Abstract
Recurrent spontaneous miscarriage (RSM) is defined as the loss of three or more clinically recognized pregnancies before 20 weeks of gestation. Angiogenesis, a crucial process in early pregnancy, is regulated by vascular endothelial growth factor (VEGF), a protein that plays a pivotal role in successful pregnancy. Disruptions in vascular development, such as those due to variations in VEGF gene expression, may contribute to infertility and pregnancy complications. Therefore, there is a need for more studies that show the effect of VEGF on RSM. This study investigated the impact of VEGF gene polymorphisms on RSM in Saudi women. Blood samples were collected from 200 Saudi women (100 cases with RSM and 100 controls). DNA was extracted from the buffy coat and analyzed for VEGF polymorphisms (rs10434, rs3025053, rs699947, rs2010963, rs833061, and rs25648) using TaqMan Real-Time PCR. Plasma VEGF levels were measured using the Human VEGF ELISA Kit. There was no significant association between rs10434, rs833061, and rs25648 and RSM. However, rs2010963, rs3025053, and rs699947 were significantly associated with an increased risk of miscarriage (p < 0.05). Furthermore, VEGF concentrations were significantly lower in the RSM case group (both pregnant and non-pregnant) compared to the control group (p < 0.05). VEGF polymorphisms, along with reduced VEGF serum levels, are associated with an increased risk of RSM in Saudi women. Further studies are needed to explore the underlying mechanisms and potential therapeutic targets.
1. Introduction
Recurrent spontaneous miscarriage (RSM) is defined as three or more consecutive pregnancy losses before 20 weeks of gestation [1,2,3]. Genetic abnormalities are a leading cause of early pregnancy loss, with approximately 50% of first-trimester miscarriages exhibiting chromosomal abnormalities [4,5].
Various factors contribute to RSM, including immunological disorders, antiphospholipid syndrome, thrombophilia, smoking, alcohol consumption, age, environmental pollutants, radiation exposure, uterine abnormalities, infections, and endocrine imbalances [6,7]. RSM is associated with adverse pregnancy outcomes, including placental abruption, fetal growth restriction, stillbirth, and preterm birth [8].
Vascular endothelial growth factor (VEGF) is a widely recognized angiogenic factor that plays a crucial role in various physiological and pathological processes [9]. The vascular endothelial growth factor (VEGF) gene is located within a specific region of chromosome 6p21.3 and has a complex structural composition consisting of eight exons interspersed with seven introns [10].
VEGF is a key regulator of angiogenesis and is critical for embryo implantation, placental development, and endothelial cell function. Alterations in VEGF expression have been linked to recurrent implantation failure and RSM [11]. In 2021, a study was conducted to examine the relationships between recurrent implantation failure (RIF) and vascular endothelial growth factor (VEGF) polymorphisms. It was determined that women with VEGF 1154 AA/GA genotypes were at higher risk of recurrent implantation failure (RIF). The VEGF-1154A allele may serve as one of the predisposing factors of RIF [12]. In another study, patients with the ID genetic model of -2549I/D polymorphisms in the promoter region of the VEGFA gene, along with the presence of the D allele, are at a greater risk for idiopathic recurrent spontaneous miscarriage (IRSM) [13]. In one study conducted on Iranian women, researchers found that the VEGF coding gene polymorphism rs10434 significantly differed between the patient and control groups, and this variation may have an impact on the prevalence of pre-eclampsia (PE), which is considered a pregnancy complication [14].
Many studies have shown that certain vascular endothelial growth factor polymorphisms are associated with gastrointestinal tract cancer risk. Researchers have discovered a significant association between gallbladder cancer risk and VEGF-2578C/A polymorphism. On the other hand, VEGF-460T/C polymorphism was associated with higher risks of colorectal, gastric, and hepatocellular cancers [15]. Also, in another study, the results showed that according to the study’s findings, blood VEGF levels and the rs2010963 VEGF gene polymorphism may be risk factors for breast cancer. In comparison to patients with the CC genotype, individuals with the GG genotype had the highest serum VEGF levels, bigger tumors, more advanced tumor stages, and a shorter survival time [16]. In a study involving Pakistani patients, the results indicated a strong association between the rs699947 polymorphism and the risk of developing proliferative diabetic retinopathy (PDR) in type 2 diabetes patients [17].
An in vitro study investigated endothelial reactivity to VEGF, which showed that VEGF strongly induces in vitro angiogenesis in pregnant human uterine artery endothelial cells (P-hUAECs) but not in non-pregnant human uterine artery endothelial cells (NP-hUAECs) [18]. In humans, the VEGF family consists of various members, including VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF) [19,20].
Tyrosine kinase cell receptors (VEGFRs) are bound by VEGF and include VEGFR-1 (also known as Fms-like tyrosine kinase 1 (Flt-1)), VEGFR-2 (also known as CD309, fetal liver kinase 1 (Flk-1), or kinase insert domain receptor (KDR)), and VEGFR-3 (also known as Fms-like tyrosine kinase 4 (Flt-4)). Endothelial cells contain the co-receptors neuropilin-1 (NP-1) and neuropilin-2 (NP-2), which regulate tyrosine kinase receptor function. It is important to note that the expression of both vascular endothelial growth factor (VEGF) and its receptors (VEGFRs) is not limited to endothelial cells; they are also expressed in various non-endothelial cell types [19]. Another receptor, the soluble form of VEGFR-1 (sFlt-1), is strongly associated with recurrent miscarriage [21], adverse pregnancy outcomes [22], and unexplained infertility [23,24]. This is linked to its ability to trap PlGF, VEGF, and VEGF-B, preventing them from attaching to membrane receptors [9].
It has been established that the most significant and powerful factor in angiogenesis is VEGF-A, also known as VEGF. VEGF-B has been documented to possess significant antioxidant properties through its ability to enhance the production of critical antioxidant enzymes. Moreover, VEGF-C and VEGF-D have essential roles in lymphangiogenesis. In addition, VEGF-D binds to VEGFR-2 and VEGFR-3 and can control angiogenesis, lymphangiogenesis, fibrogenesis, and apoptosis; VEGF-C binds to VEGFR-2 and VEGFR-3 to control angiogenesis, inflammation, lymphangiogenesis, apoptosis, and fibrogenesis [25,26,27].
VEGF exerts its effects through its receptors VEGFR-1, VEGFR-2, and VEGFR-3, and its activity is modulated by several genetic polymorphisms [19]. Numerous studies have examined the genetic association of polymorphisms of VEGF, especially rs10434, rs3025053, rs699947, rs2010963, rs833061, and rs25648, which have been reported to have an effect on miscarriage in women of ethnically diverse groups, but there are no studies that specifically investigate these particular SNPs and their impact on miscarriage in Saudi women. Therefore, the purpose of this study was to determine whether the susceptibility of Saudi women to RSM might be influenced by these polymorphisms mentioned above.
2. Results
2.1. Demographic Data of Subjects Included in This Study
Table 1a displays the demographic data of the pregnant women (patients vs. controls).

Table 1.
(a). The demographic data of the pregnant women (patients vs. controls). (b). The demographic data of the non-pregnant women (patients vs. controls).
The body mass index (BMI) and number of children were similar in both groups (p > 0.05), while there was a significant difference in age and the number of pregnancies (p < 0.05).
The demographic information of the non-pregnant women (patients vs. controls) is shown in Table 1b.
Age and BMI were matched in both groups (p > 0.05); however, a significant disparity was seen in the number of pregnancies and the number of children (p < 0.05).
2.2. Genotypic and Allelic Frequencies
2.2.1. rs699947, (-2578C/A)
The (-2578C/A) polymorphism in the promoter region of the vascular endothelial growth factor (VEGF) gene and wild-type homozygous (CC) forms were identified.
Table 2a presents the genotype and allele frequency of (-2578C/A) polymorphism in pregnant patients and pregnant controls. We observed no statistically significant association between the wild-type CC (p = 0.15) and AA (p = 0.48) genotype and RSM in pregnant Saudi women. However, the -2578 AC genotype was linked to a lower risk of RSM development and had an odds ratio (OR) of 0.43 in pregnant women, and the frequency between case and control is significant (p = 0.04).

Table 2.
(a). The genotype and allele frequencies of VEGF rs699947 (-2578C/A)—(pregnant). (b). The genotype and allele frequencies of VEGF rs699947 (-2578C/A)—(non-pregnant).
There were no notable variations between the non-pregnant patients and non-pregnant control groups (Table 2b).
2.2.2. rs2010963, (-634G/C)
Table 3a (pregnant, patients vs. controls) and Table 3b (non-pregnant, patients vs. controls) show the genotype and allele frequencies for the -634G/C (rs2010963) polymorphism in the 5′-untranslated region (5′-UTR) of the VEGF gene, and the wild type is homozygous (CC).

Table 3.
(a). The genotype and allele frequencies of VEGF rs2010963, (-634G/C)—(pregnant). (b). The genotype and allele frequencies of VEGF rs2010963, (-634G/C)—(non-pregnant).
The results show that the rs2010963 (-634G/C) genotype wild-type homozygous CC (OR = 4.95, p = 0.02) was associated with the risk of recurrent spontaneous miscarriage, while the genotype GG (OR = 0.32, p = 0.01) showed less association with RSM. Additionally, there was a statistically significant difference (p = 0.001) in the frequencies of the G and C alleles for rs2010963 (-634G/C) in pregnant patients compared to pregnancy controls. However, there was no statistically significant difference in the genotype CG (p = 0.35) (Table 3a). There were no significant differences between the non-pregnant patient group and the relevant controls (Table 3b).
2.2.3. rs3025053, (1725G/A)
The genotype and allele frequencies for the 1725G/A (rs3025053) polymorphism in the 3′-untranslated region (3′-UTR) of the VEGF gene are displayed in Table 4a for the pregnant group (patients vs. controls) and in Table 4b for the non-pregnant group (patients vs. controls). Most of the pregnant and non-pregnant women were homozygous for the wild-type genotype (GG).

Table 4.
(a). The genotype and allele frequencies of VEGF rs3025053, (1725G>A)—(pregnant). (b). The genotype and allele frequencies of VEGF rs3025053, (1725G>A)—(non-pregnant).
The results show that the frequency of the genotypes wild-type GG and GA (p = 0.01) for rs3025053 (1725G>A) was statistically significantly different between the pregnant patient group and the pregnant control group. Furthermore, the adjusted OR for RSM for the GG genotype was 3.58, and that for the GA genotype was 0.26. Importantly, the allele frequencies (G and A) demonstrated a significant correlation with RSM (p = 0.02). For the genotype AA, there was no difference between the two groups of pregnant women (Table 4a).
Many patients and controls were homozygous for the wild-type genotype (GG); however, there were no significant differences between the non-pregnant patient and non-pregnant control groups (Table 4b).
2.2.4. rs25648, rs833061, and rs10434
In contrast to the findings reported above, rs25648 (-7C>T) is located at the 5′-untranslated region (5′-UTR). As shown in Table 5a (pregnant) and Table 5b (non-pregnant), patients and controls showed higher frequencies of the homozygous wild-type genotype (CC). However, there were no statistically significant differences in either the pregnant (patients vs. controls) or non-pregnant (patients vs. controls) groups.

Table 5.
(a). The genotype and allele frequencies of VEGF rs25648 (-7C>T)—(pregnant). (b). The genotype and allele frequencies of VEGF rs25648 (-7C>T)—(non-pregnant).
In addition, Table 6a (pregnant) and Table 6b (non-pregnant) present the polymorphism -460T>C (rs833061) located in the promoter region; the homozygous wild type was TT, and there were no significant differences between the pregnant and non-pregnant groups (patients vs. controls).

Table 6.
(a). The genotype and allele frequencies of VEGF rs833061, (-460T>C)—(pregnant). (b). The genotype and allele frequencies of VEGF rs833061, (-460T>C)—(non-pregnant).
Also, the polymorphism rs10434 (1612G>A) was identified in the 3′-untranslated region (3′-UTR) of the VEGF gene. The homozygous wild-type genotype (GG) was more common in patients and controls in Table 7a (pregnant) and Table 7b (non-pregnant). Nevertheless, neither the pregnant (patients vs. controls) nor the non-pregnant (patients vs. controls) groups showed any statistically significant changes.

Table 7.
(a). The genotype and allele frequencies of VEGF rs10434, (1612G>A)—(pregnant). (b). The genotype and allele frequencies of VEGF rs10434, (1612G>A)—(non-pregnant).
2.3. VEGF Plasma Levels
2.3.1. Comparison of VEGF Plasma Levels in Pregnant Patients (Patients vs. Controls)
Figure 1a presents the results of the comparison between pregnant women (patients vs. controls). The results showed a statistically significant difference between pregnant women (patients vs. controls), with a p-value = 0.003.
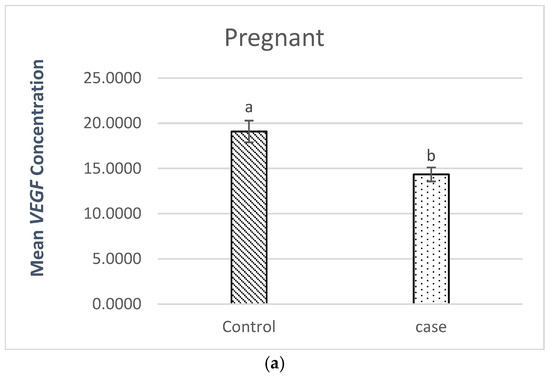
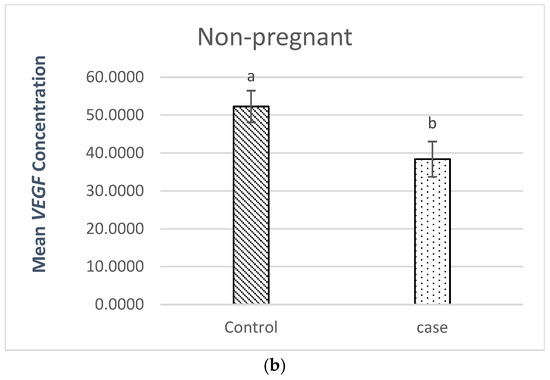
Figure 1.
(a). Comparison of VEGF plasma levels in pregnant patients (patients vs. controls), where a, b stand for different superscripts within columns that are significantly different, p < 0.05. (b). Comparison of VEGF plasma levels in non-pregnant patients (patients vs. controls), where a, b stand for different superscripts within columns that are significantly different, p < 0.05.
The level of VEGF plasma in the pregnant patients (14.34 pg/mL) was lower than the level (19.09 pg/mL) found in the pregnant controls.
2.3.2. Comparison of VEGF Plasma Levels in Non-Pregnant Patients (Patients vs. Controls)
The comparison of non-pregnant women (patients vs. controls) is shown in Figure 1b. The results demonstrated a statistically significant difference in the p-value (p = 0.034) in non-pregnant women (patients vs. controls).
The non-pregnant patients’ VEGF plasma level (38.39 pg/mL) was lower than the non-pregnant controls’ level (52.28 pg/mL).
The observed lower VEGF levels in cases with RSM (pregnant and non-pregnant) suggest impaired angiogenesis as a contributing factor to recurrent spontaneous miscarriage.
3. Discussion
Vascular endothelial growth factor (VEGF) is recognized as an important growth factor due to its crucial pro-angiogenic properties. It exerts both a mitogenic influence and has an anti-apoptotic impact on endothelial cells. Moreover, VEGF is a multifunctional factor that controls vascular permeability and the proliferation, differentiation, and survival of endothelial cells [28].
Numerous single-nucleotide polymorphisms (SNPs) in the vascular endothelial growth factor (VEGF) gene have been documented, including rs699947 and rs833061, located in the promoter region of the VEGF gene [29]; rs3025053 and rs10434, situated in the 3′-untranslated region (3′-UTR) [30]; and rs2010963 and rs25648, found in the 5′-untranslated region (5′-UTR) [31,32], which are correlated with an elevated risk of miscarriage.
Our findings align with previous studies that reported increased prevalences of the rs2010963 C allele and rs699947 AC genotype in women with recurrent pregnancy loss [33,34]. Similar studies in Korean and Egyptian populations have also reported associations between VEGF polymorphisms and pregnancy complications [30,35]. However, some studies have reported conflicting results. For example, Elmi et al. (2023) found no significant association between rs2010963 and implantation failure [36].
In contrast, several studies involving Korean women have shown that 1612G>A polymorphisms are related to recurrent pregnancy loss (RPL) [30]. Additionally, the VEGF rs3025020/rs833061 genotype allele was found to be linked to recurrent pregnancy loss (RPL) and the levels of hematocrit (HCT) in the blood of RPL patients [37]. Moreover, the rs25648 T allele and the rs833061 C allele have been linked to recurrent implantation failure (RIF) [38].
Furthermore, Yalcintepe et al. found that when comparing fetal genotypes to their mothers and healthy controls, the VEGF A rs833061, rs2010963, and rs3025039 fetal genotypes are risk factors for spontaneous abortion [39]. In addition, VEGF expression and its relationship with recurrent spontaneous miscarriage (RSM) are affected by the VEGFA SNPs + 398G/A, 583T/C, and -460T/C, and there is also a weak correlation with 634G/C [40]. These discrepancies may be attributed to genetic differences across populations or variations in study design.
Deficiencies in the formation of blood vessels and blood vessel growth contribute to an increased risk of pregnancy loss [2]. Reduced VEGF levels have been implicated in pregnancy loss, as VEGF plays a critical role in vascular remodeling and endometrial receptivity [41]. Our results confirm a significant decrease in VEGF concentrations in RSM cases, supporting the hypothesis that VEGF deficiency contributes to pregnancy failure.
In a study to detect the level of VEGF in different stages of taken throughout pregnancy and postpartum, where blood samples were collected at 12, 20, 24, 28, 32, 36, and 40 gestational weeks, and in the period from 8 to 10 weeks after birth, the results showed that compared to the percentage seen 9–10 weeks postpartum (64%), the percentage of women with levels over the detection limit (9 pg/mL) in gestational weeks 9–10 (10%) and 39–40 (15%) was significantly lower. All samples obtained at 9–10 weeks postpartum had a median VEGF-A level of 76.4 pg/mL [42].
Furthermore, previous studies have observed elevated levels of VEGF in the serum of non-pregnant women compared to normal pregnant women during the third trimester of pregnancy [43]. In addition, another study noted that the level of VEGF in the plasma of the non-pregnant rats was significantly higher compared to the term pregnant ones [44].
Modifying the concentrations of growth factors has the potential to influence the intricate mechanisms involved in programmed cell death, cell division, and the formation of new blood vessels through angiogenesis. This could have profound implications on cellular functions and tissue development [45]. However, patients with various cardiovascular diseases (CVDs) have been discovered to have high levels of vascular endothelial growth factor-A (VEGF-A), which is commonly associated with adverse disease prognosis and severity [46]. An optimal level of VEGF-A is advantageous in preserving the structure of the glomerulus, whereas an excessive amount may result in aberrant angiogenesis [47].
4. Materials and Methods
4.1. The Study Design and Participants
This case–control study was conducted at King Khalid University Hospital, Riyadh, Saudi Arabia, and included 200 Saudi women aged 18–45 years. The case group consisted of 100 women (50 pregnant and 50 non-pregnant) with a history of unexplained recurrent spontaneous miscarriage (RSM). The control group included 100 healthy women (50 pregnant and 50 non-pregnant) with no history of miscarriage. All participants signed an informed consent form to participate in this study. The Medical Ethics Committee at King Khalid University Hospital and the Ethics Committee at King Saud University approved this study (protocol No. E-24-8865 and date of approval 15 July 2024). Women who had miscarriages with known causes, such as infections, anatomical abnormalities, hormonal imbalances, and chromosomal disorders, were excluded.
4.2. DNA Extraction and Genotyping
Five milliliters of blood was collected in EDTA tubes for DNA extraction using the GeneJET™ Whole Blood Genomic DNA Purification Mini Kit. Genotyping was performed using TaqMan SNP Genotyping Assays on the Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). The genotyping assay targeted VEGF polymorphisms (rs10434, rs3025053, rs699947, rs2010963, rs833061, and rs25648).
4.3. Plasma VEGF Measurement
Two milliliters of blood were collected in sodium citrate tubes for plasma isolation. VEGF-A levels were measured using the Human VEGF ELISA Kit (Thermo Fisher Scientific, Catalog No. KHG0111, Waltham, MA, USA), which has an assay sensitivity of <5 pg/mL.
4.4. Statistical Analysis
Statistical analyses were performed using SPSS 22. The Chi-square test was used to compare genotype and allele frequencies. Independent t-tests were used to compare VEGF levels between groups. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. A p-value < 0.05 was considered statistically significant.
5. Conclusions
The vascular endothelial growth factor (VEGF) polymorphisms rs699947, rs2010963, and rs3025053, along with reduced VEGF serum levels, are associated with an increased risk of RSM in Saudi women. In this study, no notable associations were found between the VEGF polymorphisms rs25648, rs10434, and rs833061 and recurrent spontaneous miscarriage.
Further investigations are imperative and should be conducted in order to thoroughly examine and elucidate the intricate underlying mechanisms that cause RSM, and identify and assess the potential therapeutic targets that may offer viable avenues for intervention and treatment.
Author Contributions
Conceptualization, W.K.A.-Q. and A.F.A.; methodology, W.K.A.-Q., A.F.A. and Z.A.B.; software, W.K.A.-Q. and A.M.H.K.; validation, W.K.A.-Q. and A.M.H.K.; formal analysis, W.K.A.-Q. and N.M.A.-M.; investigation, J.O.A.; resources, A.F.A. and Z.A.B.; data curation, W.K.A.-Q. and A.M.H.K.; writing—original draft preparation, W.K.A.-Q.; writing—review and editing, J.O.A.; visualization, A.F.A.; supervision, A.F.A.; project administration, A.F.A. and Z.A.B.; funding acquisition, A.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
The current investigation received funding from the Researchers Supporting Project number (RSP2025R97), affiliated with King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
This study was approved by the Medical Ethics Committee at King Khalid University Hospital and the Ethics Committee at King Saud University (protocol No. E-24-8865 and date of approval 15 July 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors extend their thanks and gratitude to the Central Laboratory in the female students’ campus at King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| RSM | Recurrent spontaneous miscarriage |
| VEGF | Vascular endothelial growth factor |
| SNP | Single-nucleotide polymorphism |
| OR | Odds ratio |
| CI | Confidence interval |
| X2 | Chi-square |
References
- Al-Khateeb, G.M.; Mustafa, F.E.; Sater, M.S.; Almawi, W.Y. Effect of the functional VEGFA− 583C/T variant on vascular endothelial growth factor levels and the risk of recurrent spontaneous miscarriage. Fertil. Steril. 2011, 95, 2471–2473. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, E.; Tazik, K.; Taheri, F.; Shayankia, G.; Gheibihayat, S.M.; Saberi, A. Abnormal angiogenesis associated with HIF-1α/VEGF signaling pathway in recurrent miscarriage along with therapeutic goals. Gene Rep. 2022, 26, 101483. [Google Scholar] [CrossRef]
- Abu-Ghazaleh, N.; Brennecke, S.; Murthi, P.; Karanam, V. Association of vascular endothelial growth factors (VEGFs) with recurrent miscarriage: A systematic review of the literature. Int. J. Mol. Sci. 2023, 24, 9449. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Gimenez, C.; Alijotas-Reig, J. Recurrent miscarriage: Causes, evaluation and management. Postgrad. Med. J. 2015, 91, 151–162. [Google Scholar] [CrossRef]
- Blyth, U.; Craciunas, L.; Hudson, G.; Choudhary, M. Maternal germline factors associated with aneuploid pregnancy loss: A systematic review. Hum. Reprod. Update 2021, 27, 866–884. [Google Scholar] [CrossRef]
- Bilibio, J.P.; Gama, T.B.; Nascimento, I.C.M.; Meireles, A.J.C.; de Aguiar, A.S.C.; do Nascimento, F.C.; Lorenzzoni, P.L. Causes of recurrent miscarriage after spontaneous pregnancy and after in vitro fertilization. Am. J. Reprod. Immunol. 2020, 83, e13226. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, A.C.; Edwards, D.R.V.; Slaughter, J.C.; Wu, P.; Jones, S.H.; Torstenson, E.S.; Hartmann, K.E. Week-by-week alcohol consumption in early pregnancy and spontaneous abortion risk: A prospective cohort study. Am. J. Obstet. Gynecol. 2021, 224, 97.e1–97.e16. [Google Scholar] [CrossRef]
- Quenby, S.; Gallos, I.D.; Dhillon-Smith, R.K.; Podesek, M.; Stephenson, M.D.; Fisher, J.; Brosens, J.J.; Brewin, J.; Ramhorst, R.; Lucas, E.S. Miscarriage matters: The epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 2021, 397, 1658–1667. [Google Scholar] [CrossRef]
- Guo, X.; Yi, H.; Li, T.C.; Wang, Y.; Wang, H.; Chen, X. Role of vascular endothelial growth factor (VEGF) in human embryo implantation: Clinical implications. Biomolecules 2021, 11, 253. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Ewadh, M.J.; Jeddoa, Z.M.A. The association of vascular endothelial growth factor polymorphism (rs699947) with diabetic foot ulcer and oxidative status. Gene Rep. 2020, 19, 100606. [Google Scholar] [CrossRef]
- Boldeanu, L.; Dijmărescu, A.L.; Radu, M.; Siloşi, C.A.; Popescu-Drigă, M.V.; Poenariu, I.S.; Siloşi, I.; Boldeanu, M.V.; Novac, M.B.; Novac, L.V. The role of mediating factors involved in angiogenesis during implantation. Rom. J. Morphol. Embryol. 2020, 61, 665. [Google Scholar] [CrossRef]
- Zeng, H.; Hu, L.; Xie, H.; Ma, W.; Quan, S. Polymorphisms of vascular endothelial growth factor and recurrent implantation failure: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2021, 304, 297–307. [Google Scholar] [CrossRef]
- Azzam, O.; Mahgoub, S.; Farhan, S. Is there an Association Between-2549 Insertion/Deletion Polymorphisms in the Promotor Region of the Gene Encoding for VEGFA as a Risk Factor and the Idiopathic Recurrent Spontaneous Miscarriage in a Sample of Jordanian Women. Prensa Med. Argent (Genet.) 2020, 106, 3. [Google Scholar]
- Niktalab, R.; Piravar, Z.; Behzadi, R. Different polymorphisms of vascular endothelial growth factor gene in patients with pre-eclampsia among the Iranian women population. Int. J. Fertil. Steril. 2020, 14, 41. [Google Scholar]
- Mahajan, D.; Sambyal, V.; Uppal, M.S.; Sudan, M.; Guleria, K. VEGF-2578C/A,-460T/C polymorphisms and gastrointestinal tract cancer risk: An updated meta-analysis. Genet. Test. Mol. Biomark. 2024, 28, 176–188. [Google Scholar] [CrossRef]
- El-Hefnawy, S.M.; Naidany, S.S.E.; Alhanafy, A.M.; Badr, N.; Ellaithy, M.A. Prognostic impact of serum vascular endothelial growth factor and VEGF gene polymorphism (rs2010963) in breast cancer patients. Hum. Gen. 2023, 36, 201168. [Google Scholar] [CrossRef]
- Qayyum, S.; Afzal, M.; Naveed, A.K.; Butt, I.A.; Sajjad, M.; Azam, M. Association of vascular endothelial growth factor a gene (VEGFA) polymorphisms, rs699947 and rs1570360, with diabetic retinopathy and altered VEGF secretion in the Pakistani patients with type 2 diabetes mellitus: A casecontrol study. J. Pak. Med. Assoc. 2023, 73, 2348–2356. [Google Scholar] [CrossRef]
- Zhang, H.-h.; Chen, J.C.; Sheibani, L.; Lechuga, T.J.; Chen, D.-b. Pregnancy augments VEGF-stimulated in vitro angiogenesis and vasodilator (NO and H2S) production in human uterine artery endothelial cells. J. Clin. Endocrinol. Metab. 2017, 102, 2382–2393. [Google Scholar] [CrossRef]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.-M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)-key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar] [PubMed]
- Mamer, S.B.; Wittenkeller, A.; Imoukhuede, P. VEGF-A splice variants bind VEGFRs with differential affinities. Sci. Rep. 2020, 10, 14413. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Wei, Z.; Li, O.; Huang, R.; Qin, J.; Chen, H.; Fan, X.; Chen, Z.-J. An increase in vascular endothelial growth factor (VEGF) and VEGF soluble receptor-1 (sFlt-1) are associated with early recurrent spontaneous abortion. PLoS ONE 2013, 8, e75759. [Google Scholar] [CrossRef]
- Woo, I.; Chan, Y.; Sriprasert, I.; Louie, K.; Ingles, S.; Stanczyk, F.; McGinnis, L.K.; Chung, K. The role of angiogenic markers in adverse perinatal outcomes: Fresh versus frozen embryo transfers. J. Assist. Reprod. Genet. 2017, 34, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Wathén, K.-A.; Unkila-Kallio, L.; Isaksson, R.; Tiitinen, A.; Stenman, U.-H.; Vuorela, P. Is serum-soluble vascular endothelial growth factor receptor-1 of importance in unexplained infertility? Acta Obstet. Gynecol. Scand. 2008, 87, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Edgell, T.A.; Evans, J.; Lazzaro, L.; Boyes, K.; Sridhar, M.; Catt, S.; Rombauts, L.J.; Vollenhoven, B.J.; Salamonsen, L.A. Assessment of potential biomarkers of pre-receptive and receptive endometrium in uterine fluid and a functional evaluation of the potential role of CSF3 in fertility. Cytokine 2018, 111, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Astin, J.W.; Haggerty, M.J.; Okuda, K.S.; Le Guen, L.; Misa, J.P.; Tromp, A.; Hogan, B.M.; Crosier, K.E.; Crosier, P.S. Vegfd can compensate for loss of Vegfc in zebrafish facial lymphatic sprouting. Development 2014, 141, 2680–2690. [Google Scholar] [CrossRef]
- Bui, H.M.; Enis, D.; Robciuc, M.R.; Nurmi, H.J.; Cohen, J.; Chen, M.; Yang, Y.; Dhillon, V.; Johnson, K.; Zhang, H. Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD. J. Clin. Investig. 2016, 126, 2167–2180. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Cui, H.; Shi, J.; Yuan, G.; Shi, S.; Hu, Y. The role of the VEGF family in coronary heart disease. Front. Cardiovasc. Med. 2021, 8, 738325. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in signaling and disease: Beyond discovery and development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Tu, J.; Wang, S.; Zhao, J.; Zhu, J.; Sheng, L.; Sheng, Y.; Chen, H.; Tian, J. rs833061 and rs699947 on promoter gene of vascular endothelial growth factor (VEGF) and associated lung cancer susceptibility and survival: A meta-analysis. Med. Sci. Monit. 2014, 20, 2520. [Google Scholar]
- An, H.J.; Kim, J.H.; Ahn, E.H.; Kim, Y.R.; Kim, J.O.; Park, H.S.; Ryu, C.S.; Kim, E.-G.; Cho, S.H.; Lee, W.S. 3′-UTR polymorphisms in the vascular endothelial growth factor gene (VEGF) contribute to susceptibility to recurrent pregnancy loss (RPL). Int. J. Mol. Sci. 2019, 20, 3319. [Google Scholar] [CrossRef]
- García-Closas, M.; Malats, N.; Real, F.X.; Yeager, M.; Welch, R.; Silverman, D.; Kogevinas, M.; Dosemeci, M.; Figueroa, J.; Chatterjee, N. Large-scale evaluation of candidate genes identifies associations between VEGF polymorphisms and bladder cancer risk. PLoS Genet. 2007, 3, e29. [Google Scholar] [CrossRef]
- Scartozzi, M.; Faloppi, L.; Svegliati Baroni, G.; Loretelli, C.; Piscaglia, F.; Iavarone, M.; Toniutto, P.; Fava, G.; De Minicis, S.; Mandolesi, A. VEGF and VEGFR genotyping in the prediction of clinical outcome for HCC patients receiving sorafenib: The ALICE-1 study. Int. J. Cancer 2014, 135, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, M.S.; Ghandil, P.; Shahbazian, N.; Saberi, A. Association of vascular endothelial growth factor A polymorphisms and aberrant expression of connexin 43 and VEGFA with idiopathic recurrent spontaneous miscarriage. J. Obstet. Gynaecol. Res. 2020, 46, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Amin, I.; Pandith, A.A.; Manzoor, U.; Mir, S.H.; Koul, A.M.; Wani, S.; Ahmad, A.; Qasim, I.; Rashid, M.; Wani, U.M. Implications of VEGF gene sequence variations and its expression in recurrent pregnancy loss. Reprod. Biomed. Online 2021, 43, 1035–1044. [Google Scholar] [CrossRef]
- El-baz, R.; Bedairy, M.H.; Saad, A.M.; El-gareeb, M.S. Study of genetic polymorphisms of Vascular Endothelial Growth Factor in women with recurrent abortion. J. Pharm. Biol. Sci. 2014, 9, 51–55. [Google Scholar] [CrossRef]
- Elmi, M.; Ghandil, P.; Hemadi, M.; Birgani, M.T.; Saberi, A. Association of rs1570360 and rs2010963 in VEGF and rs2279744 in the MDM2 gene with Recurrent Implantation Failure in Iranian Women. JBRA Assist. Reprod. 2023, 27, 342. [Google Scholar] [CrossRef]
- Jung, Y.W.; Ahn, E.H.; Kim, J.O.; An, H.J.; Cho, S.H.; Kim, Y.R.; Lee, W.S.; Kim, N.K. Association of genetic polymorphisms in VEGF-460,-7 and-583 and hematocrit level with the development of idiopathic recurrent pregnancy loss and a meta-analysis. J. Gene Med. 2018, 20, e3048. [Google Scholar] [CrossRef]
- Jung, Y.W.; Kim, J.O.; Rah, H.; Kim, J.H.; Kim, Y.R.; Lee, Y.; Lee, W.S.; Kim, N.K. Genetic variants of vascular endothelial growth factor are associated with recurrent implantation failure in Korean women. Reprod. Biomed. Online 2016, 32, 190–196. [Google Scholar] [CrossRef]
- Yalcintepe, S.A.; Silan, F.; Hacivelioglu, S.O.; Uludag, A.; Cosar, E.; Ozdemir, O. Fetal Vegf genotype is more important for abortion risk than mother genotype. Int. J. Mol. Cell Med. 2014, 3, 88. [Google Scholar]
- Almawi, W.Y.; Saldanha, F.L.; Mahmood, N.A.; Al-Zaman, I.; Sater, M.S.; Mustafa, F.E. Relationship between VEGFA polymorphisms and serum VEGF protein levels and recurrent spontaneous miscarriage. Hum. Reprod. 2013, 28, 2628–2635. [Google Scholar] [CrossRef]
- Gupta, P.; Deo, S.; Jaiswar, S.; Sankhwar, P. Case control study to compare serum vascular endothelial growth factor (VEGF) level in women with recurrent pregnancy loss (RPL) compared to women with term pregnancy. J. Obstet. Gynaecol. India 2019, 69, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Palm, M.; Basu, S.; Larsson, A.; Wernroth, L.; Åkerud, H.; Axelsson, O. A longitudinal study of plasma levels of soluble fms-like tyrosine kinase 1 (sFlt1), placental growth factor (PlGF), sFlt1: PlGF ratio and vascular endothelial growth factor (VEGF-A) in normal pregnancy. Acta Obstet. Gynecol. Scand. 2011, 90, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Lyall, F.; Greer, I.A.; Boswell, F.; Fleming, R. Suppression of serum vascular endothelial growth factor immunoreactivity in normal pregnancy and in pre-eclampsia. Br. J. Obstet. Gynaecol. 1997, 104, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Alotaibi, M.A. Maternal plasma levels of vascular endothelial growth factor and interleukin-6 in the term-pregnant and non-pregnant rats. Anim. Reprod. (AR) 2018, 14, 1259–1263. [Google Scholar] [CrossRef]
- Marakhovskaya, T.A.; Butenko, E.V.; Kovalenko, K.A.; Mashkina, E.V. Association of growth factors genes with miscarriage. J. Reprod. Infertil. 2018, 19, 219. [Google Scholar]
- Braile, M.; Marcella, S.; Cristinziano, L.; Galdiero, M.R.; Modestino, L.; Ferrara, A.L.; Varricchi, G.; Marone, G.; Loffredo, S. VEGF-A in cardiomyocytes and heart diseases. Int. J. Mol. Sci. 2020, 21, 5294. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, H.; Chen, J.; He, L.; Chen, Y. Role of VEGF-A and LRG1 in abnormal angiogenesis associated with diabetic nephropathy. Front. Physiol. 2020, 11, 1064. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).