In Silico Screening and Identification of Functional Peptides from Yak Bone Collagen Hydrolysates: Antioxidant and Osteoblastic Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening Antioxidant Peptides from Yak Bone Collagen Hydrolysates
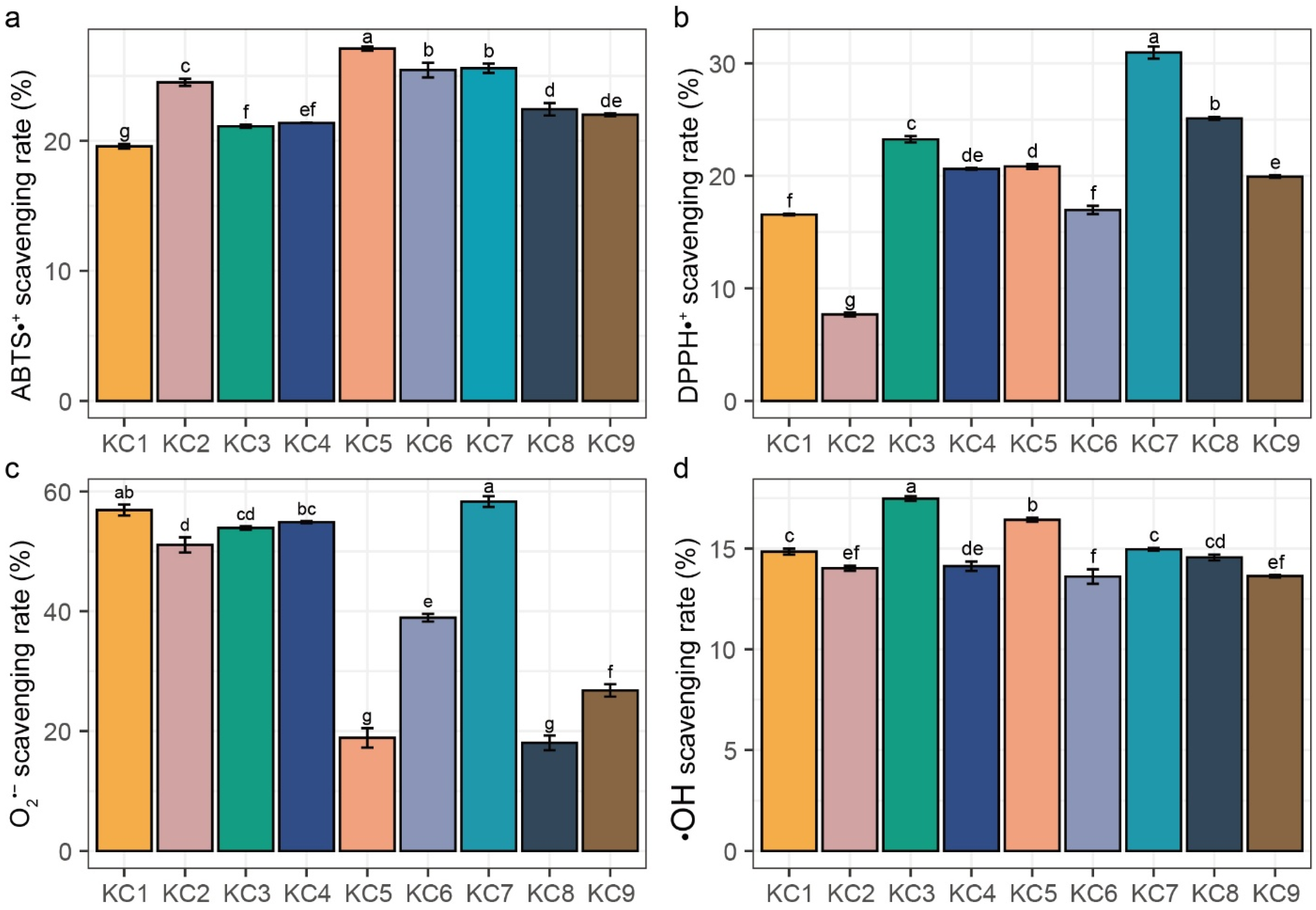
2.2. In Vitro Antioxidant Activity Assays
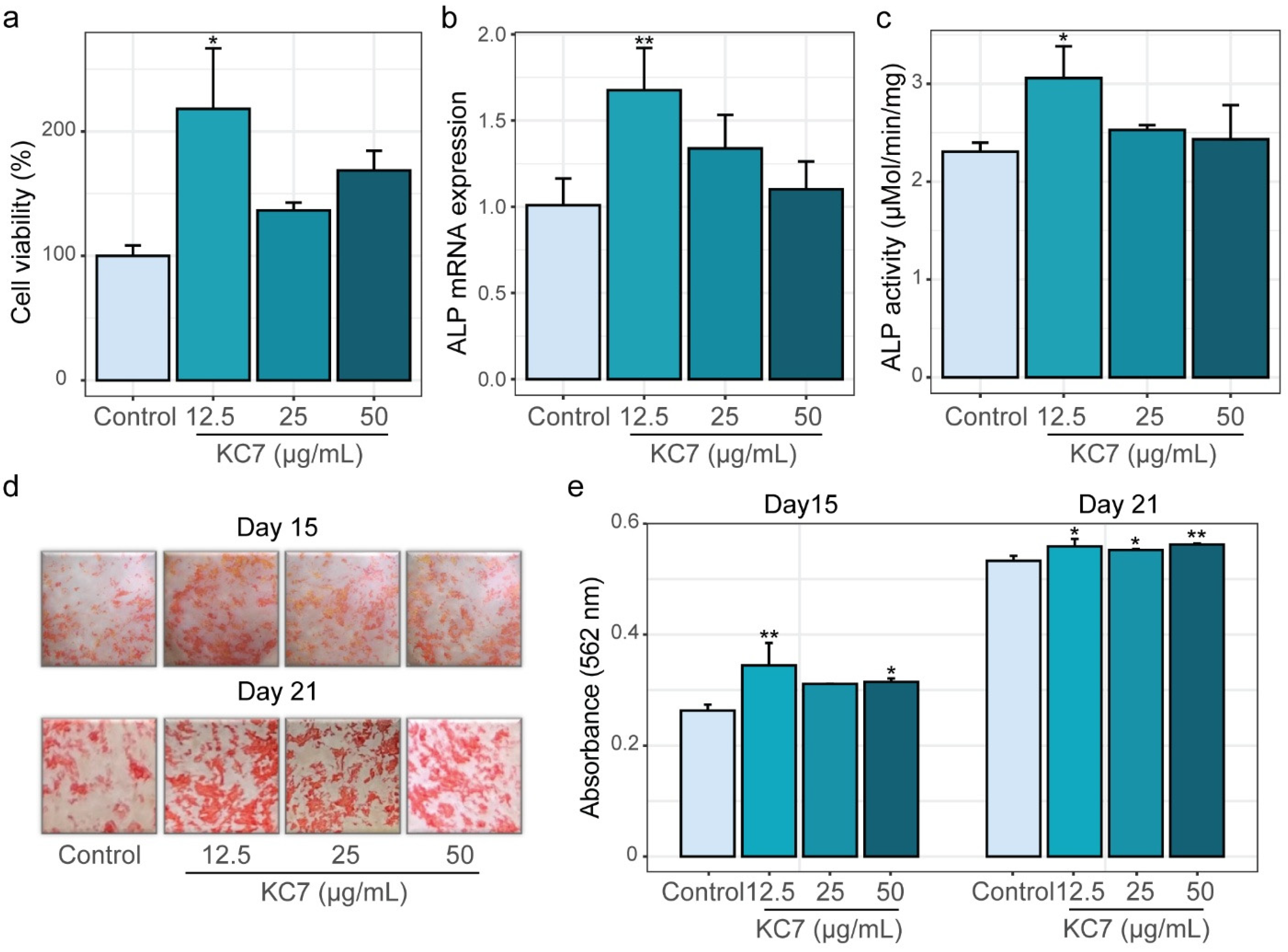
2.3. Osteogenic Effects of Peptide KC7 on MC3T3-E1 Cells
2.3.1. Assessment of Proliferation, ALP Activity, and Mineralization Capacity
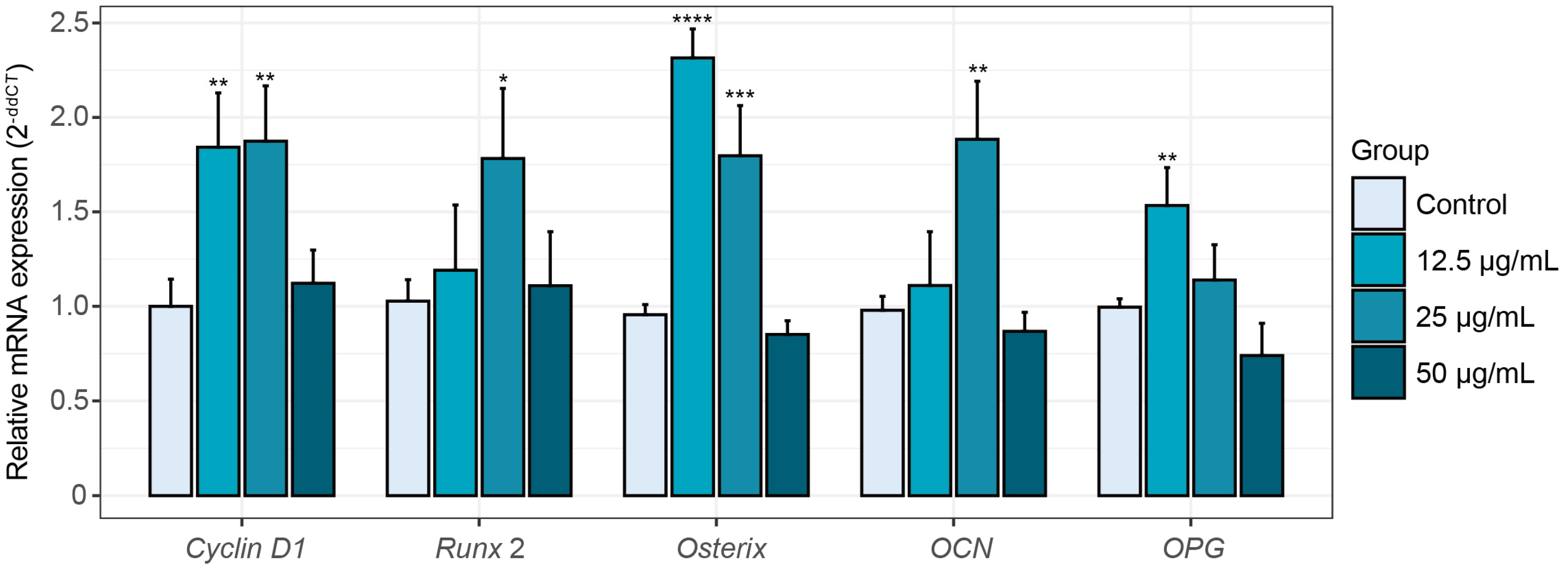
2.3.2. Alterations in Osteogenesis-Related Gene Expression Induced by Peptide KC7
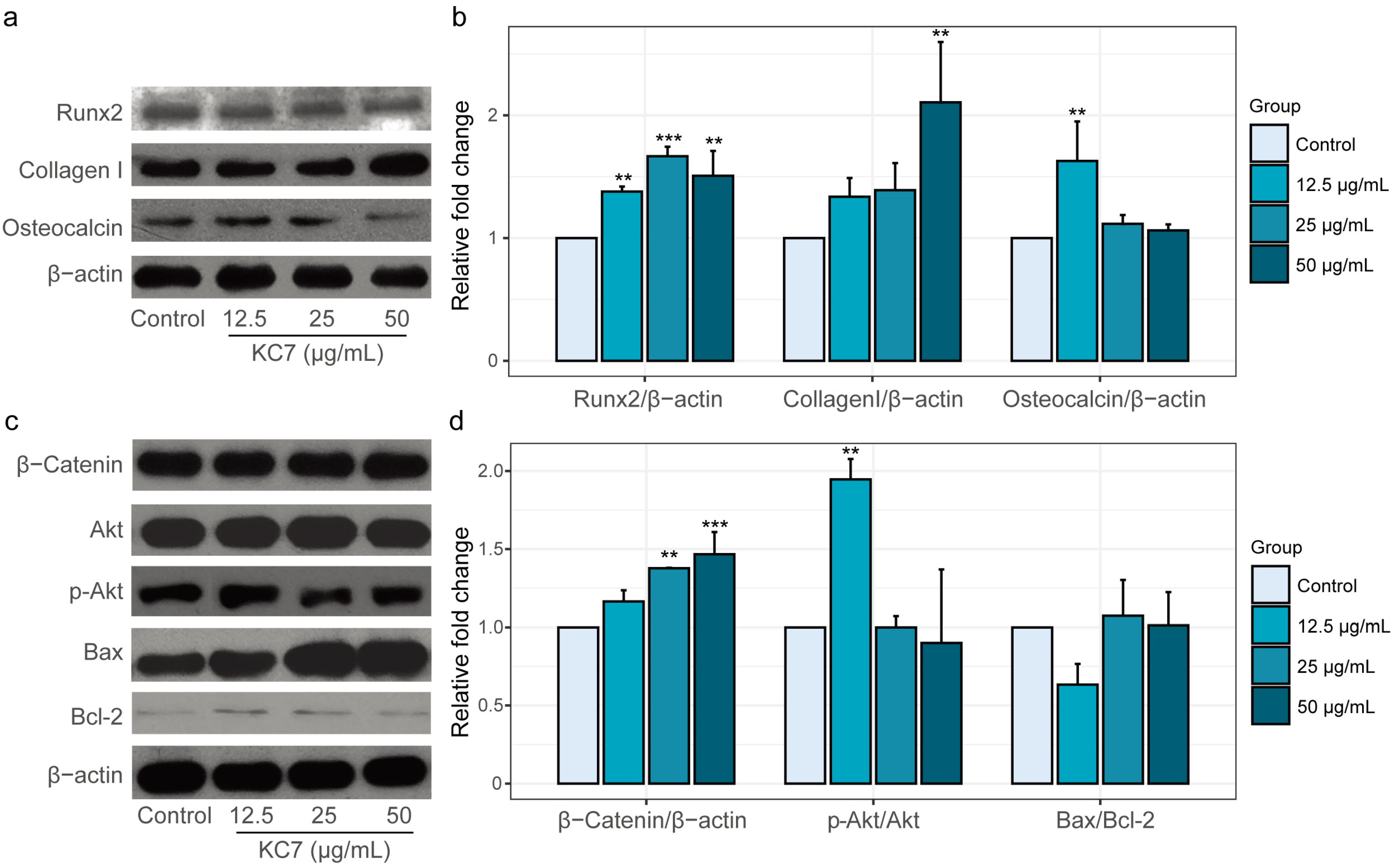
2.3.3. Changes in Osteogenesis-Related Protein Expression Mediated by Peptide KC7
3. Materials and Methods
3.1. Molecular Docking Analysis
3.2. In Vitro Antioxidant Activity Assay
3.3. MC3T3-E1 Cell Culture
3.4. Cell Viability Assay
3.5. Alkaline Phosphatase (ALP) Assay
3.6. Mineralization Assay
3.7. RNA Isolation and Real-Time Quantitative PCR
3.8. Western Blotting Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, M.I.; Li, Y.; Pan, J.; Liu, F.; Dai, H.; Fu, Y.; Huang, T.; Farooq, S.; Zhang, H. Collagen and Gelatin: Structure, Properties, and Applications in Food Industry. Int. J. Biol. Macromol. 2024, 254, 128037. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, Z.; Ge, C.; Wu, Y. Animal By-Products Collagen and Derived Peptide, as Important Components of Innovative Sustainable Food Systems—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8703–8727. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.-Y.; Zhou, J.; Liu, G.-G.; Zhang, M.-Y.; Li, X.-Z.; Wang, Y. Anti-Inflammatory Activity of Collagen Peptide in Vitro and Its Effect on Improving Ulcerative Colitis. NPJ Sci. Food 2025, 9, 1. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Quan, Z.; Xiao, P.; Duan, J.-A. New Insights into Antioxidant Peptides: An Overview of Efficient Screening, Evaluation Models, Molecular Mechanisms, and Applications. Antioxidants 2024, 13, 203. [Google Scholar] [CrossRef]
- Delikanlı Kıyak, B.; İnan Çınkır, N.; Çelebi, Y.; Durgut Malçok, S.; Çalışkan Koç, G.; Adal, S.; Yüksel, A.N.; Süfer, Ö.; Özkan Karabacak, A.; Ramniwas, S.; et al. Advanced Technologies for the Collagen Extraction from Food Waste—A Review on Recent Progress. Microchem. J. 2024, 201, 110404. [Google Scholar] [CrossRef]
- Collagen Peptides Market Size, Share & Growth Report 2030. Available online: https://www.grandviewresearch.com/industry-analysis/collagen-peptides-market-report (accessed on 17 February 2025).
- König, D.; Oesser, S.; Scharla, S.; Zdzieblik, D.; Gollhofer, A. Specific Collagen Peptides Improve Bone Mineral Density and Bone Markers in Postmenopausal Women-A Randomized Controlled Study. Nutrients 2018, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef]
- Iantomasi, T.; Romagnoli, C.; Palmini, G.; Donati, S.; Falsetti, I.; Miglietta, F.; Aurilia, C.; Marini, F.; Giusti, F.; Brandi, M.L. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with microRNAs. Int. J. Mol. Sci. 2023, 24, 3772. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Li, J.; Hu, J.; Yang, K.; Tao, L. Oxidative Stress: A Common Pathological State in a High-Risk Population for Osteoporosis. Biomed. Pharmacother. 2023, 163, 114834. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Liu, J.; Wang, H. Antioxidant Scaffolds for Enhanced Bone Regeneration: Recent Advances and Challenges. BioMedical Eng. 2025, 24, 41. [Google Scholar] [CrossRef]
- Hong, H.; Fan, H.; Chalamaiah, M.; Wu, J. Preparation of Low-Molecular-Weight, Collagen Hydrolysates (Peptides): Current Progress, Challenges, and Future Perspectives. Food Chem. 2019, 301, 125222. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hou, H.; Li, Y.; Tang, W.; Gao, D.; Liu, Z.; Gao, X.; Zhao, F.; Sun, F.; Tan, H.; et al. Isolation, Purification, and Antiosteoporosis Activity of Donkey Bone Collagen from Discarded Bone and Its Antioxidant Peptides. Heliyon 2024, 10, e23531. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, Y.; Liu, Y.; Zhang, C.; Huang, F.; Chen, L. Identification of Di/Tripeptide(s) With Osteoblasts Proliferation Stimulation Abilities of Yak Bone Collagen by in Silico Screening and Molecular Docking. Front. Nutr. 2022, 9, 874259. [Google Scholar] [CrossRef]
- Hassan, M.T.; Tayara, H.; Chong, K.T. iAnOxPep: A Machine Learning Model for the Identification of Anti-Oxidative Peptides Using Ensemble Learning. IEEE/ACM Trans. Comput. Biol. Bioinform. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Wang, B.; Lin, P.; Zhong, Y.; Tan, X.; Shen, Y.; Huang, Y.; Jin, K.; Zhang, Y.; Zhan, Y.; Shen, D.; et al. Explainable Deep Learning and Virtual Evolution Identifies Antimicrobial Peptides with Activity against Multidrug-Resistant Human Pathogens. Nat. Microbiol. 2025, 10, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, X.; Liu, Y.; Zheng, Z. High-Accuracy Identification and Structure–Activity Analysis of Antioxidant Peptides via Deep Learning and Quantum Chemistry. J. Chem. Inf. Model. 2025, 65, 603–612. [Google Scholar] [CrossRef]
- Xu, B.; Dong, Q.; Yu, C.; Chen, H.; Zhao, Y.; Zhang, B.; Yu, P.; Chen, M. Advances in Research on the Activity Evaluation, Mechanism and Structure-Activity Relationships of Natural Antioxidant Peptides. Antioxidants 2024, 13, 479. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Han, Y.; Sun, Y.; Zhang, T.; Tu, M.; Du, L.; Pan, D. Preparation and Characterization of Duck Liver-Derived Antioxidant Peptides Based on LC-MS/MS, Molecular Docking, and Machine Learning. LWT 2023, 175, 114479. [Google Scholar] [CrossRef]
- Hesamzadeh, P.; Seif, A.; Mahmoudzadeh, K.; Ganjali Koli, M.; Mostafazadeh, A.; Nayeri, K.; Mirjafary, Z.; Saeidian, H. De Novo Antioxidant Peptide Design via Machine Learning and DFT Studies. Sci. Rep. 2024, 14, 6473. [Google Scholar] [CrossRef]
- Song, Y.; Fu, Y.; Huang, S.; Liao, L.; Wu, Q.; Wang, Y.; Ge, F.; Fang, B. Identification and Antioxidant Activity of Bovine Bone Collagen-Derived Novel Peptides Prepared by Recombinant Collagenase from Bacillus Cereus. Food Chem. 2021, 349, 129143. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Wang, X.; Wang, Y.; Liao, L.; Zhang, Y.; Fang, B.; Fu, Y. Novel Antioxidant Peptides from Yak Bones Collagen Enhanced the Capacities of Antiaging and Antioxidant in Caenorhabditis elegans. J. Funct. Foods 2022, 89, 104933. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhuang, X.; Zhang, Y.; Fang, B.; Fu, Y. Unraveling the Osteogenic Activity and Molecular Mechanism of an Antioxidant Collagen Peptide in MC3T3-E1 Cells. Nutrients 2025, 17, 824. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Deng, W.; Chen, Y.; Sun, X.; Wang, L. AODB: A Comprehensive Database for Antioxidants Including Small Molecules, Peptides and Proteins. Food Chem. 2023, 418, 135992. [Google Scholar] [CrossRef]
- Qin, D.; Jiao, L.; Wang, R.; Zhao, Y.; Hao, Y.; Liang, G. Prediction of Antioxidant Peptides Using a Quantitative Structure−activity Relationship Predictor (AnOxPP) Based on Bidirectional Long Short-Term Memory Neural Network and Interpretable Amino Acid Descriptors. Comput. Biol. Med. 2023, 154, 106591. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.H.; Yesiltas, B.; Marin, F.I.; Pertseva, M.; García-Moreno, P.J.; Gregersen, S.; Overgaard, M.T.; Jacobsen, C.; Lund, O.; Hansen, E.B.; et al. AnOxPePred: Using Deep Learning for the Prediction of Antioxidative Properties of Peptides. Sci. Rep. 2020, 10, 21471. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef]
- Obeme-Nmom, J.I.; Abioye, R.O.; Flores, S.S.R.; Udenigwe, C.C. Regulation of Redox Enzymes by Nutraceuticals: A Review of the Roles of Antioxidant Polyphenols and Peptides. Food Funct. 2024, 15, 10956–10980. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bhullar, K.S.; Fu, J.; Chen, B.; Liu, H.; Su, D.; Wu, S.; He, H.; Wang, Q.; Qiao, Y.; et al. Unraveling Novel Antioxidant Peptides from Asian Swamp Eel: Identification, in Silico Selection, and Mechanistic Insights through Quantum Chemical Calculation and Molecular Docking. Food Chem. 2025, 464, 141668. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Trivedi, S.; Srivastava, K.; Gupta, A.; Saluja, T.S.; Kumar, S.; Mehrotra, D.; Singh, S.K. A Quantitative Method to Determine Osteogenic Differentiation Aptness of Scaffold. J. Oral Biol. Craniofacial Res. 2020, 10, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Bonewald, L.F. The Osteocyte: New Insights. Annu. Rev. Physiol. 2020, 82, 485–506. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.; Wesner, A.A.; Abuhattab, M.; Kutty, R.G.; Premnath, P. Cell Cycle Regulators and Bone: Development and Regeneration. Cell Biosci. 2023, 13, 35. [Google Scholar] [CrossRef]
- Glowacki, J.; Rey, C.; Glimcher, M.J.; Cox, K.A.; Lian, J. A Role for Osteocalcin in Osteoclast Differentiation. J. Cell. Biochem. 1991, 45, 292–302. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast Differentiation by RANKL and OPG Signaling Pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Sekaran, S.; Dhanasekaran, A.; Warrier, S. Type 1 Collagen: Synthesis, Structure and Key Functions in Bone Mineralization. Differentiation 2024, 136, 100757. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, W.; Masson, A.; Li, Y.-P. Cell Signaling and Transcriptional Regulation of Osteoblast Lineage Commitment, Differentiation, Bone Formation, and Homeostasis. Cell Discov. 2024, 10, 71. [Google Scholar] [CrossRef]
- McGonnell, I.M.; Grigoriadis, A.E.; Lam, E.W.-F.; Price, J.S.; Sunters, A. A Specific Role for Phosphoinositide 3-Kinase and AKT in Osteoblasts? Front. Endocrinol. 2012, 3, 88. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT Pathway as a Key Link Modulates the Multidrug Resistance of Cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
- Vaskivuo, T.E.; Stenbäck, F.; Tapanainen, J.S. Apoptosis and Apoptosis-Related Factors Bcl-2, Bax, Tumor Necrosis Factor-Alpha, and NF-kappaB in Human Endometrial Hyperplasia and Carcinoma. Cancer 2002, 95, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Kma, L.; Baruah, T.J. The Interplay of ROS and the PI3K/Akt Pathway in Autophagy Regulation. Biotechnol. Appl. Biochem. 2022, 69, 248–264. [Google Scholar] [CrossRef]
- Deng, S.; Dai, G.; Chen, S.; Nie, Z.; Zhou, J.; Fang, H.; Peng, H. Dexamethasone Induces Osteoblast Apoptosis through ROS-PI3K/AKT/GSK3β Signaling Pathway. Biomed. Pharmacother. 2019, 110, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jin, Z.; Xu, Z.; Yang, H.; Li, L.; Li, G.; Li, F.; Gu, S.; Zong, S.; Zhou, J.; et al. Antioxidant Effects of Ginkgolides and Bilobalide against Cerebral Ischemia Injury by Activating the Akt/Nrf2 Pathway in Vitro and in Vivo. Cell Stress Chaperones 2019, 24, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Song, X.-N.; Lin, Y.; Xiao, Q.; Du, X.-P.; Chen, Y.-H.; Xiao, A.-F. Antioxidant Capacity and Prebiotic Effects of Gracilaria Neoagaro Oligosaccharides Prepared by Agarase Hydrolysis. Int. J. Biol. Macromol. 2019, 137, 177–186. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
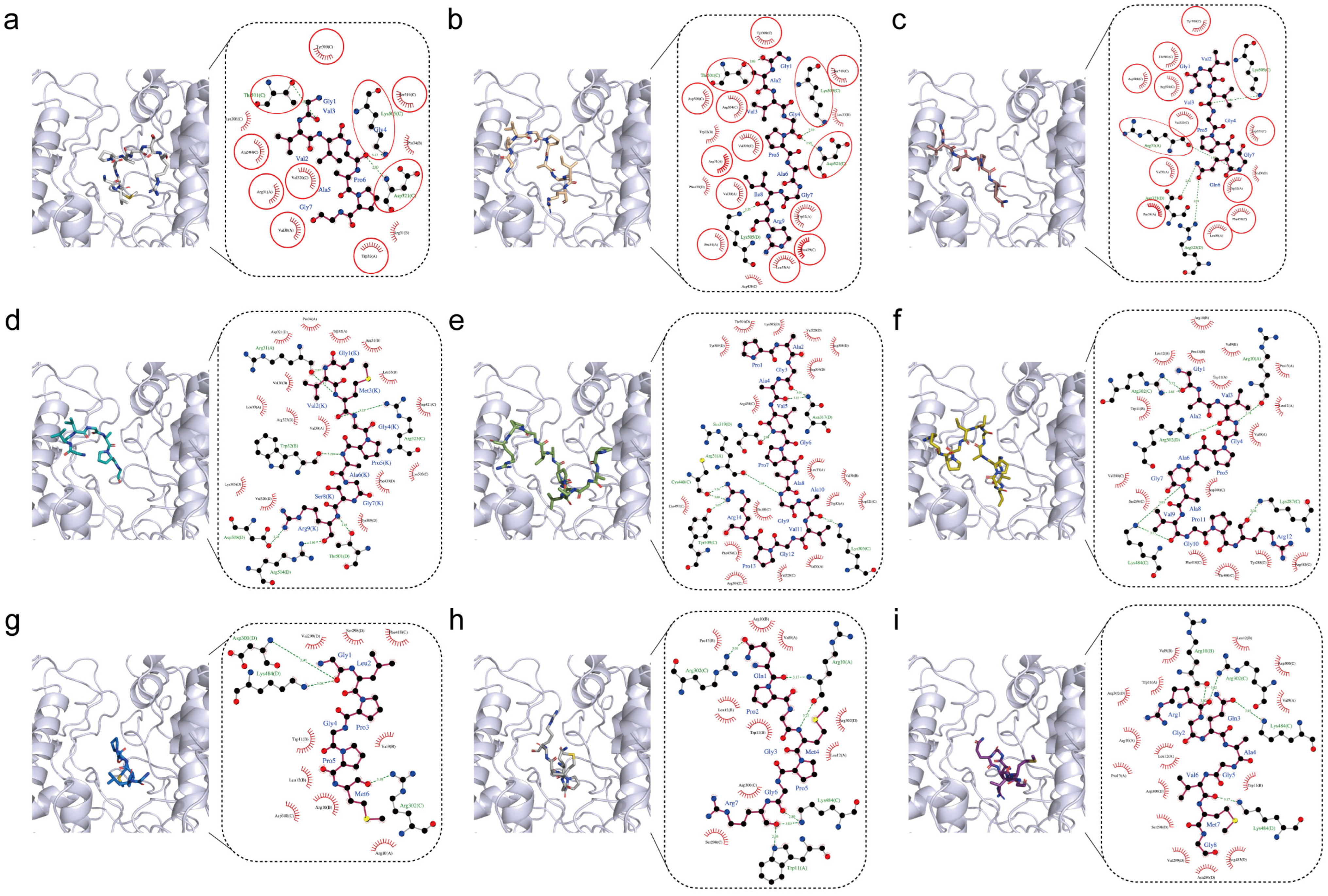
| Name | Peptide Sequence | Molecular Weight | Yak Collagen Resource | AnOxPP | AnOxPePred | AutoDock Vina | ||
|---|---|---|---|---|---|---|---|---|
| Pre-Score (10−2) | Scavenger Score (10−2) | Chelator Score (10−2) | Affinity (kcal/mol) |
Per-Residue Affinity (kcal/mol) | ||||
| KC1 | GVVGAPG | 555.30 | alpha-2(I) | 99.81 | 43.12 | 21.58 | −6.7 | −0.96 |
| KC2 | GAVGPAGIR | 796.46 | alpha-2(I) | 99.99 | 45.10 | 22.69 | −7.2 | −0.80 |
| KC3 | GVVGPQG | 612.32 | alpha-2(I) | 99.80 | 45.22 | 23.80 | −6.9 | −0.99 |
| KC4 | GVMGPAGSR | 830.41 | alpha-2(I) | 99.99 | 46.74 | 21.54 | −7.0 | −0.78 |
| KC5 | PAGAVGPAGAVGPR | 1217.65 | alpha-2(I) | 99.99 | 50.96 | 19.70 | −8.6 | −0.61 |
| KC6 | GAVGPAGAVGPR | 1007.55 | alpha-2(I) | 99.99 | 47.57 | 18.18 | −8.1 | −0.68 |
| KC7 | GLPGPM | 570.71 | alpha-1(XIII) | 99.93 | 52.80 | 29.33 | −6.6 | −1.10 |
| KC8 | QPGMPGR | 782.82 | alpha-1(IV) | 99.98 | 47.04 | 26.20 | −7.2 | −1.03 |
| KC9 | RGQAGVMG | 774.90 | alpha-1(I) | 99.16 | 38.12 | 20.15 | −6.5 | −0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Y.; Fang, B.; Fu, Y. In Silico Screening and Identification of Functional Peptides from Yak Bone Collagen Hydrolysates: Antioxidant and Osteoblastic Activities. Int. J. Mol. Sci. 2025, 26, 4570. https://doi.org/10.3390/ijms26104570
Wang Y, Wang Y, Fang B, Fu Y. In Silico Screening and Identification of Functional Peptides from Yak Bone Collagen Hydrolysates: Antioxidant and Osteoblastic Activities. International Journal of Molecular Sciences. 2025; 26(10):4570. https://doi.org/10.3390/ijms26104570
Chicago/Turabian StyleWang, Yali, Yue Wang, Baishan Fang, and Yousi Fu. 2025. "In Silico Screening and Identification of Functional Peptides from Yak Bone Collagen Hydrolysates: Antioxidant and Osteoblastic Activities" International Journal of Molecular Sciences 26, no. 10: 4570. https://doi.org/10.3390/ijms26104570
APA StyleWang, Y., Wang, Y., Fang, B., & Fu, Y. (2025). In Silico Screening and Identification of Functional Peptides from Yak Bone Collagen Hydrolysates: Antioxidant and Osteoblastic Activities. International Journal of Molecular Sciences, 26(10), 4570. https://doi.org/10.3390/ijms26104570








