−)-21 and its enantiomer (+)-21, were performed on a Chirasil-DEX CB column (2500 × 0.25 mm I.D.) on a Perkin-Elmer Autosystem XL GC consisting of a Flame Ionization Detector (Perkin-Elmer Corp., Norwalk, CT, USA) and a Turbochrom Workstation data system (Perkin-Elmer Corp., Norwalk, CT, USA). Melting points were determined on a Kofler apparatus (Nagema, Dresden, Germany) and they are uncorrected. 1H NMR, 13C NMR, and 19F NMR were recorded on a Brucker Avance DRX 500 spectrometer [500 MHz (1H), 471 MHz (19F), and 125 MHz (13C)]. The 1H and 13C chemical shifts are given relative to TMS and 19F to CFCl3 (0.00 ppm). J values are given in Hz. Images of NMR spectra are found in Figures S1–S86 in the Supplementary Materials.
(−)-Isopulegol (−)-21 is available commercially from Merck Co with ee% = 95%. (+)-Neoisopulegol (+)-22 [36,38], (−)-α-methylene-γ-butyrolactone (–)-20 [36,37], and cysteine derivatives (WO 2015148880 A1) were prepared according to modified literature procedures.
3.2. Preparation of (+)-Neoisopulegol (+)-22
Oxidation of (−)-isopulegol was based on Ref. [38]. To a slurry of PCC (27.6 g, 2 equiv.) and silica gel (55 g) in DCM (200 mL), (−)-isopulegol 9 (10 g, 64 mmol) was added. The reaction mixture was stirred at room temperature for 48 h. Upon completion of the reaction (as monitored by TLC), the mixture was filtered and the filtrate was evaporated to dryness. The crude product was purified by column chromatography on silica gel using n-hexane/EtOAc 9:1 as the eluent. This provided (–)-isopulegone as a pale yellow oil (yield: 80%).
Reduction of (–)-isopulegone was based on Ref. [36]. (–)-Isopulegone (2 g, 12.5 mmol) was dissolved in dry THF (40 mL). L-Selectride (15 mL, 1.2 equiv.) was dissolved in dry THF (20 mL), and added dropwise to the previous solution under an argon atmosphere at −78 °C. After 1–2 h, the reaction mixture was allowed to warm up to RT, and then it was treated with H2O2 in 5% NaOH (40 mL) and extracted with Et2O (3 × 100 mL). The organic layer was dried over Na2SO4 and evaporated to dryness. The crude product was purified by column chromatography on silica gel using n-hexane/EtOAc 9:1 as the eluent. This provided (+)-neoisopulegol (+)-22 as a pale yellow oil (yield: 95%).
3.3. Preparation of (–)-α-Methylene-γ-Butyrolactone (−)-20
Hydroxylation of (+)-neoisopulegol was based on Ref. [37]. t-BuOK (2.2 g, 1.5 equiv.) was dissolved in dry n-hexane (15 mL). Then, n-BuLi (2 g, 2 equiv.) was added dropwise under an argon atmosphere at 0 °C. The mixture was stirred for one hour, during which the color changed from white to light orange. After the dropwise addition of (+)-neoisopulegol (+)-22 (2 g, 13 mmol), the reaction mixture was stirred under an argon atmosphere at RT overnight. B(OMe)3 (4.5 mL, 3 equiv.) was added dropwise at −78 °C and stirred with the mixture for one hour. Upon reaching −20 °C, 30% aqueous H2O2 (7 mL) was introduced into the mixture and stirred for one hour. Water was then added to quench the reaction, and the mixture was extracted with Et2O (3 × 100 mL). The organic layer was dried over Na2SO4, filtered, and evaporated to dryness. The crude product was crystallized from n-hexane to give (1S,2S,5R)-2-(3-hydroxyprop-1-en-2-yl)-5-methylcyclohexanol as white crystals (yield: 50%).
Oxidation of (1S,2S,5R)-2-(3-hydroxyprop-1-en-2-yl)-5-methylcyclohexanol is based on Ref. [36]. To a suspension of MnO2 (29.6 g, 20 equiv.) in DCM (50 mL), a solution of (1S,2S,5R)-2-(3-hydroxyprop-1-en-2-yl)-5-methylcyclohexanol (3 g, 17 mmol) in DCM (50 mL) was added. The reaction mixture was vigorously stirred at 50–60 °C under reflux for 48 h. Upon completion of the reaction (monitored by TLC), the mixture was filtered through a Celite pad, and the filtrate was concentrated to afford the corresponding lactone (−)-20 as a yellow oil (yield: 60%).
3.4. General Procedure for the Preparation of (+)-Neoisopulegol-Based β-Aminolactone Compounds 23–30
To a solution of compound (−)-20 (50 mg, 0.3 mmol) in dry EtOH (1.0 mL), the appropriate amines (0.45 mmol, 1.5 equiv.) were added. The reaction mixture was stirred at room temperature for 24 h. After the completion of the reaction (as monitored by TLC), the mixture was evaporated to dryness. The crude product was purified by column chromatography on silica gel using CHCl3:MeOH = 39:1 as the eluent.
(3S,3aS,6R,7aS)-3-((Benzylamino)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (23): The reaction was accomplished with benzylamine. Yield: 89%; yellow oil; = −44.45 (c = 0.15, MeOH). 1H NMR (500 MHz, CDCl3): δ = 7.34–7.30 (m, 4H), 7.28–7.22 (m, 1H), 4.45 (d, 1H, J = 2.7 Hz), 3.87–3. 76 (m, 2H), 2.99 (dd, J = 11.8, 7.1 Hz, 1H), 2.94–2.87 (1H, m), 2.69 (dd, J = 11.8, 7.4 Hz, 1H), 2.32 (ddd, J = 11.6, 9.9, 5.7 Hz, 1H), 2.22 (1H, d, J = 15 Hz), 1.66–1.60 (m, 2H), 1.60–1.51 (m, 1H), 1.08–1.28 (m, 2H), 0.94–0.81 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 178.6, 140.2, 128.6, 128.3, 127.2, 78.8, 54.4, 48.3, 44.9, 38.0, 36.2, 32.1, 26.4, 23.1, 22.1. HRMS (ESI): m/z calcd for C17H24NO2 [M+H]+: 274.1807; found: 274.1798.
(3S,3aS,6R,7aS)-3-(((2-Fluorobenzyl)amino)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (24): The reaction was accomplished with 2-fluorobenzylamine. Yield: 42%; yellow oil; = −33.80 (c = 0.17, MeOH). ¹H NMR (500 MHz, CDCl3): δ = 7.34 (t, J = 7.5 Hz, 1H), 7.25–7.21 (m, 1H), 7.11 (t, J = 7.4 Hz, 1H), 7.07–7.01 (m, 1H), 4.45 (d, J = 2.6 Hz, 1H), 3.92–3.82 (m, 2H), 3.00 (dd, J = 11.8, 6.9 Hz, 1H), 2.91 (dd, J = 13.7, 6.8 Hz, 1H), 2.68 (dd, J = 11.7, 7.7 Hz, 1H), 2.33 (ddd, J = 11.4, 10.0, 5.7 Hz, 1H), 2.22 (d, J = 14.9 Hz, 1H), 1.69–1.61 (m, 2H), 1.61–1.51 (m, 1H), 1.23–1.08 (m, 2H), 0.94–0.81 (m, 4H). 13C NMR (125 MHz, CDCl3): δ 178.5, 161.4 (d, 1JC-F = 245.1 Hz), 130.5 (d, 3JC-F = 4.6 Hz), 129 (d, 3JC-F = 8.1 Hz), 127 (d, 2JC-F = 14.5 Hz), 124.3 (d, 4JC-F = 3.6 Hz), 115.5 (d, 2JC-F = 21.8 Hz), 78.8, 48.2, 47.6 (d, 3JC-F = 2.9 Hz), 44.7, 37.9, 36.2, 32.1, 26.4, 23.1, 22.1. 19F NMR (470 MHz, CDCl3) δ = −119.46. HRMS (ESI): m/z calcd for C17H23FNO2 [M+H]+: 292.1713; found: 292.1701.
(3S,3aS,6R,7aS)-3-(((3-Fluorobenzyl)amino)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (25): The reaction was accomplished with 3-fluorobenzylamine. Yield: 39%; yellow oil; = −36.11 (c = 0.18, MeOH). ¹H NMR (500 MHz, CDCl3): δ = 7.31–7.26 (m, 1H), 7.12–7.03 (m, 2H), 6.94 (td, J = 8.5, 2.1 Hz, 1H), 4.46 (d, J = 2.6 Hz, 1H), 3.87–3.75 (m, 2H), 2.98 (dd, J = 11.6, 7.3 Hz, 1H), 2.90 (dd, J = 13.6, 6.8 Hz, 1H), 2.67 (dd, J = 11.6, 7.1 Hz, 1H), 2.32 (ddd, J = 11.4, 10.0, 5.7 Hz, 1H), 2.22 (d, J = 14.9 Hz, 1H), 1.70–1.63 (m, 2H), 1.59–1.51 (m, 1H), 1.25–1.09 (m, 2H), 0.95–0.81 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 178.5, 163.0 (d, 1JC-F = 245.7 Hz), 142.7 (d, 3JC-F = 6.9 Hz), 129.9 (d, 3JC-F = 8.2 Hz), 123.5 (d, 4JC-F = 2.7 Hz), 114.8 (d, 2JC-F = 21.2 Hz), 113.9 (d, 2JC-F = 21.1 Hz), 78.7, 53.6 (d, 4JC-F = 1.4 Hz), 48.1, 44.7, 37.9, 36.0, 31.9, 26.2, 23.0, 21.9. 19F NMR (470 MHz, CDCl3) δ = −113.46. HRMS (ESI): m/z calcd for C17H23FNO2 [M+H]+: 292.1713; found: 292.1703.
(3S,3aS,6R,7aS)-3-(((4-Fluorobenzyl)amino)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (26): The reaction was accomplished with 4-fluorobenzylamine. Yield: 52%; yellow oil; = −44.45 (c = 0.16, MeOH). 1H NMR (500 MHz, CDCl3): δ = 7.29 (dd, J = 8.3, 5.6 Hz, 2H), 7.01 (t, J = 8.7 Hz, 2H), 4.46 (d, J = 2.7 Hz, 1H), 3.84–3.73 (m, 2H), 2.97 (dd, J = 11.5, 7.3 Hz, 1H), 2.91 (dd, J = 13.4, 6.7 Hz, 1H), 2.68 (dd, J = 11.5, 6.9 Hz, 1H), 2.32 (ddd, J = 11.5, 9.8, 5.7 Hz, 1H), 2.22 (d, J = 15.2 Hz, 1H), 1.69–1.59 (m, 2H), 1.59–1.51 (m, 1H), 1.28–1.07 (m, 2H), 0.94–0.81 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 178.5, 162.0 (d, 1JC-F = 245.7 Hz), 135.5 (d, 4JC-F = 3.2 Hz), 129.7 (d, 3JC-F = 8.4 Hz), 115.3 (d, 2JC-F = 21.3 Hz), 78.7, 53.4, 47.9, 44.6, 37.9, 36.0, 31.9, 26.2, 23.0, 21.9. 19F NMR (470 MHz, CDCl3) δ = −115.78. HRMS (ESI): m/z calcd for C17H23FNO2 [M+H]+: 292.1713; found: 292.1703.
(3S,3aS,6R,7aS)-3-(((2-Methoxybenzyl)amino)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (27): The reaction was accomplished with 2-methoxybenzylamine. Yield: 48% (35 mg); white powder, mp. 95.8–97.1 °C; = −31.60 (c = 0.19, MeOH). 1H NMR (500 MHz, CDCl3): δ = 7.25–7.21 (m, 2H), 6.91 (t, J = 7.4 Hz, 1H), 6.88–6.85 (m, 1H), 4.44 (d, J = 2.6 Hz, 1H), 3.84 (s, 3H), 3.81 (s, 2H), 2.98 (dd, J = 11.7, 6.5 Hz, 1H), 2.92 (dd, J = 6.5, 13.9 Hz, 1H), 2.66 (dd, J = 11.7, 7.9 Hz, 1H), 2.33 (ddd, J = 11.4, 9.9, 5.6 Hz, 1H), 2.21 (d, J = 13.3 Hz, 1H), 1.67–1.64 (m, 3H), 1.23–1.16 (m, 1H), 1.15–1.07 (m, 1H), 0.93–0.82 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 178.3, 129.8, 128.4, 128.0, 120.5, 110.3, 78.6, 55.3, 49.6, 48.1, 44.5, 37.8, 36.1, 32.0, 26.2, 22.8, 21.9. HRMS (ESI): m/z calcd for C18H26NO3 [M+H]+: 304.1913; found: 304.1901.
(3S,3aS,6R,7aS)-3-(((3-Methoxybenzyl)amino)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (28): The reaction was accomplished with 3-methoxybenzylamine. Yield: 66%; yellow oil; = −37.92 (c = 0.19, MeOH). ¹H NMR (500 MHz, CDCl3): δ = 7.23 (t, J = 7.9 Hz, 1H), 6.92–6.88 (m, 2H), 6.81–6.78 (m, 1H), 4.45 (d, J = 2.7 Hz, 1H), 3.84–3.74 (m, 5H), 2.99 (dd, J = 11.8, 7.1 Hz, 1H), 2.90 (q, J = 6.8 Hz, 1H), 2.68 (dd, J = 11.8, 7.3 Hz, 1H), 2.35–2.28 (m, 1H), 2.21 (d, J = 15.1 Hz, 1H), 1.69–1.63 (m, 2H), 1.58–1.51 (m, 1H), 1.24–1.09 (m, 2H), 0.91 (d, J = 6.6 Hz, 3H), 0.89–0.82 (m, 1H). 13C NMR (125 MHz, CDCl3): δ = 178.6, 160.0, 141.9, 129.6, 120.6, 113.7, 112.7, 78.8, 55.4, 54.3, 48.3, 44.9, 38.1, 36.2, 32.1, 26.4, 23.2, 22.1. HRMS (ESI): m/z calcd for C18H26NO3 [M+H]+: 304.1913; found: 304.1902.
(3S,3aS,6R,7aS)-3-(((4-Methoxybenzyl)amino)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (29): The reaction was accomplished with 4-methoxybenzylamine. Yield: 69%; white powder, mp. 48.5–50.5 °C; = −10.96 (c = 0.17, MeOH). 1H NMR (500 MHz, CDCl3): δ = 7.23 (d, J = 8.4 Hz, 2H), 6.86 (d, J = 8.5 Hz, 2H), 4.45 (d, J = 2.6 Hz, 1H), 3.80 (s, 3H), 3.79–3.70 (m, 2H), 2.97 (dd, J = 11.7, 7.0 Hz, 1H), 2.89 (dd, J = 13.5, 6.9Hz, 1H), 2.67 (dd, J = 11.7, 7.4Hz, 1H), 2.34–2.27 (m, 1H), 2.21 (d, J = 14.9 Hz, 1H), 1.68–1.63 (m, 2H), 1.58–1.50 (m, 1H), 1.23–1.08 (m, 2H), 0.91 (d, J = 6.5 Hz, 3H), 0.88–0.81 (m, 1H). 13C NMR (125 MHz, CDCl3): δ = 178.6, 158.9, 132.3, 129.4, 114.0, 78.8, 55.4, 53.8, 48.3, 44.7, 38.0, 36.2, 32.1, 26.4, 23.1, 22.1. HRMS (ESI): m/z calcd for C18H26NO3 [M+H]+: 304.1913; found: 304.1902.
(3S,3aS,6R,7aS)-6-Methyl-3-(((naphthalen-1-ylmethyl)amino)methyl)hexahydro-benzofuran-2(3H)-one (30): The reaction was accomplished with 1-naphthylmethylamine. Yield: 90%; white crystals, mp. 62.1–64.5 °C; = −40.88 (c = 0.18, MeOH). 1H NMR (500 MHz, CDCl3): δ = 8.13 (d, J = 8.3 Hz, 1H), 7.86 (d, J = 8.0 Hz, 1H), 7.77 (d, J = 8.1 Hz, 1H), 7.56–7.45 (m, 3H), 7.45–7.39 (m, 1H), 4.46–4.41 (m, 1H), 4.33–4.19 (m, 2H), 3.11 (dd, J = 11.8, 6.8Hz, 1H), 2.93 (dd, J = 13.8, 6.8 Hz, 1H), 2.80 (dd, J = 11.8, 7.8 Hz, 1H), 2.33–2.26 (m, 1H), 2.21 (d, J = 14.8 Hz, 1H), 1.65–1.48 (m, 3H), 1.23–1.10 (m, 2H), 0.93–0.80 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 178.6, 135.6, 134.1, 131.9, 128.9, 128.0, 126.3, 126.2, 125.8, 125.5, 123.8, 78.8, 52.1, 48.3, 45.3, 38.0, 36.2, 32.1, 26.4, 23.1, 22.1. HRMS (ESI): m/z calcd for C21H26NO2 [M+H]+: 324.1964; found: 324.1953.
3.5. General Procedure for the Preparation of (+)-Neoisopulegol-Based Thiol Adducts 33–35 and 38–43
To a solution of compound (−)-20 (50 mg, 0.3 mmol) in dry EtOH (1.0 mL), the appropriate benzyl mercaptan derivatives or cysteine derivatives (0.45 mmol, 1.5 equiv.) and Et3N (0.84 mmol, 2.8 equiv.) were added. After stirring for 24 h at room temperature (as monitored by TLC), the mixture was evaporated to dryness. The crude product was purified by column chromatography on silica gel, eluted with appropriate solvent.
(3R,3aS,6R,7aS)-3-((Benzylthio)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (33): The reaction was accomplished with benzyl mercaptan and chromatographed by n-hexane/EtOAc 9:1. Yield: 80%, white powder, mp. 88.7–90.1 °C; = −116.51 (c = 0.17, MeOH). 1H NMR (500 MHz, CDCl3): δ = 7.31 (d, J = 4.3 Hz, 4H), 7.27–7.22 (m, 1H), 4.37 (dd, J = 6.0, 3.2 Hz, 1H), 3.75 (s, 2H), 2.91 (dd, J = 13.0, 4.1 Hz, 1H), 2.78–2.71 (m, 1H), 2.46 (t, J = 12.3 Hz, 1H), 2.35–2.27 (m, 1H), 2.19 (d, J = 15.0 Hz, 1H), 1.68–1.59 (m, 2H), 1.56–1.48 (m, 1H), 1.22–1.14 (m, 1H), 1.02–0.92 (m, 1H), 0.91–0.80 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 177.3, 138.2, 128.9, 128.8, 127.4, 78.6, 47.7, 37.6, 37.2, 36.2, 31.9, 26.8, 26.3, 22.4, 22.0. HRMS (ESI): m/z calcd for C17H23O2S [M+H]+: 291.1419; found: 291.1410.
(3R,3aS,6R,7aS)-3-(((4-Fluorobenzyl)thio)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (34): The reaction was accomplished with 4-fluorobenzyl mercaptan and chromatographed by n-hexane/EtOAc 9:1. Yield: 76%; white powder, mp. 58.4–61.2 °C; = −107.25 (c = 0.16, MeOH). 1H NMR (500 MHz, CDCl3): δ = 7.31–7.26 (m, 2H), 7.00 (t, J = 8.6 Hz, 2H), 4.40 (d, J = 2.5 Hz, 1H), 3.72 (s, 2H), 2.89 (dd, J = 12.9, 4.1 Hz, 1H),), 2.82–2.73 (m, 1H), 2.45 (t, J = 12.2 Hz, 1H), 2.32 (ddd, J = 11.5, 9.9, 5.5 Hz, 1H), 2.20 (d, J = 14.9 Hz, 1H), 1.70–1.61 (m, 2H), 1.54–1.49 (m, 1H), 1.24–1.14 (m, 1H), 1.05–0.93 (m, 1H), 0.92–0.83 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 177.2, 162.1 (d, 1JC-F = 245.8 Hz), 133.9 (d, 4JC-F = 3.0 Hz), 130.5 (d, 3JC-F = 8.2 Hz), 115.6 (d, 2JC-F = 21.5 Hz), 78.7, 47.7, 37.6, 36.5, 36.2, 31.9, 26.8, 26.3, 22.4, 22.0. 19F NMR (470 MHz, CDCl3) δ = −115.20. HRMS (ESI): m/z calcd for C17H22FO2S [M+H]+: 309.1325; found: 309.1317.
(3R,3aS,6R,7aS)-3-(((4-Methoxybenzyl)thio)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (35): The reaction was accomplished with 4-methoxybenzyl mercaptan and chromatographed by n-hexane/EtOAc 9:1. Yield: 73%; white powder, mp. 60.7–62.3 °C; = −114.78 (c = 0.15, MeOH). 1H NMR (500 MHz, CDCl3): δ = 7.22 (d, J = 8.5 Hz, 2H), 6.84 (d, J = 8.5 Hz, 2H), 4.39 (d, J = 2.6 Hz, 1H), 3.80 (s, 3H), 3.70 (s, 2H), 2.90 (dd, J = 13.0, 4.1 Hz, 1H), 2.80–2.72 (m, 1H), 2.45 (t, J = 12.3 Hz, 1H), 2.37–2.28 (m, 1H), 2.20 (d, J = 15.1 Hz, 1H), 1.69–1.63 (m, 2H), 1.53–1.50 (m, 1H), 1.23–1.14 (m, 1H), 1.02–0.94 (m, 1H), 0.93–0.82 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 177.3, 159.0, 130.1, 130.0, 114.2, 78.7, 55.5, 47.8, 37.6, 36.6, 36.2, 31.9, 26.7, 26.3, 22.4, 22.0. HRMS (ESI): m/z calcd for C18H25O3S [M+H]+: 321.1524; found: 321.1514.
(R)-2-Acetamido-3-((((3R,3aS,6R,7aS)-6-methyl-2-oxooctahydrobenzofuran-3-yl)-methyl)-thio)propanoic acid (38): The reaction was accomplished with N-acetyl-L-cysteine and chromatographed by CHCl3/CH3OH 19:1. Yield: 71%; white powder, mp. 136.2–138.3 °C; = −51.80 (c = 0.17, MeOH). 1H NMR (500 MHz, DMSO-d6): δ = 7.63 (d, J = 6.5 Hz, 1H), 4.51 (s, 1H), 4.12 (d, J = 3.8 Hz, 1H), 3.13–3.06 (m, 1H), 3.04 (dd, J = 13.3, 3.7 Hz, 1H), 2.79 (dd, J =13.1, 3.9 Hz, 1H), 2.71 (dd, J = 13.2, 7.2 Hz, 1H),), 2.42 (t, J = 12.3 Hz, 1H), 2.34 (d, J = 4.6 Hz, 1H), 2.04 (d, J = 14.5 Hz, 1H), 1.84 (s, 3H), 1.67 (s, 1H), 1.58 (s, 1H), 1.38 (s, 1H), 1.23 (t, J = 12.1 Hz, 1H), 1.09 (t, J = 6.9 Hz, 1H), 0.87 (d, J = 6.3 Hz, 5H). 13C NMR (125 MHz, DMSO-d6): δ = 177.7, 173.4, 169.1, 78.1, 54.3, 47.2, 37.2, 35.8, 35.3, 31.8, 27.3, 26.5, 23.3, 22.5, 22.3. HRMS (ESI): m/z calcd for C15H24NO5S [M+H]+: 330.1375; found: 330.1367.
(R)-Methyl 2-amino-3-((((3R,3aS,6R,7aS)-6-methyl-2-oxooctahydrobenzofuran-3-yl)-methyl)thio)propanoate (39): The reaction was accomplished with L-cysteine methyl ester and chromatographed by CHCl3/CH3OH 19:1. Yield: 50%; orange powder, mp. 78.4–80.3 °C; = −134.73 (c = 0.15, MeOH). 1H NMR (500 MHz, CDCl3): δ =4.46 (dd, J = 5.9, 3.1 Hz, 1H), 3.75 (s, 3H), 3.68 (dd, J = 7.4, 4.6 Hz, 1H), 2.98 (ddd, J = 13.5, 6.7, 4.4 Hz, 2H), 2.94–2.88 (m, 1H), 2.80 (dd, J = 13.5, 7.4 Hz, 1H), 2.60 (dd, J = 12.8, 11.5 Hz, 1H), 2.40 (ddd, J = 11.9, 9.8, 5.9 Hz, 1H), 2.26–2.19 (m, 1H), 1.82–1.74 (m, 1H), 1.74–1.65 (m, 1H), 1.61–1.51 (m, 1H), 1.21 (ddd, J = 15.6, 12.5, 3.5 Hz, 1H), 1.06 (ddd, J = 25.6, 13.3, 3.3 Hz, 1H), 0.95–0.84 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 177.1, 174.5, 78.7, 54.3, 52.5, 48.0, 38.0, 37.5, 36.2, 31.9, 27.8, 26.3, 22.5, 22.0. HRMS (ESI): m/z calcd for C14H24NO4S [M+H]+: 302.1426; found: 302.1420.
(R)-Methyl 2-acetamido-3-((((3R,3aS,6R,7aS)-6-methyl-2-oxooctahydrobenzofuran-3-yl)methyl)thio)propanoate (40): The reaction was accomplished with N-acetyl-L-cysteine methyl ester and chromatographed by n-hexane/EtOAc 1:2. Yield: 62%; white powder, mp. 105.4–107.6 °C; = −104.50 (c = 0.16, MeOH). 1H NMR (500 MHz, CDCl3): δ = 6.32 (d, J = 6.9 Hz, 1H), 4.85 (dt, J = 7.4, 4.9 Hz, 1H), 4.46 (dd, J1 = 5.8, 3.1 Hz, 1H), 3.79 (s, 3H), 3.13 (dd, J = 13.9, 4.8 Hz, 1H), 3.03–2.92 (m, 2H), 2.87 (ddd, J = 10.7, 5.9, 4.5 Hz, 1H), 2.58 (dd, J = 12.9, 11.1 Hz, 1H), 2.37 (ddd, J = 11.9, 9.8, 5.9 Hz, 1H), 2.26–2.18 (m, 1H), 2.06 (s, 3H), 1.78–1.66 (m, 2H), 1.60–1.51 (m, 1H), 1.25–1.17 (m, 1H), 1.06 (ddd, J = 25.4, 13.2, 3.2 Hz, 1H), 0.95–0.82 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 177.0, 171.3, 170.0, 78.6, 53.0, 52.4, 48.0, 37.6, 36.1, 34.9, 31.9, 28.1, 26.3, 23.3, 22.5, 22.0. HRMS (ESI): m/z calcd for C16H26NO5S [M+H]+: 344.1532; found: 344.1522.
(R)-2-Acetamido-3-((((3R,3aS,6R,7aS)-6-methyl-2-oxooctahydrobenzofuran-3-yl)-methyl)thio)propanamide (41):The reaction was accomplished with N-acetyl-L-cysteine amide and chromatographed by n-hexane/EtOAc 1:2. Yield: 41%; white powder, mp. 99.3–101.2 °C; = −116.83 (c = 0.12, MeOH). 1H NMR (500 MHz, DMSO-d6): δ = 8.04 (d, J = 8.3 Hz, 1H), 7.52 (s, 1H), 7.12 (s, 1H), 4.51 (s, 1H), 4.38 (dd, J = 12.6, 6.8 Hz, 1H), 3.15–3.08 (m, 1H), 2.88 (ddd, J = 17.5, 13.4, 4.7 Hz, 2H), 2.61 (dd, J = 13.6, 8.8 Hz, 1H), 2.46 (d, J = 12.4 Hz, 1H), 2.39–2.30 (m, 1H), 2.05 (d, J = 14.6 Hz, 1H), 1.85 (s, 3H), 1.74–1.62 (m, 1H), 1.58 (d, J = 7.9 Hz, 1H), 1.39 (s, 1H), 1.29–1.19 (m, 1H), 0.95–0.82 (m, 5H). 13C NMR (125 MHz, DMSO-d6): δ = 177.5, 172.7, 169.7, 78.1, 52.3, 47.0, 37.2, 35.8, 34.4, 31.8, 27.0, 26.5, 23.0, 22.5, 22.3. HRMS (ESI): m/z calcd for C15H25N2O4S [M+H]+: 329.1535; found: 329.1525.
(R)-Ethyl 2-acetamido-3-((((3R,3aS,6R,7aS)-6-methyl-2-oxooctahydrobenzofuran-3-yl)-methyl)thio)propanoate (42): The reaction was accomplished with N-acetyl-L-cysteine ethyl ester and chromatographed by n-hexane/EtOAc 1:2. Yield: 93%; white powder, mp. 98.3–100.7 °C; = −95.53 (c = 0.17, MeOH). 1H NMR (500 MHz, CDCl3): δ = 6.31 (d, J = 6.9 Hz, 1H), 4.82 (dt, J = 7.3, 4.9 Hz,1H), 4.46 (d, J = 2.8 Hz, 1H), 4.24 (q, J = 7.1 Hz, 2H), 3.13 (dd, J = 13.9, 4.7 Hz, 1H), 2.97 (dd, J1 = 13.2, 4.5 Hz, 2H), 2.88–2.84 (m, 1H), 2.58 (dd, J = 12.9, 11.3 Hz, 1H), 2.37 (ddd, J = 11.9, 9.8, 5.8 Hz, 1H), 2.22 (dd, J = 15.0, 3.0 Hz, 1H), 2.05 (s, 3H), 1.77–1.72 (m, 1H), 1.72–1.65 (m, 1H), 1.60–1.50 (m, 1H), 1.31 (t, J = 7.1 Hz, 3H), 1.24–1.18 (m, 1H), 1.05 (qd, J = 13.2, 3.1 Hz, 1H), 0.96–0.84 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 177.0, 170.8, 170.0, 78.6, 62.2, 52.5, 48.0, 37.6, 36.2, 34.8, 31.9, 28.1, 26.3, 23.3, 22.5, 22.0, 14.3. HRMS (ESI): m/z calcd for C17H28NO5S [M+H]+: 358.1688; found: 358.1677.
(R)-Methyl 2-((tert-butoxycarbonyl)amino)-3-((((3R,3aS,6R,7aS)-6-methyl-2-oxooctahydrobenzofuran-3-yl)methyl)thio)propanoate (43): The reaction was accomplished with N-Boc-L-cysteine methyl ester and chromatographed by n-hexane/EtOAc 2:1. Yield: 54%; white powder, mp. 88.3–90.3 °C; = −82.13 (c = 0.19, MeOH). 1H NMR (500 MHz, CDCl3): δ = 5.33 (d, J = 6.2 Hz, 1H), 4.56 (d, J = 3.2 Hz, 1H), 4.45 (d, J = 2.7 Hz, 1H), 3.78 (s, 3H), 3.08 (dd, J = 13.8, 4.2 Hz, 1H), 3.01–2.92 (m, 2H), 2.91–2.84 (m, 1H), 2.65–2.58 (m, 1H), 2.37 (ddd, J = 11.8, 9.8, 5.8 Hz, 1H), 2.25–2.19 (m, 1H), 1.81–1.74 (m, 1H), 1.72–1.64 (m, 1H), 1.61–1.51 (m, 1H), 1.45 (s, 9H), 1.25–1.16 (m, 1H), p1.06 (ddd, J =25.7, 13.2, 3.2 Hz, 1H), 0.97–0.84 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 176.8, 171.3, 155.1, 80.3, 78.5, 53.5, 52.7, 47.9, 37.4, 36.0, 35.3, 31.8, 28.3, 28.0, 26.2, 22.3, 21. 9. HRMS (ESI): m/z calcd for C19H32NO6S [M+H]+: 402.1950; found: 402.1941.
3.6. General Procedure for the Preparation of (+)-Neoisopulegol-Based Azole Adducts 44–48
Compound (−)-20 (50 mg, 0.3 mmol) and the azole derivative (0.6 mmol, 2 equiv.) were dissolved in acetonitrile (5.0 mL), and then DBU (0.3 mmol, 1 equiv.) was added. The mixture was stirred at 70 °C overnight. Then, it was evaporated to dryness, and the crude product was purified by column chromatography on silica gel with an appropriate solvent mixture.
(3S,3aS,6R,7aS)-3-((1H-Imidazol-1-yl)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (44a): The reaction was accomplished with imidazole and chromatographed by CHCl3/isopropanol 19:1. Yield: 50%; white crystals, mp. 43.5–44.3 °C; = −73.36 (c = 0.15, MeOH). 1H NMR (500 MHz, CDCl3): δ = 7.52 (s, 1H), 7.10 (s, 1H), 6.98 (s, 1H), 4.48 (d, J =2.7 Hz, 1H), 4.41 (dd, J = 14.7, 4.4 Hz, 1H), 4.07 (dd, J = 14.7, 9.8 Hz, 1H), 3.14–3.08 (m, 1H), 2.33–2.22 (m, 2H), 1.83–1.71 (m, 2H), 1.63–1.61 (m, 1H), 1.28–1.18 (m, 2H), 0.98–0.86 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 130.4, 78.7, 49.9, 42.5, 37.4, 36.0, 31.8, 26.1, 23.2, 21.9. HRMS (ESI): m/z calcd for C13H19N2O2 [M+H]+: 235.1447; found: 235.1438.
(3R,3aS,6R,7aS)-3-((1H-Imidazol-1-yl)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (44b): The reaction was accomplished with imidazole and chromatographed by CHCl3/isopropanol 19:1. Yield: 14%; white crystals, mp. 150.2–152.3 °C; = −45.30 (c = 0.20, MeOH). 1H NMR (500 MHz, CDCl3): δ =7.5 (s, 1H), 7.11 (s, 1H), 6.96 (s, 1H), 4.31–4.21 (m, 2H), 4.18 (q, J= 4.2 Hz, 1H), 2.71–2.65 (m, 1H), 2.09–2.02 (m, 2H), 1.82–1.71 (m, 1H), 1.71–1.56 (m, 2H), 1.33–1.19 (m, 2H), 1.00–0.87 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 176.5, 130.8, 78.0, 50.8, 45.5, 37.3, 35.7, 31.0, 27.0, 25.9, 21.2. HRMS (ESI): m/z calcd for C13H19N2O2 [M+H]+: 235.1447; found: 235.1438.
(3S,3aS,6R,7aS)-3-((1H-1,2,4-Triazol-1-yl)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (45a): The reaction was accomplished with 1,2,4-triazole and chromatographed by CHCl3/isopropanol 19:1. Yield: 57%; colorless oil; = −75.33 (c = 0.18, MeOH). 1H NMR (500 MHz, CDCl3): δ = 8.17 (s, 1H), 7.95 (s, 1H), 4.58 (dd, J = 14.3, 4.8 Hz, 1H), 4.51 (d, J=15.0 Hz, 1H), 4.28 (dd, J = 14.3, 9.4 Hz, 1H), 3.34–3.30 (m, 1H), 2.43–2.34 (m, 1H), 2.25 (d, J = 15.0 Hz, 1H), 1.92–1.87 (m, 1H), 1.77–1.70 (m, 1H), 1.64–1.56 (m, 1H), 1.26 (s, 1H), 1.24–1.18 (m, 1H), 0.98–0.86 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 175.9, 152.6, 78.9, 48.5, 45.0, 37.6, 36.0, 31.8, 26.2, 23.1, 21.9. HRMS (ESI): m/z calcd for C12H18N3O2 [M+H]+: 236.1399; found: 236.1390.
(3R,3aS,6R,7aS)-3-((1H-1,2,4-Triazol-1-yl)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (45b): The reaction was accomplished with 1,2,4-triazole and chromatographed by CHCl3/isopropanol 19:1. Yield: 18%; white crystals, mp. 102.5–103.1 °C; = −42.11 (c =0.17, MeOH). 1H NMR (500 MHz, CDCl3): δ = 8.12 (s, 1H), 7.96 (s, 1H), 4.52–4.41 (m, 2H), 4.38 (q, J= 4.3 Hz, 1H), 2.86–2.80 (m, 1H), 2.35–2.30 (m, 1H), 2.07 (d, J= 14.7 Hz, 1H), 1.81–1.75 (m, 1H), 1.72–1.60 (m, 2H), 1.36–1.22 (m, 2H), 1.01–0.96 (m, 1H), 0.93 (d, J= 6.58 Hz, 3H). 13C NMR (125 MHz, CDCl3): δ = 176.2, 152.6, 143.7, 78.1, 50.0, 47.7, 37.2, 35.6, 30.9, 26.8, 26.0, 21.2. HRMS (ESI): m/z calcd for C12H18N3O2 [M+H]+: 236.1399; found: 236.1390.
(3S,3aS,6R,7aS)-3-((1H-1,2,3-Triazol-1-yl)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (46a): The reaction was accomplished with 1,2,3-triazole and chromatographed by CHCl3/isopropanol 19:1. Yield: 53%; white crystals, mp. 99.3–101.2 °C; = −82.49 (c =0.18, MeOH). 1H NMR (500 MHz, CDCl3): δ = 7.71 (d, J= 11.5 Hz, 2H), 4.69 (dd, J= 14.4, 4.9 Hz, 1H), 4.57 (dd, J= 14.4, 9.1 Hz, 1H), 4.51 (d, J= 2.7 Hz, 1H), 3.34 (dt, J= 9.2, 5.5 Hz, 1H), 2.45 (ddd, J = 11.9, 9.9, 5.9 Hz, 1H), 2.26–2.25 (d, J= 15.2 Hz, 1H), 1.98–1.93 (m, 1H), 1.77–1.70 (m, 1H), 1.65–1.57 (m, 1H), 1.29–1.16 (m, 2H), 0.98–0.87 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 175.9, 134.1, 124.4, 79.0, 49.02, 45.7, 37.9, 36.0, 31.8, 26.2, 23.1, 21.9. HRMS (ESI): m/z calcd for C12H18N3O2 [M+H]+: 236.1399; found: 236.1390.
(3R,3aS,6R,7aS)-3-((1H-1,2,3-Triazol-1-yl)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (46b): The reaction was accomplished with 1,2,3-triazole and chromatographed by CHCl3/isopropanol 19:1. Yield: 28%; white crystals, mp. 101.8–104.3 °C; = −46.11 (c = 0.15, MeOH). 1H NMR (500 MHz, CDCl3): δ = 7.73 (s, 1H), 7.64 (s, 1H), 4.75–4.67 (m, 2H), 4.23 (dd, J = 8.7, 4.3 Hz, 1H), 2.80 (td, J = 6.2, 3.0 Hz, 1H), 2.36–2.31 (m, 1H), 2.03 (d, J= 15.0 Hz, 1H), 1.81–1.75 (m, 1H), 1.70–1.58 (m, 2H), 1.35–1.22 (m, 2H), 1.02–0.94 (m, 1H), 0.91 (d, J= 6.6 Hz, 3H). 13C NMR (125 MHz, CDCl3): δ = 176.3, 134.7, 124.1, 78.3, 50.3, 48.2, 37.1, 35.6, 30.8, 26.8, 26.0, 21.1. HRMS (ESI): m/z calcd for C12H18N3O2 [M+H]+: 236.1399; found: 236.1390.
(3S,3aS,6R,7aS)-3-((1H-Benzo[d]imidazol-1-yl)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (47a): The reaction was accomplished with benzimidazole and chromatographed by CHCl3/t-BuOH 19:1. Yield: 20%; white crystals, mp. 120.1–122.9 °C; = −74.60 (c = 0.22, MeOH). 1H NMR (500 MHz, CDCl3): δ = 7.97 (s, 1H), 7.83 (d, J = 7.3 Hz, 1H), 7.42 (d, J = 7.9 Hz, 1H), 7.36–7.29 (m, 2H), 4.64 (dd, J = 15.0, 4.6 Hz, 1H), 4.45 (d, J = 2.65 Hz, 1H), 4.31 (dd, J = 15.0, 9.3 Hz, 1H), 3.31–3.27 (m, 1H), 2.28–2.23 (m, 2H), 1.89–1.86 (m, 1H), 1.77–1.75 (m, 1H), 1.67–1.60 (m, 1H), 1.37–1.16 (m, 2H), 0.94–0.87 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 176.0, 144.1, 143.0, 133.4, 123.5, 122.7, 121.0, 109.4, 78.7, 48.1, 40.6, 37.4, 36.0, 31.8, 26.1, 23.3, 21.9. HRMS (ESI): m/z calcd for C17H21N2O2 [M+H]+: 285.1603; found: 285.1592.
(3R,3aS,6R,7aS)-3-((1H-Benzo[d]imidazol-1-yl)methyl)-6-methylhexahydrobenzofuran-2(3H)-one (47b): The reaction was accomplished with benzimidazole and chromatographed by CHCl3/t-BuOH 19:1. Yield: 12%; white powder, mp. 125.3–126.5 °C; = −22.77 (c = 0.21, MeOH). 1H NMR (500 MHz, CDCl3): δ = 7.92 (s, 1H), 7.83 (d, J = 7.5 Hz, 1H), 7.44 (d, J = 7.8 Hz, 1H), 7.36–7.31 (m, 2H), 4.54–4.40 (m, 3H), 2.87 (ddd, J = 7.9, 5.1, 2.7 Hz, 1H), 2.14–2.02 (m, 2H), 1.71–1.62 (m, 2H), 1.58 (d, J = 3.4 Hz, 1H), 1.32–1.17 (m, 2H), 0.95–0.86 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 142.8, 141.7, 123.6, 122.7, 120.8, 109.2, 77.8, 49.7, 43.1, 37.3, 35.5, 30.7, 26.7, 25.7, 20.9. HRMS (ESI): m/z calcd for C17H21N2O2 [M+H]+: 285.1603; found: 285.1592.
(3S,3aS,6R,7aS)-3-((1H-Benzo[d][1,2,3]triazol-1-yl)methyl)-6-methylhexahydrobenzo-furan-2(3H)-one (48a): The reaction was accomplished with benzotriazole and chromatographed by CHCl3/t-BuOH 19:1. Yield: 21%; white powder, mp. 129.8–131.7 °C; = −91.04 (c = 0.15, MeOH). 1H NMR (500 MHz, CDCl3): δ = 8.09 (d, J = 8.35 Hz, 1H), 7.59 (d, J = 8.3 Hz, 1H), 7.54 (t, J = 7.6 Hz, 1H), 7.41 (t, J = 7.6 Hz, 1H), 4.94 (dd, J = 14.6, 3.8 Hz, 1H), 4.77 (dd, J = 14.6, 10.7 Hz, 1H), 4.52 (d, J = 2.6 Hz, 1H), 3.58–3.52 (m, 1H), 2.41 (ddd, J = 11.8, 9.9, 5.9 Hz, 1H), 2.26 (d, J = 15.2 Hz, 1H), 2.08–2.02 (m, 1H), 1.78–1.70 (m, 1H), 1.68–1.58 (m, 1H), 1.34–1.18 (m, 2H), 0.97–0.86 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 176.0, 127.9, 124.4, 120.4, 109.2, 79.1, 48.3, 43.4, 37.6, 36.0, 31.8, 26.2, 23.1, 22.0. HRMS (ESI): m/z calcd for C16H20N3O2 [M+H]+: 286.1556; found: 286.1546.
(3R,3aS,6R,7aS)-3-((1H-Benzo[d][1,2,3]triazol-1-yl)methyl)-6-methylhexahydrobenzo-furan-2(3H)-one (48b): The reaction was accomplished with benzotriazole and chromatographed by CHCl3/t-BuOH 19:1. Yield: 12%; white crystals, mp. 135.9–137.5 °C; = −38.03 (c = 0.19, MeOH). 1H NMR (500 MHz, CDCl3): δ = 8.08 (d, J= 8.4 Hz, 1H), 7.61 (d, J = 8.3 Hz, 1H), 7.54 (t, J = 7.6 Hz, 1H), 7.41 (t, J = 7.6 Hz, 1H), 4.94 (qd, J = 14.6, 6.7 Hz, 2H), 4.36 (q, J = 3.9 Hz, 1H), 2.94–2.92 (m, 1H), 2.4–2.36 (m, 1H), 2.08 (d, J = 15.5 Hz, 1H), 1.69–1.58 (m, 2H), 1.28–1.16 (m, 3H), 0.94–0.86 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 128.3, 124.6, 120.4, 109.4, 78.5, 50.3, 46.2, 37.2, 35.7, 31.3, 27.3, 25.9, 21.4. HRMS (ESI): m/z calcd for C16H20N3O2 [M+H]+: 286.1556; found: 286.1545.
3.7. General Procedure for the Ring Opening of Lactones with NH3 (Synthesis of 31, 36, and 49a–b)
To a solution of lactone 23, 33, or 47a–b (0.36 mmol) in MeOH (2.0 mL), a solution of 25% NH3 in MeOH (5.0 mL) was added. The mixture was stirred at room temperature for 12 h. After the completion of the reaction (as monitored by TLC), the mixture was evaporated to dryness. The crude product was purified by column chromatography on silica gel then recrystallized in Et2O, resulting in compounds 31, 36, and 49a–b, respectively.
(S)-3-(Benzylamino)-2-((1S,2S,4R)-2-hydroxy-4-methylcyclohexyl)propanamide (31): Recrystallized in Et2O. Yield: 94%; white powder; mp. 140.3–142.5 °C; = +27.52 (c = 0.20, MeOH). ¹H NMR (500 MHz, CD3OD): δ = 7.31 (dd, J = 7.3, 5.9 Hz, 4H), 7.27–7.22 (m, 1H), 3.81–3.70 (m, 2H), 3.40 (td, J = 10.6, 4.2 Hz, 1H), 2.98–2. 89 (m, 2H), 2.71–2.65 (m, 1H), 1.95 (d, J = 12.2 Hz, 1H), 1.71–1.55 (m, 3H), 1.46–1.34 (m, 1H), 1.15–1.04 (m, 1H), 0.96 (dd, J = 23.4, 12.0 Hz, 1H), 0.91 (d, J = 6.5 Hz, 3H), 0.89–0.79 (m, 1H).¹³C NMR (125 MHz, CD3OD) δ = 129.5, 129.5, 128.2, 71.6, 54.6, 48.3, 47.1, 46.7, 45.7, 35.7, 32.8, 27.8, 22.5. HRMS (ESI) m/z: calcd for C17H27N2O2[M+H]+: 291.2073; found: 291.2063.
(R)-3-(Benzylthio)-2-((1S,2S,4R)-2-hydroxy-4-methylcyclohexyl)propanamide (36): Chromatographed by CHCl3/MeOH 19:1. Yield: 95%; white powder, mp. 156.3–158.6 °C; = +86.75 (c = 0.16, MeOH). 1H NMR (500 MHz, CDCl3): δ = 7.36–7.29 (m, 4H), 7.27–7.23 (m, 1H), 5.62 (s, 1H), 5.39 (s, 1H), 3.94 (d, J = 1.8 Hz, 1H), 3.78–3.69 (m, 2H), 2.71 (d, J = 7.1 Hz, 2H), 2.32 (dd, J = 15.5, 7.1, 1H), 1.79 (ddd, J = 13.8, 5.9, 3.3 Hz, 1H), 1.75–1.63 (m, 2H), 1.59–1.51 (m, 1H), 1.51–1.44 (m, 1H), 1.44–1.33 (m, 1H), 1.17–1.10 (m, 1H), 0.94–0.83 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 176.6, 138.7, 129.2, 128.7, 127.3, 67.5, 49.4, 43.3, 42.5, 37.3, 34.6, 31.9, 25.9, 24.6, 22.3. HRMS (ESI) m/z: calcd for C17H26NO2S [M+H]+: 308.1684; found: 308.1675.
(S)-3-(1H-Benzo[d]imidazol-1-yl)-2-((1S,2S,4R)-2-hydroxy-4-methylcyclohexyl)-propanamide (49a): Chromatographed by CHCl3/acetone 9:1. Yield: 59%; mp. 263.3–265.0 °C; = +89.60 (c = 0.20, MeOH). 1H NMR (500 MHz, CD3OD): δ = 8.00 (s, 1H), 7.64 (t, J = 8.2 Hz, 2H), 7.31 (t, J = 7.6 Hz, 1H), 7.26 (t, J = 7.6 Hz, 1H), 4.58 (dd, J = 13.9, 3.8 Hz, 1H), 4.47 (dd, J = 22.0, 10.3 Hz, 2H), 4.22 (s, 1H), 2.99 (td, J1 = 10.9, 3.7 Hz, 1H), 1.94 (dd, J = 13.9, 2.4 Hz, 1H), 1.89–1.79 (m, 1H), 1.76–1.67 (m, 1H), 1.60–1.52 (m, 2H), 1.52–1.45 (m, 1H), 1.32–1.20 (m, 1H), 0.98 (td, J = 12.6, 3.9 Hz, 1H),
0.95–0.90 (m, 3H). 13C NMR (125 MHz, CD3OD): δ = 124.2, 123.5, 120.0, 111.8, 66.9, 50.3, 43.7, 42.6, 35.7, 26.9, 26.4, 22.7. HRMS (ESI) m/z: calcd for C17H24N3O2 [M+H]+: 302.1868; found: 302.1859.
(R)-3-(1H-Benzo[d]imidazol-1-yl)-2-((1S,2S,4R)-2-hydroxy-4-methylcyclohexyl)-propanamide (49b): Chromatographed by CHCl3/acetone 9:1. Yield: 69%; white crystals, mp. 240.4–243.3 °C; = −48.20 (c = 0.20, MeOH). 1H NMR (500 MHz, CD3OD): δ = 0.92 (d, J = 6.3 Hz, 3H), 0.99–1.11 (m, 1H), 1.15–1.21 (m, 1H), 1.66–1.80 (m, 2H), 1.80–1.95 (m, 4H), 3.03 (ddd, J1 = 4.5 Hz, J2 = 8.0 Hz, J3 = 12.0 Hz, 1H), 4.01 (s, 1H), 4.50–4.62 (m, 2H), 7.28 (t, J = 7.6 Hz, 1H), 7.34 (t, J = 7.6 Hz, 1H), 7.60 (d, J = 8.1 Hz, 1H), 7.66 (d, J = 8.0 Hz, 1H), 8.04 (s, 1H). 13C NMR (125 MHz, CD3OD): δ = 124.3, 123.5, 120.1, 111.6, 68.9, 50.8, 46.3, 43.7, 43.5, 35.9, 26.8, 24.8, 22.6. HRMS (ESI) m/z: calcd for C17H24N3O2 [M+H]+: 302.1868; found: 302.1859.
3.8. General Procedure for the Ring Opening of Lactones with Benzylamine (Synthesis of 32, 37, and 50a–b)
To a solution of lactone 23, 33, or 47a–b (0.36 mmol) in dry THF (5.0 mL), benzylamine (0.72 mmol) was added. The mixture was stirred at 70 °C for 48 h. When the reaction was completed, the mixture was evaporated to dryness. The crude product was purified by column chromatography on silica gel mixture, then recrystallized in n-hexane/Et2O, resulting in compounds 32, 37, and 50a–b, respectively.
(S)-N-Benzyl-3-(benzylamino)-2-((1S,2S,4R)-2-hydroxy-4-methylcyclohexyl)-propanamide (32): Chromatographed by CHCl3/CH3OH 19:1. Yield: 70%; white powder, mp. 211.0–212.7 °C; = +21.05 (c = 0.20, MeOH). 1H NMR (500 MHz, DMSO-d6): δ = 9.18 (s, 1H), 9.06 (s, 1H), 8.75 (t, J = 5.7 Hz, 1H), 7.54 (d, J = 4.0 Hz, 2H), 7.42 (d, J = 4.9 Hz, 3H), 7.35–7.20 (m, 5H), 4.74 (s, 1H), 4.43–4.15 (m, 2H), 4.11 (s, 2H), 3.80 (s, 1H), 3.23–3.12 (m, 1H), 3.05 (d, J = 11.8 Hz, 1H), 2.79–2.71 (m, 1H), 1.75–1.63 (d, J = 11.2 Hz, 2H), 1.60–1.50 (m, 2H), 1.47–1.36 (m, 1H), 1.18 (d, J = 10.2 Hz, 1H), 1.01 (t, J = 13.0 Hz, 1H), 0.80–0.74 (m, 4H). 13C NMR (125 MHz, DMSO-d6): δ = 172.3, 139.0, 131.8, 130.1, 128.9, 128.6, 128.2, 127.5, 126.9, 64.0, 50.1, 45.3, 44.3, 42.9, 41.9, 41.3, 34.3, 25.1, 24.4, 22.2. HRMS (ESI) m/z: calcd for C24H33N2O2 [M+H]+: 381.2542; found: 381.2530.
(R)-N-Benzyl-3-(benzylthio)-2-((1S,2S,4R)-2-hydroxy-4-methylcyclohexyl)propanamide (37): Chromatographed by n-hexane/EtOAc 2:1. Yield: 54%; white powder, mp. 172.1–175.4 °C; = +84.00 (c = 0.17, MeOH). 1H NMR (500 MHz, CDCl3): δ = 7.38–7.16 (m, 10H), 5.77 (t, J = 4.8 Hz, 1H), 4.48 (dd, J = 14.7, 5.7 Hz, 1H), 4.39 (dd, J = 14.7, 5.5 Hz, 1H), 3.94 (s, 1H), 3.74–3.64 (m, 2H), 2.74 (d, J = 7.2 Hz, 2H), 2.20 (dd, J = 15.9, 7.2 Hz, 1H), 1.78 (ddd, J = 13.8, 5.7, 3.2 Hz, 1H), 1.72–1.58 (m, 3H), 1.44–1.37 (m, 1H), 1.36–1.27 (m, 1H), 1.18–1.06 (m, 1H), 0.93–0.82 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 174.0, 138.9, 138.3, 129.1, 128.8, 128.7, 128.2, 127.6, 127.3, 67.2, 50.3, 43.8, 43.5, 42.5, 37.4, 34.6, 32.2, 25.9, 24.8, 22.3. HRMS (ESI) m/z: calcd for C24H32NO2S [M+H]+: 398.2154; found: 398.2144.
(S)-3-(1H-Benzo[d]imidazol-1-yl)-N-benzyl-2-((1S,2S,4R)-2-hydroxy-4-methylcyclo-hexyl)propanamide (50a): Chromatographed by CHCl3/t-BuOH 19:1. Yield: 39%; white powder, mp. 181.0–184.2 °C; = −52.53 (c = 0.23, MeOH). 1H NMR (500 MHz, CDCl3): δ = 7.86 (s, 1H), 7.84–7.77 (m, 1H), 7.42–7.36 (m, 1H), 7.28 (d, J = 4.5 Hz, 2H), 7.18 (d, J = 3.3 Hz, 3H), 6.89–6.82 (m, 2H), 5.78 (s, 1H), 4.65 (dd, J = 13.5, 11.9 Hz, 1H), 4.35 (dd, J = 14.0, 3.1 Hz, 1H), 4.26 (dd, J = 14.7, 5.6 Hz, 1H), 4.15 (dd, J = 14.7, 5.6 Hz, 1H), 3.96 (s, 1H), 2.80–2.70 (m, 1H), 2.05 (s, 1H), 1.86–1.73 (m, 5H), 1.72–1.60 (m, 1H), 1.19 (t, J = 13.6 Hz, 1H), 1.06–0.95 (m, 1H), 0.91 (d, J = 6.1 Hz, 3H). 13C NMR (125 MHz, CDCl3): δ = 173.0, 144.0, 143.9, 137.4, 133.6, 128.8, 127.7, 127.6, 123.2, 122.4, 120.7, 109.6, 67.6, 51.3, 45.6, 43.9, 42.4, 42.5, 34.7, 25.8, 24.8, 22.2. HRMS (ESI) m/z: calcd for C24H30N3O2 [M+H]+: 392.2338; found: 392.2326.
(R)-3-(1H-Benzo[d]imidazol-1-yl)-N-benzyl-2-((1S,2S,4R)-2-hydroxy-4-methylcyclo-hexyl)propanamide (50b): Chromatographed by CHCl3/t-BuOH 19:1. Yield: 60%; white powder, mp. 258.2–260.2 °C; = +41.23 (c = 0.21, MeOH). 1H NMR (500 MHz, CD3OD): δ = 7.97 (s, 1H), 7.69 (d, J = 7.5 Hz, 1H), 7.65 (d, J = 7.8 Hz, 1H), 7.34–7.26 (m, 2H), 7.14–7.06 (m, 3H), 6.71–6.66 (m, 2H), 4.60 (dd, J = 13.8, 3.9 Hz, 1H), 4.50 (t, J = 12.7 Hz, 1H), 4.26–4.15 (m, 2H), 3.95 (d, J = 14.9 Hz, 1H), 3.01 (td, J = 11.0, 3.9 Hz, 1H), 1.93 (dd, J = 13.8, 1.9 Hz, 1H), 1.88–1.80 (m, 1H), 1.80–1.72 (m, 1H), 1.69 (d, J = 12.9 Hz, 1H), 1.50 (qd, J = 13.0, 3.2 Hz, 1H), 1.37 (dd, J = 13.0, 3.2 Hz, 1H), 1.39–1.21 (m, 1H), 1.00–0.88 (m, 4H). 13C NMR (125 MHz, CD3OD): δ = 175.4, 145.0, 144.0, 139.1, 134.9, 129.3, 128.2, 128.0, 124.3, 123.5, 120.1, 111.9, 66.9, 50.8, 46.0, 43.9, 43.6, 42.9, 35.6, 26.9, 26.4, 22.7. HRMS (ESI) m/z: calcd for C24H30N3O2 [M+H]+: 392.2338; found: 392.2327.
3.9. Determination of the Antimicrobial Effect
3.9.1. Reagents and Media
DMSO (Sigma-Aldrich, St Louis, MO, USA), phosphate-buffered saline (PBS; pH 7.4), Mueller–Hinton (MH) broth, tryptic soy broth (TSB), tryptic soy agar (TSA), Luria–Bertani broth (LBB), and Luria–Bertani agar (LBA) were used. All reagents were purchased from Sigma.
3.9.2. Bacterial Strains
Escherichia coli ATCC 25922 was used as the Gram-negative strain. Gram-positive strains investigated in this study included the following: Staphylococcus aureus American Type Culture Collection (ATCC) 25923 as a methicillin-susceptible reference strain, and the methicillin- and oxacillin-resistant S. aureus MRSA ATCC 43300 strain.
3.9.3. Antibacterial Activity
The minimum inhibitory concentrations (MICs) of the compounds were determined according to the Clinical and Laboratory Standard Institute guidelines [41]. Two-fold serial dilutions of the compounds were prepared in Mueller–Hinton broth in 96-well plates, and the starting concentration of the compounds was 100 µM (Sigma-Aldrich, St. Louis, MO, USA). The MIC values of the compounds were determined by visual inspection. DMSO as a solvent was also assayed to ensure that it had no antibacterial effect.
3.10. Computational Studies
The crystal structures of the protein templates were obtained from PDB (protein data bank), and the tested structures were drawn by ChemBioDraw Ultra 12.0. The Accelrys Discovery Studio 3.5 software was used to perform the docking study and the in silico ADMET prediction.
3.10.1. Preparation of the Crystal Structures of the Protein Targets
The structures were prepared by using the Accelrys Discovery Studio 3.5 software’s Clean Geometry option after eliminating the water molecules. Then, the absent hydrogen atoms were furnished by applying the CHARMm force field. Adopted Basis minimization was utilized to minimize the complex energy and to get the most stable structure without altering the basic protein skeleton. Thereafter, a 10 Å radius sphere was created to define the active site residues [46].
3.10.2. Docking Study (CDOCKER)
The CDOCKER method enables the generation of all possible conformations of the compound within the protein’s active site. These conformations were then assessed according to both the CDOCKER energy and the interactions observed between the ligand and the active site. This approach requires preparing the crystal structure (as previously described) and the examined compound, which involves using the Accelrys Discovery Studio protocol and applying a force field. Before initiating this study, it is crucial to validate the method by comparing the conformation of the reference compound to the conformations generated by the docking approach, ensuring that the RMSD (Root Mean Square Deviation) remains within the acceptable range.
3.10.3. In Silico ADMET prediction
The ADMET properties of compounds 28 and 30 were predicted using ADMET descriptors in the Accelrys Discovery Studio 3.5 software. Multiple mathematical models were employed for quantitative prediction. These models included the following: aqueous solubility (predict solubility in water at 25 °C), blood–brain barrier (BBB) penetration, cytochrome P450 (CYP2D6) inhibition, human intestinal absorption (HIA), and plasma protein binding (PPB) [47]. An ADMET model was also generated to predict the human intestinal absorption (HIA) and blood–brain barrier (BBB) penetration of the tested compounds. The model shows the ADMET_PSA_2D and ADMET_ALogP98 plot with 95 and 99% confidence limit ellipses (Figure S87 in Supplementary Materials).
4. Conclusions
In summary, starting from the commercially available (−)-isopulegol, a new family of Michael-type amino and thiol adducts were prepared and characterized through chiral (+)-neoisopulegol as a key intermediate via stereoselective transformations.
The resulting β-aminolactones exert selective inhibition for the Gram-positive bacteria S. aureus. In vitro pharmacological studies have clearly shown that the N-naphthylmethyl substituent on the β-aminolactone function is essential.
Finally, molecular docking results exemplified that the β-aminolactones 28 and 30 could foster potent affinity by forming significant interactions with DNA gyrase (PDB code: 3U2D) and C(30) carotenoid dehydrosqualene synthase (PDB code: 2ZCQ). Hence, N-naphthylmethyl-substituted β-aminolactone has the potential to be developed into clinically important therapeutic choices for the treatment of infections caused by S. aureus.
While the thiol and azole Michael adducts showed no notable antimicrobial activity in our assays, their structurally diverse and heteroatom-rich frameworks suggest potential for alternative bioactivities. This motivates further pharmacological screening, especially given prior reports of such scaffolds in biomedical research.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms26104791/s1.
Author Contributions
T.M.L. and G.S. conceived and designed the experiments. R.M. and N.S. performed the experiments, analyzed the data, and wrote the experimental part. Z.S., T.M.L. and A.M.R. discussed the results and contributed to the writing of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for financial support from the Hungarian Research Foundation (NKFI K138871). Project no. TKP2021-EGA-32 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund and also by the University of Szeged Open Access Fund (grant No. 7172). N.S. was supported by the ÚNKP-23-4-SZTE-347 New National Excellence Program of the Ministry for Innovation and Technology sourced from the National Research, Development and Innovation Fund. G.S. was supported by the János Bolyai Research Scholarship (BO/00158/22/5) of the Hungarian Academy of Sciences and by the ÚNKP-23-5-SZTE-677 New National Excellence Program of the Ministry for Culture and Innovation sourced from the National Research, Development and Innovation Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The HR-MS analysis was performed by Róbert Berkecz.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wetzel, S.; Bon, R.S.; Kumar, K.; Waldmann, H. Biology-Oriented Synthesis. Angew. Chem. Int. Ed. 2011, 50, 10800–10826. [Google Scholar] [CrossRef] [PubMed]
- Karageorgis, G.; Waldmann, H. Guided by Evolution: Biology-Oriented Synthesis of Bioactive Compound Classes. Synthesis 2019, 51, 55–66. [Google Scholar] [CrossRef]
- Gaich, T.; Mulzer, J. Chiral Pool Synthesis: Starting from Terpenes. In Comprehensive Chirality; Mulzer, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 2, pp. 163–206. [Google Scholar]
- Pacuła-Miszewska, A.J.; Obieziurska-Fabisiak, M.; Ścianowski, J. Monoterpenes as Chiral Building Blocks. In Chiral Building Blocks in Asymmetric Synthesis: Synthesis and Applications; Wojaczýnska, E., Wojaczýnski, J., Eds.; Wiley-VCH: Weinheim, Germany, 2022; pp. 235–266. [Google Scholar]
- Le, M.T.; Szakonyi, Z. Enantiomeric Isopulegol as the Chiral Pool in the Total Synthesis of Bioactive Agents. Chem. Rec. 2022, 22, e202100194. [Google Scholar] [CrossRef]
- Szakonyi, Z.; Fülöp, F. Monoterpene-based chiral β-amino acid derivatives prepared from natural sources: Syntheses and applications. Amino Acids 2011, 41, 597–608. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef]
- Picman, A.K. Biological Activities of Sesquiterpene Lactones. Biochem. Syst. Ecol. 1986, 14, 255–281. [Google Scholar] [CrossRef]
- Schmidt, T.J. Structure-Activity Relationships of Sesquiterpene Lactones. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 33, pp. 309–392. [Google Scholar]
- Paço, A.; Brás, T.; Santos, J.O.; Sampaio, P.; Gomes, A.C.; Duarte, M.F. Anti-Inflammatory and Immunoregulatory Action of Sesquiterpene Lactones. Molecules 2022, 27, 1142. [Google Scholar] [CrossRef]
- Woods, J.R.; Mo, H.; Bieberich, A.A.; Alavanja, T.; Colby, D.A. Amino-Derivatives of the Sesquiterpene Lactone Class of Natural Products as Prodrugs. Med. Chem. Commun. 2013, 4, 27–33. [Google Scholar] [CrossRef]
- Li, X.; Payne, D.T.; Ampolu, B.; Bland, N.; Brown, J.T.; Dutton, M.J.; Fitton, C.A.; Gulliver, A.; Hale, L.; Hamza, D.; et al. Derivatisation of Parthenolide to Address Chemoresistant Chronic Lymphocytic Leukaemia. Med. Chem. Commun. 2019, 10, 1379–1390. [Google Scholar] [CrossRef]
- Cala, A.; Zorrilla, J.G.; Rial, C.; Molinillo, J.M.G.; Varela, R.M.; Macías, F.A. Easy Access to Alkoxy, Amino, Carbamoyl, Hydroxy, and Thiol Derivatives of Sesquiterpene Lactones and Evaluation of Their Bioactivity on Parasitic Weeds. J. Agric. Food Chem. 2019, 67, 10764–10773. [Google Scholar] [CrossRef]
- Kumar, C.; Kumar, A.; Nalli, Y.; Lone, W.I.; Satti, N.K.; Verma, M.K.; Ahmed, Z.; Ali, A. Design, Synthesis and Biological Evaluation of Alantolactone Derivatives as Potential Anti-Inflammatory Agents. Med. Chem. Res. 2019, 28, 849–856. [Google Scholar] [CrossRef]
- Quy, A.S.; Li, X.; Male, L.; Stankovic, T.; Agathanggelou, A.; Fossey, J.S. Aniline-containing derivatives of parthenolide: Synthesis and anti-chronic lymphocytic leukaemia activity. Tetrahedron 2020, 76, 131631. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Jiang, L.; Li, T.; Song, L.; Wu, L.; Cao, H.; Yang, C. Natural Product-Based Semisynthesis and Biological Evaluation of Thiol/Amino-Michael Adducts of Xanthatin Derived from Xanthium Strumarium as Potential Pesticidal Agents. Bioinorg. Chem. 2020, 97, 103696. [Google Scholar] [CrossRef]
- Klochkov, S.G.; Neganova, M.E.; Pukhov, S.A.; Afanas’eva, S.V.; Aleksandrova, Y.R.; Yandulova, E.Y. New Arteannuin B Derivatives and Their Cytotoxic Activity. Chem. Nat. Compd. 2020, 56, 445–451. [Google Scholar] [CrossRef]
- Ordóñez, P.E.; Mery, D.E.; Sharma, K.K.; Nemu, S.; Reynolds, W.F.; Enriquez, R.G.; Burns, D.C.; Malagón, O.; Jones, D.E.; Guzman, M.L.; et al. Synthesis, Crystallography, and Anti-Leukemic Activity of the Amino Adducts of Dehydroleucodine. Molecules 2020, 25, 4825. [Google Scholar] [CrossRef]
- Zaki, M.; Loubidi, M.; Bilgiç, T.; Birim, D.; Akssira, M.; Dagcı, T.; Berteina-Raboin, S.; Saso, L.; Khouili, M.; Armagan, G. Design, Synthesis, and Biological Evaluation of Novel Tomentosin Derivatives in NMDA-Induced Excitotoxicity. Pharmaceuticals 2022, 15, 421. [Google Scholar] [CrossRef]
- Patrushev, S.S.; Vasil’eva, D.O.; Burova, L.G.; Bondareva, E.A.; Zakharova, L.N.; Evstropov, A.N.; Shults, E.E. Synthesis and Evaluation of Antibacterial Activity of Bis-Eudesmanolides Connected by Nitrogen-Containing Linkers. Russ. Chem. Bull. 2023, 72, 2513–2524. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Li, Z.-H.; Liu, J.-K. A new menthane-type monoterpene from Pleurotus eryngii. Chin. J. Nat. Med. 2013, 11, 71–73. [Google Scholar] [CrossRef]
- Wang, S.; Bao, L.; Zhao, F.; Wang, Q.; Li, S.; Ren, J.; Li, L.; Wen, H.; Guo, L.; Liu, H. Isolation, Identification, and Bioactivity of Monoterpenoids and Sesquiterpenoids from the Mycelia of Edible Mushroom Pleurotus cornucopiae. J. Agric. Food Chem. 2013, 61, 5122–5129. [Google Scholar] [CrossRef]
- Poli, J.-P.; Guinoiseau, E.; de Rocca Serra, D.; Sutour, S.; Paoli, M.; Tomi, F.; Quilichini, Y.; Berti, L.; Lorenzi, V. Anti-Quorum Sensing Activity of 12 Essential Oils on chromobacterium violaceum and Specific Action of cis-cis-p-Menthenolide from Corsican Mentha suaveolens ssp. Molecules 2018, 23, 2125. [Google Scholar] [CrossRef]
- Hayashi, T.; Shinbo, T.; Shimizu, M.; Arisawa, M.; Morita, N.; Kimura, M.; Matsuda, S.; Kikuchi, T. Paeonilactone-A, -B, and -C, New Monoterpenoids from Paeony Root. Tetrahedron Lett. 1985, 26, 3699–3702. [Google Scholar] [CrossRef]
- Qin, X.-Y.; Chen, B.-Y.; Fu, J.-J.; Shan, L.; Lei, X.-G.; Zhang, W.-D. Synthesis, cytotoxicity and inhibition of NO production of ivangustin enantiomer analogues. Eur. J. Med. Chem. 2015, 102, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Touge, T.; Sakaguchi, K.; Tamaki, N.; Nara, H.; Yokozawa, T.; Matsumura, K.; Kayaki, Y. Multiple Absolute Stereocontrol in Cascade Lactone Formation via Dynamic Kinetic Resolution Driven by the Asymmetric Transfer Hydrogenation of Keto Acids with Oxo-Tethered Ruthenium Catalysts. J. Am. Chem. Soc. 2019, 141, 16354–16361. [Google Scholar] [CrossRef] [PubMed]
- Serra, S.; Fuganti, C. Natural p-Menthene Monoterpenes: Synthesis of the Enantiomeric Forms of Wine Lactone, Epi-wine Lactone, Dill Ether, and Epi-dill Ether Starting from a Common Intermediate. Helv. Chim. Acta 2004, 87, 2100–2109. [Google Scholar] [CrossRef]
- Serra, S.; Fuganti, C. Enzyme-Mediated Preparation of Enantiomerically Pure p-Menthan-3,9-diols and Their Use for the Synthesis of Natural p-Menthane Lactones and Ethers. Helv. Chim. Acta 2002, 85, 2489–2502. [Google Scholar] [CrossRef]
- Le, M.T.; Bérdi, P.; Zupkó, I.; Fülöp, F.; Szakonyi, Z. Synthesis and Transformation of (-)-Isopulegol-Based Chiral β-Aminolactones and β-Aminoamides. Int. J. Mol. Sci. 2018, 19, 3522. [Google Scholar] [CrossRef]
- Le, M.T.; Huynh, T.; Endre, G.; Szekeres, A.; Fülöp, F.; Szakonyi, Z. Stereoselective synthesis and application of isopulegol-based bi- and trifunctional chiral compounds. RSC Adv. 2020, 10, 38468–38477. [Google Scholar] [CrossRef]
- Bajtai, A.; Németi, G.; Le, T.M.; Szakonyi, Z.; Péter, A.; Ilisz, I. Enantiomeric Separation of Newly Synthesized Amino, Thio, and Oxy Derivatives of Monoterpene Lactones, Amides, and Ester Applying Polysaccharide-Based Chiral Stationary Phases in Normal-Phase Mode. J. Chromatogr. A 2022, 1672, 463050. [Google Scholar] [CrossRef]
- Németi, G.; Berkecz, R.; Le, T.M.; Szakonyi, Z.; Péter, A.; Ilisz, I. High-Performance Liquid Chromatographic Enantioseparation of Azole Analogs of Monoterpene Lactones and Amides Focusing on the Separation Characteristics of Polysaccharide-Based Chiral Stationary Phases. J. Chromatogr. A 2024, 1717, 464660. [Google Scholar] [CrossRef]
- Le, M.T.; Szilasi, T.; Volford, B.; Szekeres, A.; Fülöp, F.; Szakonyi, Z. Stereoselective Synthesis and Investigation of Isopulegol-Based Chiral Ligands. Int. J. Mol. Sci. 2019, 20, 4050. [Google Scholar] [CrossRef]
- Bal, B.S.; Pinnick, H.W. Total Synthesis of p-Menthenolides. Heterocycles 1981, 16, 2091–2104. [Google Scholar] [CrossRef]
- Brocksom, T.J.; dos Santos, R.B.; Varanda, N.A.; Brocksom, U. An Efficient Synthesis of Monoterpene α-Methylene-γ-Butyrolactones. Synth. Commun. 1988, 18, 1403–1410. [Google Scholar] [CrossRef]
- Friedrich, D.; Bohlmann, F. Total Synthesis of Various Elemanolides. Tetrahedron 1988, 44, 1369–1392. [Google Scholar] [CrossRef]
- Schlosser, M.; Kotthaus, M. Isopulegol as a Model Compound: Metalation and Substitution of an Allylic Position in the Presence of an Unprotected Hydroxy Function. Eur. J. Org. Chem. 1999, 1999, 459–462. [Google Scholar] [CrossRef]
- Moreira, J.A.; Corrêa, A.G. Enantioselective synthesis of three stereoisomers of 5,9-dimethylpentadecane, sex pheromone component of Leucoptera coffeella, from (−)-isopulegol. Tetrahedron Asymmetry 2003, 14, 3787–3795. [Google Scholar] [CrossRef]
- Escobar, Z.; Bjartell, A.; Canesin, G.; Evans-Axelsson, S.; Sterner, O.; Hellsten, R.; Johansson, M.H. Preclinical Characterization of 3β-(N-Acetyl L-Cysteine Methyl Ester)-2aβ,3-Dihydrogaliellalactone (GPA512), a Prodrug of a Direct STAT3 Inhibitor for the Treatment of Prostate Cancer. J. Med. Chem. 2016, 59, 4551–4562. [Google Scholar] [CrossRef]
- Lone, S.H.; Bhat, K.A.; Shakeel-u-Rehman; Majeed, R.; Hamid, A.; Khuroo, M.A. Synthesis and Biological Evaluation of Amino Analogs of Ludartin: Potent and Selective Cytotoxic Agents. Bioorg. Med. Chem. Lett. 2013, 23, 4931–4934. [Google Scholar] [CrossRef]
- Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: M07-A10, Clinical and Laboratory Standards Institute, Ed.; Documents/Clinical and Laboratory Standards Institute, 10th ed.; Approved Standard; Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2015; Volume 35, ISBN 978-1-56238-987-1PA. [Google Scholar]
- Bouley, R.; Kumarasiri, M.; Peng, Z.; Otero, L.H.; Song, W.; Suckow, M.A.; Schroeder, V.A.; Wolter, W.R.; Lastochkin, E.; Antunes, N.T.; et al. Discovery of Antibiotic (E)-3-(3-Carboxyphenyl)-2-(4-Cyanostyryl)Quinazolin-4(3 H)-One. J. Am. Chem. Soc. 2015, 137, 1738–1741. [Google Scholar] [CrossRef]
- Heaslet, H.; Harris, M.; Fahnoe, K.; Sarver, R.; Putz, H.; Chang, J.; Subramanyam, C.; Barreiro, G.; Miller, J.R. Structural Comparison of Chromosomal and Exogenous Dihydrofolate Reductase from Staphylococcus Aureus in Complex with the Potent Inhibitor Trimethoprim. Proteins 2009, 76, 706–717. [Google Scholar] [CrossRef]
- Eakin, A.E.; Green, O.; Hales, N.; Walkup, G.K.; Bist, S.; Singh, A.; Mullen, G.; Bryant, J.; Embrey, K.; Gao, N.; et al. Pyrrolamide DNA Gyrase Inhibitors: Fragment-Based Nuclear Magnetic Resonance Screening To Identify Antibacterial Agents. Antimicrob. Agents Chemother. 2012, 56, 1240–1246. [Google Scholar] [CrossRef]
- Liu, C.-I.; Liu, G.Y.; Song, Y.; Yin, F.; Hensler, M.E.; Jeng, W.-Y.; Nizet, V.; Wang, A.H.-J.; Oldfield, E. A Cholesterol Biosynthesis Inhibitor Blocks Staphylococcus Aureus Virulence. Science 2008, 319, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Rampogu, S.; Baek, A.; Son, M.; Park, C.; Yoon, S.; Parate, S.; Lee, K.W. Discovery of Lonafarnib-Like Compounds: Pharmacophore Modeling and Molecular Dynamics Studies. ACS Omega 2020, 5, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, J.; Hu, C.Q.; Zhang, X.; Ma, B.; Zhang, P. In Silico ADME and Toxicity Prediction of Ceftazidime and Its Impurities. Front. Pharmacol. 2019, 10, 434. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).