Assessment of the Curative Anti-Glycation Properties of a Novel Injectable Formulation Combining Dual-Weight Hyaluronic Acid (Low- and Mid/High-Molecular Weight) with Trehalose on Human Skin Ex Vivo
Abstract
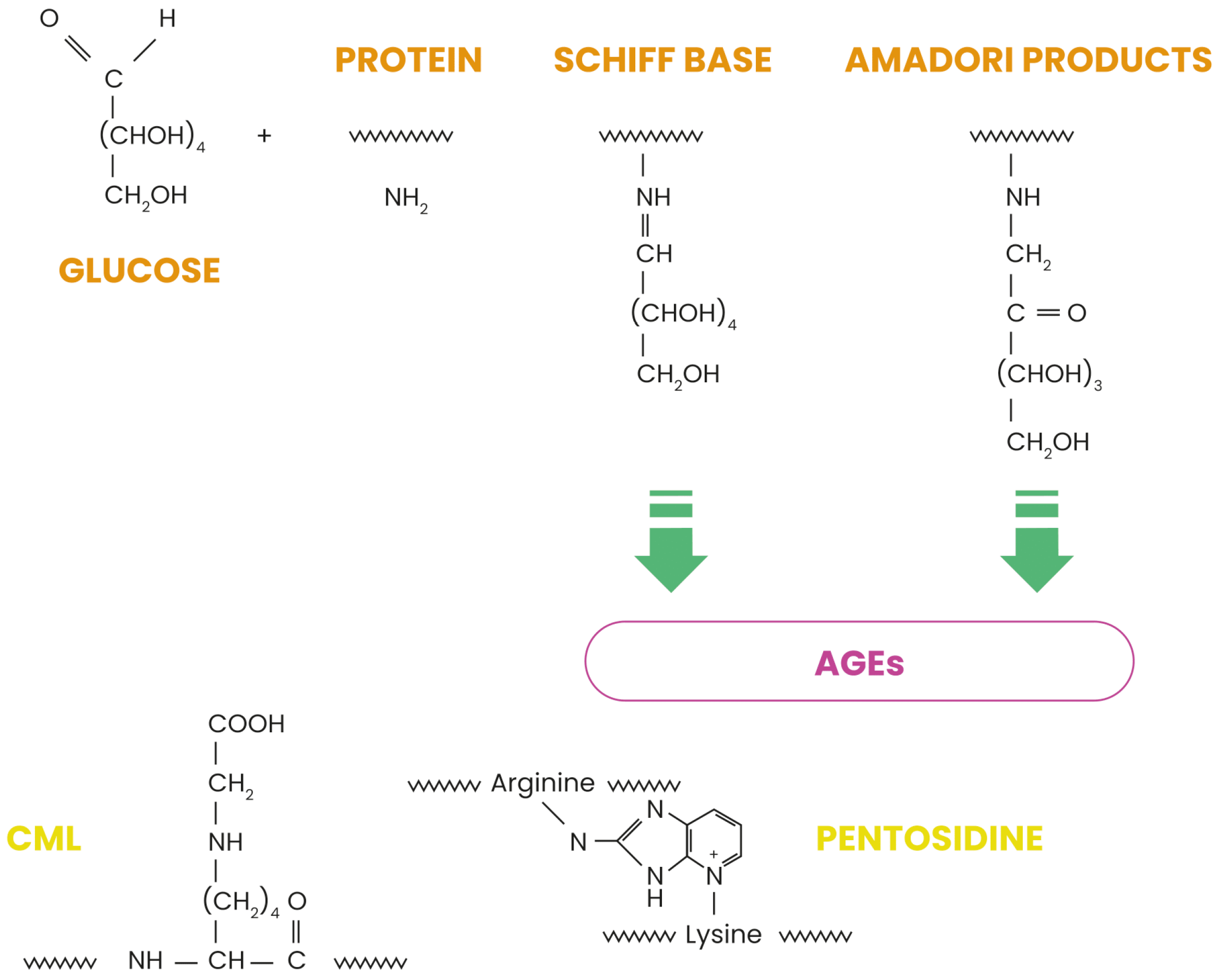
1. Introduction
2. Results
2.1. Cell Viability
2.2. Results of CML Immunohistochemical Analysis
2.3. Quantification of CML Immunostaining in Human Skin Explants
3. Discussion
4. Materials and Methods
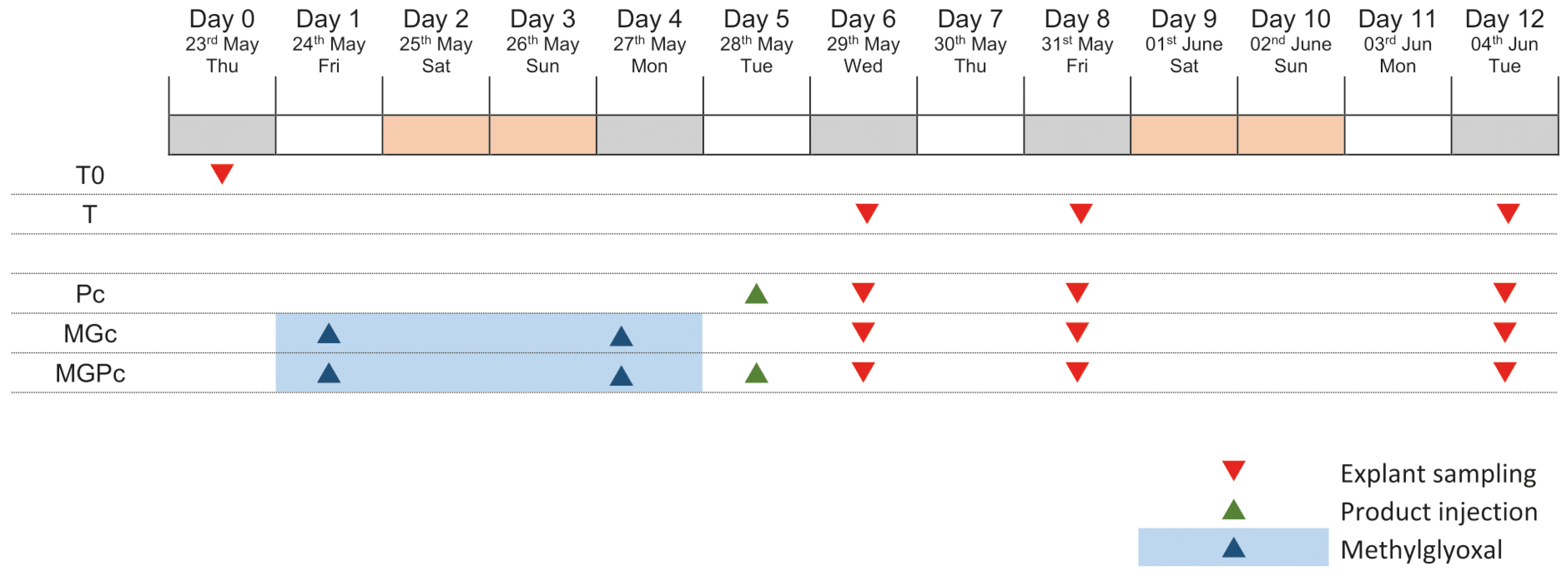
4.1. Schedule of the Study
4.2. Tested Product
4.3. Explants Sampling
4.4. Treatment Protocol
4.5. Induction of Glycation
4.6. Sample Collection
4.7. Histological Methods
4.7.1. Viability Assessment
4.7.2. CML Detection and Quantification
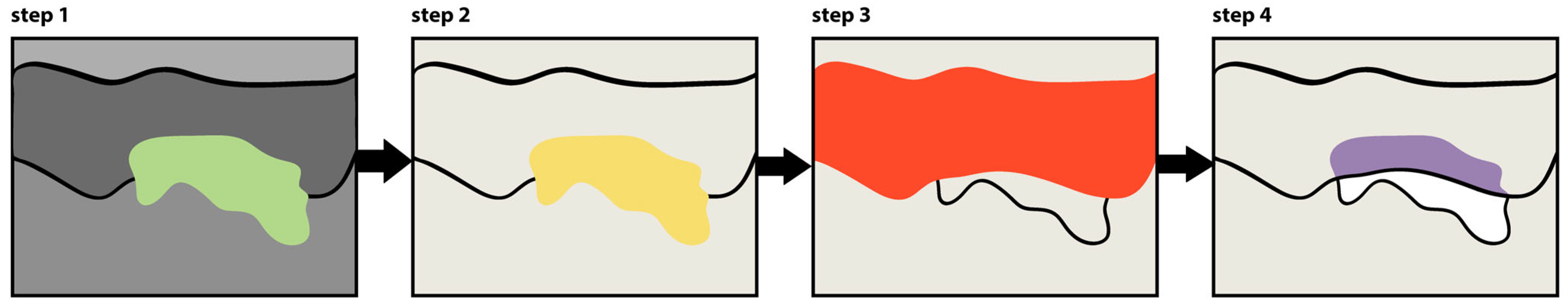
4.8. Image Analysis Protocol
4.9. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MW | Molecular Weight |
| AGEs | Advanced Glycation End-products |
| CML | Nε-(carboxymethyl)lysine |
| ECM | extracellular matrix |
| RAGE | receptor for advanced glycation end-products |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| HA | Hyaluronic acid |
| Pc | curative product |
| TJ6 | control samples on day 6 |
| T0 | initial timepoint |
| TJ8 | control samples on day 8 |
| TJ12 | control samples on day 12 |
| PcJ6 | curative product treatment on day 6 |
| PcJ8 | curative product treatment on day 8 |
| PcJ12 | curative product treatment on day 12 |
| MGpJ6 | glycation-inducing stressor methylglyoxal on day 6 |
| MGcJ8 | glycation-inducing stressor methylglyoxal on day 8 |
| MGcJ12 | glycation-inducing stressor methylglyoxal on day 12 |
| MGPcJ6 | combined treatment with curative product and glycation-inducing methylglyoxal on day 6 |
| MGPcJ8 | combined treatment with curative product and glycation-inducing methylglyoxal on day 8 |
| MGPcJ12 | combined treatment with curative product and glycation-inducing methylglyoxal on day 12 |
| FFPE | formalin-fixed paraffin-embedded |
| ROI | region of interest |
References
- Chen, C.Y.; Zhang, J.Q.; Li, L.; Guo, M.M.; He, Y.F.; Dong, Y.M.; Meng, H.; Yi, F. Advanced glycation end products in the skin: Molecular mechanisms, methods of measurement, and inhibitory pathways. Front. Med. 2022, 9, 837222. [Google Scholar] [CrossRef] [PubMed]
- Damasiewicz-Bodzek, A.; Nowak, A. Concentrations of N6-carboxymethyllysine (CML), N6-carboxyethyllysine (CEL), and soluble receptor for Advanced Glycation End-Products (sRAGE) are increased in psoriatic patients. Biomolecules 2022, 12, 1870. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, S.R.; Baynes, J.W. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids 2003, 25, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Bhadada, S.K. AGEs accumulation with vascular complications, glycemic control and metabolic syndrome: A narrative review. Bone 2023, 176, 116884. [Google Scholar] [CrossRef]
- Abdelkader, H.; Mustafa, W.W.; Alqahtani, A.M.; Alsharani, S.; Al Fatease, A.; Alany, R.G. Glycation-induced age-related illnesses, antiglycation and drug delivery strategies. J. Pharm. Pharmacol. 2022, 74, 1546–1567. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products: Key players in skin aging? Dermato-Endocrinology 2012, 4, 259–270. [Google Scholar] [CrossRef]
- Maria-Engler, S.S. Assessing the effects of advanced glycation end products in the skin. Br. J. Dermatol. 2017, 176, 12–13. [Google Scholar] [CrossRef]
- Lohwasser, C.; Neureiter, D.; Weigle, B.; Kirchner, T.; Schuppan, D. The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha. J. Investig. Dermatol. 2006, 126, 291–299. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Hori, O.; Cao, R.; Yan, S.D.; Brett, J.; Wautier, J.L.; Ogawa, S.; Kuwabara, K.; Matsumoto, M.; Stern, D. RAGE: A novel cellular receptor for advanced glycation end products. Diabetes 1996, 45, S77–S80. [Google Scholar] [CrossRef]
- Zheng, W.; Li, H.; Go, Y.; Chan, X.H.; Huang, Q.; Wu, J. Research advances on the damage mechanism of skin glycation and related inhibitors. Nutrients 2022, 14, 4588. [Google Scholar] [CrossRef]
- Chmielewski, R.; Lesiak, A. Mitigating Glycation and Oxidative Stress in Aesthetic Medicine: Hyaluronic Acid and Trehalose Synergy for Anti-AGEs Action in Skin Aging Treatment. Clin. Cosmet. Investig. Dermatol. 2024, 17, 2701–2712. [Google Scholar] [CrossRef] [PubMed]
- Yung, S.; Chan, T.M. Pathophysiology of the peritoneal membrane during peritoneal dialysis: The role of hyaluronan. BioMed Res. Int. 2011, 2011, 180594. [Google Scholar] [CrossRef] [PubMed]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of hyaluronic acid and its combination with other bioactive ingredients in cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic acid: Redefining its role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Hyaluronic acid: A powerful biomolecule with wide-ranging applications—A comprehensive review. Int. J. Mol. Sci. 2023, 24, 10296. [Google Scholar] [CrossRef]
- Muhammad, P.; Novianto, E.; Setyorini, M.; Legiawati, L.; Yusharyahya, S.N.; Menaldi, S.L.; Budianti, W.K. Effectiveness of topical hyaluronic acid of different molecular weights in xerosis cutis treatment in elderly: A double-blind, randomized controlled trial. Arch. Dermatol. Res. 2024, 316, 329. [Google Scholar] [CrossRef]
- Li, L.; Chen, H.; Chen, X.; Chen, S.; Gu, H. Trehalose Protects Keratinocytes against Ultraviolet B Radiation by Activating Autophagy via Regulating TIMP3 and ATG9A. Oxidative Med. Cell. Longev. 2022, 2022, 9366494. [Google Scholar] [CrossRef]
- Laughlin, T.; Tan, Y.; Jarrold, B.; Chen, J.; Li, L.; Fang, B.; Zhao, W.; Tamura, M.; Matsubara, A.; Deng, G.; et al. Autophagy activators stimulate the removal of advanced glycation end products in human keratinocytes. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 12–18. [Google Scholar] [CrossRef]
- Chmielewski, R.; Lebiedowska, A.; Barańska-Rybak, W. Evaluation of the Anti-Glycation Protective Effect of an Injectable Product Based on a Combination of Two Different Ranges of Molecular Weights of Hyaluronic Acid and Trehalose on Human Skin Explants. Int. J. Mol. Sci. 2025, 26, 3217. [Google Scholar] [CrossRef]
- Lain, E.; Mariwalla, K.; Zeichner, J.; Kirchner, F.; Ruvolo, E.; Draelos, Z.D. Clinical Evaluation of Next-generation, Multi-weight Hyaluronic Acid Plus Antioxidant Complex-based Topical Formulations with Targeted Delivery to Enhance Skin Rejuvenation. J. Clin. Aesthet. Dermatol. 2024, 17, 12–16. [Google Scholar]
- Neumann, A.; Schinzel, R.; Palm, D.; Riederer, P.; Münch, G. High molecular weight hyaluronic acid inhibits advanced glycation endproduct-induced NF-κB activation and cytokine expression. FEBS Lett. 1999, 453, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Suji, G.; Sivakami, S. Glucose, glycation and aging. Biogerontology 2004, 5, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Fournet, M.; Bonté, F.; Desmoulière, A. Glycation damage: A possible hub for major pathophysiological disorders and aging. Aging Dis. 2018, 9, 880. [Google Scholar] [CrossRef]
- Mizunoe, Y.; Kobayashi, M.; Sudo, Y.; Watanabe, S.; Yasukawa, H.; Natori, D.; Hoshino, A.; Negishi, A.; Okita, N.; Komatsu, M.; et al. Trehalose protects against oxidative stress by regulating the Keap1–Nrf2 and autophagy pathways. Redox Biol. 2018, 15, 115–124. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, D.; Chen, X.; Bian, F.; Qin, W.; Gao, N.; Xiao, Y.; Li, J.; Pflugfelder, S.C.; Li, D.Q. Trehalose induces autophagy against inflammation by activating TFEB signaling pathway in human corneal epithelial cells exposed to hyperosmotic stress. Investig. Ophthalmol. Vis. Sci. 2020, 61, 26. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Cho, E.D.; Jung Ahn, W.; Won Lee, K.; Lee, S.J.; Lee, H.J. Is trehalose an autophagic inducer? Unraveling the roles of non-reducing disaccharides on autophagic flux and alpha-synuclein aggregation. Cell Death Dis. 2017, 8, e3091. [Google Scholar] [CrossRef]
- Hosseinpour-Moghaddam, K.; Caraglia, M.; Sahebkar, A. Autophagy induction by trehalose: Molecular mechanisms and therapeutic impacts. J. Cell. Physiol. 2018, 233, 6524–6543. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced glycation end-products (AGEs): Formation, chemistry, classification, receptors, and diseases related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Ciccone, V.; Zazzetta, M.; Morbidelli, L. Comparison of the effect of two hyaluronic acid preparations on fibroblast and endothelial cell functions related to angiogenesis. Cells 2019, 8, 1479. [Google Scholar] [CrossRef]
- Panzieri, F. Injectable Composition Including Hyaluronic Acid and Use of the Said Composition. U.S. Patent 20210315804A1, 14 October 2021. [Google Scholar]
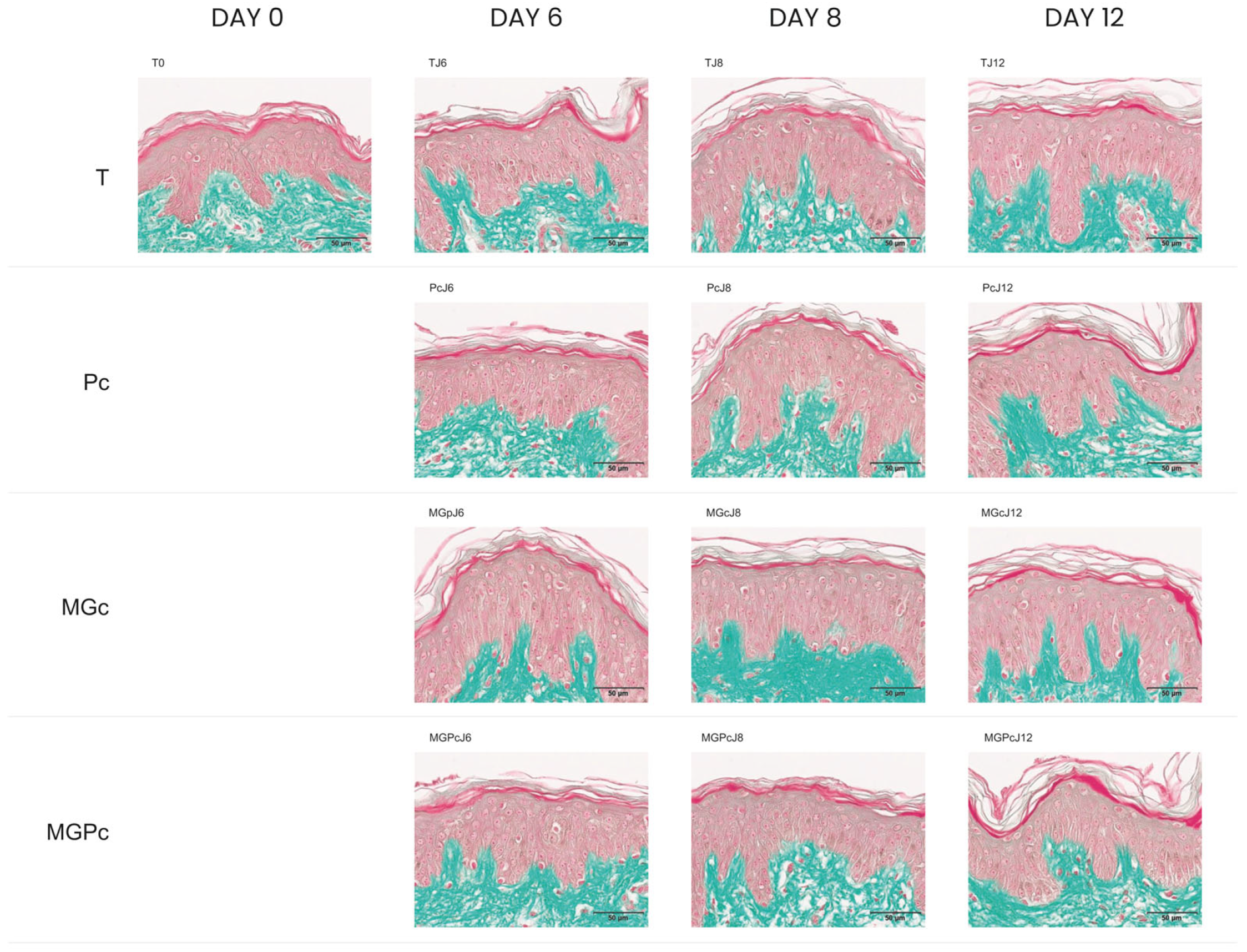


| T0 | TJ6 | PcJ6 | MGpJ6 | MGPcJ6 | TJ8 | PcJ8 | MGcJ8 | MGPcJ8 | TJ12 | PcJ12 | MGcJ12 | MGPcJ12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 4.7 | 5.0 | 2.0 | 7.0 | 2.2 | 2.5 | 1.8 | 9.2 | 3.2 | 3.2 | 2.4 | 9.6 | 3.7 |
| SD | 1.7 | 0.9 | 0.9 | 2.4 | 1.4 | 1.1 | 2.0 | 3.6 | 0.6 | 0.6 | 1.3 | 3.2 | 1.0 |
| Control (Untreated) Condition | |||
|---|---|---|---|
| Batch | Conditions | Nb of Explants | Sampling Day |
| T0 * | Tissue control | 3 | 0 |
| T ** | Non-treated control | 3, 3, 3 | 6, 8, 12 |
| Curative Effect | |||
|---|---|---|---|
| Batch | Conditions | Nb of Explants | Sampling Time |
| Pc | Injection of product | 3, 3, 3 | Day 6, day 8, day 12 |
| MGc | Methylglyoxal | 3, 3 | Day 8, day 12 |
| MGPc | Injectable product + Methylglyoxal | 3, 3, 3 | Day 6, day 8, day 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmielewski, R.; Lebiedowska, A.; Barańska-Rybak, W. Assessment of the Curative Anti-Glycation Properties of a Novel Injectable Formulation Combining Dual-Weight Hyaluronic Acid (Low- and Mid/High-Molecular Weight) with Trehalose on Human Skin Ex Vivo. Int. J. Mol. Sci. 2025, 26, 4747. https://doi.org/10.3390/ijms26104747
Chmielewski R, Lebiedowska A, Barańska-Rybak W. Assessment of the Curative Anti-Glycation Properties of a Novel Injectable Formulation Combining Dual-Weight Hyaluronic Acid (Low- and Mid/High-Molecular Weight) with Trehalose on Human Skin Ex Vivo. International Journal of Molecular Sciences. 2025; 26(10):4747. https://doi.org/10.3390/ijms26104747
Chicago/Turabian StyleChmielewski, Robert, Agata Lebiedowska, and Wioletta Barańska-Rybak. 2025. "Assessment of the Curative Anti-Glycation Properties of a Novel Injectable Formulation Combining Dual-Weight Hyaluronic Acid (Low- and Mid/High-Molecular Weight) with Trehalose on Human Skin Ex Vivo" International Journal of Molecular Sciences 26, no. 10: 4747. https://doi.org/10.3390/ijms26104747
APA StyleChmielewski, R., Lebiedowska, A., & Barańska-Rybak, W. (2025). Assessment of the Curative Anti-Glycation Properties of a Novel Injectable Formulation Combining Dual-Weight Hyaluronic Acid (Low- and Mid/High-Molecular Weight) with Trehalose on Human Skin Ex Vivo. International Journal of Molecular Sciences, 26(10), 4747. https://doi.org/10.3390/ijms26104747







