Influence of Atherosclerosis-Associated Risk Factors on Expression of Endothelin Receptors in Advanced Atherosclerosis
Abstract
1. Introduction
2. Results
2.1. Cohort Data and Pathological State of the Collected Specimen
2.2. Histologic Examination of Atherosclerotic Plaques and Arterial Controls
2.3. Endothelin Receptor Expression Is Reduced in Advanced Atherosclerotic Plaques
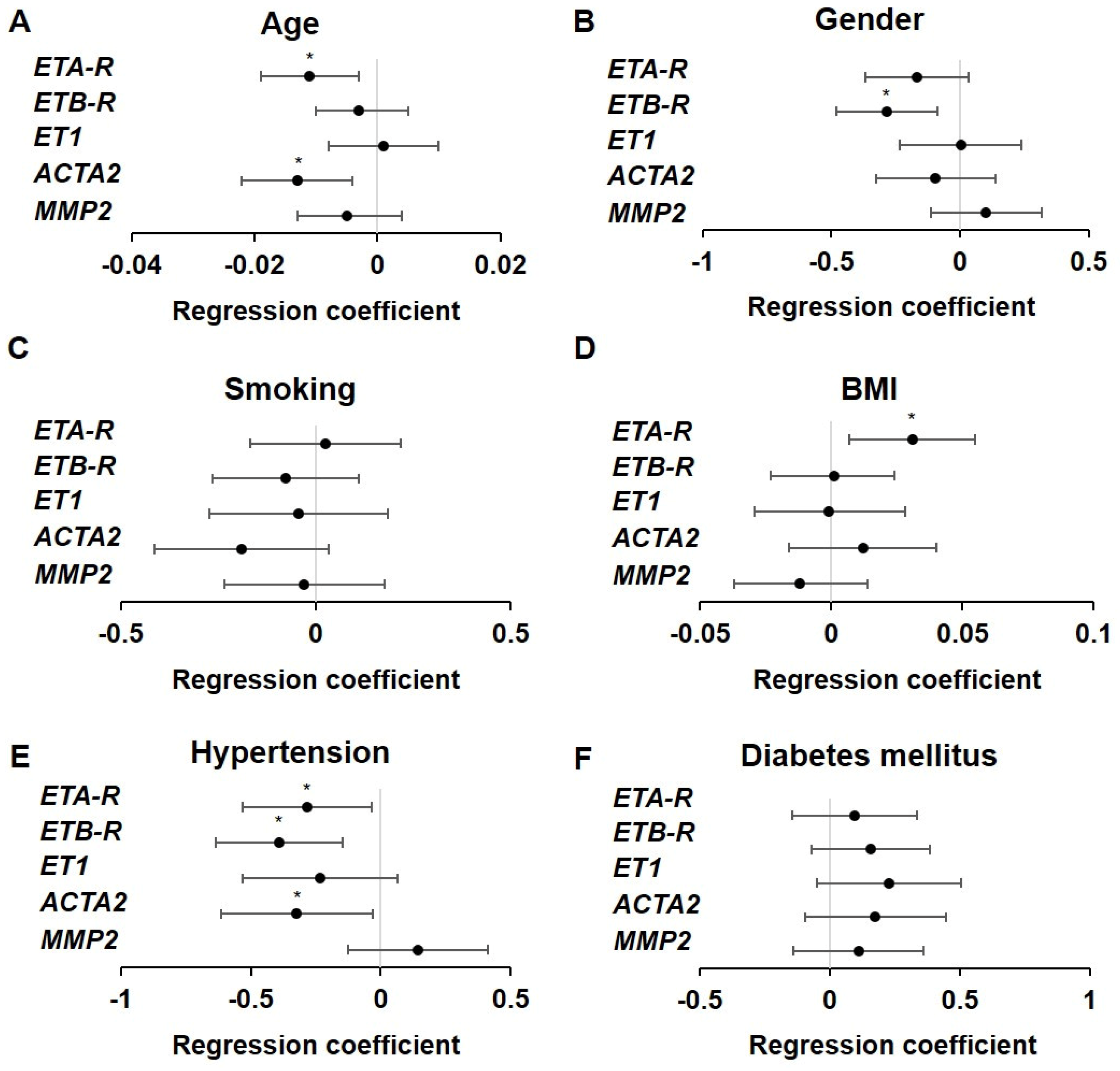
2.4. Influence of Atherosclerotic Risk Factors on ET Receptor Expression
3. Discussion
4. Materials and Methods
4.1. Subject Recruitment and Questionnaire
4.2. Surgical Procedures
4.3. RNA Isolation
4.4. Quantitative Real-Time PCR
4.5. Histology and Immunohistochemistry (IHC)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CI | Confidence interval |
| CT | Computer tomography |
| DAPI | 4′,6-diamidino-2-phenylindole |
| ETA/BR | Endothelin receptor type A/B |
| ET-1 | Endothelin 1 |
| ETR | Endothelin receptor |
| ERA | Endothelin receptor antagonist |
| MMP-2 | Matrix metalloproteinase-2 |
| MRI | Magnetic resonance imaging |
| NASCET | North American Symptomatic Carotid Endarterectomy Trial |
| NO | Nitric oxide |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| RNA | Ribonucleic acid |
| SMA | α-Smooth muscle actin |
| VSMC | Vascular smooth muscle cell |
References
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Sukhorukov, V.N.; Eremin, I.I.; Nadelyaeva, I.I.; Orekhov, A.N. Diagnostics of atherosclerosis: Overview of the existing methods. Front. Cardiovasc. Med. 2023, 10, 1134097. [Google Scholar] [CrossRef] [PubMed]
- Sosnovik, D.E.; Nahrendorf, M.; Weissleder, R. Molecular Magnetic Resonance Imaging in Cardiovascular Medicine. Circulation 2007, 115, 2076–2086. [Google Scholar] [CrossRef]
- Dixon, D.B. Non-Invasive Techniques in Pediatric Dyslipidemia. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Hong, Y.M. Atherosclerotic Cardiovascular Disease Beginning in Childhood. Korean Circ. J. 2010, 40, 1–9. [Google Scholar] [CrossRef]
- Raitakari, O.; Pahkala, K.; Magnussen, C.G. Prevention of atherosclerosis from childhood. Nat. Rev. Cardiol. 2022, 19, 543–554. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Tousoulis, D.; Androulakis, E.; Giotakis, A.; Siasos, G.; Latsios, G.; Stefanadis, C. Lifestyle factors and endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 94–106. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef]
- Shaw, J.; Anderson, T. Coronary endothelial dysfunction in non-obstructive coronary artery disease: Risk, pathogenesis, diagnosis and therapy. Vasc. Med. 2016, 21, 146–155. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Dhaun, N.; Webb, D.J. Endothelins in cardiovascular biology and therapeutics. Nat. Rev. Cardiol. 2019, 16, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.J.; Davenport, A.P. Endothelin Receptors and Their Antagonists. Semin. Nephrol. 2015, 35, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Babaei, S.; Picard, P.; Ravandi, A.; Monge, J.C.; Lee, T.C.; Cernacek, P.; Stewart, D.J. Blockade of endothelin receptors markedly reduces atherosclerosis in LDL receptor deficient mice: Role of endothelin in macrophage foam cell formation. Cardiovasc. Res. 2000, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Haudenschild, C.C.; d’Uscio, L.V.; Shaw, S.; Münter, K.; Lüscher, T.F. Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 14367–14372. [Google Scholar] [CrossRef]
- Winkles, J.A.; Alberts, G.F.; Brogi, E.; Libby, P. Endothelin-1 and Endothelin Receptor mRNA Expression in Normal and Atherosclerotic Human Arteries. Biochem. Biophys. Res. Commun. 1993, 191, 1081–1088. [Google Scholar] [CrossRef]
- Yoon, M.H.; Reriani, M.; Mario, G.; Rihal, C.; Gulati, R.; Lennon, R.; Tilford, J.M.; Lerman, L.O.; Lerman, A. Long-term endothelin receptor antagonism attenuates coronary plaque progression in patients with early atherosclerosis. Int. J. Cardiol. 2013, 168, 1316–1321. [Google Scholar] [CrossRef]
- Iwasa, S.; Fan, J.; Shimokama, T.; Nagata, M.; Watanabe, T. Increased immunoreactivity of endothelin-1 and endothelin B receptor in human atherosclerotic lesions. A possible role in atherogenesis. Atherosclerosis 1999, 146, 93–100. [Google Scholar] [CrossRef]
- Mazzuca, M.Q.; Khalil, R.A. Vascular endothelin receptor type B: Structure, function and dysregulation in vascular disease. Biochem. Pharmacol. 2012, 84, 147–162. [Google Scholar] [CrossRef]
- Lerman, A.; Webster, M.W.; Chesebro, J.H.; Edwards, W.D.; Wei, C.M.; Fuster, V.; Burnett, J.C., Jr. Circulating and tissue endothelin immunoreactivity in hypercholesterolemic pigs. Circulation 1993, 88, 2923–2928. [Google Scholar] [CrossRef]
- Best, P.J.; McKenna, C.J.; Hasdai, D.; Holmes, D.R.; Lerman, A. Chronic Endothelin Receptor Antagonism Preserves Coronary Endothelial Function in Experimental Hypercholesterolemia. Circulation 1999, 99, 1747–1752. [Google Scholar] [CrossRef]
- Campia, U.; Tesauro, M.; Di Daniele, N.; Cardillo, C. The vascular endothelin system in obesity and type 2 diabetes: Pathophysiology and therapeutic implications. Life Sci. 2014, 118, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Higashi, Y. Pathophysiological Association between Diabetes Mellitus and Endothelial Dysfunction. Antioxidants 2021, 10, 1306. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Yanagisawa, M. Endothelin: 30 Years From Discovery to Therapy. Hypertension 2019, 74, 1232–1265. [Google Scholar] [CrossRef] [PubMed]
- Emoto, N.; Vignon-Zellweger, N.; Lopes, R.A.M.; Cacioppo, J.; Desbiens, L.; Kamato, D.; Leurgans, T.; Moorhouse, R.; Straube, J.; Wurm, R.; et al. 25 Years of endothelin research: The next generation. Life Sci. 2014, 118, 77–86. [Google Scholar] [CrossRef][Green Version]
- Kohan, D.E. Endothelin, hypertension and chronic kidney disease: New insights. Curr. Opin. Nephrol. Hypertens. 2010, 19, 134–139. [Google Scholar] [CrossRef]
- Schiffrin, E.L.; Pollock, D.M. Endothelin System in Hypertension and Chronic Kidney Disease. Hypertension 2024, 81, 691–701. [Google Scholar] [CrossRef]
- Liu, R.; Yuan, T.; Wang, R.; Gong, D.; Wang, S.; Du, G.; Fang, L. Insights into Endothelin Receptors in Pulmonary Hypertension. Int. J. Mol. Sci. 2023, 24, 10206. [Google Scholar] [CrossRef]
- Herbet, A.; Costa, N.; Leventoux, N.; Mabondzo, A.; Couraud, J.-Y.; Borrull, A.; Hugnot, J.-P.; Boquet, D. Antibodies Targeting Human Endothelin-1 Receptors Reveal Different Conformational States in Cancer Cells. Physiol. Res. 2018, 67 (Suppl. S1), S257–S264. [Google Scholar] [CrossRef]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef]
- Olejarz, W.; Łacheta, D.; Kubiak-Tomaszewska, G. Matrix Metalloproteinases as Biomarkers of Atherosclerotic Plaque Instability. Int. J. Mol. Sci. 2020, 21, 3946. [Google Scholar] [CrossRef]
- Rafnsson, A.; Matic, L.P.; Lengquist, M.; Mahdi, A.; Shemyakin, A.; Paulsson-Berne, G.; Hansson, G.K.; Gabrielsen, A.; Hedin, U.; Yang, J.; et al. Endothelin-1 increases expression and activity of arginase 2 via ETB receptors and is co-expressed with arginase 2 in human atherosclerotic plaques. Atherosclerosis 2020, 292, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Reriani, M.; Raichlin, E.; Prasad, A.; Mathew, V.; Pumper, G.M.; Nelson, R.E.; Lennon, R.; Rihal, C.; Lerman, L.O.; Lerman, A. Long-Term Administration of Endothelin Receptor Antagonist Improves Coronary Endothelial Function in Patients with Early Atherosclerosis. Circulation 2010, 122, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Raichlin, E.; Prasad, A.; Mathew, V.; Kent, B.; Holmes, D.R.; Pumper, G.M.; Nelson, R.E.; Lerman, L.O.; Lerman, A. Efficacy and Safety of Atrasentan in Patients with Cardiovascular Risk and Early Atherosclerosis. Hypertension 2008, 52, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Kowala, M.C.; Rose, P.M.; Stein, P.D.; Goller, N.; Recce, R.; Beyer, S.; Valentine, M.; Barton, D.; Durham, S.K. Selective blockade of the endothelin subtype A receptor decreases early atherosclerosis in hamsters fed cholesterol. Am. J. Pathol. 1995, 146, 819–826. [Google Scholar]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Oh, S.; Son, M.; Park, C.-H.; Jang, J.T.; Son, K.H.; Byun, K. Pyrogallol-Phloroglucinol-6,6-Bieckolon Attenuates Vascular Smooth Muscle Cell Proliferation and Phenotype Switching in Hyperlipidemia through Modulation of Chemokine Receptor 5. Mar. Drugs 2020, 18, 393. [Google Scholar] [CrossRef]
- Kuczmarski, A.V.; Welti, L.M.; Moreau, K.L.; Wenner, M.M. ET-1 as a Sex-Specific Mechanism Impacting Age-Related Changes in Vascular Function. Front. Aging 2021, 2, 727416. [Google Scholar] [CrossRef]
- Wenner, M.M.; Sebzda, K.N.; Kuczmarski, A.V.; Pohlig, R.T.; Edwards, D.G. ETB receptor contribution to vascular dysfunction in postmenopausal women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R51–R57. [Google Scholar] [CrossRef]
- Shoemaker, L.N.; Haigh, K.M.; Kuczmarski, A.V.; McGinty, S.J.; Welti, L.M.; Hobson, J.C.; Edwards, D.G.; Feinberg, R.F.; Wenner, M.M. ETB receptor-mediated vasodilation is regulated by estradiol in young women. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H592–H598. [Google Scholar] [CrossRef]
- Miyagawa, K.; Emoto, N. Current state of endothelin receptor antagonism in hypertension and pulmonary hypertension. Ther. Adv. Cardiovasc. Dis. 2014, 8, 202–216. [Google Scholar] [CrossRef]
- Kumagae, S.; Adachi, H.; Jacobs, D.R.; Hirai, Y.; Enomoto, M.; Fukami, A.; Otsuka, M.; Nanjo, Y.; Esaki, E.; Kumagai, E.; et al. High Level of Plasma Endothelin-1 Predicts Development of Hypertension in Normotensive Subjects. Am. J. Hypertens. 2010, 23, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.; Hoeper, M.M. Endothelin receptor antagonists in pulmonary arterial hypertension. Eur. Respir. J. 2008, 31, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Enevoldsen, F.C.; Sahana, J.; Wehland, M.; Grimm, D.; Infanger, M.; Krüger, M.J. Endothelin Receptor Antagonists: Status Quo and Future Perspectives for Targeted Therapy. Clin. Med. 2020, 9, 824. [Google Scholar] [CrossRef]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus–Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [PubMed]
- Yugar-Toledo, J.C.; Modolo, R.; Moreno, H. Smoking and the Endothelium. In Endothelium and Cardiovascular Diseases; Elsevier: Amsterdam, The Netherlands, 2018; pp. 537–554. [Google Scholar]
- Mather, K.J.; Mirzamohammadi, B.; Lteif, A.; Steinberg, H.O.; Baron, A.D. Endothelin Contributes to Basal Vascular Tone and Endothelial Dysfunction in Human Obesity and Type 2 Diabetes. Diabetes 2002, 51, 3517–3523. [Google Scholar] [CrossRef]
- Schneider, J.G.; Tilly, N.; Hierl, T.; Sommer, U.; Hamann, A.; Dugi, K.; Leidig-Bruckner, G.; Kasperk, C. Elevated plasma endothelin-1 levels in diabetes mellitus. Am. J. Hypertens. 2002, 15, 967–972. [Google Scholar] [CrossRef]
- Cardillo, C.; Campia, U.; Bryant, M.B.; Panza, J.A. Increased Activity of Endogenous Endothelin in Patients with Type II Diabetes Mellitus. Circulation 2002, 106, 1783–1787. [Google Scholar] [CrossRef]
- Cardillo, C.; Campia, U.; Kilcoyne, C.M.; Bryant, M.B.; Panza, J.A. Improved Endothelium-Dependent Vasodilation After Blockade of Endothelin Receptors in Patients with Essential Hypertension. Circulation 2002, 105, 452–456. [Google Scholar] [CrossRef][Green Version]
- Rodella, L.F.; Favero, G.; Foglio, E.; Rossini, C.; Castrezzati, S.; Lonati, C.; Rezzani, R. Vascular endothelial cells and dysfunctions: Role of melatonin. Front. Biosci. (Elite Ed.) 2013, 5, 119–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.-B. The roles of endothelin and its receptors in cigarette smoke-associated pulmonary hypertension with chronic lung disease. Pathol. Res. Pract. 2020, 216, 153083. [Google Scholar] [CrossRef]
- Haak, T.; Jungmann, E.; Raab, C.; Usadel, K.H. Elevated endothelin-1 levels after cigarette smoking. Metab. Clin. Exp. 1994, 43, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Tanus-Santos, J.E.; Sampaio, R.C.; Hyslop, S.; Franchini, K.G.; Moreno, H. Endothelin ET(A) receptor antagonism attenuates the pressor effects of nicotine in rats. Eur. J. Pharmacol. 2000, 396, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Derella, C.C.; Blanks, A.M.; Wang, X.; Tucker, M.A.; Horsager, C.; Jeong, J.H.; Rodriguez-Miguelez, P.; Looney, J.; Thomas, J.; Pollock, D.M.; et al. Endothelin receptor blockade blunts the pressor response to acute stress in men and women with obesity. J. Appl. Physiol. 2022, 132, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Weil, B.R.; Westby, C.M.; van Guilder, G.P.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Enhanced endothelin-1 system activity with overweight and obesity. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H689–H695. [Google Scholar] [CrossRef]
- Vivier, D.; Hautière, M.; Pineau, D.; Dancer, P.-A.; Herbet, A.; Hugnot, J.-P.; Bernhard, C.; Goncalves, V.; Truillet, C.; Boquet, D.; et al. Synthesis and Preclinical Fluorescence Imaging of Dually Functionalized Antibody Conjugates Targeting Endothelin Receptor-Positive Tumors. Bioconjug. Chem. 2023, 34, 2144–2153. [Google Scholar] [CrossRef]
- Höltke, C.; Enders, L.; Stölting, M.; Geyer, C.; Masthoff, M.; Kuhlmann, M.T.; Wildgruber, M.; Helfen, A. Detection of Early Endothelial Dysfunction by Optoacoustic Tomography. Int. J. Mol. Sci. 2023, 24, 8627. [Google Scholar] [CrossRef]
- Bartnett, H.J.M. North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke 1991, 22, 711–720. [Google Scholar] [CrossRef]
- Schoenefeld, E.; Donas, K.P.; Schönefeld, T.; Osada, N.; Austermann, M.; Torsello, G. Mid-term outcome after endovascular therapy in the superficial femoral and popliteal artery using long stents. VASA. Z. Gefasskrankh. 2012, 41, 49–56. [Google Scholar] [CrossRef]
- Schmedt, C.-G.; Dikic, S.; Demhasaj, S.; Küspert, T. Diagnostik und Therapie der Stammveneninsuffizienz. Gefässchirurgie 2015, 20, 589–607. [Google Scholar] [CrossRef]
- Stölting, M.; Geyer, C.; Helfen, A.; Hahnenkamp, A.; Usai, M.V.; Wardelmann, E.; Kuhlmann, M.T.; Wildgruber, M.; Höltke, C. Monitoring Endothelin—A Receptor Expression During the Progression of Atherosclerosis; Universitäts- und Landesbibliothek Münster: Münster, Germany, 2022. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

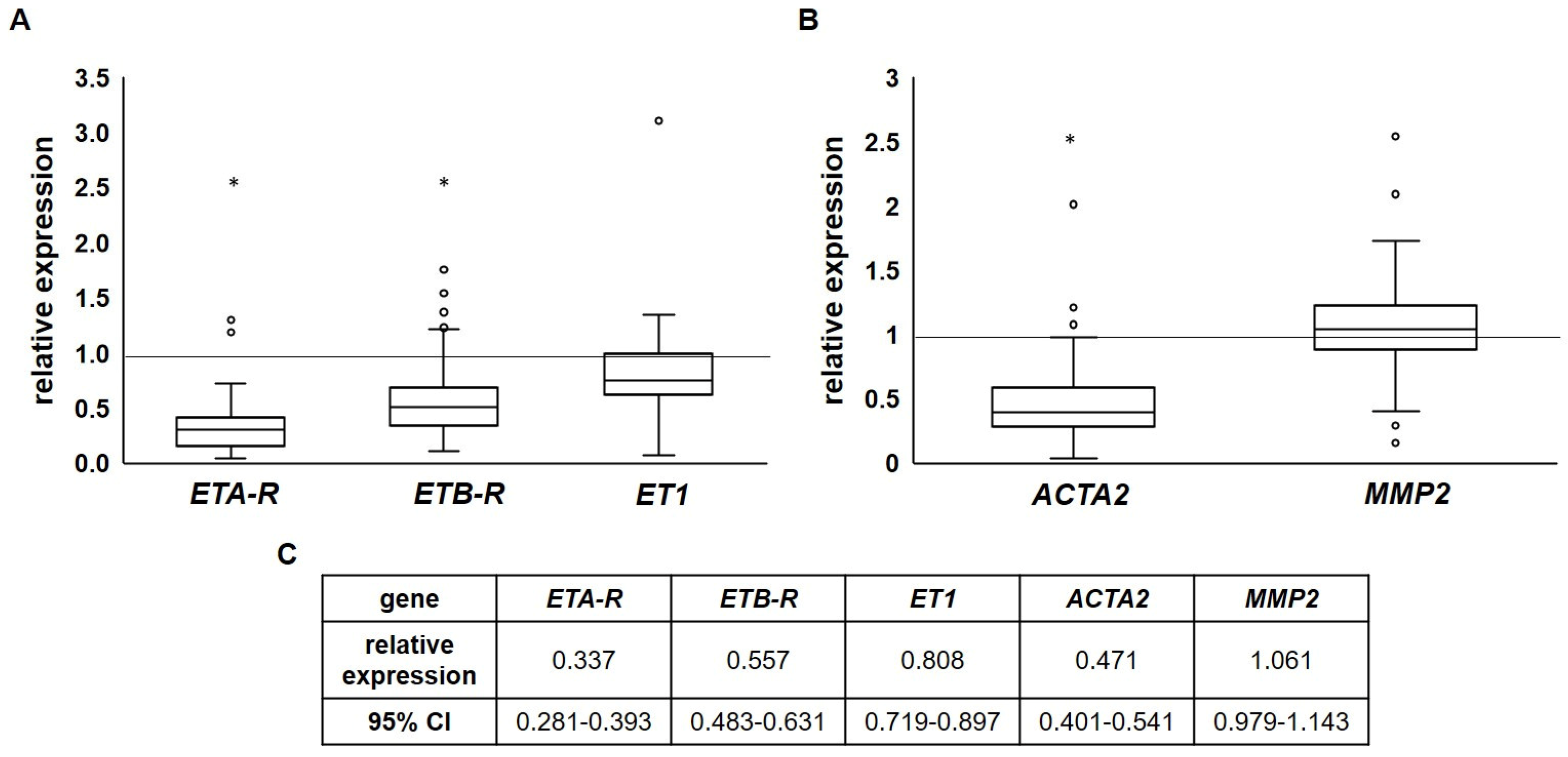
| Type of Specimen | Healthy Artery * | Atherosclerotic Carotid Artery | Atherosclerotic Femoral Artery | Total * |
|---|---|---|---|---|
| female | 17 | 17 | 2 | 36 |
| male | 8 | 39 | 14 | 61 |
| mean age | 49 | 69 | 70 | 63 |
| NASCET Score (%) | 50 | 60 | 70 | 80 | 90 | w/o Score but Symptomatic | Total |
|---|---|---|---|---|---|---|---|
| number of specimens | 1 | 3 | 8 | 30 | 9 | 15 | 66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbers, O.; Höltke, C.; Usai, M.V.; Hochhalter, J.; Mallik, M.; Wildgruber, M.; Helfen, A.; Stölting, M. Influence of Atherosclerosis-Associated Risk Factors on Expression of Endothelin Receptors in Advanced Atherosclerosis. Int. J. Mol. Sci. 2025, 26, 2310. https://doi.org/10.3390/ijms26052310
Herbers O, Höltke C, Usai MV, Hochhalter J, Mallik M, Wildgruber M, Helfen A, Stölting M. Influence of Atherosclerosis-Associated Risk Factors on Expression of Endothelin Receptors in Advanced Atherosclerosis. International Journal of Molecular Sciences. 2025; 26(5):2310. https://doi.org/10.3390/ijms26052310
Chicago/Turabian StyleHerbers, Oliver, Carsten Höltke, Marco Virgilio Usai, Jana Hochhalter, Moushami Mallik, Moritz Wildgruber, Anne Helfen, and Miriam Stölting. 2025. "Influence of Atherosclerosis-Associated Risk Factors on Expression of Endothelin Receptors in Advanced Atherosclerosis" International Journal of Molecular Sciences 26, no. 5: 2310. https://doi.org/10.3390/ijms26052310
APA StyleHerbers, O., Höltke, C., Usai, M. V., Hochhalter, J., Mallik, M., Wildgruber, M., Helfen, A., & Stölting, M. (2025). Influence of Atherosclerosis-Associated Risk Factors on Expression of Endothelin Receptors in Advanced Atherosclerosis. International Journal of Molecular Sciences, 26(5), 2310. https://doi.org/10.3390/ijms26052310







