CRISPR/Cas9-Mediated Knockout of the White Gene in Agasicles hygrophila
Abstract
1. Introduction
2. Results
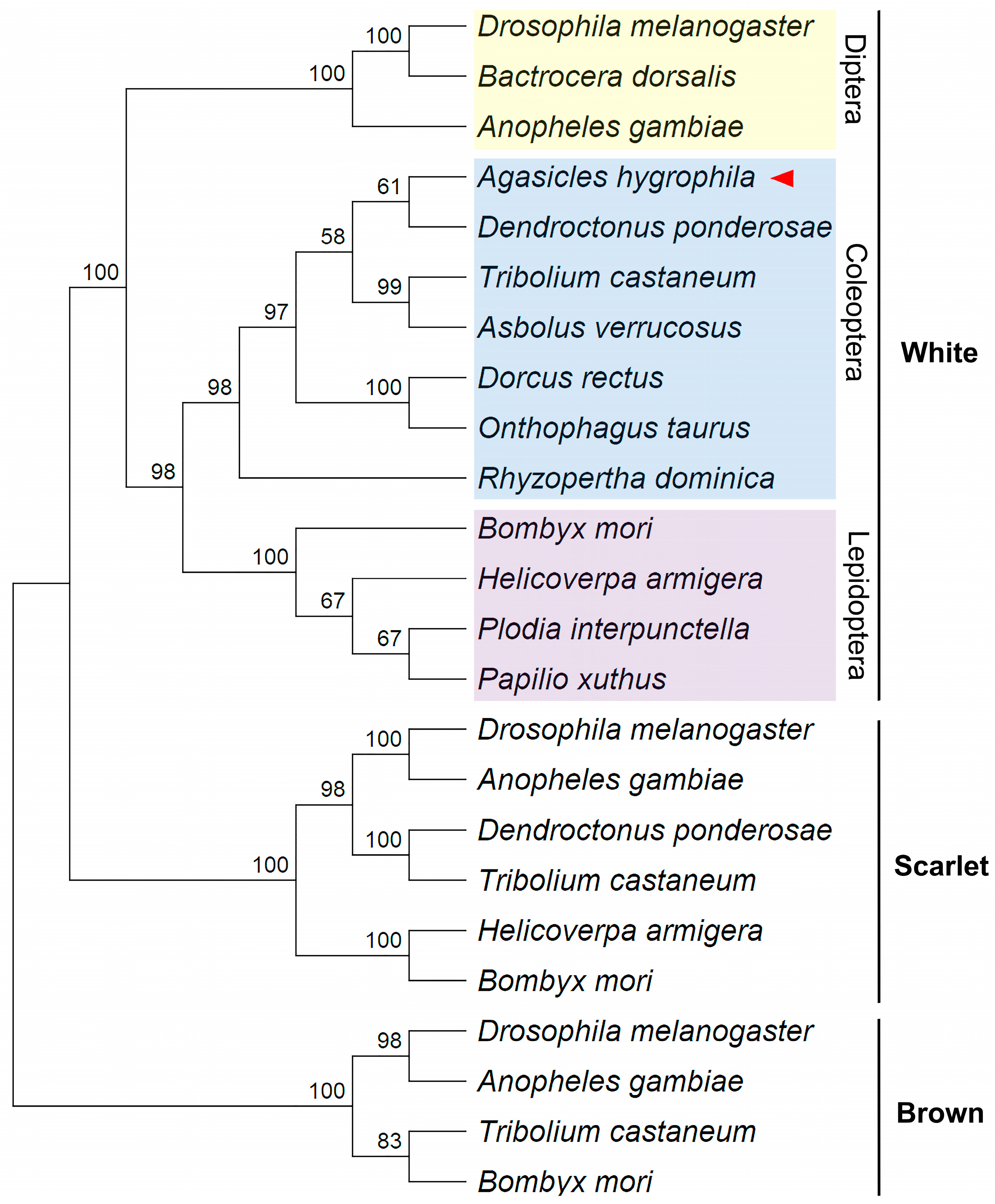
2.1. Phylogenetic Identification of the A. hygrophila White (AhW) Gene
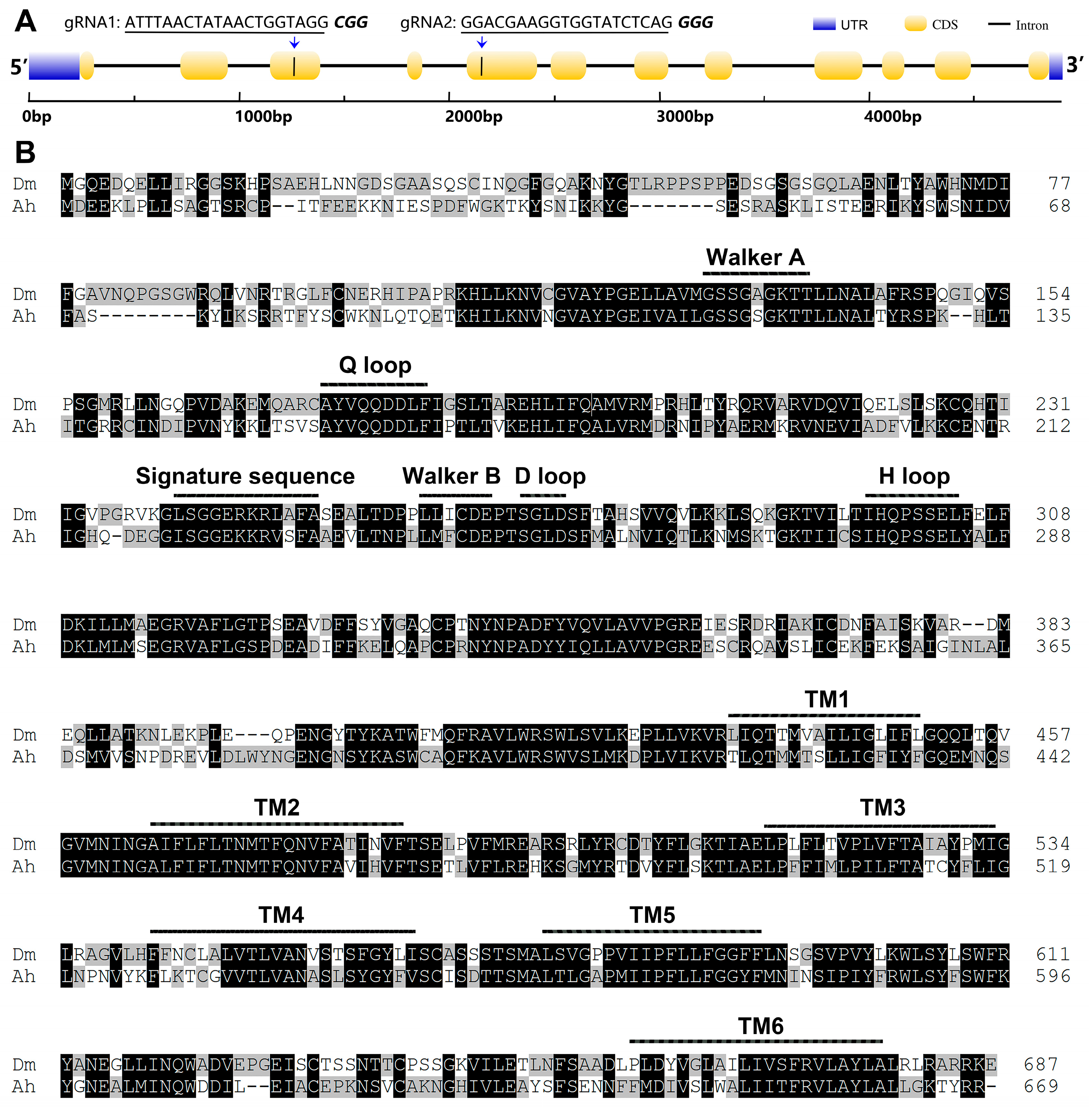
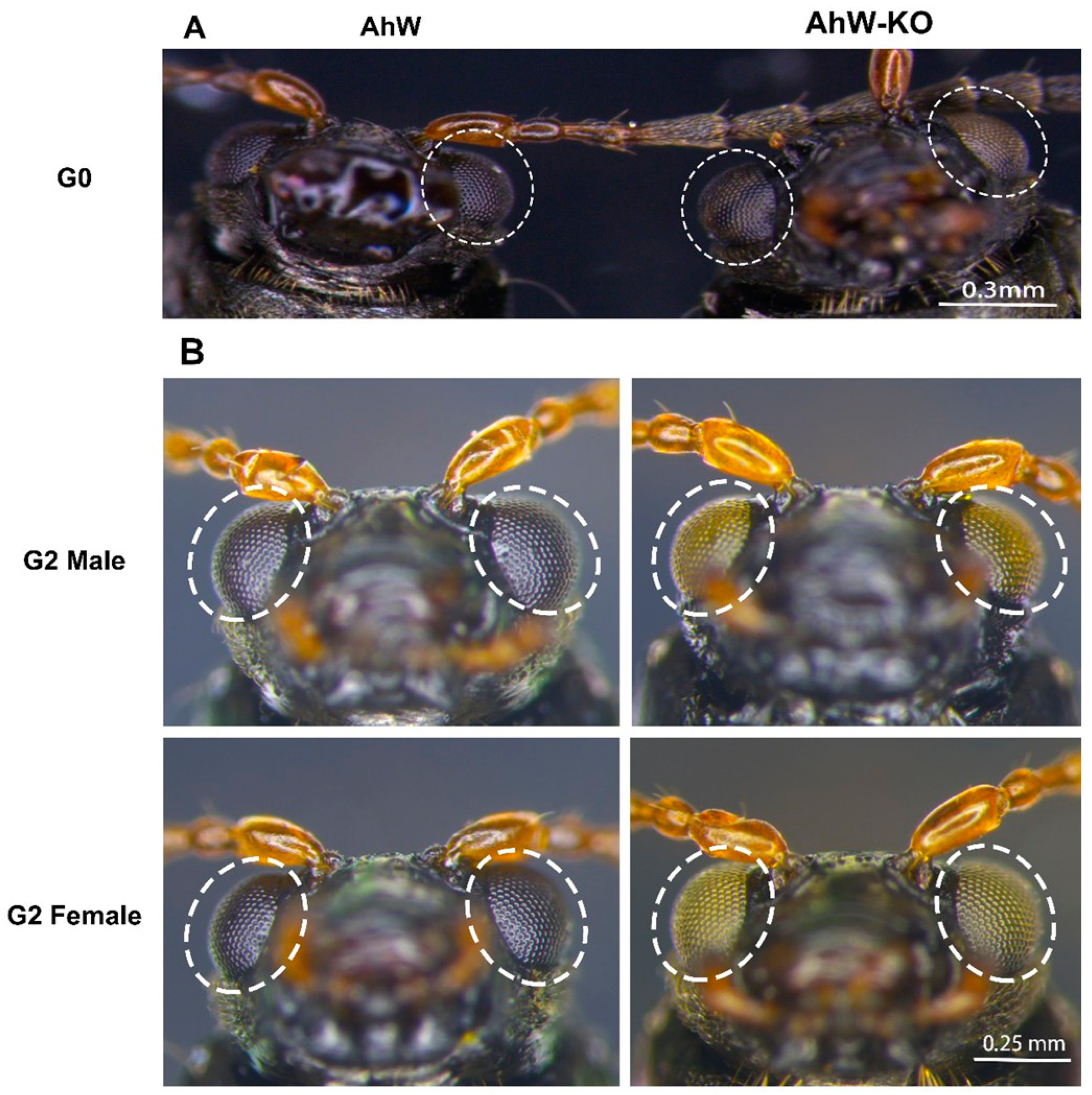
2.2. Creation of an AhW Knockout White-Eyed Strain
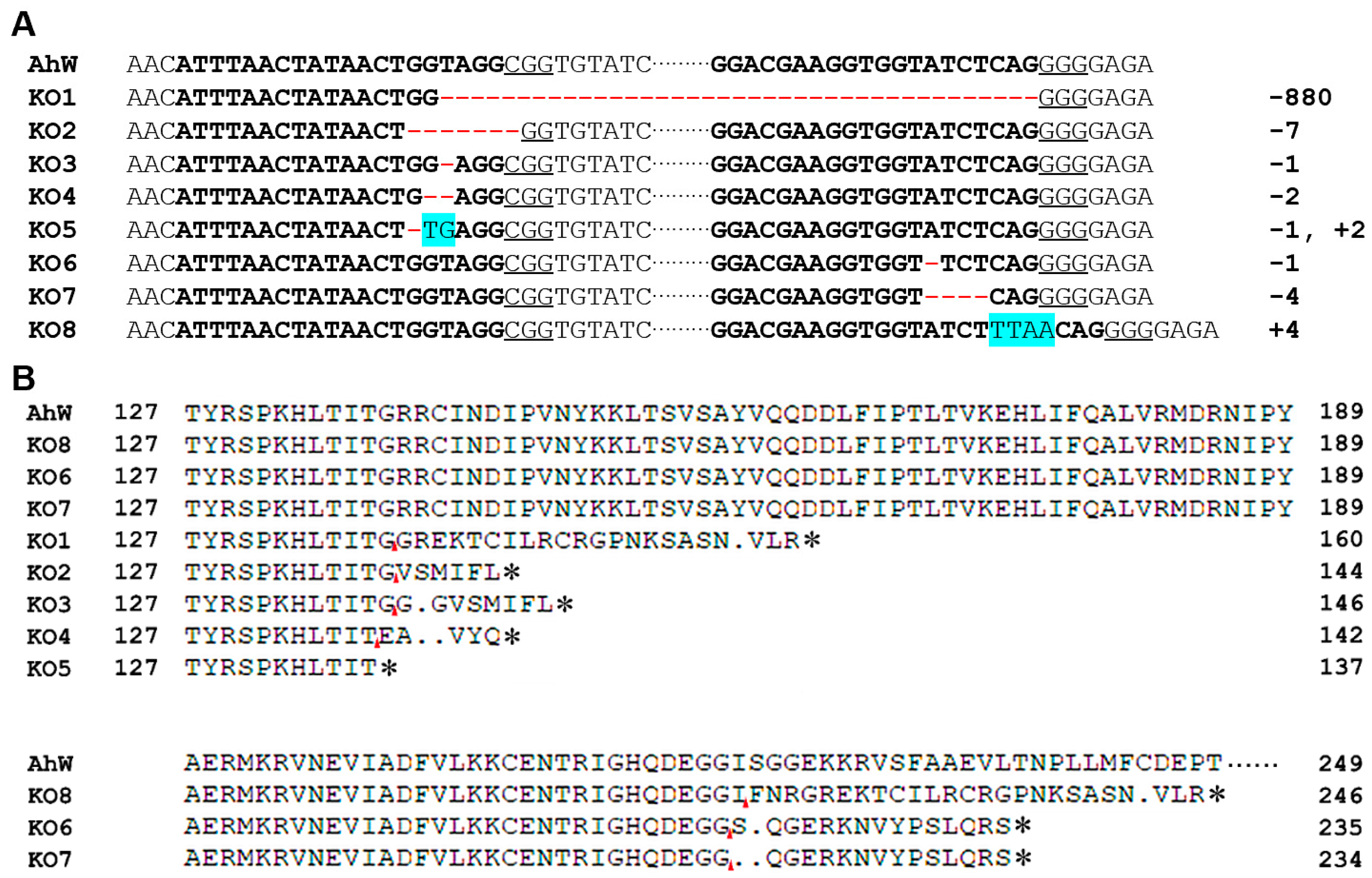
2.3. Mutations in the Two sgRNA Target Sites in AhW gDNA from the AhW-KO Strain
2.4. Inheritance of Cas9-Introduced White Eye Mutations
2.5. Time Course of AhW Expression and Blackening of Eyes
3. Discussion
4. Materials and Methods
4.1. Insects
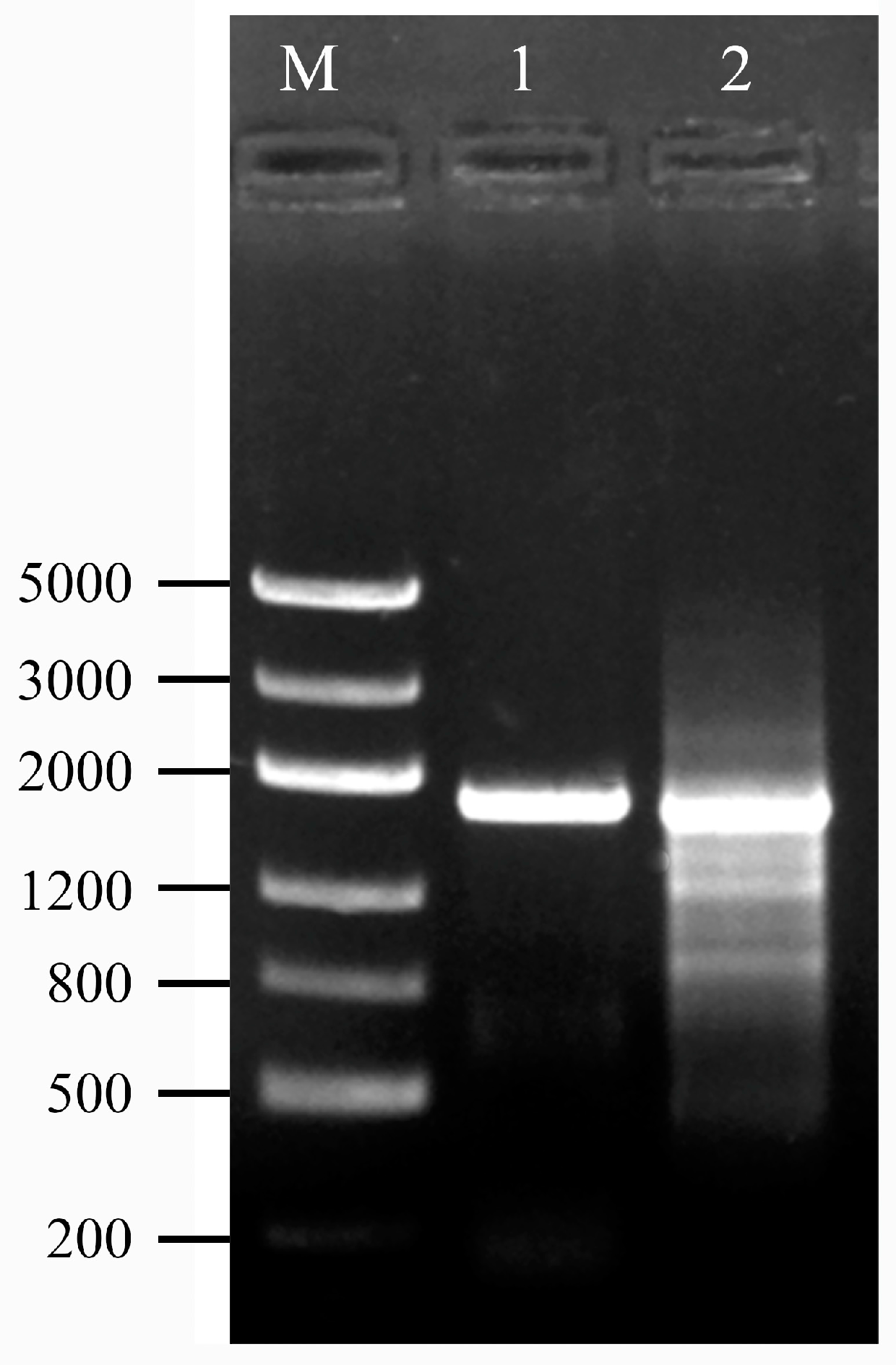
4.2. Sequence Amplification and Analysis
4.3. sgRNA Design and Synthesis
4.4. Embryonic Microinjection
4.5. Mutagenesis Analysis
4.6. Reverse Transcription Quantitative PCR (RT-qPCR)
4.7. Eye Pigment Observation
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sander, J.D.; Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–355. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Wang, J.Y.; Doudna, J.A. CRISPR technology: A decade of genome editing is only the beginning. Science 2023, 379, eadd8643. [Google Scholar] [CrossRef] [PubMed]
- Gilles, A.F.; Schinko, J.B.; Averof, M. Efficient CRISPR-mediated gene targeting and transgene replacement in the beetle Tribolium castaneum. Development 2015, 142, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Gui, S.; Taning, C.N.T.; Wei, D.; Smagghe, G. First report on CRISPR/Cas9-targeted mutagenesis in the Colorado potato beetle, Leptinotarsa decemlineata. J. Insect Physiol. 2020, 121, 104013. [Google Scholar] [CrossRef]
- Chu, F.C.; Wu, P.S.; Pinzi, S.; Grubbs, N.; Lorenzen, M.D. Microinjection of western corn rootworm, Diabrotica virgifera virgifera, embryos for germline transformation, or CRISPR/Cas9 genome editing. J. Vis. Exp. 2018, 27, 57497. [Google Scholar] [CrossRef]
- Wu, M.M.; Chen, X.; Xu, Q.X.; Zang, L.S.; Wang, S.; Li, M.; Xiao, D. Melanin synthesis pathway Interruption: CRISPR/Cas9-mediated knockout of dopa decarboxylase (DDC) in Harmonia axyridis (Coleoptera: Coccinellidae). J. Insect Sci. 2022, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, S.M.; Brooker, M.R.; Gill, T.R.; Cox, G.B.; Howells, A.J.; Ewart, G.D. Mutations in the white gene of Drosophila melanogaster affecting ABC transporters that determine eye colouration. Biochim. Biophys. Acta 1999, 1419, 173–185. [Google Scholar] [CrossRef]
- Mackenzie, S.M.; Howells, A.J.; Cox, G.B.; Ewart, G.D. Sub-cellular localisation of the white/scarlet ABC transporter to pigment granule membranes within the compound eye of Drosophila melanogaster. Genetica 2000, 108, 239–252. [Google Scholar] [CrossRef]
- Ewart, G.D.; Howells, A.J. ABC transporters involved in transport of eye pigment precursors in Drosophila melanogaster. Methods Enzymol. 1998, 292, 213–224. [Google Scholar] [CrossRef]
- Dreesen, T.D.; Johnson, D.H.; Henikoff, S. The brown protein of Drosophila melanogaster is similar to the white protein and to components of active transport complexes. Mol. Cell. Biol. 1988, 8, 5206–5215. [Google Scholar] [CrossRef] [PubMed]
- Howells, A.J. Isolation and biochemical analysis of a temperature-sensitive scarlet eye color mutant of Drosophila melanogaster. Biochem. Genet. 1979, 17, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.H.; Tsai, C.J.; Green, M.M.; Chao, J.L.; Yu, C.T.; Jaw, T.J.; Yeh, J.Y.; Bolshakov, V.N. White as a reporter gene to detect transcriptional silencers specifying position-specific gene expression during Drosophila melanogaster eye development. Genetics 1995, 141, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Siegal, M.L.; Hartl, D.L. Application of Cre/loxP in Drosophila. Site-specific recombination and transgene coplacement. Methods Mol. Biol. 2000, 136, 487–495. [Google Scholar] [CrossRef]
- Voutev, R.; Mann, R.S. Robust ΦC31-mediated genome engineering in Drosophila melanogaster using minimal attP/attB phage sites. G3 2018, 8, 1399–1402. [Google Scholar] [CrossRef]
- Oberstein, A.; Pare, A.; Kaplan, L.; Small, S. Site-specific transgenesis by Cre-mediated recombination in Drosophila. Nat. Methods 2005, 2, 583–585. [Google Scholar] [CrossRef]
- Grubbs, N.; Haas, S.; Beeman, R.W.; Lorenzen, M.D. The ABCs of eye color in Tribolium castaneum: Orthologs of the Drosophila white, scarlet, and brown Genes. Genetics 2015, 199, 749–759. [Google Scholar] [CrossRef]
- Khan, S.A.; Reichelt, M.; Heckel, D.G. Functional analysis of the ABCs of eye color in Helicoverpa armigera with CRISPR/Cas9-induced mutations. Sci. Rep. 2017, 7, 40025. [Google Scholar] [CrossRef]
- Xue, W.H.; Xu, N.; Yuan, X.B.; Chen, H.H.; Zhang, J.L.; Fu, S.J.; Zhang, C.X.; Xu, H.J. CRISPR/Cas9-mediated knockout of two eye pigmentation genes in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Insect Biochem. Mol. Biol. 2018, 93, 19–26. [Google Scholar] [CrossRef]
- Shi, M.; Fu, J. Alligator Weed Alternanthera philoxeroides (Mart.) Griseb. In Biological Invasions and Its Management in China; Wan, F., Jiang, M., Zhan, A., Eds.; Springer: Singapore, 2017; Volume 2, pp. 163–173. [Google Scholar]
- Zhang, H.; Liu, Y.; Jin, J.; Zhou, Z.; Guo, J. Identification and characterization of the vitellogenin receptor gene and its role in reproduction in the alligatorweed flea beetle, Agasicles hygrophila. Front. Physiol. 2019, 10, 969. [Google Scholar] [CrossRef]
- Shirai, Y.; Piulachs, M.D.; Belles, X.; Daimon, T. DIPA-CRISPR is a simple and accessible method for insect gene editing. Cell Rep. Methods 2022, 2, 100215. [Google Scholar] [CrossRef] [PubMed]
- Adrianos, S.; Lorenzen, M.; Oppert, B. Metabolic pathway interruption: CRISPR/Cas9-mediated knockout of tryptophan 2,3-dioxygenase in Tribolium castaneum. J. Insect Physiol. 2018, 107, 104–109. [Google Scholar] [CrossRef]
- Reding, K.; Lê, M.; Pick, L. Genome editing of the vermilion locus generates a visible eye color marker for Oncopeltus fasciatus. Sci. Rep. 2023, 13, 4188. [Google Scholar] [CrossRef]
- Yan, Y.; Ziemek, J.; Schetelig, M.F. CRISPR/Cas9 mediated disruption of the white gene leads to pigmentation deficiency and copulation failure in Drosophila suzukii. J. Insect Physiol. 2020, 126, 104091. [Google Scholar] [CrossRef]
- Chaverra-Rodriguez, D.; Macias, V.M.; Hughes, G.L.; Pujhari, S.; Suzuki, Y.; Peterson, D.R.; Kim, D.; McKeand, S.; Rasgon, J.L. Targeted delivery of CRISPR-Cas9 ribonucleoprotein into arthropod ovaries for heritable germline gene editing. Nat. Commun. 2018, 9, 3008. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Daimon, T. Mutations in cardinal are responsible for the red-1 and peach eye color mutants of the red flour beetle Tribolium castaneum. Biochem. Biophys. Res. Commun. 2020, 529, 372–378. [Google Scholar] [CrossRef]
- Heryanto, C.; Mazo-Vargas, A.; Martin, A. Efficient hyperactive piggyBac transgenesis in Plodia pantry moths. Front. Genome Ed. 2022, 4, 1074888. [Google Scholar] [CrossRef] [PubMed]
- Berghammer, A.J.; Klingler, M.A.; Wimmer, E. A universal marker for transgenic insects. Nature 1999, 402, 370–371. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.B. Gal4 system in Drosophila: A fly geneticist’s swiss army knife. Genesis 2002, 34, 1–15. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Concordet, J.-P.; Haeussler, M. CRISPOR: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018, 46, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Wang, Y.; Liu, Y.; Hu, J.; Guo, Y.; Gao, L.; Ma, R. SMRT sequencing of full-length transcriptome of flea beetle Agasicles hygrophila (Selman and Vogt). Sci. Rep. 2018, 8, 2197. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O.; et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Kistler, K.E.; Vosshall, L.B.; Matthews, B.J. Genome engineering with CRISPR-Cas9 in the mosquito Aedes aegypti. Cell Rep. 2015, 11, 51–60. [Google Scholar] [CrossRef]


| Gene | Total Score | Query Covery | E Value | Identity |
|---|---|---|---|---|
| Dm-white | 694 | 100% | 0 | 49.35% |
| Dm-scarlet | 399 | 85% | 5 × 10−130 | 36.12% |
| Dm-brown | 250 | 86% | 2 × 10−73 | 27.26% |
| Generations | Parents | Total Adult Progeny | Female Progeny | Male Progeny | Females with Black Eyes | Females with White Eyes | Males with Black Eyes | Males with White Eyes | Black/White Ratio for All Progeny | Black/White Ratio for Females | Black/White Ratio for Males |
|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 hybrid | 1 ♀AhW × 1 ♂AhW-KO | 83 | 44 | 39 | 44 | 0 | 39 | 0 | 1.0:0 | 1.0:0 | 1.0:0 |
| G1′ hybrid | 1 ♀AhW-KO × 1 ♂AhW | 72 | 38 | 34 | 38 | 0 | 0 | 34 | 1.1:1 | 1:0 | 0:1.0 |
| Self-inbred G2 | 44 ♀G1 × 39 ♂ G1 | 486 | 232 | 254 | 232 | 0 | 130 | 124 | 2.92:1 | 1:0 | 1.05:1.0 |
| Self-inbred G2′ | 38 ♀G1′ × 34 ♂ G1′ | 433 | 226 | 207 | 115 | 111 | 108 | 99 | 1.06:1.0 | 1.04:1.0 | 1.09:1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, L.; Li, P.; Rui, Z.; Sun, J.; Yang, J.; Wang, Y.; Jia, D.; Hu, J.; Li, X.; Ma, R. CRISPR/Cas9-Mediated Knockout of the White Gene in Agasicles hygrophila. Int. J. Mol. Sci. 2025, 26, 4586. https://doi.org/10.3390/ijms26104586
Fu L, Li P, Rui Z, Sun J, Yang J, Wang Y, Jia D, Hu J, Li X, Ma R. CRISPR/Cas9-Mediated Knockout of the White Gene in Agasicles hygrophila. International Journal of Molecular Sciences. 2025; 26(10):4586. https://doi.org/10.3390/ijms26104586
Chicago/Turabian StyleFu, Li, Penghui Li, Zhiyi Rui, Jiang Sun, Jun Yang, Yuanxin Wang, Dong Jia, Jun Hu, Xianchun Li, and Ruiyan Ma. 2025. "CRISPR/Cas9-Mediated Knockout of the White Gene in Agasicles hygrophila" International Journal of Molecular Sciences 26, no. 10: 4586. https://doi.org/10.3390/ijms26104586
APA StyleFu, L., Li, P., Rui, Z., Sun, J., Yang, J., Wang, Y., Jia, D., Hu, J., Li, X., & Ma, R. (2025). CRISPR/Cas9-Mediated Knockout of the White Gene in Agasicles hygrophila. International Journal of Molecular Sciences, 26(10), 4586. https://doi.org/10.3390/ijms26104586







