Obesity-Related Serum Monocyte Chemoattractant Protein-1 (MCP-1) as a Biomarker of Plaque Instability in Patients Undergoing Carotid Endarterectomy
Abstract
:1. Introduction
2. Results
3. Discussion
Limitations
4. Materials and Methods
4.1. Patients
4.2. Biochemical Assessment
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CEA | Carotid endarterectomy |
| ELISA | Enzyme-linked immunosorbent assay |
| EVAR | Endovascular aneurysm repair |
| GSM | Gray-scale median |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| TC | Total cholesterol |
| HDL | High-density lipoprotein cholesterol |
| LDL | Low-density lipoprotein cholesterol |
| TG | Triglycerides |
| RAAS | Renin–angiotensin–aldosterone system |
| CRP | C-reactive protein |
| CKD | Chronic kidney disease |
| IHD | Ischemic disease |
| DM | Diabetes |
| CCA | Calcium channel antagonist |
References
- Peycheva, M.; Deneva, T.; Zlatareva, D.; Zdravkova, T.; Chervenkov, L.; Harizanova, Z. Image and Laboratory Aspects of Carotid Atherosclerosis. Exp. Appl. Biomed. Res. 2023, 24, 135–144. [Google Scholar] [CrossRef]
- Sarraju, A.; Nissen, S.E. Atherosclerotic Plaque Stabilization and Regression: A Review of Clinical Evidence. Nat. Rev. Cardiol. 2024, 21, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Vergallo, R.; Crea, F. Atherosclerotic Plaque Healing. N. Engl. J. Med. 2020, 383, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Arakelyan, A.; Petrkova, J.; Hermanova, Z.; Boyajyan, A.; Lukl, J.; Petrek, M. Serum Levels of the MCP-1 Chemokine in Patients with Ischemic Stroke and Myocardial Infarction. Mediators Inflamm. 2005, 2005, 175–179. [Google Scholar] [CrossRef]
- Barber, R.M.; Platt, S.R.; De Risio, L.; Barber, J.; Robinson, K.R. Multiplex Analysis of Cytokines in the Cerebrospinal Fluid of Dogs after Ischemic Stroke Reveals Elevations in Chemokines CXCL1 and MCP-1. Front. Vet. Sci. 2023, 10, 1169617. [Google Scholar] [CrossRef]
- Basurto, L.; Gregory, M.A.; Hernández, S.B.; Sánchez-Huerta, L.; Martínez, A.D.; Manuel-Apolinar, L.; Avelar, F.J.; Alonso, L.A.M.; Sánchez-Arenas, R. Monocyte Chemoattractant Protein-1 (MCP-1) and Fibroblast Growth Factor-21 (FGF-21) as Biomarkers of Subclinical Atherosclerosis in Women. Exp. Gerontol. 2019, 124, 110624. [Google Scholar] [CrossRef]
- Blanco-Colio, L.M.; Méndez-Barbero, N.; Pello Lázaro, A.M.; Aceña, Á.; Tarín, N.; Cristóbal, C.; Martínez-Milla, J.; González-Lorenzo, Ó.; Martín-Ventura, J.L.; Huelmos, A.; et al. MCP-1 Predicts Recurrent Cardiovascular Events in Patients with Persistent Inflammation. J. Clin. Med. 2021, 10, 1137. [Google Scholar] [CrossRef]
- Kim, C.-S.; Park, H.-S.; Kawada, T.; Kim, J.-H.; Lim, D.; Hubbard, N.E.; Kwon, B.-S.; Erickson, K.L.; Yu, R. Circulating Levels of MCP-1 and IL-8 Are Elevated in Human Obese Subjects and Associated with Obesity-Related Parameters. Int. J. Obes. 2006, 30, 1347–1355. [Google Scholar] [CrossRef]
- Dragomir, E.; Simionescu, M. Monocyte Chemoattractant Protein-1—A Major Contributor to the Inflammatory Process Associated with Diabetes. Arch. Physiol. Biochem. 2006, 112, 239–244. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, Regulation, and Involvement in Disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Strazzullo, P.; D’Elia, L.; Cairella, G.; Garbagnati, F.; Cappuccio, F.P.; Scalfi, L. Excess Body Weight and Incidence of Stroke: Meta-Analysis of Prospective Studies with 2 Million Participants. Stroke 2010, 41, e418–e426. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.B.; Cole, J.W.; McArdle, P.F.; Cheng, Y.-C.; Ryan, K.A.; Sparks, M.J.; Mitchell, B.D.; Kittner, S.J. Obesity Increases Risk of Ischemic Stroke in Young Adults. Stroke 2015, 46, 1690–1692. [Google Scholar] [CrossRef] [PubMed]
- Rovella, V.; Anemona, L.; Cardellini, M.; Scimeca, M.; Saggini, A.; Santeusanio, G.; Bonanno, E.; Montanaro, M.; Legramante, I.M.; Ippoliti, A.; et al. The Role of Obesity in Carotid Plaque Instability: Interaction with Age, Gender, and Cardiovascular Risk Factors. Cardiovasc. Diabetol. 2018, 17, 46. [Google Scholar] [CrossRef]
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef]
- Bonifačić, D.; Toplak, A.; Benjak, I.; Tokmadžić, V.S.; Lekić, A.; Kučić, N. Monocytes and Monocyte Chemoattractant Protein 1 (MCP-1) as Early Predictors of Disease Outcome in Patients with Cerebral Ischemic Stroke. Wien. Klin. Wochenschr. 2016, 128, 20–27. [Google Scholar] [CrossRef]
- Worthmann, H.; Tryc, A.B.; Goldbecker, A.; Ma, Y.T.; Tountopoulou, A.; Hahn, A.; Dengler, R.; Lichtinghagen, R.; Weissenborn, K. The Temporal Profile of Inflammatory Markers and Mediators in Blood after Acute Ischemic Stroke Differs Depending on Stroke Outcome. Cerebrovasc. Dis. 2010, 30, 85–92. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Malik, R.; Björkbacka, H.; Pana, T.A.; Demissie, S.; Ayers, C.; Elhadad, M.A.; Fornage, M.; Beiser, A.S.; Benjamin, E.J.; et al. Circulating Monocyte Chemoattractant Protein-1 and Risk of Stroke: Meta-Analysis of Population-Based Studies Involving 17,180 Individuals. Circ. Res. 2019, 125, 773–782. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Gill, D.; Rannikmäe, K.; Traylor, M.; Anderson, C.D.; MEGASTROKE Consortium of the International Stroke Genetics Consortium (ISGC); Lee, J.-M.; Kamatani, Y.; Hopewell, J.C.; Worrall, B.B.; et al. Genetically Determined Levels of Circulating Cytokines and Risk of Stroke: Role of Monocyte Chemoattractant Protein-1. Circulation 2019, 139, 256–268. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Y.; Tian, X.; Xia, X.; Zhang, Y.; Zhang, X.; Wang, Y.; Sun, P.; Meng, X.; Wang, A. Monocyte Chemoattractant Protein-1, Inflammatory Biomarkers, and Prognosis of Patients with Ischemic Stroke or Transient Ischemic Attack: Fndings from a Nationwide Registry Study. J. Am. Heart Assoc. 2024, 13, e035820. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Van Der Laan, S.W.; Asare, Y.; Mekke, J.M.; Haitjema, S.; Schoneveld, A.H.; De Jager, S.C.A.; Nurmohamed, N.S.; Kroon, J.; Stroes, E.S.G.; et al. Monocyte-Chemoattractant Protein-1 Levels in Human Atherosclerotic Lesions Associate with Plaque Vulnerability. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Madycki, G.; Gabriel, M.; Hawro, P.; Pawlaczyk, K.; Kuczmik, W.; Urbanek, T. Duplex Doppler Ultrasound Examination of Carotid and Vertebral Arteries: Guidelines of the Polish Society for Vascular Surgery. Kardiol. Pol. 2014, 72, 288–309. [Google Scholar] [CrossRef]
- Ibrahimi, P.; Jashari, F.; Johansson, E.; Gronlund, C.; Bajraktari, G.; Wester, P.; Henein, M.Y. Vulnerable Plaques in the Contralateral Carotid Arteries in Symptomatic Patients: A Detailed Ultrasound Analysis. Atherosclerosis 2014, 235, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Płoński, A.; Pawlak, D.; Płoński, A.F.; Głowiński, J.; Madycki, G.; Pawlak, K. Gray-Scale Median in Patients with Symptomatic and Asymptomatic Carotid Atherosclerosis—Risk Factors and Diagnostic Potential. Biomedicines 2024, 12, 1594. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr. Pract. 2016, 22, 1–203. [Google Scholar] [CrossRef]
- Ovbiagele, B.; Bath, P.M.; Cotton, D.; Vinisko, R.; Diener, H.-C. Obesity and Recurrent Vascular Risk After a Recent Ischemic Stroke. Stroke 2011, 42, 3397–3402. [Google Scholar] [CrossRef]
- Vemmos, K.; Ntaios, G.; Spengos, K.; Savvari, P.; Vemmou, A.; Pappa, T.; Manios, E.; Georgiopoulos, G.; Alevizaki, M. Association Between Obesity and Mortality After Acute First-Ever Stroke: The Obesity–Stroke Paradox. Stroke 2011, 42, 30–36. [Google Scholar] [CrossRef]
- Barba, R.; Marco, J.; Ruiz, J.; Canora, J.; Hinojosa, J.; Plaza, S.; Zapatero-Gaviria, A. The Obesity Paradox in Stroke: Impact on Mortality and Short-Term Readmission. J. Stroke Cerebrovasc. Dis. 2015, 24, 766–770. [Google Scholar] [CrossRef]
- Kanda, H. MCP-1 Contributes to Macrophage Infiltration into Adipose Tissue, Insulin Resistance, and Hepatic Steatosis in Obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef]
- Salcedo, R.; Ponce, M.L.; Young, H.A.; Wasserman, K.; Ward, J.M.; Kleinman, H.K.; Oppenheim, J.J.; Murphy, W.J. Human Endothelial Cells Express CCR2 and Respond to MCP-1: Direct Role of MCP-1 in Angiogenesis and Tumor Progression. Blood 2000, 96, 34–40. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Shang, H.; Xu, Y.; Qian, M. Monocyte chemoattractant protein-1 induces endothelial cell apoptosis in vitro through a p53-dependent mitochondrial pathway. Acta Biochim. Biophys. Sin. 2011, 43, 787–795. [Google Scholar] [CrossRef] [PubMed]
- De Lemos, J.A.; Morrow, D.A.; Sabatine, M.S.; Murphy, S.A.; Gibson, C.M.; Antman, E.M.; McCabe, C.H.; Cannon, C.P.; Braunwald, E. Association Between Plasma Levels of Monocyte Chemoattractant Protein-1 and Long-Term Clinical Outcomes in Patients with Acute Coronary Syndromes. Circulation 2003, 107, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Guo, L.; Chen, H.; Gao, Y.; Guo, F.; Huang, J.; Jiang, C.; Wang, Z. Serum Chemokines Combined with Multi-Modal Imaging to Evaluate Atherosclerotic Plaque Stability in Patients Undergoing Carotid Endarterectomy. Front. Neurol. 2025, 16, 1537161. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.E.; Singhateh, Y.; Mackay, D.; Huxley, R.R.; Woodward, M. Total Cholesterol as a Risk Factor for Coronary Heart Disease and Stroke in Women Compared with Men: A Systematic Review and Meta-Analysis. Atherosclerosis 2016, 248, 123–131. [Google Scholar] [CrossRef]
- Lindenstrom, E.; Boysen, G.; Nyboe, J. Influence of Total Cholesterol, High Density Lipoprotein Cholesterol, and Triglycerides on Risk of Cerebrovascular Disease: The Copenhagen City Heart Study. BMJ 1994, 309, 11–15. [Google Scholar] [CrossRef]
- Benfante, R.; Yano, K.; Hwang, L.J.; Curb, J.D.; Kagan, A.; Ross, W. Elevated Serum Cholesterol Is a Risk Factor for Both Coronary Heart Disease and Thromboembolic Stroke in Hawaiian Japanese Men. Implications of Shared Risk. Stroke 1994, 25, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R. Blood Cholesterol and Vascular Mortality by Age, Sex, and Blood Pressure: A Meta-Analysis of Individual Data from 61 Prospective Studies with 55 000 Vascular Deaths. Lancet 2007, 370, 1829–1839. [Google Scholar] [CrossRef]
- Harmsen, P.; Lappas, G.; Rosengren, A.; Wilhelmsen, L. Long-Term Risk Factors for Stroke: Twenty-Eight Years of Follow-Up of 7457 Middle-Aged Men in Göteborg, Sweden. Stroke 2006, 37, 1663–1667. [Google Scholar] [CrossRef]
- Nago, N.; Ishikawa, S.; Goto, T.; Kayaba, K. Low Cholesterol Is Associated with Mortality from Stroke, Heart Disease, and Cancer: The Jichi Medical School Cohort Study. J. Epidemiol. 2011, 21, 67–74. [Google Scholar] [CrossRef]
- Zhao, W.; An, Z.; Hong, Y.; Zhou, G.; Guo, J.; Zhang, Y.; Yang, Y.; Ning, X.; Wang, J. Low Total Cholesterol Level Is the Independent Predictor of Poor Outcomes in Patients with Acute Ischemic Stroke: A Hospital-Based Prospective Study. BMC Neurol. 2016, 16, 36. [Google Scholar] [CrossRef]
- Yi, S.-W.; Shin, D.-H.; Kim, H.; Yi, J.-J.; Ohrr, H. Total Cholesterol and Stroke Mortality in Middle-Aged and Elderly Adults: A Prospective Cohort Study. Atherosclerosis 2018, 270, 211–217. [Google Scholar] [CrossRef]
- Li, B.; McIntyre, M.; Gandhi, C.; Halabi, M.; Long, A.; Van Hoof, A.; Afridi, A.; Schmidt, M.; Cole, C.; Santarelli, J.; et al. Low Total Cholesterol and High Density Lipoprotein Are Independent Predictors of Poor Outcomes Following Aneurysmal Subarachnoid Hemorrhage: A Preliminary Report. Clin. Neurol. Neurosurg. 2020, 197, 106062. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Yang, S.-S.; Pan, C.-J.; Wang, J.-H.; Li, H.-W.; Chen, S.-M.; Hao, J.-K.; Li, X.-H.; Li, R.-R.; Li, B.-Y.; et al. Cholesterol Paradox in the Community-Living Old Adults: Is Higher Better? J. Geriatr. Cardiol. 2023, 20, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Khattab, E.; Velidakis, N.; Patsourakos, N.; Lambadiari, V. A New Approach of Statin Therapy in Carotid Atherosclerosis: Targeting Indices of Plaque Vulnerability on the Top of Lipid-Lowering. A Narrative Review. Kardiol. Pol. 2022, 80, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Hou, J.; Xing, L.; Jia, H.; Hu, S.; Soeda, T.; Minami, Y.; Ong, D.; Vergallo, R.; Zhang, S.; et al. Is Age an Important Factor for Vascular Response to Statin Therapy? A Serial Optical Coherence Tomography and Intravascular Ultrasound Study. Coron. Artery Dis. 2017, 28, 209–217. [Google Scholar] [CrossRef]
- Płoński, A.; Krupa, A.; Pawlak, D.; Sokołowska, K.; Sieklucka, B.; Gabriel, M.; Płoński, A.F.; Głowiński, J.; Pawlak, K. The Metabolism of Big Endothelin-1 Axis and Lipids Affects Carotid Atherosclerotic Plaque Stability—The Possible Opposite Effects of Treatment with Statins and Aspirin. Pharmacol. Rep. 2025, 77, 739–750. [Google Scholar] [CrossRef]
- Ishida, M.; Sakai, C.; Kobayashi, Y.; Ishida, T. Cigarette Smoking and Atherosclerotic Cardiovascular Disease. J. Atheroscler. Thromb. 2024, 31, 189–200. [Google Scholar] [CrossRef]
- Gorgui, J.; Gorshkov, M.; Khan, N.; Daskalopoulou, S.S. Hypertension as a Risk Factor for Ischemic Stroke in Women. Can. J. Cardiol. 2014, 30, 774–782. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, L.; Chen, Y.; Xie, Q.; Yan, Z.; Liu, Y.; Kang, J.; Li, S. Risk Factors for Ischemic Stroke: Differences between Cerebral Small Vessel and Large Artery Atherosclerosis Aetiologies. Folia Neuropathol. 2021, 59, 378–385. [Google Scholar] [CrossRef]
- Fassaert, L.M.M.; Timmerman, N.; Van Koeverden, I.D.; Pasterkamp, G.; De Kleijn, D.P.V.; De Borst, G.J. Preoperative Hypertension Is Associated with Atherosclerotic Intraplaque Hemorrhage in Patients Undergoing Carotid Endarterectomy. Atherosclerosis 2019, 290, 214–221. [Google Scholar] [CrossRef]
- Chhatriwalla, A.K.; Nicholls, S.J.; Wang, T.H.; Wolski, K.; Sipahi, I.; Crowe, T.; Schoenhagen, P.; Kapadia, S.; Tuzcu, E.M.; Nissen, S.E. Low Levels of Low-Density Lipoprotein Cholesterol and Blood Pressure and Progression of Coronary Atherosclerosis. J. Am. Coll. Cardiol. 2009, 53, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Alfaddagh, A.; Elajami, T.K.; Ashfaque, H.; Haj-Ibrahim, H.; Welty, F.K. Diastolic Blood Pressure Predicts Coronary Plaque Volume in Patients with Coronary Artery Disease. Atherosclerosis 2018, 277, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Sakyi, S.A.; Opoku, A.S.; Amoani, B.; Afranie, B.O.; Kwarteng, A.; Ephriam, R.D.; Opoku, S.; Senu, E.; Aidoo, E.; Sarfo, F.S. Assessing the Variability and the Role of Inflammatory Cytokines and Monocyte Chemoattractant Protein-1 (MCP-1) in Predicting Stroke among Hypertensives: A Case-Control Study. Dialogues Health 2022, 1, 100086. [Google Scholar] [CrossRef]
- Kremer, C.; Lorenzano, S.; Bejot, Y.; Lal, A.; Epple, C.; Gdovinova, Z.; Mono, M.-L.; Karapanayiotides, T.; Jovanovic, D.; Dawson, J.; et al. Sex Differences in Outcome after Carotid Revascularization in Symptomatic and Asymptomatic Carotid Artery Stenosis. J. Vasc. Surg. 2023, 78, 817–827.e10. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.G.; Eliasziw, M.; Barr, H.W.K.; Clagett, G.P.; Barnes, R.W.; Wallace, M.C.; Taylor, D.W.; Haynes, R.B.; Finan, J.W.; Hachinski, V.C.; et al. The North American Symptomatic Carotid Endarterectomy Trial: Surgical Results in 1415 Patients. Stroke 1999, 30, 1751–1758. [Google Scholar] [CrossRef]
- Bonati, L.H.; Kakkos, S.; Berkefeld, J.; De Borst, G.J.; Bulbulia, R.; Halliday, A.; Van Herzeele, I.; Koncar, I.; McCabe, D.J.; Lal, A.; et al. European Stroke Organisation Guideline on Endarterectomy and Stenting for Carotid Artery Stenosis. Eur. Stroke J. 2021, 6, I–XLVII. [Google Scholar] [CrossRef]
- Klinghammer, L.; Urschel, K.; Cicha, I.; Lewczuk, P.; Raaz-Schrauder, D.; Achenbach, S.; Garlichs, C.D. Impact of Telmisartan on the Inflammatory State in Patients with Coronary Atherosclerosis—Influence on IP-10, TNF-α and MCP-1. Cytokine 2013, 62, 290–296. [Google Scholar] [CrossRef]
- Bogacka, J.; Pawlik, K.; Ciapała, K.; Ciechanowska, A.; Mika, J. CC Chemokine Receptor 4 (CCR4) as a Possible New Target for Therapy. Int. J. Mol. Sci. 2022, 23, 15638. [Google Scholar] [CrossRef]
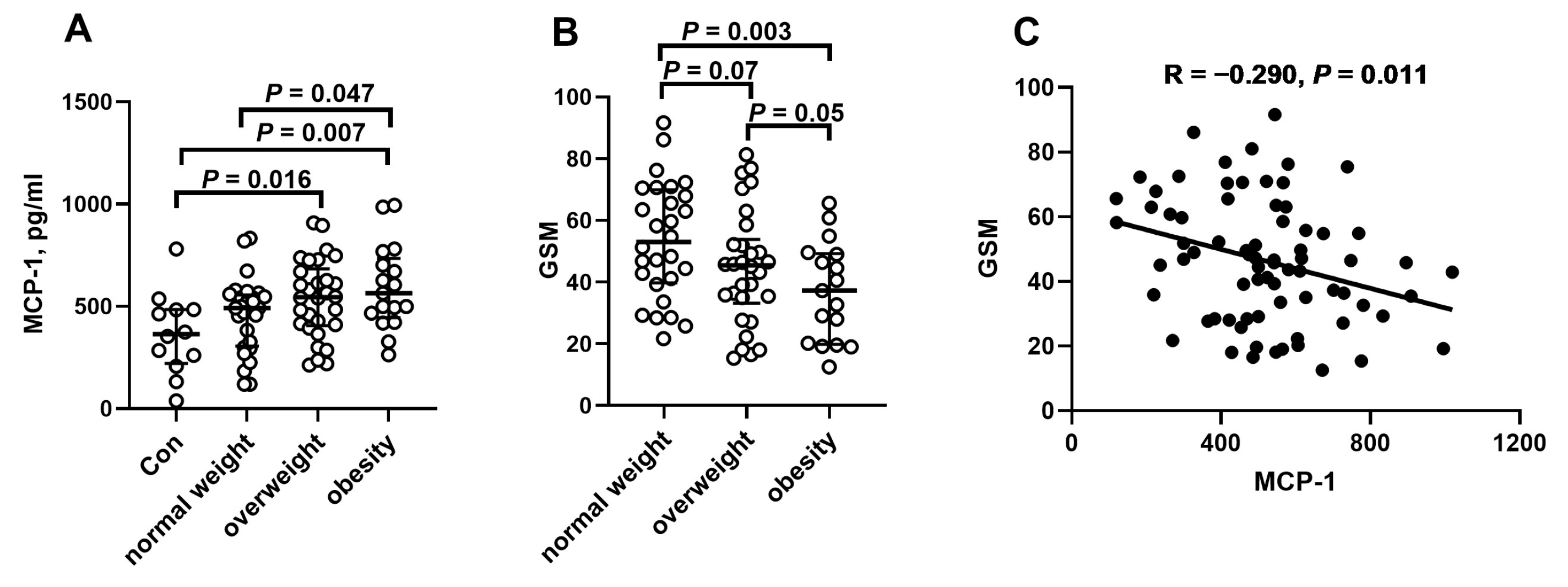
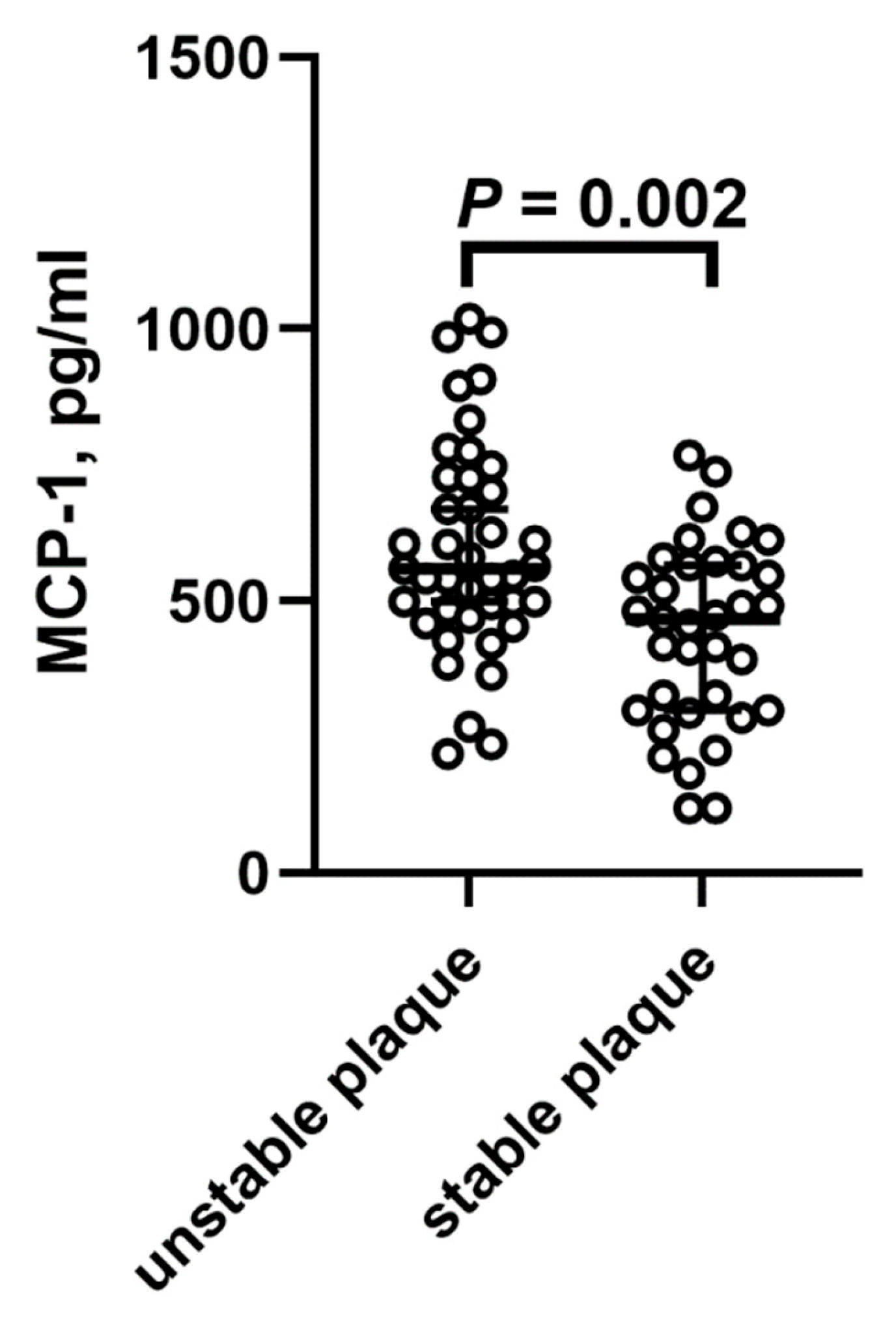
| Controls n = 12 | Normal Weight n = 28 | Overweight n = 32 | Obesity n = 17 | |
|---|---|---|---|---|
| Age, years | 73.5 (8.39) | 66.46 (7.55) a | 69.48 (10.09) | 67.41 (7.09) |
| Male sex, % | 83.3 | 60.7 | 83.9 | 88.2 |
| BMI, kg/m2 | 24.43 (1.43) | 22.52 (1.85) | 27.51 (1.41) a,d | 32.68 (2.16) b,d,e |
| Fibrinogen, g/L | 3.45 (2.93–4.18) | 3.91 (3.21–4.66) | 3.76 (3.42–4.09) | 3.95 (3.50–4.69) |
| D-dimers, μg/mL | 0.40 (0.19–0.63) | 0.49 (0.35–0.71) | 0.38 (0.27–0.72) | 0.35 (0.27–0.62) c |
| TC, mmol/L | 4.44 (3.57–4.84) | 4.19 (3.54–5.35) | 3.88 (3.42–4.94) | 3.74 (2.91–4.58) |
| LDL, mmol/L | 2.59 (1.73–2.82) | 2.35 (1.93–3.49) | 2.15 (1.79–3.21) | 1.99 (1.65–2.56) |
| HDL, mmol/L | 1.31 (0.75–1.45) | 1.19 (0.96–1.58) | 1.06 (0.98–1.27) | 0.92 (0.80–1.24) |
| TG, mmol/L | 1.08 (0.91–2.15) | 1.37 (0.93–1.66) | 1.25 (1.05–1.57) | 1.52 (1.26–1.87) |
| CRP, mg/L | 3.40 (1.50–8.10) | 2.70 (0.80–10.20) | 2.45 (1.20–4.60) | 3.40 (2.40–6.65) |
| Glucose, mmol/L | 4.22 (3.64–5.11) | 4.78 (4.03–5.58) | 5.06 (4.06–6.06) | 5.00 (4.67–5.94) |
| Hypertension, % | 75.0 | 85.7 | 81.3 | 100 |
| Grade 1 hypertension, % | 16.7 | 35.7 | 21.9 | 47.1 |
| Grade 2 hypertension, % | 58.3 | 35.7 | 46.9 | 47.0 |
| Grade 3 hypertension, % | 0 | 14.3 | 12.5 | 5.9 |
| CKD, % | 16.7 | 10.7 | 19.4 | 11.8 |
| IHD, % | 25.0 | 21.5 | 25.8 | 17.6 |
| DM, % | 16.7 | 25.0 | 32.3 | 41.2 a |
| Smoking, % | 75.0 | 71.4 | 80.6 | 82.4 |
| Diuretics, % | 33.3 | 21.4 | 41.9 | 29.4 |
| RAAS inhibitor, % | 83.3 | 67.9 | 67.7 | 88.2 |
| β-blockers, % | 58.3 | 39.3 | 48.4 | 47.1 |
| CCA, % | 16.7 | 39.3 | 38.7 | 23.5 |
| Aspirin, % | 83.3 | 85.7 | 64.5 | 52.9 c |
| Statins, % | 41.7 | 67.9 | 61.3 | 64.7 |
| OR | (95% CI) | p | |
|---|---|---|---|
| MCP-1 | 1.03 | 1.01–1.04 | 0.002 |
| Total cholesterol | 0.52 | 0.31–0.85 | 0.009 |
| Grade 2 hypertension | 3.52 | 1.35–9.20 | 0.010 |
| Overweight + obesity | 3.44 | 1.28–9.26 | 0.014 |
| Statin | 3.45 | 1.25–9.36 | 0.015 |
| Sulfonylurea derivatives | 0.22 | 0.04–1.13 | 0.063 |
| BMI | 1.09 | 0.97–1.22 | 0.149 |
| Fibrynogen | 1.003 | 0.99–1.01 | 0.304 |
| D-dimers | 0.74 | 0.33–1.65 | 0.742 |
| OR | (95% CI) | p | |
|---|---|---|---|
| MCP-1 | 1.04 | 1.03–1.07 | 0.009 |
| Total cholesterol | 0.51 | 0.28–0.93 | 0.028 |
| Grade 2 hypertension | 3.22 | 0.99–10.39 | 0.050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płoński, A.; Krupa, A.; Płoński, A.F.; Pawlak, D.; Gabriel, M.; Sieklucka, B.; Głowiński, J.; Pawlak, K. Obesity-Related Serum Monocyte Chemoattractant Protein-1 (MCP-1) as a Biomarker of Plaque Instability in Patients Undergoing Carotid Endarterectomy. Int. J. Mol. Sci. 2025, 26, 4731. https://doi.org/10.3390/ijms26104731
Płoński A, Krupa A, Płoński AF, Pawlak D, Gabriel M, Sieklucka B, Głowiński J, Pawlak K. Obesity-Related Serum Monocyte Chemoattractant Protein-1 (MCP-1) as a Biomarker of Plaque Instability in Patients Undergoing Carotid Endarterectomy. International Journal of Molecular Sciences. 2025; 26(10):4731. https://doi.org/10.3390/ijms26104731
Chicago/Turabian StylePłoński, Adam, Anna Krupa, Adam Filip Płoński, Dariusz Pawlak, Marcin Gabriel, Beata Sieklucka, Jerzy Głowiński, and Krystyna Pawlak. 2025. "Obesity-Related Serum Monocyte Chemoattractant Protein-1 (MCP-1) as a Biomarker of Plaque Instability in Patients Undergoing Carotid Endarterectomy" International Journal of Molecular Sciences 26, no. 10: 4731. https://doi.org/10.3390/ijms26104731
APA StylePłoński, A., Krupa, A., Płoński, A. F., Pawlak, D., Gabriel, M., Sieklucka, B., Głowiński, J., & Pawlak, K. (2025). Obesity-Related Serum Monocyte Chemoattractant Protein-1 (MCP-1) as a Biomarker of Plaque Instability in Patients Undergoing Carotid Endarterectomy. International Journal of Molecular Sciences, 26(10), 4731. https://doi.org/10.3390/ijms26104731






