Leptin and PAI-1 Levels Are Decreased After a Dietary Intervention in Patients with Irritable Bowel Syndrome
Abstract
1. Introduction
2. Results
2.1. Basal Characteristics
2.2. Dietary Changes and Weight
2.3. IBS Symptoms
2.4. Hormonal Changes
2.5. Comparison of Changes in Leptin and PAI-1 with Changes in Diet and Symptoms
2.6. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Study Design
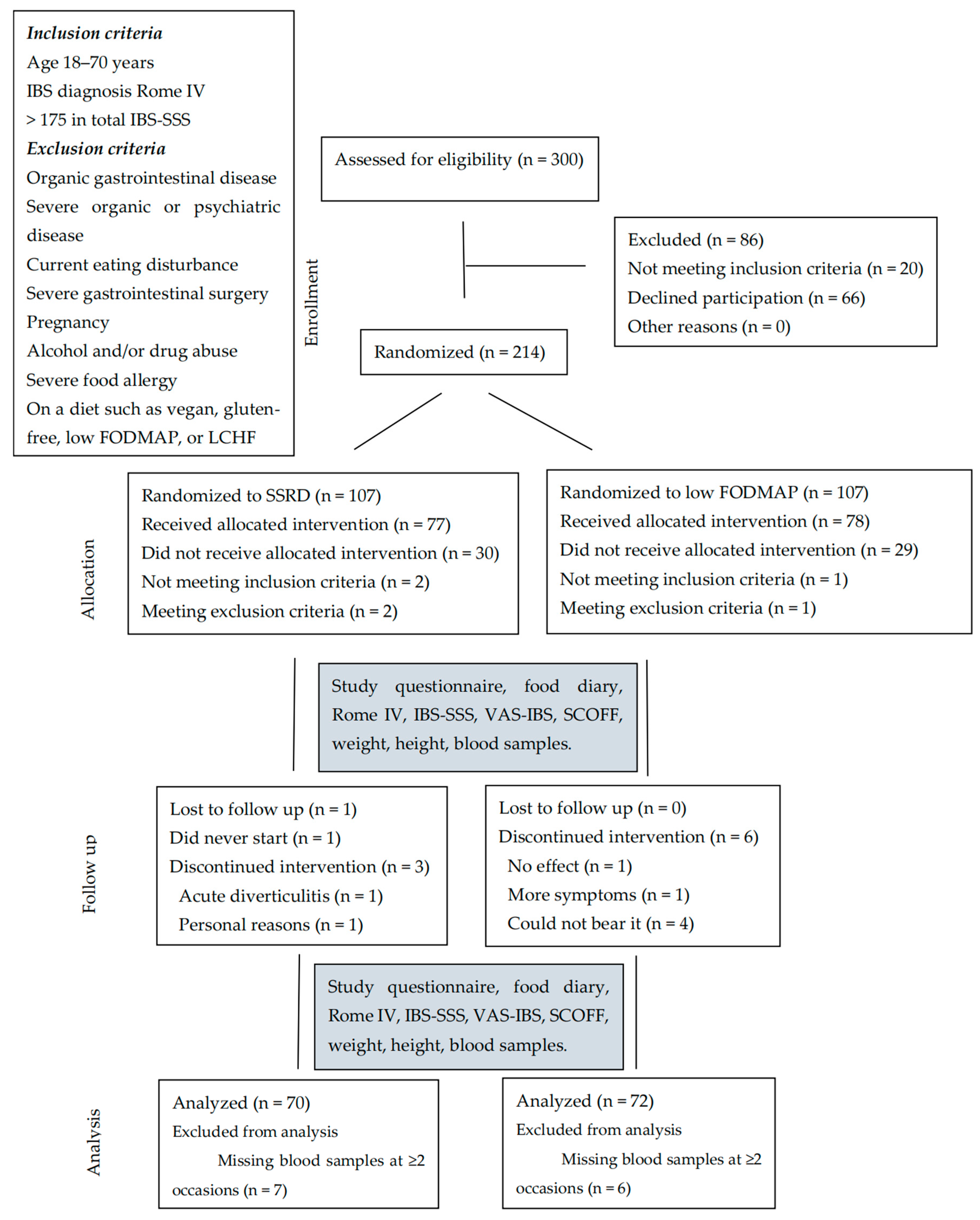
4.2. Participants
4.3. Dietary Advice
4.4. Questionnaires
4.4.1. Study Questionnaire
4.4.2. ROME IV Questionnaire
4.4.3. Irritable Bowel Syndrome Severity Scoring System
4.4.4. Visual Analog Scale for Irritable Bowel Syndrome
4.5. Blood Samples
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology 2021, 160, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Gambaccini, D.; Stasi, C.; Urbano, M.T.; Marchi, S.; Usai-Satta, P. Irritable bowel syndrome: A disease still searching for pathogenesis, diagnosis and therapy. World J. Gastroenterol. 2014, 20, 8807–8820. [Google Scholar] [PubMed]
- Ng, J.J.J.; Loo, W.M.; Siah, K.T.H. Associations between irritable bowel syndrome and non-alcoholic fatty liver disease: A systematic review. World J. Hepatol. 2023, 15, 925–938. [Google Scholar] [CrossRef]
- Roth, B.; Ohlsson, B. Overweight and vitamin D deficiency are common in patients with irritable bowel syndrome—A cross-sectional study. BMC Gastroenterol. 2024, 24, 296. [Google Scholar] [CrossRef]
- Hayes, P.A.; Fraher, M.H.; Quigley, E.M. Irritable bowel syndrome: The role of food in pathogenesis and management. Gastroenterol. Hepatol. 2014, 10, 164–174. [Google Scholar]
- Algera, J.; Colomier, E.; Simrén, M. The Dietary Management of Patients with Irritable Bowel Syndrome: A Narrative Review of the Existing and Emerging Evidence. Nutrients 2019, 11, 2162. [Google Scholar] [CrossRef]
- Mitchell, H.; Porter, J.; Gibson, P.R.; Barrett, J.; Garg, M. Review article: Implementation of a diet low in FODMAPs for patients with irritable bowel syndrome-directions for future research. Aliment. Pharmacol. Ther. 2019, 49, 124–139. [Google Scholar] [CrossRef]
- Henström, M.; Diekmann, L.; Bonfiglio, F.; Hadizadeh, F.; Kuech, E.M.; von Köckritz-Blickwede, M.; Thingholm, L.B.; Zheng, T.; Assadi, G.; Dierks, C.; et al. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut 2018, 67, 263–270. [Google Scholar] [CrossRef]
- Nilholm, C.; Roth, B.; Ohlsson, B. A Dietary Intervention with Reduction of Starch and Sucrose Leads to Reduced Gastrointestinal and Extra-Intestinal Symptoms in IBS Patients. Nutrients 2019, 11, 1662. [Google Scholar] [CrossRef]
- Roth, B.; Nseir, M.; Jeppsson, H.; D’Amato, M.; Sundquist, K.; Ohlsson, B. A Starch- and Sucrose-Reduced Diet Has Similar Efficiency as Low FODMAP in IBS-A Randomized Non-Inferiority Study. Nutrients 2024, 16, 3039. [Google Scholar] [CrossRef]
- Treem, W.R. Congenital sucrase-isomaltase deficiency. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 1–14. [Google Scholar] [PubMed]
- Gibson, P.R.; Shepherd, S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Wahren, J.; Ekberg, K.; Johansson, J.; Henriksson, M.; Pramanik, A.; Johansson, B.L.; Rigler, R.; Jörnvall, H. Role of C-peptide in human physiology. Am. J. Physiol. Metab. 2000, 278, E759–E768. [Google Scholar] [CrossRef]
- Drucker, D.J. The role of gut hormones in glucose homeostasis. J. Clin. Investig. 2007, 117, 24–32. [Google Scholar] [CrossRef]
- Mathews, S.G.; Krishna, R.B.D.; Lavanya, M.; Nandini, K.; Murali, S.; Agarwal, P.; Rani, E.; F, A.M. The Role of the Plasminogen Activator Inhibitor 1 (PAI1) in Ovarian Cancer: Mechanisms and Therapeutic Implications. Glob. Med. Genet. 2024, 11, 358–365. [Google Scholar] [CrossRef]
- Wang, B.; Gu, B.; Zhang, T.; Li, X.; Wang, N.; Ma, C.; Xiang, L.; Wang, Y.; Gao, L.; Yu, Y.; et al. Good or bad: Paradox of plasminogen activator inhibitor 1 (PAI-1) in digestive system tumors. Cancer Lett. 2023, 559, 216117. [Google Scholar] [CrossRef]
- Fenske, W.K.; Schnyder, I.; Koch, G.; Walti, C.; Pfister, M.; Kopp, P.; Fassnacht, M.; Strauss, K.; Christ-Crain, M. Release and decay kinetics of copeptin vs AVP in response to osmotic alterations in healthy volunteers. J. Clin. Endocrinol. Metab. 2018, 103, 505–513. [Google Scholar] [CrossRef]
- Enhörning, S.; Wang, T.J.; Nilsson, P.M.; Almgren, P.; Hedblad, B.; Berglund, G.; Struck, J.; Morgenthaler, N.G.; Bergmann, A.; Lindholm, E.; et al. Plasma copeptin and the risk of diabetes mellitus. Circulation 2010, 121, 2102–2108. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Li, X.; Cui, W.; Zhang, S. Serum copeptin in women with gestational diabetes mellitus: A meta-analysis. Biomol. Biomed. 2024, 24, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Guler, B.; Ertuglu, L.A.; Dagel, T.; Afsar, B.; Incir, S.; Baygul, A.; Covic, A.; Andres-Hernando, A.; Sánchez-Lozada, L.G.; et al. The Speed of Ingestion of a Sugary Beverage Has an Effect on the Acute Metabolic Response to Fructose. Nutrients 2021, 13, 1916. [Google Scholar] [CrossRef] [PubMed]
- Al-Suhaimi, E.A.; Shehzad, A. Leptin, resistin and visfatin: The missing link between endocrine metabolic disorders and immunity. Eur. J. Med. Res. 2013, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Bado, A.; Levasseur, S.; Attoub, S.; Kermorgant, S.; Laigneau, J.P.; Bortoluzzi, M.N.; Moizo, L.; Lehy, T.; Guerre-Millo, M.; Le Marchand-Brustel, Y.; et al. The stomach is a source of leptin. Nature 1998, 394, 790–793. [Google Scholar] [CrossRef]
- Williams, K.W.; Scott, M.M.; Elmquist, J.K. From observation to experimentation: Leptin action in the mediobasal hypothalamus. Am. J. Clin. Nutr. 2009, 89, 985S–990S. [Google Scholar] [CrossRef]
- Howard, J.M.; Pidgeon, G.P.; Reynolds, J.V. Leptin and gastro-intestinal malignancies. Obes. Rev. 2010, 11, 863–874. [Google Scholar] [CrossRef]
- Ahima, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Flier, J.S. Role of leptin in the neuroendocrine response to fasting. Nature 1996, 382, 250–252. [Google Scholar] [CrossRef]
- Flier, J.S. Clinical review 94: What’s in a name? In search of leptin’s physiologic role. J. Clin. Endocrinol. Metab. 1998, 83, 1407–1413. [Google Scholar]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Cummings, D.E.; Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Investig. 2007, 117, 13–23. [Google Scholar] [CrossRef]
- Saidi, K.; Nilholm, C.; Roth, B.; Ohlsson, B. A carbohydrate-restricted diet for patients with irritable bowel syndrome lowers serum C-peptide, insulin, and leptin without any correlation with symptom reduction. Nutr. Res. 2021, 86, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.; Myllyvainio, J.; D’Amato, M.; Larsson, E.; Ohlsson, B. A Starch- and Sucrose-Reduced Diet in Irritable Bowel Syndrome Leads to Lower Circulating Levels of PAI-1 and Visfatin: A Randomized Controlled Study. Nutrients 2022, 14, 1688. [Google Scholar] [CrossRef]
- Guo, Y.; Niu, K.; Momma, H.; Kobayashi, Y.; Chujo, M.; Otomo, A.; Fukudo, S.; Nagatomi, R. Irritable bowel syndrome is positively related to metabolic syndrome: A population-based cross-sectional study. PLoS ONE 2014, 9, e112289. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Gilja, O.H.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. Interaction between ingested nutrients and gut endocrine cells in patients with irritable bowel syndrome. Int. J. Mol. Med. 2014, 34, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, M.; Ohlsson, B.; Ulander, K. Development and psychometric testing of the Visual Analogue Scale for Irritable Bowel Syndrome (VAS-IBS). BMC Gastroenterol. 2007, 7, 16. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- Lindqvist, A.; Baelemans, A.; Erlanson-Albertsson, C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul. Pept. 2008, 150, 26–32. [Google Scholar] [CrossRef]
- Russo, F.; Chimienti, G.; Riezzo, G.; Linsalata, M.; D’Attoma, B.; Clemente, C.; Orlando, A. Adipose tissue-derived biomarkers of intestinal barrier functions for the characterization of diarrhoea-predominant IBS. Dis. Markers 2018, 2018, 1827937. [Google Scholar] [CrossRef]
- Kim, J.; Oh, C.M.; Kim, H. The Interplay of Adipokines and Pancreatic Beta Cells in Metabolic Regulation and Diabetes. Biomedicines 2023, 11, 2589. [Google Scholar] [CrossRef]
- Kieffer, T.J.; Habener, J.F. The adipoinsular axis: Effects of leptin on pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E1–E14. [Google Scholar] [CrossRef]
- Lee, Y.H.; Magkos, F.; Mantzoros, C.S.; Kang, E.S. Effects of leptin and adiponectin on pancreatic β-cell function. Metabolism 2011, 60, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.; Asakawa, A.; Amitani, H.; Inui, A. Stimulation of leptin secretion by insulin. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. S3), S543–S548. [Google Scholar] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Le Drean, G.; Haure-Mrande, V.; Ferrier, L.; Bonnet, C.; Hulin, P.; de Coppet, P.; Segain, J.P. Visceral adipose tissue and leptin increase colonic epithelial tight junction permeability via a RhoA-ROCK-dependent pathway. FASEB J. 2014, 28, 1059–1070. [Google Scholar] [CrossRef]
- Yanping, W.; Gao, X.; Cheng, Y.; Liu, M.; Liao, S.; Zhou, J.; Hao, J.; Jiang, G.; Lu, Y.; Qu, T.; et al. The interaction between obesity and visceral hypersensitivity. J. Gastroenterol. Hepatol. 2023, 38, 370–377. [Google Scholar] [CrossRef]
- Jamialahamdi, T.; Mirhadi, E.; Almahmeed, W.; Sukhorukov, V.N.; Eid, A.H.; Salehabadi, S.; Sahebkar, A. The Effect of Bariatric Surgery on PAI-1 Levels: A Systematic Review and Meta-analysis. Obes. Surg. 2024, 34, 3876–3887. [Google Scholar] [CrossRef]
- Walti, C.; Siegenthaler, J.; Christ-Crain, M. Copeptin levels are independent of ingested nutrient type after standardized meal administration--the CoMEAL study. Biomarkers 2014, 19, 557–562. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Liang, S.; Zhou, X.; Guo, X.; Huang, B.; Pan, H.; Zhu, H.; Chen, S. Impact Factors of Blood Copeptin Levels in Health and Disease States. Endocr. Pract. 2024, 30, 1197–1205. [Google Scholar] [CrossRef]
- Roth, B.; Ohlsson, B. Challenges of recruitment processes to a randomized dietary trial in irritable bowel syndrome. F1000Research 2024, 13, 323. [Google Scholar] [CrossRef]
- Available online: www.livsmedelsverket.se (accessed on 22 October 2024).
- Available online: https://labbutbud.vgregion.se/katalog/s-copeptin-proavp-b7c0 (accessed on 21 April 2025).
- Available online: https://www.sahlgrenska.se/for-dig-som-ar/vardgivare/tillvaxtlaboratoriet/analyser/ (accessed on 21 April 2025).
- Available online: http://www.analysportalen-labmedicin.skane.se/default.asp (accessed on 30 October 2024).
- Staudacher, H.M.; Rossi, M.; Kaminski, T.; Dimidi, E.; Ralph, F.S.E.; Wilson, B.; Martin, L.D.; Louis, P.; Lomer, M.C.E.; Irving, P.M.; et al. Long-term personalized low FODMAP diet improves symptoms and maintains luminal Bifidobacteria abundance in irritable bowel syndrome. Neurogastroenterol. Motil. 2022, 34, e14241. [Google Scholar] [CrossRef]
- Choosing Your Foods—CSID Cares. Available online: https://www.csidcares.org (accessed on 24 October 2024).
- Danialifar, T.F.; Chumpitazi, B.P.; Mehta, D.I.; Di Lorenzo, C. Genetic and acquired sucrase-isomaltase deficiency: A clinical review. J. Pediatr. Gastroenterol. Nutr. 2024, 78, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Nilholm, C.; Larsson, E.; Sonestedt, E.; Roth, B.; Ohlsson, B. Assessment of a 4-Week Starch- and Sucrose-Reduced Diet and Its Effects on Gastrointestinal Symptoms and Inflammatory Parameters among Patients with Irritable Bowel Syndrome. Nutrients 2021, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Ralph, F.S.E.; Irving, P.M.; Whelan, K.; Lomer, M.C.E. Nutrient intake, diet quality, and diet diversity in irritable bowel syndrome and the impact of the low FODMAP diet. J. Acad. Nutr. Diet. 2020, 120, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.; Morgan, J.; Reid, F.; Brunton, J.; O’Brien, A.; Luck, A.; Lacey, H. Screening for symptoms of eating disorders: Reliability of the SCOFF screening tool with written compared to oral delivery. Int. J. Eat. Disord. 2002, 32, 466–472. [Google Scholar] [CrossRef]
- Palsson, O.S.; Whitehead, W.E.; Van Tilburg, M.A.L.; Chang, L.; Chey, W.; Crowell, M.D.; Keefer, L.; Lembo, A.J.; Parkman, H.P.; Rao, S.S.C.; et al. Development and validation of the Rome IV diagnostic questionnaire for adults. Gastroenterology 2016, 150, 1481–1491. [Google Scholar] [CrossRef]
- Bengtsson, M.; Persson, J.; Sjölund, K.; Ohlsson, B. Further validation of the visual analogue scale for irritable bowel syndrome after use in clinical practice. Gastroenterol. Nurs. 2013, 36, 188–198. [Google Scholar] [CrossRef]
| Parameter | SSRD N = 70 | Low FODMAP N = 72 | p-Value |
|---|---|---|---|
| Sex (women/men; n, %) | 57 (81)/13 (19) | 64 (89)/8 (11) | 0.243 |
| Age (year) | 42.4 ± 14.0 | 45.2 ± 14.2 | 0.234 |
| Disease duration (year) Missing | 19.5 (7.8–28.8) | 20.0 (10.5–33.5) 3 | 0.313 |
| Education (n, %) | 0.578 | ||
| Primary school | 5 (7.1) | 2 (2.8) | |
| Secondary school | 10 (14.3) | 13 (18.1) | |
| Education after secondary school | 18 (25.7) | 16 (22.2) | |
| Examination at university | 37 (52.9) | 41 (56.9) | |
| Occupation (n, %) | 0.684 | ||
| Working full-time | 44 (62.9) | 45 (62.5) | |
| Working 99–51% | 6 (8.6) | 8 (11.1) | |
| Working 50% | 1 (1.4) | 1 (1.4) | |
| Studying | 8 (11.4) | 9 (12.5) | |
| Sick leave | 3 (4.3) | 0 (0) | |
| Unemployment | 2 (2.9) | 1 (1.4) | |
| Retirement | 6 (8.6) | 8 (11.1) | |
| Marital status (n, %) | 0.595 | ||
| Living alone | 15 (21.4) | 12 (16.7) | |
| Living together | 50 (71.4) | 52 (72.2) | |
| Other | 5 (7.1) | 8 (11.1) | |
| Smoking (n, %) | 0.131 | ||
| Never | 38 (54.3) | 39 (54.2) | |
| Former | 24 (34.3) | 26 (36.1) | |
| Present irregular | 2 (2.9) | 6 (8.3) | |
| Present regular | 6 (8.6) | 1 (1.4) | |
| Alcohol intake for 1 week (standard glass) (n, %) | 0.882 | ||
| Missing | - | 1 | |
| <1 | 30 (42.9) | 29 (40.8) | |
| 1–4 | 26 (37.1) | 29 (40.8) | |
| 5–9 | 12 (17.1) | 12 (16.9) | |
| 10–14 | 1 (1.4) | 1 (1.4) | |
| ≥15 | 1 (1.4) | 0 (0) | |
| Physical activity for 1 week (n, %) | 0.274 | ||
| Missing | - | 1 | |
| No time | 10 (14.3) | 5 (7.0) | |
| <30 min | 10 (14.3) | 12 (16.9) | |
| 30–60 min | 13 (18.6) | 14 (19.7) | |
| 60–90 min | 7 (10.0) | 16 (22.5) | |
| 90–120 min | 8 (11.4) | 8 (11.3) | |
| >120 min | 22 (31.4) | 16 (22.5) |
| SSRD N = 70 | Low FODMAP N = 72 | (§) p-Value | Effect Size | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Median (IQR) | p-Value | Effect Size | Median (IQR) | p-Value | Effect Size | ||
| S-Copeptin (pmol/L) | ||||||||
| Baseline Missing | 4.0 (2.8–6.3) | - | 3.0 (2.0–4.0) 1 | - | 0.044 | 0.17 | ||
| 4 weeks | 4.0 (3.0–6.0) | 0.466 | 0.07 | 3.0 (2.0–4.0) | 0.802 | −0.02 | 0.101 | 0.14 |
| 6 months Missing | 4.0 (2.0–5.0) 21 | 0.081 | 0.25 | 3.0 (2.0–4.0) 24 | 0.893 | −0.02 | 0.118 | 0.16 |
| S-Leptin (µg/L) | ||||||||
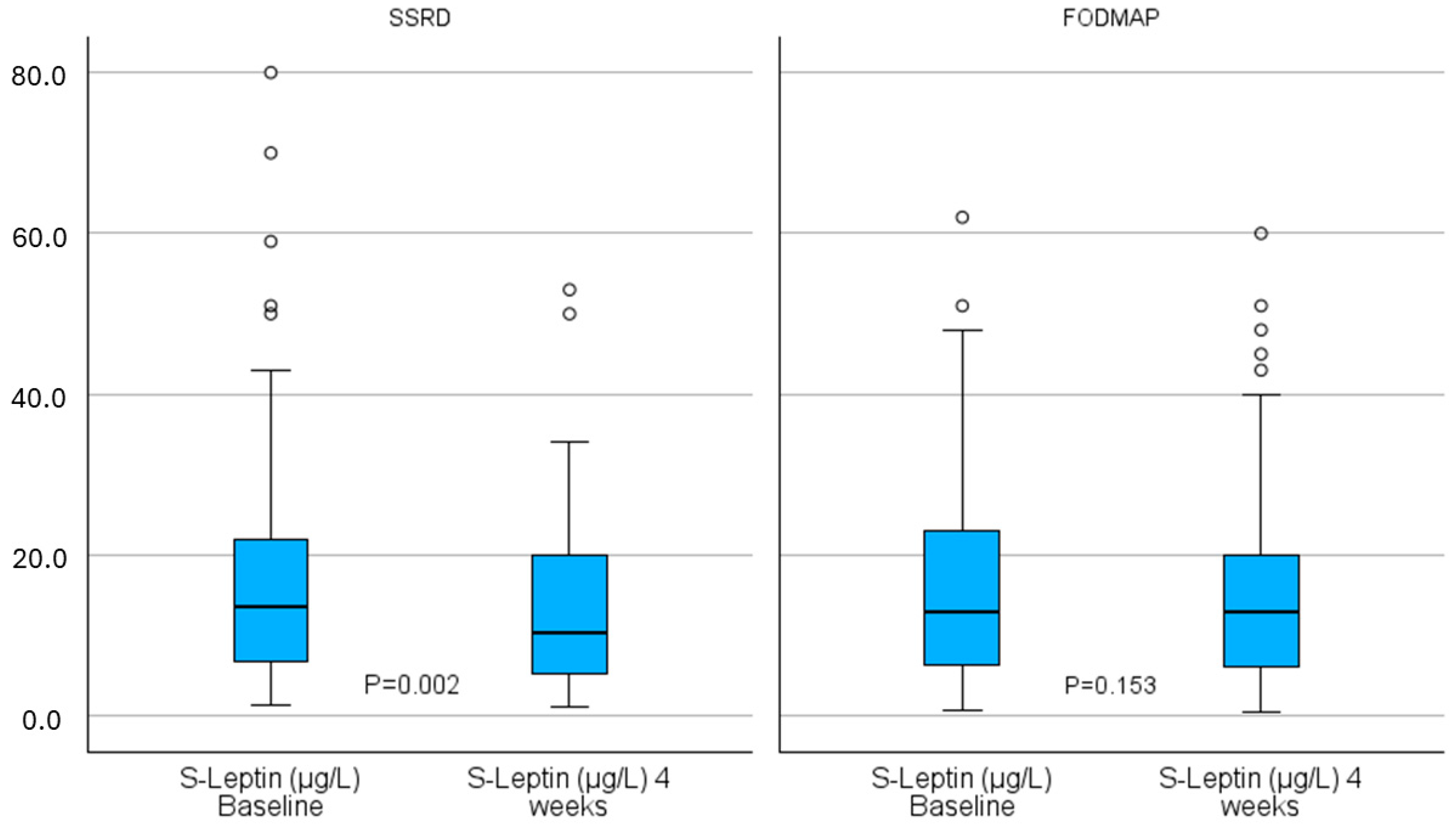
| Baseline Missing | 13.5 (6.8–22.0) | - | 13.0 (6.2–23.0) 1 | - | 0.790 | 0.02 | ||
| 4 weeks | 10.4 (5.1–20.3) | 0.002 | 0.37 | 13.0 (6.2–20.0) | 0.153 | 0.18 | 0.349 | −0.08 |
| 6 months Missing | 16.0 (6.5–25.5) 21 | 0.670 | 0.06 | 16.0 (7.6–23.5) 24 | 0.917 | 0.01 | 0.848 | 0.02 |
| P-PAI-1 (ng/mL) | ||||||||
| Baseline Missing | 14.1 (8.7–21.6) | - | 13.7 (7.4–26.6) 1 | - | 0.910 | 0.01 | ||
| 4 weeks | 11.6 (6.9–19.3) | 0.056 | 0.23 | 12.5 (5.6–21.5) | 0.171 | 0.16 | 0.845 | −0.02 |
| 6 months Missing | 9.9 (6.3–19.2) 21 | 0.551 | 0.08 | 9.9 (7.7–21.2) 24 | 0.262 | 0.16 | 0.823 | −0.02 |
| P-C-Peptide (nmol/L) | ||||||||
| Baseline Missing | 1.0 (0.8–1.5) | - | 1.1 (0.7–1.5) 1 | - | 0.867 | −0.01 | ||
| 4 weeks | 1.0 (0.8–1.3) | 0.237 | 0.14 | 1.1 (0.8–1.4) | 0.551 | −0.08 | 0.070 | −0.15 |
| 6 months Missing | 1.1 (0.7–1.4) 21 | 0.970 | −0.006 | 1.0 (0.7–1.4) 24 | 0.788 | −0.04 | 0.621 | 0.05 |
| P-Insulin (mIE/L) | ||||||||
| Baseline Missing | 13.0 (7.8–26.0) | - | 14.0 (7.0–26.0) 1 | - | 0.845 | −0.02 | ||
| 4 weeks | 13.0 (8.0–20.0) | 0.210 | 0.14 | 13.0 (8.0–23.3) | 0.701 | −0.05 | 0.451 | −0.06 |
| 6 months Missing | 15.0 (7.0–28.5) 21 | 0.795 | −0.04 | 11.0 (6.3–28.0) 24 | 0.436 | −0.11 | 0.453 | 0.08 |
| Independent Variable | β-Coefficient and 95% CI | p-Value |
|---|---|---|
| Weight (kg) | 1.036 (−0.325–2.398) | 0.136 |
| Kilocalorie intake (kcal) | 0.004 (0.001–0.008) | 0.020 |
| Carbohydrate intake (g) | 0.033 (0.003–0.064) | 0.033 |
| Protein intake (g) | −0.007 (−0.091–0.078) | 0.879 |
| Fat intake (g) | 0.056 (0.001–0.110) | 0.044 |
| Fiber intake (g) | 0.177 (−0.119–0.474) | 0.241 |
| Monosaccharide intake (g) | 0.117 (−0.026–0.260) | 0.109 |
| Disaccharide intake (g) | 0.023 (−0.050–0.097) | 0.536 |
| Sucrose intake (g) | 0.042 (−0.019–0.012) | 0.357 |
| Starch intake (g) | 0.064 (0.004–0.124) | 0.037 |
| Added sugar intake (g) | 0.030 (−0.039–0.099) | 0.392 |
| Adjusted Independent Variable | β-Coefficient and 95% CI | p-Value |
|---|---|---|
| Kilocalorie intake (kcal) | 0.004 (0.000–0.008) | 0.028 |
| Carbohydrate intake (g) | 0.031 (−0.001–0.062) | 0.059 |
| Fat intake changes (g) | 0.057 (0.002–0.111) | 0.041 |
| Fiber intake (g) | 0.179 (−0.116–0.473) | 0.235 |
| Monosaccharide intake (g) | 0.128 (−0.015–0.271) | 0.080 |
| Disaccharide intake (g) | 0.018 (−0.056–0.092) | 0.630 |
| Sucrose intake (g) | 0.036 (−0.054–0.126) | 0.433 |
| Starch intake (g) | 0.059 (−0.002–0.120) | 0.058 |
| Added sugar intake (g) | 0.023 (−0.047–0.093) | 0.517 |
| Symptom Change (Dependent Variable) | Leptin Change β-Coefficient and 95% CI; p-Value | Weight Change β-Coefficient and 95% CI; p-Value | Fat Intake Change β-Coefficient and 95% CI; p-Value |
|---|---|---|---|
| Abdominal pain | 0.098 (−0.288–0.485); 0.619 | −0.774 (−3.926–2.378); 0.630 | 0.035 (−0.090–0.160); 0.583 |
| Diarrhea | 0.456 (−0.035–0.948); 0.069 | −2.186 (−6.193–1.822); 0.285 | −0.002 (−0.161–0.157); 0.980 |
| Constipation | −0.105 (−0.547–0.337); 0.642 | −0.761 (−4.366–2.845); 0.679 | −0.062 (−0.206–0.081); 0.392 |
| Bloating and flatulence | −0.007 (−0.446–0.432); 0.975 | 1.337 (−2.236–4.909); 0.463 | −0.011 (−0.154–1.33); 0.883 |
| Vomiting and nausea | −0.234 (−0.540–0.072); 0.134 | −0.382 (−2.873–2.108); 0.764 | 0.004 (−0.095–0.103); 0.937 |
| Intestinal symptoms’ influence on daily life | 0.033 (−0.381–0.447); 0.877 | 1.354 (−2.023–4.731); 0.432 | 0.030 (−0.104–0.164); 0.663) |
| Psychological well-being | 0.061 (−0.310–0.431); 0.749 | −1.040 (−4.064–1.985); 0.500 | −0.108 (−0.228–0.012); 0.077 |
| Difficulties eating a whole meal | −0.118 (−0.413–0.178); 0.434 | −1.624 (−4.032–0.784); 0.186 | 0.027 (−0.068–0.123); 0.578 |
| Headache | −0.108 (−0.463–0.248); 0.553 | 2.045 (−0.854–4.943); 0.167 | 0.038 (−0.077–0.153); 0.518 |
| Back pain | 0.038 (−0.322–0.398); 0.837 | 1.807 (−1.127–4.742); 0.227 | 0.066 (−0.051–0.182); 0.270 |
| Fatigue | 0.115 (−0.246–0.477); 0.532 | 1.484 (−1.463–4.432); 0.324 | −0.025 (−0.142–0.092); 0.676 |
| Belching/excess wind | −0.084 (−0.539–0.371); 0.718 | 3.844 (0.132–7.556); 0.042 | 0.042 (−0.105–0.190); 0.574 |
| Reflux | −0.123 (−0.503–0.258); 0.527 | 2.464 (−0.637–5.565); 0.119 | −0.012 (−0.135–0.111); 0.848 |
| Urinary urgency | −0.324 (−0.705–0.057); 0.096 | 3.530 (0.421–6.638); 0.026 | 0.012 (−0.111–0.136); 0.845 |
| Leg pain | −0.167 (−0.421–0.086); 0.195 | 1.254 (−0.813–3.320); 0.234 | −0.001 (−0.083–0.081); 0.982 |
| Muscle/joint pain | −0.008 (−0.367–0.351); 0.964 | −1.787 (−4.713–1.139); 0.231 | 0.041 (−0.075–0.157); 0.486 |
| Total IBS-SSS | 0.154 (−1.346–1.653); 0.841 | 0.223 (−12.007–12.453); 0.971 | 0.336 (−0.150–0.821); 0.175 |
| Total extraintestinal IBS-SSS | −0.510 (−1.462–0.443); 0.294 | 6.335 (−1.433–14.104); 0.110 | 0.097 (−0.212–0.405); 0.538 |
| Nutrient Intake Change | Component 1 | Component 2 | Component 3 |
|---|---|---|---|
| Kilocalories | 0.40 | 0.30 | −0.01 |
| Carbohydrates | 0.42 | −0.10 | 0.19 |
| Protein | 0.18 | 0.48 | −0.05 |
| Fat | 0.26 | 0.42 | −0.18 |
| Fiber | 0.22 | 0.42 | 0.07 |
| Monosaccharides | 0.19 | 0.03 | −0.44 |
| Disaccharide | 0.40 | −0.29 | −0.08 |
| Sucrose | 0.39 | −0.33 | −0.06 |
| Starch | 0.16 | 0.05 | 0.85 |
| Added sugar | 0.38 | −0.35 | −0.06 |
| Proportion of variance explained | 46% | 21% | 11% |
| Change | All N = 133 | SSRD N = 66 | Low FODMAP N = 67 | Difference in Association Between Groups |
|---|---|---|---|---|
| Changes in ln S-Leptin (β; p-value) | ||||
| Component 1 | 0.89 (0.06) | 1.04 (0.17) | 0.26 (0.68) | −0.78 (0.44) |
| Adjusted for component 2 | 0.88 (0.06) | 1.02 (0.18) | 0.11 (0.87) | −0.93 (0.35) |
| Adjusted for component 3 | 0.88 (0.05) | 1.15 (0.14) | 0.27 (0.68) | −0.83 (0.41) |
| Adjusted for change in weight | 0.81 (0.08) | 0.99 (0.19) | 0.32 (0.61) | −0.79 (0.43) |
| Changes in ln P-PAI-1 (β; p-value) | ||||
| Component 1 | 0.85 (0.43) | 2.12 (0.12) | −0.35 (0.86) | −2.46 (0.29) |
| Adjusted for component 2 | 0.86 (0.43) | 2.03 (0.12) | −0.38 (0.85) | −2.80 (0.23) |
| Adjusted for component 3 | 0.85 (0.44) | 2.27 (0.10) | −0.42 (0.83) | −2.37 (0.31) |
| Adjusted for change in weight | 0.82 (0.46) | 2.08 (0.13) | −0.33 (0.87) | −2.47 (0.29) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szekely, A.-B.; Nseir, M.; Roth, B.; Ohlsson, B. Leptin and PAI-1 Levels Are Decreased After a Dietary Intervention in Patients with Irritable Bowel Syndrome. Int. J. Mol. Sci. 2025, 26, 4607. https://doi.org/10.3390/ijms26104607
Szekely A-B, Nseir M, Roth B, Ohlsson B. Leptin and PAI-1 Levels Are Decreased After a Dietary Intervention in Patients with Irritable Bowel Syndrome. International Journal of Molecular Sciences. 2025; 26(10):4607. https://doi.org/10.3390/ijms26104607
Chicago/Turabian StyleSzekely, Andreas-Balázs, Mohamed Nseir, Bodil Roth, and Bodil Ohlsson. 2025. "Leptin and PAI-1 Levels Are Decreased After a Dietary Intervention in Patients with Irritable Bowel Syndrome" International Journal of Molecular Sciences 26, no. 10: 4607. https://doi.org/10.3390/ijms26104607
APA StyleSzekely, A.-B., Nseir, M., Roth, B., & Ohlsson, B. (2025). Leptin and PAI-1 Levels Are Decreased After a Dietary Intervention in Patients with Irritable Bowel Syndrome. International Journal of Molecular Sciences, 26(10), 4607. https://doi.org/10.3390/ijms26104607







