Oxidative Damage Under Microgravity Conditions: Response Mechanisms, Monitoring Methods and Countermeasures on Somatic and Germ Cells
Abstract
1. Introduction
1.1. Damage to Somatic Cells from Different Systems in Microgravity
1.2. Damage of Germ Cells in Microgravity
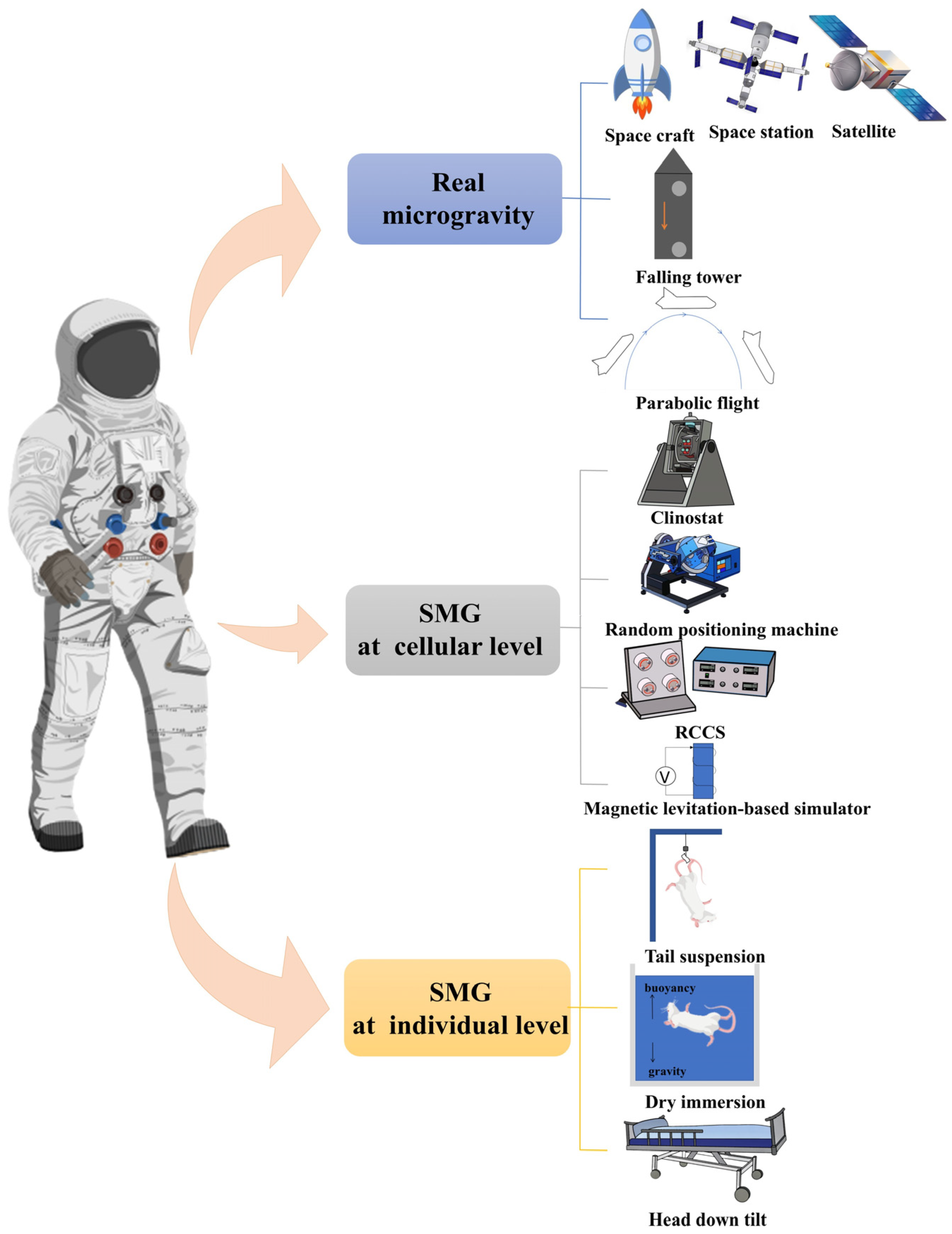
1.3. Methods of Simulated Microgravity
1.3.1. Real (Simulated) Microgravity Environment
1.3.2. Cellular-Level Simulation Methods
1.3.3. Organism-Level Simulation Methods
1.4. Oxidative Stress (OS) in Microgravity
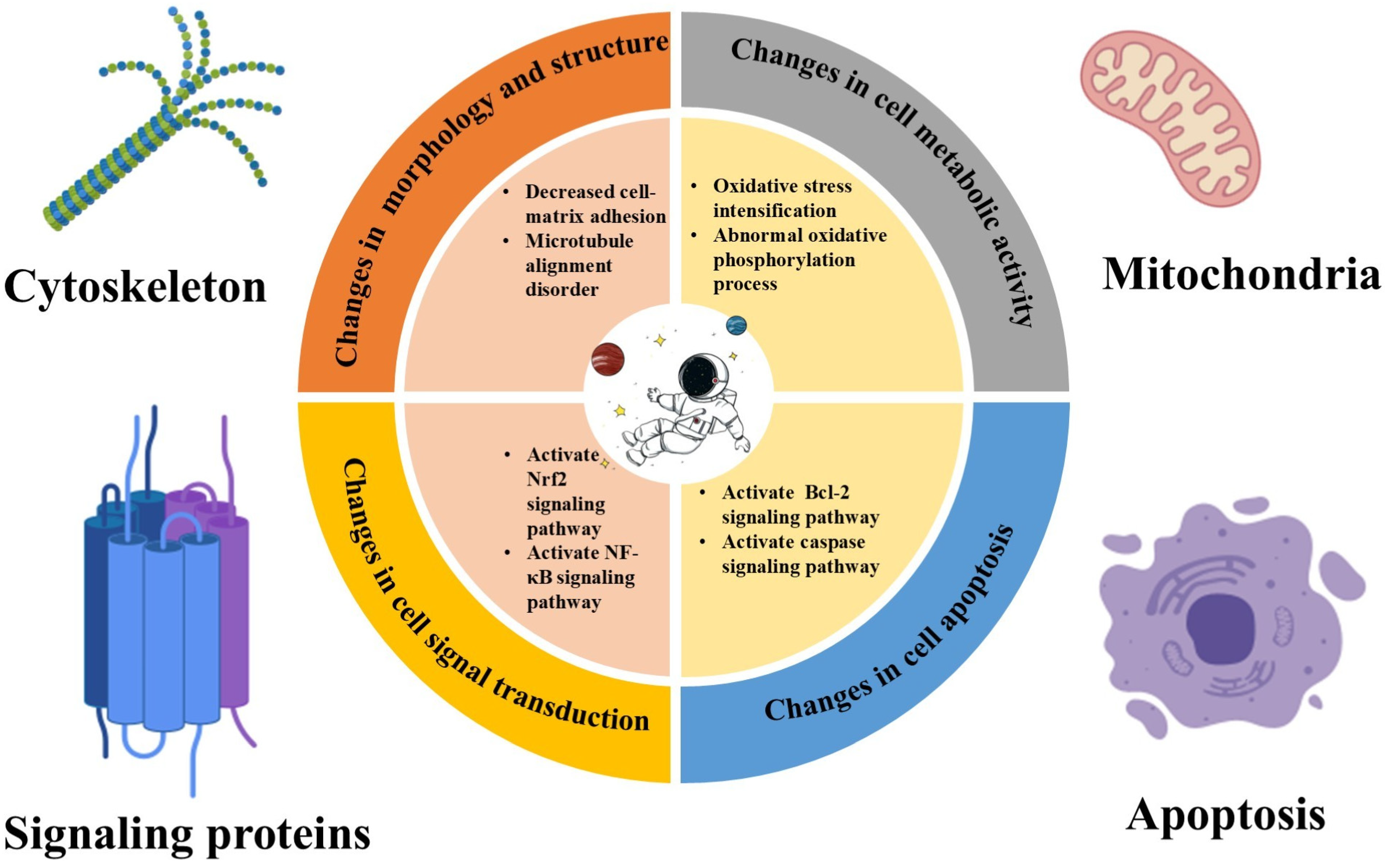
2. Effect of Microgravity on Cellular Physiology
2.1. Microgravity’s Influence on Cellular Gene Expression
2.2. Microgravity’s Influence on Cellular Protein Synthesis
2.3. Microgravity’s Influence on Cellular Metabolic Pathways
2.4. Microgravity’s Influence on the Mechanisms of ROS Generation
2.4.1. Mitochondrial Dysfunction
2.4.2. Altered Enzyme Activity
3. Oxidative Damage to Cells in Microgravity
3.1. Direct Damaging Effects of ROS on Germ Cells
3.1.1. DNA Damage
3.1.2. Lipid Peroxidation
3.1.3. Protein Modification
3.2. Somatic Cell Antioxidant Enzyme Systems in Microgravity
3.2.1. Superoxide Dismutase
3.2.2. Catalase
3.2.3. Glutathione Peroxidase
3.2.4. Effect of Microgravity on Antioxidant Enzymes
3.3. Cell Fate Reprogramming Triggered by Oxidative Stress in Microgravity
3.3.1. Apoptosis in Germ Cells
3.3.2. Autophagy in Somatic Cells and Germ Cells
3.3.3. Cell Cycle Checkpoint Changed in Somatic Cells and Germ Cells
3.4. Gene Expression Remodeling by OS in Microgravity
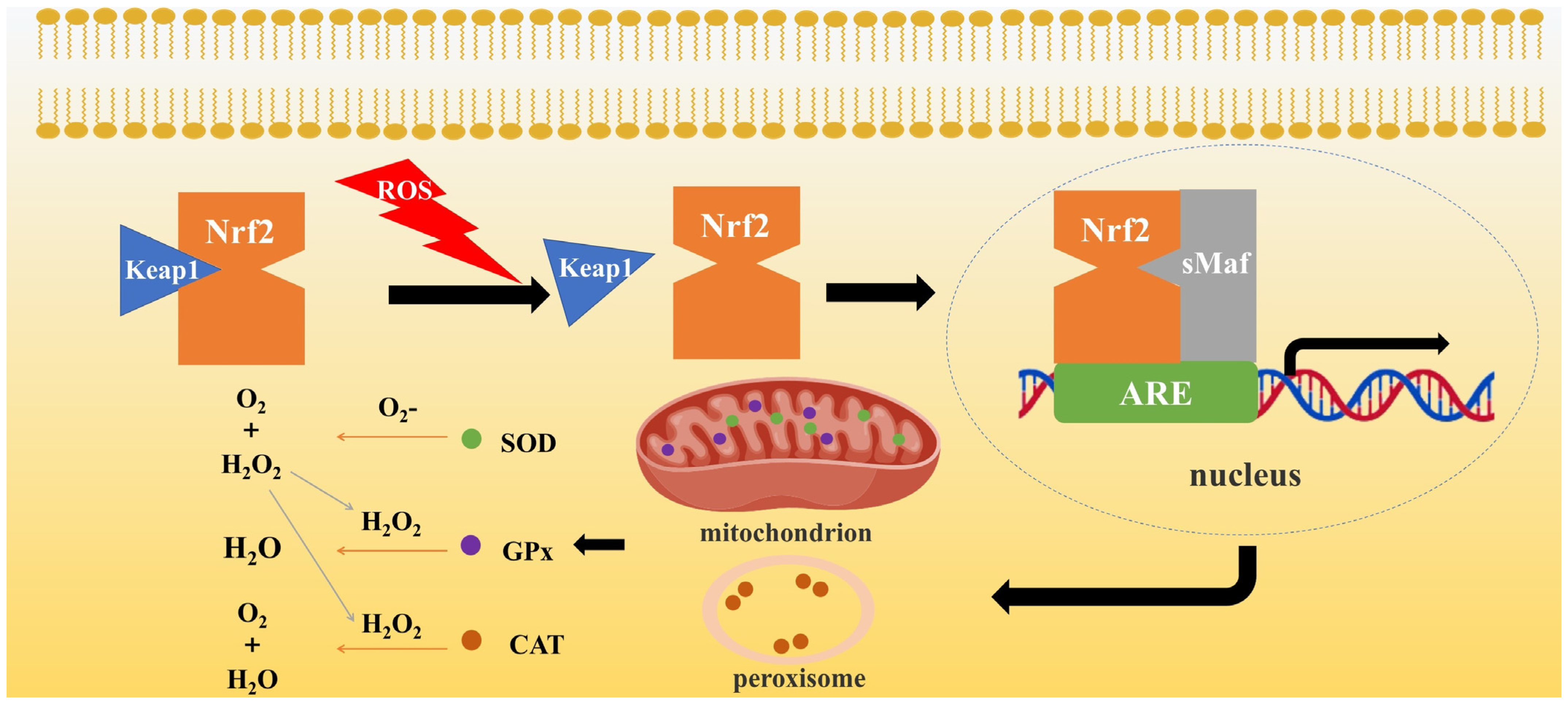
3.4.1. Nrf2-Related Signaling Pathway
3.4.2. Heat Shock Proteins
3.4.3. Apoptosis-Related Genes
3.5. Risks to Other Aspects of the Reproductive System
3.5.1. Microgravity-Induced ROS on Female Reproductive System
3.5.2. Microgravity-Induced ROS on Male Reproductive System
3.5.3. Microgravity-Induced ROS on Embryonic Development
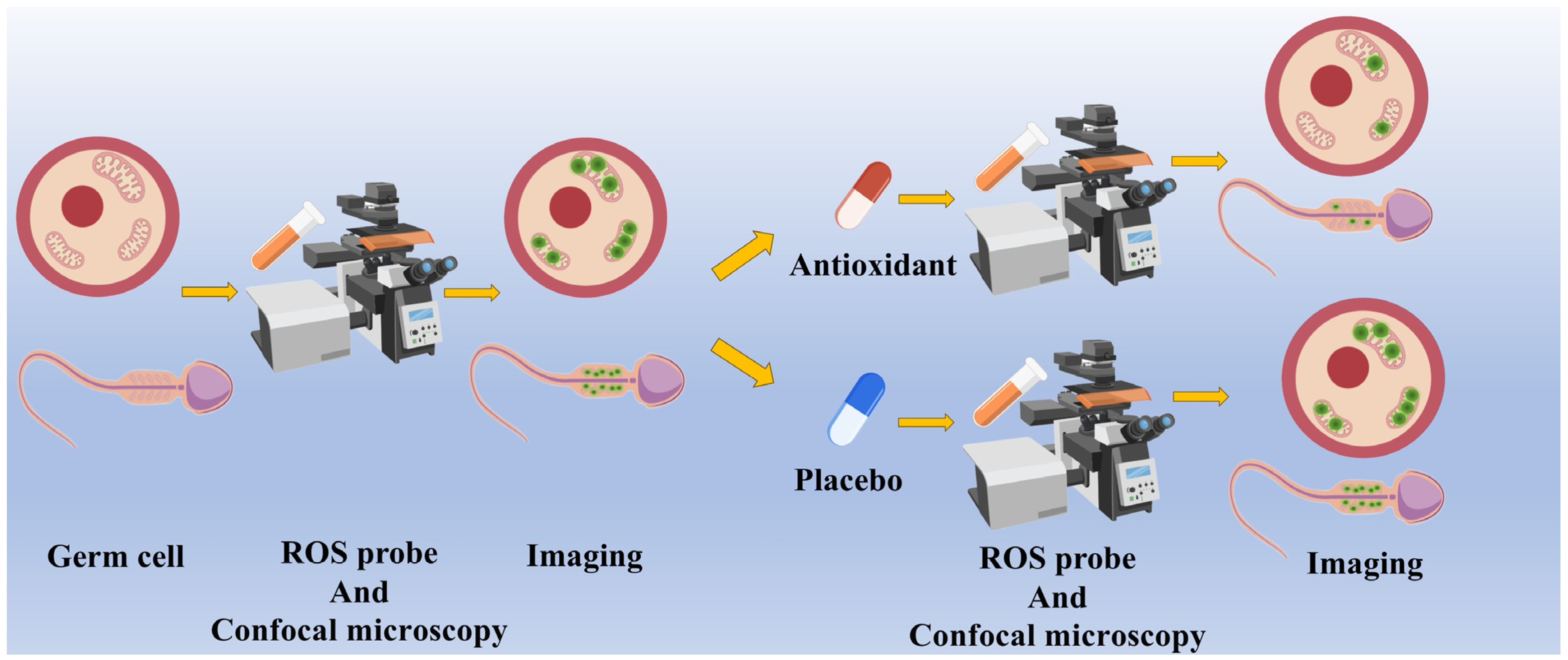
4. Detection of Oxidative Stress Using Fluorescence Probes
4.1. Fluorescent Probes for the Detection of ROS
4.1.1. DCFH-DA
4.1.2. MitoSOX
4.1.3. CellROX
4.2. ROS Fluorescent Probes in Germ Cell Research
4.3. Limitations and Challenges of Fluorescent Probes to Detect ROS in Germ Cells in Microgravity
5. Protective Countermeasures for Oxidative Stress in Microgravity
5.1. Antioxidant Supplementation
5.1.1. Vitamin C
5.1.2. Vitamin E
5.1.3. Coenzyme Q10
5.1.4. Controversy over Conventional Antioxidants
5.2. Gene Editing
5.3. Exercise and Physical Intervention
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, L.; Nie, L.; Xie, S.; Li, M.; Zhu, C.; Qiu, X.; Kuang, J.; Liu, C.; Lu, C.; Li, W.; et al. Attenuation of Antiviral Immune Response Caused by Perturbation of TRIM25-Mediated RIG-I Activation under Simulated Microgravity. Cell Rep. 2021, 34, 108600. [Google Scholar] [CrossRef] [PubMed]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; McCoy, T.; Gazda, D.; Morgan, J.L.; Heer, M.; Zwart, S.R. Space flight calcium: Implications for astronaut health, spacecraft operations, and Earth. Nutrients 2012, 4, 2047–2068. [Google Scholar] [CrossRef] [PubMed]
- Gioia, M.; Michaletti, A.; Scimeca, M.; Marini, M.; Tarantino, U.; Zolla, L.; Coletta, M. Simulated microgravity induces a cellular regression of the mature phenotype in human primary osteoblasts. Cell Death Discov. 2018, 4, 59. [Google Scholar] [CrossRef]
- Smith, J.K. Osteoclasts and Microgravity. Life 2020, 10, 207. [Google Scholar] [CrossRef]
- Williams, D.; Kuipers, A.; Mukai, C.; Thirsk, R. Acclimation during space flight: Effects on human physiology. Cmaj 2009, 180, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X.; Zhou, Q.; Deng, C.; Yang, Y.; Huang, D.; Luo, H.; Zhang, S.; Li, Y.; Xu, J.; et al. Mechanisms and Countermeasures for Muscle Atrophy in Microgravity. Cells 2024, 13, 2120. [Google Scholar] [CrossRef]
- Stervbo, U.; Roch, T.; Kornprobst, T.; Sawitzki, B.; Grütz, G.; Wilhelm, A.; Lacombe, F.; Allou, K.; Kaymer, M.; Pacheco, A.; et al. Gravitational stress during parabolic flights reduces the number of circulating innate and adaptive leukocyte subsets in human blood. PLoS ONE 2018, 13, e0206272. [Google Scholar] [CrossRef]
- Ludtka, C.; Silberman, J.; Moore, E.; Allen, J.B. Macrophages in microgravity: The impact of space on immune cells. NPJ Microgravity 2021, 7, 13. [Google Scholar] [CrossRef]
- Ogneva, I.V.; Usik, M.A.; Biryukov, N.S.; Zhdankina, Y.S. Sperm Motility of Mice under Simulated Microgravity and Hypergravity. Int. J. Mol. Sci. 2020, 21, 5054. [Google Scholar] [CrossRef]
- Ricci, G.; Esposito, R.; Catizone, A.; Galdieri, M. Direct effects of microgravity on testicular function: Analysis of hystological, molecular and physiologic parameters. J. Endocrinol. Investig. 2008, 31, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Feng, X.; Yang, C.; Ma, C.; Niu, S.; Jia, L.; Yang, X.; Liang, J.; Bo, Y.; Geng, K.; et al. Simulated microgravity reduces quality of ovarian follicles and oocytes by disrupting communications of follicle cells. NPJ Microgravity 2023, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; An, L.; Jiang, Y.; Hang, H. Effects of simulated microgravity on embryonic stem cells. PLoS ONE 2011, 6, e29214. [Google Scholar] [CrossRef]
- Li, F.; Ye, Y.; Lei, X.; Zhang, W. Effects of Microgravity on Early Embryonic Development and Embryonic Stem Cell Differentiation: Phenotypic Characterization and Potential Mechanisms. Front. Cell Dev. Biol. 2021, 9, 797167. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Cao, Y.; Ma, B.; Zhang, Y.; Ning, L.; Qian, J.; Zhang, L.; Qu, Y.; Zhang, T.; Li, D.; et al. Development of mouse preimplantation embryos in space. Natl. Sci. Rev. 2020, 7, 1437–1446. [Google Scholar] [CrossRef]
- Lei, X.; Cao, Y.; Zhang, Y.; Qian, J.; Zhao, Q.; Liu, F.; Zhang, T.; Zhou, J.; Gu, Y.; Xia, G.; et al. Effect of microgravity on proliferation and differentiation of embryonic stem cells in an automated culturing system during the TZ-1 space mission. Cell Prolif. 2018, 51, e12466. [Google Scholar] [CrossRef]
- Wakayama, S.; Kikuchi, Y.; Soejima, M.; Hayashi, E.; Ushigome, N.; Yamazaki, C.; Suzuki, T.; Shimazu, T.; Yamamori, T.; Osada, I.; et al. Effect of microgravity on mammalian embryo development evaluated at the International Space Station. iScience 2023, 26, 108177. [Google Scholar] [CrossRef]
- Shelhamer, M. Parabolic flight as a spaceflight analog. J. Appl. Physiol. 2016, 120, 1442–1448. [Google Scholar] [CrossRef]
- Ma, C.; Xiong, Y.; Han, P.; Zhang, X.; Cao, Y.; Wang, B.; Zhao, H.; Duan, E.; Zhang, J.V.; Lei, X. Simulated Microgravity Potentiates Hematopoietic Differentiation of Human Pluripotent Stem Cells and Supports Formation of 3D Hematopoietic Cluster. Front. Cell Dev. Biol. 2021, 9, 797060. [Google Scholar] [CrossRef]
- Lei, X.H.; Ning, L.N.; Cao, Y.J.; Liu, S.; Zhang, S.B.; Qiu, Z.F.; Hu, H.M.; Zhang, H.S.; Liu, S.; Duan, E.K. NASA-approved rotary bioreactor enhances proliferation of human epidermal stem cells and supports formation of 3D epidermis-like structure. PLoS ONE 2011, 6, e26603. [Google Scholar] [CrossRef]
- Lei, X.; Deng, Z.; Zhang, H.; Zhao, H.; Zhou, J.; Liu, S.; Chen, Q.; Ning, L.; Cao, Y.; Wang, X.; et al. Rotary suspension culture enhances mesendoderm differentiation of embryonic stem cells through modulation of Wnt/β-catenin pathway. Stem Cell Rev. Rep. 2014, 10, 526–538. [Google Scholar] [CrossRef]
- Pellegrini, M.; Di Siena, S.; Claps, G.; Di Cesare, S.; Dolci, S.; Rossi, P.; Geremia, R.; Grimaldi, P. Microgravity promotes differentiation and meiotic entry of postnatal mouse male germ cells. PLoS ONE 2010, 5, e9064. [Google Scholar] [CrossRef]
- Wuest, S.L.; Richard, S.; Kopp, S.; Grimm, D.; Egli, M. Simulated microgravity: Critical review on the use of random positioning machines for mammalian cell culture. Biomed. Res. Int. 2015, 2015, 971474. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.W.; Liu, T.Z.; Sun, Y.H.; Cai, N.; Xuan, Y.Y.; Wei, Z.; Cui, B.B.; Jing, L.L.; Ma, H.P.; Xian, C.J.; et al. Simulated microgravity-induced oxidative stress and loss of osteogenic potential of osteoblasts can be prevented by protection of primary cilia. J. Cell. Physiol. 2023, 238, 2692–2709. [Google Scholar] [CrossRef] [PubMed]
- Diebold, L.; Chandel, N.S. Mitochondrial ROS regulation of proliferating cells. Free Radic. Biol. Med. 2016, 100, 86–93. [Google Scholar] [CrossRef]
- Gutti, U.; Komati, J.K.; Kotipalli, A.; Saladi, R.G.V.; Gutti, R.K. Justicia adhatoda induces megakaryocyte differentiation through mitochondrial ROS generation. Phytomedicine 2018, 43, 135–139. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, P.; Chen, Q.; Huang, Z.; Zou, D.; Zhang, J.; Gao, X.; Lin, Z. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J. Mol. Cell Biol. 2019, 11, 1069–1082. [Google Scholar] [CrossRef]
- Priya Dharshini, L.C.; Vishnupriya, S.; Sakthivel, K.M.; Rasmi, R.R. Oxidative stress responsive transcription factors in cellular signalling transduction mechanisms. Cell Signal 2020, 72, 109670. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Abdelfattah, F.; Schulz, H.; Wehland, M.; Corydon, T.J.; Sahana, J.; Kraus, A.; Krüger, M.; González-Torres, L.F.; Cortés-Sánchez, J.L.; Wise, P.M.; et al. Omics Studies of Specialized Cells and Stem Cells under Microgravity Conditions. Int. J. Mol. Sci. 2024, 25, 10014. [Google Scholar] [CrossRef]
- Fava, M.; De Dominicis, N.; Forte, G.; Bari, M.; Leuti, A.; Maccarrone, M. Cellular and Molecular Effects of Microgravity on the Immune System: A Focus on Bioactive Lipids. Biomolecules 2024, 14, 446. [Google Scholar] [CrossRef] [PubMed]
- Boonyaratanakornkit, J.B.; Cogoli, A.; Li, C.F.; Schopper, T.; Pippia, P.; Galleri, G.; Meloni, M.A.; Hughes-Fulford, M. Key gravity-sensitive signaling pathways drive T cell activation. FASEB J. 2005, 19, 2020–2022. [Google Scholar] [CrossRef]
- Martinez, E.M.; Yoshida, M.C.; Candelario, T.L.; Hughes-Fulford, M. Spaceflight and simulated microgravity cause a significant reduction of key gene expression in early T-cell activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R480–R488. [Google Scholar] [CrossRef]
- Usik, M.A.; Ogneva, I.V. DNA Methylation in Mouse Spermatozoa under Long-Term Modeling the Effects of Microgravity. Russ. J. Dev. Biol. 2019, 50, 216–224. [Google Scholar] [CrossRef]
- Sventitskaya, M.A.; Ogneva, I.V. Reorganization of the mouse oocyte’ cytoskeleton after cultivation under simulated weightlessness. Life Sci. Space Res. 2024, 40, 8–18. [Google Scholar] [CrossRef]
- Oss-Ronen, L.; Redden, R.A.; Lelkes, P.I. Enhanced Induction of Definitive Endoderm Differentiation of Mouse Embryonic Stem Cells in Simulated Microgravity. Stem Cells Dev. 2020, 29, 1275–1284. [Google Scholar] [CrossRef]
- Acharya, A.; Brungs, S.; Henry, M.; Rotshteyn, T.; Singh Yaduvanshi, N.; Wegener, L.; Jentzsch, S.; Hescheler, J.; Hemmersbach, R.; Boeuf, H.; et al. Modulation of Differentiation Processes in Murine Embryonic Stem Cells Exposed to Parabolic Flight-Induced Acute Hypergravity and Microgravity. Stem Cells Dev. 2018, 27, 838–847. [Google Scholar] [CrossRef]
- Ferranti, F.; Caruso, M.; Cammarota, M.; Masiello, M.G.; Corano Scheri, K.; Fabrizi, C.; Fumagalli, L.; Schiraldi, C.; Cucina, A.; Catizone, A.; et al. Cytoskeleton modifications and autophagy induction in TCam-2 seminoma cells exposed to simulated microgravity. Biomed. Res. Int. 2014, 2014, 904396. [Google Scholar] [CrossRef] [PubMed]
- Usik, M.A.; Golubkova, M.A.; Ogneva, I.V. State of Drosophila melanogaster Ovaries after a Full Cycle of Gametogenesis under Microgravity Modeling: Cellular Respiration and the Content of Cytoskeletal Proteins. Int. J. Mol. Sci. 2021, 22, 9234. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Tran, P.H.; Kim, K.S.; Yang, S.G. The effects of real and simulated microgravity on cellular mitochondrial function. NPJ Microgravity 2021, 7, 44. [Google Scholar] [CrossRef]
- Michaletti, A.; Gioia, M.; Tarantino, U.; Zolla, L. Effects of microgravity on osteoblast mitochondria: A proteomic and metabolomics profile. Sci. Rep. 2017, 7, 15376. [Google Scholar] [CrossRef] [PubMed]
- Feger, B.J.; Thompson, J.W.; Dubois, L.G.; Kommaddi, R.P.; Foster, M.W.; Mishra, R.; Shenoy, S.K.; Shibata, Y.; Kidane, Y.H.; Moseley, M.A.; et al. Microgravity induces proteomics changes involved in endoplasmic reticulum stress and mitochondrial protection. Sci. Rep. 2016, 6, 34091. [Google Scholar] [CrossRef]
- Higashibata, A.; Hashizume, T.; Nemoto, K.; Higashitani, N.; Etheridge, T.; Mori, C.; Harada, S.; Sugimoto, T.; Szewczyk, N.J.; Baba, S.A.; et al. Microgravity elicits reproducible alterations in cytoskeletal and metabolic gene and protein expression in space-flown Caenorhabditis elegans. NPJ Microgravity 2016, 2, 15022. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Lu, C.; Feng, L.; Fan, L.X.; Sun, J.; Fan, B.; Wang, Q.; Wang, Y.; Liu, X.M.; Wang, F.Z. Liquid chromatography-mass spectrometry-based urinary metabolomics study on a rat model of simulated microgravity-induced depression. J. Pharm. Biomed. Anal. 2019, 165, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, W.; Shi, J.; Wang, J.; Hao, J.; Pang, X.; Huang, X.; Chen, X.; Li, Y.; Jin, R.; et al. Intestinal microbiota contributes to altered glucose metabolism in simulated microgravity mouse model. FASEB J. 2019, 33, 10140–10151. [Google Scholar] [CrossRef]
- Liu, H.; Li, D.; Zhang, R.; Sun, L.; Wang, D. Lipid metabolic sensors of MDT-15 and SBP-1 regulated the response to simulated microgravity in the intestine of Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2020, 528, 28–34. [Google Scholar] [CrossRef]
- Mazat, J.P.; Devin, A.; Ransac, S. Modelling mitochondrial ROS production by the respiratory chain. Cell. Mol. Life Sci. 2020, 77, 455–465. [Google Scholar] [CrossRef]
- Zhang, R.; Ran, H.H.; Cai, L.L.; Zhu, L.; Sun, J.F.; Peng, L.; Liu, X.J.; Zhang, L.N.; Fang, Z.; Fan, Y.Y.; et al. Simulated microgravity-induced mitochondrial dysfunction in rat cerebral arteries. FASEB J. 2014, 28, 2715–2724. [Google Scholar] [CrossRef]
- Saotome, K.; Murthy, S.E.; Kefauver, J.M.; Whitwam, T.; Patapoutian, A.; Ward, A.B. Structure of the mechanically activated ion channel Piezo1. Nature 2018, 554, 481–486. [Google Scholar] [CrossRef]
- Lin, Z.; Xu, G.; Lu, X.; Wang, H.; Lu, F.; Xia, X.; Song, J.; Jiang, J.; Ma, X.; Zou, F. Piezo1 exacerbates inflammation-induced cartilaginous endplate degeneration by activating mitochondrial fission via the Ca2+/CaMKII/Drp1 axis. Aging Cell 2024, 24, e14440. [Google Scholar] [CrossRef]
- Ramzan, R.; Dolga, A.M.; Michels, S.; Weber, P.; Culmsee, C.; Rastan, A.J.; Vogt, S. Cytochrome c Oxidase Inhibition by ATP Decreases Mitochondrial ROS Production. Cells 2022, 11, 992. [Google Scholar] [CrossRef]
- Ehinger, J.K.; Piel, S.; Ford, R.; Karlsson, M.; Sjövall, F.; Frostner, E.; Morota, S.; Taylor, R.W.; Turnbull, D.M.; Cornell, C.; et al. Cell-permeable succinate prodrugs bypass mitochondrial complex I deficiency. Nat. Commun. 2016, 7, 12317. [Google Scholar] [CrossRef]
- Wyck, S.; Herrera, C.; Requena, C.E.; Bittner, L.; Hajkova, P.; Bollwein, H.; Santoro, R. Oxidative stress in sperm affects the epigenetic reprogramming in early embryonic development. Epigenet. Chromatin 2018, 11, 60. [Google Scholar] [CrossRef]
- Kordowitzki, P. Oxidative Stress Induces Telomere Dysfunction and Shortening in Human Oocytes of Advanced Age Donors. Cells 2021, 10, 1866. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, H.; Zhang, J. Oxidative Stress on the Ground and in the Microgravity Environment: Pathophysiological Effects and Treatment. Antioxidants 2025, 14, 231. [Google Scholar] [CrossRef]
- Bize, I.; Santander, G.; Cabello, P.; Driscoll, D.; Sharpe, C. Hydrogen peroxide is involved in hamster sperm capacitation in vitro. Biol. Reprod. 1991, 44, 398–403. [Google Scholar] [CrossRef]
- Rivlin, J.; Mendel, J.; Rubinstein, S.; Etkovitz, N.; Breitbart, H. Role of hydrogen peroxide in sperm capacitation and acrosome reaction. Biol. Reprod. 2004, 70, 518–522. [Google Scholar] [CrossRef]
- Sengupta, P.; Pinggera, G.M.; Calogero, A.E.; Agarwal, A. Oxidative stress affects sperm health and fertility-Time to apply facts learned at the bench to help the patient: Lessons for busy clinicians. Reprod. Med. Biol. 2024, 23, e12598. [Google Scholar] [CrossRef]
- Guz, J.; Gackowski, D.; Foksinski, M.; Rozalski, R.; Zarakowska, E.; Siomek, A.; Szpila, A.; Kotzbach, M.; Kotzbach, R.; Olinski, R. Comparison of oxidative stress/DNA damage in semen and blood of fertile and infertile men. PLoS ONE 2013, 8, e68490. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Longobardi, S.; Di Rella, F.; Adiga, S.K.; Talevi, R. Sperm Oxidative Stress during In Vitro Manipulation and Its Effects on Sperm Function and Embryo Development. Antioxidants 2021, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Said, T.M. Oxidative stress, DNA damage and apoptosis in male infertility: A clinical approach. BJU Int. 2005, 95, 503–507. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, J.; Lin, G. Advances in the study of DNA damage and repair in mammalian oocytes. Yi Chuan 2023, 45, 379–394. [Google Scholar] [CrossRef]
- Ran, F.; An, L.; Fan, Y.; Hang, H.; Wang, S. Simulated microgravity potentiates generation of reactive oxygen species in cells. Biophys. Rep. 2016, 2, 100–105. [Google Scholar] [CrossRef]
- Long, S.; Zheng, Y.; Deng, X.; Guo, J.; Xu, Z.; Scharffetter-Kochanek, K.; Dou, Y.; Jiang, M. Maintaining mitochondrial DNA copy number mitigates ROS-induced oocyte decline and female reproductive aging. Commun. Biol. 2024, 7, 1229. [Google Scholar] [CrossRef]
- Yoshida, K.; Fujita, S.I.; Isotani, A.; Kudo, T.; Takahashi, S.; Ikawa, M.; Shiba, D.; Shirakawa, M.; Muratani, M.; Ishii, S. Intergenerational effect of short-term spaceflight in mice. iScience 2021, 24, 102773. [Google Scholar] [CrossRef]
- Hirata, Y.; Cai, R.; Volchuk, A.; Steinberg, B.E.; Saito, Y.; Matsuzawa, A.; Grinstein, S.; Freeman, S.A. Lipid peroxidation increases membrane tension, Piezo1 gating, and cation permeability to execute ferroptosis. Curr. Biol. 2023, 33, 1282–1294.e5. [Google Scholar] [CrossRef]
- Evans, E.P.P.; Scholten, J.T.M.; Mzyk, A.; Reyes-San-Martin, C.; Llumbet, A.E.; Hamoh, T.; Arts, E.; Schirhagl, R.; Cantineau, A.E.P. Male subfertility and oxidative stress. Redox Biol. 2021, 46, 102071. [Google Scholar] [CrossRef]
- Aitken, R.J.; Whiting, S.; De Iuliis, G.N.; McClymont, S.; Mitchell, L.A.; Baker, M.A. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J. Biol. Chem. 2012, 287, 33048–33060. [Google Scholar] [CrossRef]
- Mihalas, B.P.; De Iuliis, G.N.; Redgrove, K.A.; McLaughlin, E.A.; Nixon, B. The lipid peroxidation product 4-hydroxynonenal contributes to oxidative stress-mediated deterioration of the ageing oocyte. Sci. Rep. 2017, 7, 6247. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef]
- Morielli, T.; O’Flaherty, C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction 2015, 149, 113–123. [Google Scholar] [CrossRef]
- Liu, X.; Li, P.; Yan, K.; Du, Y.; Peng, K.; Li, M.; Cui, K.; Zhang, H.; Yang, X.; Lu, S.; et al. Resveratrol ameliorates the defects of meiotic maturation in lipopolysaccharide exposed porcine oocytes. Reprod. Toxicol. 2023, 115, 85–93. [Google Scholar] [CrossRef]
- Luo, Y.; Che, M.J.; Liu, C.; Liu, H.G.; Fu, X.W.; Hou, Y.P. Toxicity and related mechanisms of dihydroartemisinin on porcine oocyte maturation in vitro. Toxicol. Appl. Pharmacol. 2018, 341, 8–15. [Google Scholar] [CrossRef]
- Miller, A.F. Superoxide dismutases: Ancient enzymes and new insights. FEBS Lett. 2012, 586, 585–595. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Rhee, S.G. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Zhao, J.; Shi, J.; Wang, M.; Qiu, S.; Hu, Y.; Xu, Y.; Cui, Y.; Liu, C.; et al. The Specific Inhibition of SOD1 Selectively Promotes Apoptosis of Cancer Cells via Regulation of the ROS Signaling Network. Oxid. Med. Cell. Longev. 2019, 2019, 9706792. [Google Scholar] [CrossRef]
- Glasauer, A.; Sena, L.A.; Diebold, L.P.; Mazar, A.P.; Chandel, N.S. Targeting SOD1 reduces experimental non–small-cell lung cancer. J. Clin. Investig. 2014, 124, 117–128. [Google Scholar] [CrossRef]
- Leone, A.; Roca, M.S.; Ciardiello, C.; Costantini, S.; Budillon, A. Oxidative Stress Gene Expression Profile Correlates with Cancer Patient Poor Prognosis: Identification of Crucial Pathways Might Select Novel Therapeutic Approaches. Oxidative Med. Cell Longev. 2017, 2017, 2597581. [Google Scholar] [CrossRef]
- Anwar, S.; Alrumaihi, F.; Sarwar, T.; Babiker, A.Y.; Khan, A.A.; Prabhu, S.V.; Rahmani, A.H. Exploring Therapeutic Potential of Catalase: Strategies in Disease Prevention and Management. Biomolecules 2024, 14, 697. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qiu, Z.; Wang, Y.; Guo, C.; Cai, X.; Zhang, Y.; Liu, L.; Xue, H.; Tang, J. Tea polyphenols alleviate hydrogen peroxide-induced oxidative stress damage through the Mst/Nrf2 axis and the Keap1/Nrf2/HO-1 pathway in murine RAW264.7 cells. Exp. Ther. Med. 2021, 22, 1473. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef] [PubMed]
- Luan, Z.; Fan, X.; Zhao, Y.; Song, H.; Du, W.; Xu, J.; Wang, Z.; Zhang, W.; Zhang, J. Trehalose can effectively protect sheep epididymis epithelial cells from oxidative stress. Arch. Anim. Breed. 2021, 64, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Niu, Y.; Sitia, R.; Wang, C.C. Glutathione peroxidase 7 utilizes hydrogen peroxide generated by Ero1α to promote oxidative protein folding. Antioxid. Redox Signal 2014, 20, 545–556. [Google Scholar] [CrossRef]
- Raykhel, I.; Alanen, H.; Salo, K.; Jurvansuu, J.; Nguyen, V.D.; Latva-Ranta, M.; Ruddock, L. A molecular specificity code for the three mammalian KDEL receptors. J. Cell Biol. 2007, 179, 1193–1204. [Google Scholar] [CrossRef]
- Smith, S.M.; Zwart, S.R.; Block, G.; Rice, B.L.; Davis-Street, J.E. The nutritional status of astronauts is altered after long-term space flight aboard the International Space Station. J. Nutr. 2005, 135, 437–443. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Bai, S.; Wang, G.; Mu, L.; Sun, B.; Wang, D.; Kong, Q.; Liu, Y.; Yao, X.; et al. Simulated microgravity promotes cellular senescence via oxidant stress in rat PC12 cells. Neurochem. Int. 2009, 55, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.W.; Nishiyama, N.C.; Pecaut, M.J.; Campbell-Beachler, M.; Gifford, P.; Haynes, K.E.; Becronis, C.; Gridley, D.S. Simulated Microgravity and Low-Dose/Low-Dose-Rate Radiation Induces Oxidative Damage in the Mouse Brain. Radiat. Res. 2016, 185, 647–657. [Google Scholar] [CrossRef]
- Berardini, M.; Gesualdi, L.; Morabito, C.; Ferranti, F.; Reale, A.; Zampieri, M.; Karpach, K.; Tinari, A.; Bertuccini, L.; Guarnieri, S.; et al. Simulated Microgravity Exposure Induces Antioxidant Barrier Deregulation and Mitochondria Enlargement in TCam-2 Cell Spheroids. Cells 2023, 12, 2106. [Google Scholar] [CrossRef]
- Moustafa, A. Hindlimb unloading-induced reproductive suppression via Downregulation of hypothalamic Kiss-1 expression in adult male rats. Reprod. Biol. Endocrinol. 2021, 19, 37. [Google Scholar] [CrossRef]
- Rizzo, A.M.; Montorfano, G.; Negroni, M.; Corsetto, P.; Berselli, P.; Marciani, P.; Zava, S.; Berra, B. Simulated microgravity induce glutathione antioxidant pathway in Xenopus laevis embryos. Cell Biol. Int. 2009, 33, 893–898. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhang, H.; Miao, G.Y.; Xie, Y.; Sun, C.; Di, C.X.; Liu, Y.; Liu, Y.Y.; Zhang, X.; Ma, X.F.; et al. Simulated microgravity conditions and carbon ion irradiation induce spermatogenic cell apoptosis and sperm DNA damage. Biomed. Environ. Sci. 2013, 26, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, L.; Cazzaniga, A.; De Palma, C.; Castiglioni, S.; Maier, J.A.M. Mitophagy contributes to endothelial adaptation to simulated microgravity. FASEB J. 2020, 34, 1833–1845. [Google Scholar] [CrossRef]
- Cao, Y.J.; Fan, X.J.; Shen, Z.; Ma, B.H.; Duan, E.K. Nitric oxide affects preimplantation embryonic development in a rotating wall vessel bioreactor simulating microgravity. Cell Biol. Int. 2007, 31, 24–29. [Google Scholar] [CrossRef]
- Ammar, O.; Mehdi, M.; Muratori, M. Teratozoospermia: Its association with sperm DNA defects, apoptotic alterations, and oxidative stress. Andrology 2020, 8, 1095–1106. [Google Scholar] [CrossRef]
- Sambandam, Y.; Townsend, M.T.; Pierce, J.J.; Lipman, C.M.; Haque, A.; Bateman, T.A.; Reddy, S.V. Microgravity control of autophagy modulates osteoclastogenesis. Bone 2014, 61, 125–131. [Google Scholar] [CrossRef]
- Ryu, H.W.; Choi, S.H.; Namkoong, S.; Jang, I.S.; Seo, D.H.; Choi, I.; Kim, H.S.; Park, J. Simulated microgravity contributes to autophagy induction by regulating AMP-activated protein kinase. DNA Cell Biol. 2014, 33, 128–135. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.; Zhao, Y.; Chen, H.; Yan, L.; Ge, H.; Wu, X. Activation of autophagy is required for clearance of mitochondrial ROS in patients with asthenozoospermia. PeerJ 2025, 13, e18827. [Google Scholar] [CrossRef]
- Hu, R.; Yang, X.; He, J.; Wu, S. Oxidative Stress and Autophagy: Unraveling the Hidden Threat to Boars’ Fertility. Antioxidants 2024, 14, 2. [Google Scholar] [CrossRef]
- Peters, A.E.; Mihalas, B.P.; Bromfield, E.G.; Roman, S.D.; Nixon, B.; Sutherland, J.M. Autophagy in Female Fertility: A Role in Oxidative Stress and Aging. Antioxid. Redox Signal 2020, 32, 550–568. [Google Scholar] [CrossRef]
- Sharma, P.; Kaushal, N.; Saleth, L.R.; Ghavami, S.; Dhingra, S.; Kaur, P. Oxidative stress-induced apoptosis and autophagy: Balancing the contrary forces in spermatogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166742. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, R.E.; Kaufmann, W.K.; Paules, R.S. Oxidative stress and cell cycle checkpoint function. Free Radic. Biol. Med. 2000, 28, 1387–1404. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Murray, A. Cell cycle checkpoints. Curr. Opin. Cell Biol. 1994, 6, 872–876. [Google Scholar] [CrossRef]
- Emori, C.; Boucher, Z.; Bolcun-Filas, E. CHEK2 signaling is the key regulator of oocyte survival after chemotherapy. Sci. Adv. 2023, 9, eadg0898. [Google Scholar] [CrossRef]
- Pellarin, I.; Dall’Acqua, A.; Favero, A.; Segatto, I.; Rossi, V.; Crestan, N.; Karimbayli, J.; Belletti, B.; Baldassarre, G. Cyclin-dependent protein kinases and cell cycle regulation in biology and disease. Signal Transduct. Target. Ther. 2025, 10, 11. [Google Scholar] [CrossRef]
- Morabito, C.; Guarnieri, S.; Catizone, A.; Schiraldi, C.; Ricci, G.; Mariggiò, M.A. Transient increases in intracellular calcium and reactive oxygen species levels in TCam-2 cells exposed to microgravity. Sci. Rep. 2017, 7, 15648. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Khan, A.; Huang, B.; Wei, R.; Kou, X.; Wang, X.; Chen, W.; Li, L.; Zahoor, M.; Wang, C. Bioactive Compounds Protect Mammalian Reproductive Cells from Xenobiotics and Heat Stress-Induced Oxidative Distress via Nrf2 Signaling Activation: A Narrative Review. Antioxidants 2024, 13, 597. [Google Scholar] [CrossRef]
- Li, H.; Cong, X.; Yu, W.; Jiang, Z.; Fu, K.; Cao, R.; Tian, W.; Feng, Y. Baicalin inhibits oxidative injures of mouse uterine tissue induced by acute heat stress through activating the Keap1/Nrf2 signaling pathway. Res. Vet. Sci. 2022, 152, 717–725. [Google Scholar] [CrossRef]
- Guo, S.; Wharton, W.; Moseley, P.; Shi, H. Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaperones 2007, 12, 245–254. [Google Scholar] [CrossRef]
- Parcellier, A.; Schmitt, E.; Gurbuxani, S.; Seigneurin-Berny, D.; Pance, A.; Chantôme, A.; Plenchette, S.; Khochbin, S.; Solary, E.; Garrido, C. HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation. Mol. Cell. Biol. 2003, 23, 5790–5802. [Google Scholar] [CrossRef]
- Gabai, V.L.; Meriin, A.B.; Mosser, D.D.; Caron, A.W.; Rits, S.; Shifrin, V.I.; Sherman, M.Y. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J. Biol. Chem. 1997, 272, 18033–18037. [Google Scholar] [CrossRef]
- Manna, O.M.; Burgio, S.; Picone, D.; Carista, A.; Pitruzzella, A.; Fucarino, A.; Bucchieri, F. Microgravity and Human Body: Unraveling the Potential Role of Heat-Shock Proteins in Spaceflight and Future Space Missions. Biology 2024, 13, 921. [Google Scholar] [CrossRef]
- Shimada, N.; Moorman, S.J. Changes in gravitational force cause changes in gene expression in the lens of developing zebrafish. Dev. Dyn. 2006, 235, 2686–2694. [Google Scholar] [CrossRef]
- Radmanesh, F.; Razi, M.; Shalizar-Jalali, A. Curcumin nano-micelle induced testicular toxicity in healthy rats; evidence for oxidative stress and failed homeostatic response by heat shock proteins 70-2a and 90. Biomed. Pharmacother. 2021, 142, 111945. [Google Scholar] [CrossRef]
- Zhang, W.D.; Zhang, Z.; Jia, L.T.; Zhang, L.L.; Fu, T.; Li, Y.S.; Wang, P.; Sun, L.; Shi, Y.; Zhang, H.Z. Oxygen free radicals and mitochondrial signaling in oligospermia and asthenospermia. Mol. Med. Rep. 2014, 10, 1875–1880. [Google Scholar] [CrossRef][Green Version]
- Duan, P.; Hu, C.; Butler, H.J.; Quan, C.; Chen, W.; Huang, W.; Tang, S.; Zhou, W.; Yuan, M.; Shi, Y.; et al. 4-Nonylphenol induces disruption of spermatogenesis associated with oxidative stress-related apoptosis by targeting p53-Bcl-2/Bax-Fas/FasL signaling. Environ. Toxicol. 2017, 32, 739–753. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, Y.; Li, L.; Zhou, W.; Tian, D.; Lu, C.; Yu, S.; Zhao, J.; Peng, S. PM2.5 induces embryonic growth retardation: Potential involvement of ROS-MAPKs-apoptosis and G0/G1 arrest pathways. Environ. Toxicol. 2016, 31, 2028–2044. [Google Scholar] [CrossRef]
- Yoshino, T.; Suzuki, T.; Nagamatsu, G.; Yabukami, H.; Ikegaya, M.; Kishima, M.; Kita, H.; Imamura, T.; Nakashima, K.; Nishinakamura, R.; et al. Generation of ovarian follicles from mouse pluripotent stem cells. Science 2021, 373, eabe0237. [Google Scholar] [CrossRef]
- Mikhael, S.; Punjala-Patel, A.; Gavrilova-Jordan, L. Hypothalamic-Pituitary-Ovarian Axis Disorders Impacting Female Fertility. Biomedicines 2019, 7, 5. [Google Scholar] [CrossRef]
- Gimunová, M.; Paludo, A.C.; Bernaciková, M.; Bienertova-Vasku, J. The effect of space travel on human reproductive health: A systematic review. NPJ Microgravity 2024, 10, 10. [Google Scholar] [CrossRef]
- Abudawood, M.; Tabassum, H.; Alanazi, A.H.; Almusallam, F.; Aljaser, F.; Ali, M.N.; Alenzi, N.D.; Alanazi, S.T.; Alghamdi, M.A.; Altoum, G.H.; et al. Antioxidant status in relation to heavy metals induced oxidative stress in patients with polycystic ovarian syndrome (PCOS). Sci. Rep. 2021, 11, 22935. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Meldrum, D.R.; Katz-Jaffe, M.G.; Krisher, R.L.; Schoolcraft, W.B. Oocyte environment: Follicular fluid and cumulus cells are critical for oocyte health. Fertil. Steril. 2015, 103, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Mateen, S.; Ahmad, R.; Moin, S. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J. Assist. Reprod. Genet. 2022, 39, 2439–2473. [Google Scholar] [CrossRef]
- Al-Gubory, K.H.; Garrel, C.; Faure, P.; Sugino, N. Roles of antioxidant enzymes in corpus luteum rescue from reactive oxygen species-induced oxidative stress. Reprod. Biomed. Online 2012, 25, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, L.; Xu, J.; Zhang, L.; Li, M.; Xie, X.; Xie, Y.; Luo, D.; Zhang, D.; Yu, X.; et al. Maternal exposure to perfluorooctanoic acid inhibits luteal function via oxidative stress and apoptosis in pregnant mice. Reprod. Toxicol. 2017, 69, 159–166. [Google Scholar] [CrossRef]
- Virant-Klun, I.; Imamovic-Kumalic, S.; Pinter, B. From Oxidative Stress to Male Infertility: Review of the Associations of Endocrine-Disrupting Chemicals (Bisphenols, Phthalates, and Parabens) with Human Semen Quality. Antioxidants 2022, 11, 1617. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, X.; Li, Y.; Cao, D.; Luo, C.; Zhang, Q.; Zhang, S.; Jiao, Y. Age-related testosterone decline: Mechanisms and intervention strategies. Reprod. Biol. Endocrinol. 2024, 22, 144. [Google Scholar] [CrossRef]
- Strollo, F.; Riondino, G.; Harris, B.; Strollo, G.; Casarosa, E.; Mangrossa, N.; Ferretti, C.; Luisi, M. The effect of microgravity on testicular androgen secretion. Aviat. Space Environ. Med. 1998, 69, 133–136. [Google Scholar]
- Zachwieja, J.J.; Smith, S.R.; Lovejoy, J.C.; Rood, J.C.; Windhauser, M.M.; Bray, G.A. Testosterone administration preserves protein balance but not muscle strength during 28 days of bed rest. J. Clin. Endocrinol. Metab. 1999, 84, 207–212. [Google Scholar] [CrossRef]
- Zhu, H.; Cheng, Y.; Wang, X.; Yang, X.; Liu, M.; Liu, J.; Liu, S.; Wang, H.; Zhang, A.; Li, R.; et al. Gss deficiency causes age-related fertility impairment via ROS-triggered ferroptosis in the testes of mice. Cell Death Dis. 2023, 14, 845. [Google Scholar] [CrossRef]
- Sun, T.C.; Li, D.M.; Yu, H.; Song, L.L.; Jia, Y.J.; Lin, L.; Zhou, S.J. Bilateral varicocele leads to ferroptosis, pyroptosis and necroptosis of human spermatozoa and affects semen quality in infertile men. Front. Cell Dev. Biol. 2023, 11, 1091438. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef]
- Mateo-Otero, Y.; Llavanera, M.; Torres-Garrido, M.; Yeste, M. Embryo development is impaired by sperm mitochondrial-derived ROS. Biol. Res. 2024, 57, 5. [Google Scholar] [CrossRef] [PubMed]
- Guérin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Sasaki, S.; Kubota, Y.; Ikeuchi, T.; Hayashi, Y.; Kohri, K. Effects of simulated microgravity on mammalian fertilization and preimplantation embryonic development in vitro. Fertil. Steril. 2000, 74, 1142–1147. [Google Scholar] [CrossRef]
- Rajneesh; Pathak, J.; Chatterjee, A.; Singh, S.P.; Sinha, R.P. Detection of Reactive Oxygen Species (ROS) in Cyanobacteria Using the Oxidant-sensing Probe 2′,7′-Dichlorodihydrofluorescein Diacetate (DCFH-DA). Bio-Protoc. 2017, 7, e2545. [Google Scholar] [CrossRef]
- Soh, N. Recent advances in fluorescent probes for the detection of reactive oxygen species. Anal. Bioanal. Chem. 2006, 386, 532–543. [Google Scholar] [CrossRef]
- Kauffman, M.E.; Kauffman, M.K.; Traore, K.; Zhu, H.; Trush, M.A.; Jia, Z.; Li, Y.R. MitoSOX-Based Flow Cytometry for Detecting Mitochondrial ROS. React. Oxyg. Species 2016, 2, 361–370. [Google Scholar] [CrossRef]
- Shah, A.; Dobrovolskaia, M.A. Detection of Mitochondrial Oxidative Stress in T-cells Using MitoSOX Red Dye: Version 1. In National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols; National Cancer Institute (US): Bethesda, MD, USA, 2005. [Google Scholar]
- Sun, L.; Wang, H.; Yu, S.; Zhang, L.; Jiang, J.; Zhou, Q. Herceptin induces ferroptosis and mitochondrial dysfunction in H9c2 cells. Int. J. Mol. Med. 2022, 49, 17. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, X.; Tian, X.; Zhang, J.; Xue, S.; Jiang, Y.; Liu, T.; Wang, X.; Sun, Q.; Hong, Y.; et al. Tanshinone I inhibits doxorubicin-induced cardiotoxicity by regulating Nrf2 signaling pathway. Phytomedicine 2022, 106, 154439. [Google Scholar] [CrossRef] [PubMed]
- Celeghini, E.C.C.; Alves, M.B.R.; de Arruda, R.P.; de Rezende, G.M.; Florez-Rodriguez, S.A.; de Sá Filho, M.F. Efficiency of CellROX deep red® and CellROX orange® fluorescent probes in identifying reactive oxygen species in sperm samples from high and low fertility bulls. Anim. Biotechnol. 2021, 32, 77–83. [Google Scholar] [CrossRef]
- de Castro, L.S.; de Assis, P.M.; Siqueira, A.F.; Hamilton, T.R.; Mendes, C.M.; Losano, J.D.; Nichi, M.; Visintin, J.A.; Assumpção, M.E. Sperm Oxidative Stress Is Detrimental to Embryo Development: A Dose-Dependent Study Model and a New and More Sensitive Oxidative Status Evaluation. Oxid. Med. Cell. Longev. 2016, 2016, 8213071. [Google Scholar] [CrossRef]
- Escada-Rebelo, S.; Mora, F.G.; Sousa, A.P.; Almeida-Santos, T.; Paiva, A.; Ramalho-Santos, J. Fluorescent probes for the detection of reactive oxygen species in human spermatozoa. Asian J. Androl. 2020, 22, 465–471. [Google Scholar] [CrossRef]
- Javvaji, P.K.; Dhali, A.; Francis, J.R.; Kolte, A.P.; Mech, A.; Roy, S.C.; Mishra, A.; Bhatta, R. An Efficient Nitroblue Tetrazolium Staining and Bright-Field Microscopy Based Method for Detecting and Quantifying Intracellular Reactive Oxygen Species in Oocytes, Cumulus Cells and Embryos. Front. Cell Dev. Biol. 2020, 8, 764. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ouyang, H.; Lou, X.; Xia, F.; Jin, L.; Wang, S.; Dai, J. Dual-Activated H2O2-Responsive AIE Probes for Oocyte Quality Assessment. Anal. Chem. 2024, 96, 5960–5967. [Google Scholar] [CrossRef]
- Sahoo, B.; Mishra, B.; Bhaskar, R.; Vikas, Y.N.V.; Umesh, A.; Guttula, P.K.; Gupta, M.K. Analyzing the effect of heparin on in vitro capacitation and spermatozoal RNA population in goats. Int. J. Biol. Macromol. 2023, 241, 124502. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Y.; Wang, H.; Li, H.; Zhu, J.; Cong, L.; Xu, J.; Chen, W.; Jiang, Y.; Sun, Y. NAD+ repletion attenuates obesity-induced oocyte mitochondrial dysfunction and offspring metabolic abnormalities via a SIRT3-dependent pathway. Clin. Transl. Med. 2021, 11, e628. [Google Scholar] [CrossRef]
- Baldi, E.; Traini, G.; Tamburrino, L.; Bini, F.; Raffaelli, G.; Vignozzi, L.; Marchiani, S. A fluorescent probe for Reactive Oxygen Species (ROS) detection identifies spermatozoa with a better reporductive performance. Human. Reprod. 2022, 37, 206. [Google Scholar] [CrossRef]
- Zou, J.L.; Lu, H.G.; Zhao, X.W.; Li, W.; Guan, Y.; Zheng, Y.D.; Zhang, L.J.; Gao, H. A multi-functional fluorescent probe with aggregation-induced emission characteristics: Mitochondrial imaging, photodynamic therapy and visualizing therapeutic process in zebrafish model. Dye. Pigment. 2018, 151, 45–53. [Google Scholar] [CrossRef]
- Purdey, M.S.; McLennan, H.J.; Sutton-McDowall, M.L.; Drumm, D.W.; Zhang, X.Z.; Capon, P.K.; Heng, S.; Thompson, J.G.; Abell, A.D. Biological hydrogen peroxide detection with aryl boronate and benzil BODIPY-based fluorescent probes. Sens. Actuators B Chem. 2018, 262, 750–757. [Google Scholar] [CrossRef]
- Kiani-Esfahani, A.; Tavalaee, M.; Deemeh, M.R.; Hamiditabar, M.; Nasr-Esfahani, M.H. DHR123: An alternative probe for assessment of ROS in human spermatozoa. Syst. Biol. Reprod. Med. 2012, 58, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Dietz, C.; Infanger, M.; Romswinkel, A.; Strube, F.; Kraus, A. Apoptosis Induction and Alteration of Cell Adherence in Human Lung Cancer Cells under Simulated Microgravity. Int. J. Mol. Sci. 2019, 20, 3601. [Google Scholar] [CrossRef]
- Strods, A.; Narbute, K.; Movčana, V.; Gillois, K.; Rimša, R.; Hollos, P.; Rūmnieks, F.; Spule, A.; Mozoļevskis, G.; Abols, A. Development of Organ-on-a-Chip System with Continuous Flow in Simulated Microgravity. Micromachines 2024, 15, 370. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Li, Z.; Zhang, R.; Xie, H.; Deng, Y.; Tang, B.Z. Simulated microgravity-induced endogenous H2O2 traced by an AIEgen. Sci. Bull. 2022, 67, 2513–2516. [Google Scholar] [CrossRef]
- Traini, G.; Tamburrino, L.; Vignozzi, L.; Baldi, E.; Marchiani, S. Is oxidative stress evaluated in viable human spermatozoa a marker of good semen quality? Front. Endocrinol. 2022, 13, 1012416. [Google Scholar] [CrossRef]
- Alexandru, I.; Nistor, D.; Motofelea, A.C.; Cadar Andone, B.A.; Crintea, A.; Tatu, C.; Pop, G.N.; Csep, A.N. Vitamins, Coenzyme Q10, and Antioxidant Strategies to Improve Oocyte Quality in Women with Gynecological Cancers: A Comprehensive Review. Antioxidants 2024, 13, 1567. [Google Scholar] [CrossRef]
- Noda, Y.; Ota, K.; Shirasawa, T.; Shimizu, T. Copper/zinc superoxide dismutase insufficiency impairs progesterone secretion and fertility in female mice. Biol. Reprod. 2012, 86, 1–8. [Google Scholar] [CrossRef]
- Henmi, H.; Endo, T.; Kitajima, Y.; Manase, K.; Hata, H.; Kudo, R. Effects of ascorbic acid supplementation on serum progesterone levels in patients with a luteal phase defect. Fertil. Steril. 2003, 80, 459–461. [Google Scholar] [CrossRef]
- Ovchinnikov, R.I.; Popova, A.Y.; Vtorushina, V.V.; Muradyan, A.A.; Gamidov, S.I. The use of a complex of natural antimicrobial peptides and cytokines for treatment of male infertility and chronic prostatitis. Urologiia 2022, 2, 43–53. [Google Scholar] [CrossRef]
- DiTroia, S.P.; Percharde, M.; Guerquin, M.J.; Wall, E.; Collignon, E.; Ebata, K.T.; Mesh, K.; Mahesula, S.; Agathocleous, M.; Laird, D.J.; et al. Maternal vitamin C regulates reprogramming of DNA methylation and germline development. Nature 2019, 573, 271–275. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Yilmazer, Y.; Moshfeghi, E.; Cetin, F.; Findikli, N. In vitro effects of the combination of serotonin, selenium, zinc, and vitamins D and E supplementation on human sperm motility and reactive oxygen species production. Zygote 2024, 32, 154–160. [Google Scholar] [CrossRef]
- Yamamoto, Y. Coenzyme Q10 redox balance and a free radical scavenger drug. Arch. Biochem. Biophys. 2016, 595, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Du, J.; Lian, Y.; Zhang, Y.; Li, X.; Liu, Y.; Zou, L.; Wu, T. Protective Effects of Coenzyme Q10 Against Hydrogen Peroxide-Induced Oxidative Stress in PC12 Cell: The Role of Nrf2 and Antioxidant Enzymes. Cell. Mol. Neurobiol. 2016, 36, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ben-Meir, A.; Burstein, E.; Borrego-Alvarez, A.; Chong, J.; Wong, E.; Yavorska, T.; Naranian, T.; Chi, M.; Wang, Y.; Bentov, Y.; et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 2015, 14, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Nisenblat, V.; Lu, C.; Li, R.; Qiao, J.; Zhen, X.; Wang, S. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: A randomized controlled trial. Reprod. Biol. Endocrinol. 2018, 16, 29. [Google Scholar] [CrossRef]
- Safarinejad, M.R. The effect of coenzyme Q10 supplementation on partner pregnancy rate in infertile men with idiopathic oligoasthenoteratozoospermia: An open-label prospective study. Int. Urol. Nephrol. 2012, 44, 689–700. [Google Scholar] [CrossRef]
- Steiner, A.Z.; Hansen, K.R.; Barnhart, K.T.; Cedars, M.I.; Legro, R.S.; Diamond, M.P.; Krawetz, S.A.; Usadi, R.; Baker, V.L.; Coward, R.M.; et al. The effect of antioxidants on male factor infertility: The Males, Antioxidants, and Infertility (MOXI) randomized clinical trial. Fertil. Steril. 2020, 113, 552–560.e553. [Google Scholar] [CrossRef]
- Kaltsas, A. Oxidative Stress and Male Infertility: The Protective Role of Antioxidants. Medicina 2023, 59, 1769. [Google Scholar] [CrossRef]
- Zhang, M.; ShiYang, X.; Zhang, Y.; Miao, Y.; Chen, Y.; Cui, Z.; Xiong, B. Coenzyme Q10 ameliorates the quality of postovulatory aged oocytes by suppressing DNA damage and apoptosis. Free Radic. Biol. Med. 2019, 143, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, C.; Cheng, W.; Tao, R.; Xu, H.; Liu, H. Vitamin C protects early mouse embryos against juglone toxicity. Reprod. Toxicol. 2020, 98, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Campos, A.; Soria-Meneses, P.J.; Arenas-Moreira, M.; Alonso-Moreno, C.; Bravo, I.; Rodríguez-Robledo, V.; Sánchez-Ajofrín, I.; Soler, A.J.; Garde, J.J.; Fernández-Santos, M.D.R. Vitamin E Lipid-Based Nanodevices as a Tool for Ovine Sperm Protection against Oxidative Stress: Impact on Sperm Motility. Antioxidants 2022, 11, 1988. [Google Scholar] [CrossRef]
- Sun, Y.L.; Tang, S.B.; Shen, W.; Yin, S.; Sun, Q.Y. Roles of Resveratrol in Improving the Quality of Postovulatory Aging Oocytes In Vitro. Cells 2019, 8, 1132. [Google Scholar] [CrossRef]
- Li, S.; Li, R.; Jiang, J.; Liu, L.; Ma, X.; Wang, T.; Zhao, L.; Li, W.; Niu, D. Curcumin protects porcine granulosa cells and mouse ovary against reproductive toxicity of aflatoxin B1 via PI3K/AKT signaling pathway. Environ. Pollut. 2024, 363 Pt 2, 125210. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, L.; Xu, X.; Xiao, L.; Wen, J.; Zhang, H.; Zhao, S.; Qiao, D.; Bai, J.; Liu, Y. The Antioxidant Salidroside Ameliorates the Quality of Postovulatory Aged Oocyte and Embryo Development in Mice. Antioxidants 2024, 13, 248. [Google Scholar] [CrossRef]
- Tvrdá, E.; Kováčik, A.; Tušimová, E.; Paál, D.; Mackovich, A.; Alimov, J.; Lukáč, N. Antioxidant efficiency of lycopene on oxidative stress—Induced damage in bovine spermatozoa. J. Anim. Sci. Biotechnol. 2016, 7, 50. [Google Scholar] [CrossRef]
- Mao, T.; Han, C.; Wei, B.; Zhao, L.; Zhang, Q.; Deng, R.; Liu, J.; Luo, Y.; Zhang, Y. Protective Effects of Quercetin Against Cadmium Chloride-Induced Oxidative Injury in Goat Sperm and Zygotes. Biol. Trace Elem. Res. 2018, 185, 344–355. [Google Scholar] [CrossRef]
- Yan, P.; Li, Q.; Wang, L.; Lu, P.; Suzuki, K.; Liu, Z.; Lei, J.; Li, W.; He, X.; Wang, S.; et al. FOXO3-Engineered Human ESC-Derived Vascular Cells Promote Vascular Protection and Regeneration. Cell Stem Cell 2019, 24, 447–461.e448. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, S.; Zhu, B.M. CRISPR/Cas9-Induced Loss of Keap1 Enhances Anti-oxidation in Rat Adipose-Derived Mesenchymal Stem Cells. Front. Neurol. 2019, 10, 1311. [Google Scholar] [CrossRef]
- Huang, C.; Yang, C.; Pang, D.; Li, C.; Gong, H.; Cao, X.; He, X.; Chen, X.; Mu, B.; Cui, Y.; et al. Animal models of male subfertility targeted on LanCL1-regulated spermatogenic redox homeostasis. Lab Anim. 2022, 51, 133–145. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhu, S.; Wu, J.; Zhang, M.; Xu, Y.; Xu, W.; Cui, J.; Yu, B.; Cao, W.; Liu, J. Exercise enhances cardiac function by improving mitochondrial dysfunction and maintaining energy homoeostasis in the development of diabetic cardiomyopathy. J. Mol. Med. 2020, 98, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.A.; Lee, J.H.; Song, W.; Jun, T.W. Exercise training improves the antioxidant enzyme activity with no changes of telomere length. Mech. Ageing Dev. 2008, 129, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Lisi, V.; Senesi, G.; Bertola, N.; Pecoraro, M.; Bolis, S.; Gualerzi, A.; Picciolini, S.; Raimondi, A.; Fantini, C.; Moretti, E.; et al. Plasma-derived extracellular vesicles released after endurance exercise exert cardioprotective activity through the activation of antioxidant pathways. Redox Biol. 2023, 63, 102737. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, C.; Kato, T.; Inoue-Suzuki, S.; Kikuchi, J.; Ohta, T.; Kagawa, M.; Hattori, M.; Kobayashi, H.; Shiba, D.; Shirakawa, M.; et al. Dietary intervention of mice using an improved Multiple Artificial-gravity Research System (MARS) under artificial 1 g. NPJ Microgravity 2019, 5, 16. [Google Scholar] [CrossRef]
- Hayashi, T.; Fujita, R.; Okada, R.; Hamada, M.; Suzuki, R.; Fuseya, S.; Leckey, J.; Kanai, M.; Inoue, Y.; Sadaki, S.; et al. Lunar gravity prevents skeletal muscle atrophy but not myofiber type shift in mice. Commun. Biol. 2023, 6, 424. [Google Scholar] [CrossRef]
- Mao, X.W.; Byrum, S.; Nishiyama, N.C.; Pecaut, M.J.; Sridharan, V.; Boerma, M.; Tackett, A.J.; Shiba, D.; Shirakawa, M.; Takahashi, S.; et al. Impact of Spaceflight and Artificial Gravity on the Mouse Retina: Biochemical and Proteomic Analysis. Int. J. Mol. Sci. 2018, 19, 2546. [Google Scholar] [CrossRef]
| Fluorescent Probe | Target | Excitation/Emission Wavelength (nm) | Sensitivity | Specificity | Usability | Applications on Germ Cells | Ref |
|---|---|---|---|---|---|---|---|
| DCFDA | H2O2, O2, •OH, etc. | 488/525 | Moderate | Moderate | Wide range of applications. Can be used with fluorescence microscopy or confocal microscopy, etc. | Study the effect of heparin on ROS activity in goat spermatozoa. | [148] |
| MitoSOX | O2− | 510/580 | High | High | One of the preferred tools for the detection of mitochondrial superoxide in living cells. Can be used with fluorescence microscope or confocal microscope. | Compare ROS production in the oocytes of mice on a high-fat diet and mice supplemented with nicotinamide riboside (NR). | [149] |
| CellROX Orange | H2O2, O2−, HOO−, ONOO−, etc. | 545/565 | Moderate | Moderate | Compatible with live cells and easy to operate, can be detected by fluorescence microscope, flow cytometer, and so on. | Semen parameters and ROS levels were found to have a positive correlation. | [150] |
| Nitroblue tetrazolium (NBT) | O2− | / | Moderate | Moderate | Easy to use, can be detected by bright-field microscopy, but the results are relatively crude. | Matured COC and embryos were stained with NBT to detect and quantify intracellular ROS. | [145] |
| b-PyTPA | H2O2 | 488/669 | High | High | Dual activation of oxidative stress response, can be detected by confocal microscopy. | This Dual-Activated H2O2-Responsive AIE probe can used for the detection of the ROS in oocytes, and enable the assessment of their quality. | [146] |
| AIE-FR-TPP | H2O2, O2−, •OH, etc. | 488/662–737 | High | High | Targets mitochondrial ROS with low working concentration and good biocompatibility. Can be detected by confocal laser microscopy. | AIE-FR-TPP probe is used for mitochondrial imaging, photodynamic therapy, and visualizing the therapeutic process in zebrafish embryos. | [151] |
| PeroxyBODIPY-1(PB1) | H2O2 | 490/509 | High | High | Cell-permeable, can be detected by confocal microscopy. | PB1 is able to detect H2O2 in denuded bovine oocytes. | [152] |
| DHR123 | H2O2, O2−, ONOO−, NO | 488/530 | High | Moderate | Targets mitochondria, but prolonged light exposure leads to fluorescence quenching, can be detected by flow cytometer or fluorescence microscope. | DHR123 can afford a simple method to measure OS in human spermatozoa. | [153] |
| Antioxidants | Cell Type | Findings | Ref |
|---|---|---|---|
| CoQ10 | 4–6 week-old female mice oocyte | CoQ10 can inhibit the OS caused by aging and inhibit the apoptosis of the oocytes by reducing the levels of peroxide and DNA damage in the oocytes. | [172] |
| Vitamin C | Mice 2-cell embryos | Vitamin C alleviates embryonic OS from juglone, reduces abnormal mitochondrial potentials and epigenetic modifications, and reduces excess intracellular ROS levels, DNA damage, and embryo apoptosis. | [173] |
| Vitamin E Nanoemulsion | Manchega rams’ sperm | Vitamin E nanoemulsion gives sperm greater motility than free vitamin E and also protects against the harmful effects of free radicals and lipid peroxidation caused by OS. | [174] |
| Resveratrol | Female mice MII oocytes | Resveratrol maintains homologous mitochondrial distribution in postovulatory aging (POA) oocytes and has an antiapoptotic effect on the POA oocytes, and also protects against H3K9me2 methylation deletion, protects epigenetic modifications in the POA oocytes, and improves subsequent development of blastocysts. | [175] |
| Curcumin | Porcine granulosa cells (GCs) | Curcumin attenuates afb1-induced growth inhibition and mitigates mitochondrial dysfunction, thereby reducing OS in porcine GCs. | [176] |
| Salidroside | Female mice MII oocytes | Salidroside can decreases the malformation rate and recovers mitochondrial dysfunction, relieves the OS caused by aging after ovulation, promotes protective autophagy of the senescent oocytes by activating MAPK pathway. | [177] |
| Lycopene (LYC) | Bovine sperm | LYC showed significant ROS clearance and antioxidant properties, which could improve sperm motion parameters and mitochondrial activity | [178] |
| Quercetin | Goat sperm and zygotes. | Quercetin can reduce the MDA and ROS levels of oxidized sperm, maintain sperm motility and various characteristics, and promote zygote to reduce peroxide under OS, maintain mitochondrial function and ensure embryo quality. | [179] |
| Methods | Relative Effectiveness | Feasibility in Space | Possible Side Effects. |
|---|---|---|---|
| Antioxidant Supplementation | Moderate; Conventional antioxidants are effective in scavenging free radicals, attenuating oxidative stress, and improving oocyte quality, but they cannot completely eliminate the oxidative damage caused by microgravity. | High; Traditional antioxidants have simpler preservation and use conditions, are easy to carry and use in the space environment, and are relatively inexpensive. | Single antioxidants may have limited antioxidant capacity, may be potentially toxic at high doses, and there may be interactions between different antioxidants that can affect effectiveness. |
| Gene Editing | High; Gene editing can accurately edit genes related with antioxidants to enhance the antioxidant capacity of stem cells from the root, with long-lasting and stable effects. | Low; The application of gene editing technology in the space environment faces many challenges, such as high equipment requirements, complex operation, and the risk of damage to cells, etc., and requires the support of specialized technicians and equipment, and the application in space is still in the preliminary exploration stage. | It may lead to gene mutation, off-target effects, etc., affecting the normal physiological function and genetic stability of germ cells, and is also ethically controversial. |
| Exercise | Moderate; Exercise enhances the body’s antioxidant capacity, promotes blood circulation, improves the nutrient supply and metabolic environment of germ cells, and has a positive effect on reducing oxidative stress under microgravity. | Moderate; In the space environment, exercise requires specialized equipment and space and is limited by microgravity conditions, with limited exercise modalities and intensity, but can be achieved through the design of rational exercise programs and the use of special exercise equipment. | Excessive exercise may lead to physical fatigue, injury, and increase the production of free radicals, which in turn aggravates oxidative stress, and an appropriate exercise program needs to be developed on an individual basis. |
| Physical Intervention | Theoretically the highest; 1 g mimicry restores cytomechanical signaling and maintains mitochondrial membrane potential and ROS homeostasis | Moderate; Artificial gravity devices are typically large, heavy, and energy intensive, placing extreme demands on the design and operation of spacecraft. | The effects of long-term exposure to artificial gravity on the body need to be further studied. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Xie, J.; Ma, C.; Zhang, P.; Lei, X. Oxidative Damage Under Microgravity Conditions: Response Mechanisms, Monitoring Methods and Countermeasures on Somatic and Germ Cells. Int. J. Mol. Sci. 2025, 26, 4583. https://doi.org/10.3390/ijms26104583
Chen Z, Xie J, Ma C, Zhang P, Lei X. Oxidative Damage Under Microgravity Conditions: Response Mechanisms, Monitoring Methods and Countermeasures on Somatic and Germ Cells. International Journal of Molecular Sciences. 2025; 26(10):4583. https://doi.org/10.3390/ijms26104583
Chicago/Turabian StyleChen, Zekai, Jingtong Xie, Chiyuan Ma, Pengfei Zhang, and Xiaohua Lei. 2025. "Oxidative Damage Under Microgravity Conditions: Response Mechanisms, Monitoring Methods and Countermeasures on Somatic and Germ Cells" International Journal of Molecular Sciences 26, no. 10: 4583. https://doi.org/10.3390/ijms26104583
APA StyleChen, Z., Xie, J., Ma, C., Zhang, P., & Lei, X. (2025). Oxidative Damage Under Microgravity Conditions: Response Mechanisms, Monitoring Methods and Countermeasures on Somatic and Germ Cells. International Journal of Molecular Sciences, 26(10), 4583. https://doi.org/10.3390/ijms26104583








