Profiling Tight Junction Protein Expression in Brain Vascular Malformations
Abstract
1. Introduction
2. Results
2.1. Demographic Features of the bAVM and CCM Cohort
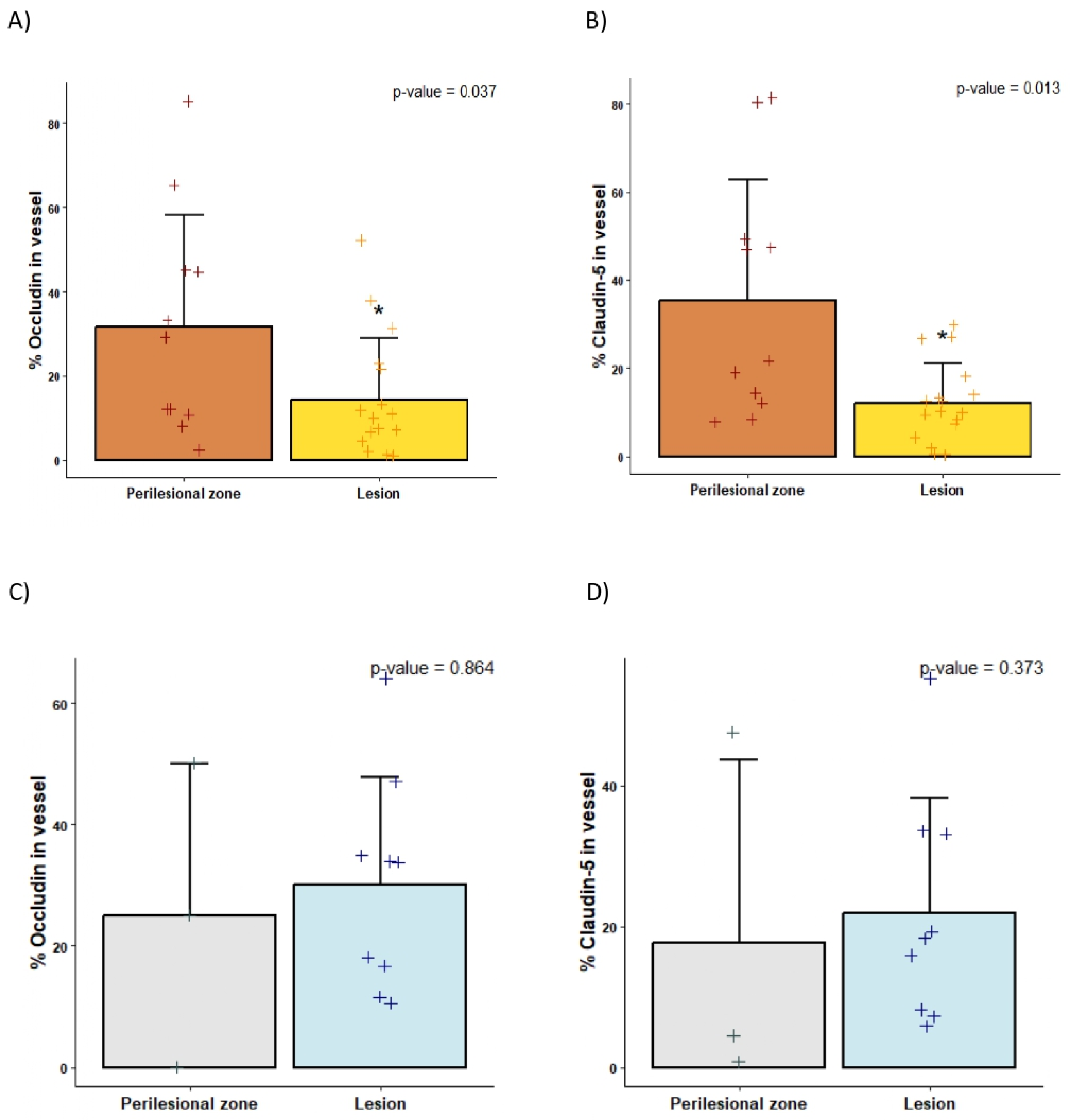
2.2. Decreased Tight Junctions in bAVMs Compared with CCMs
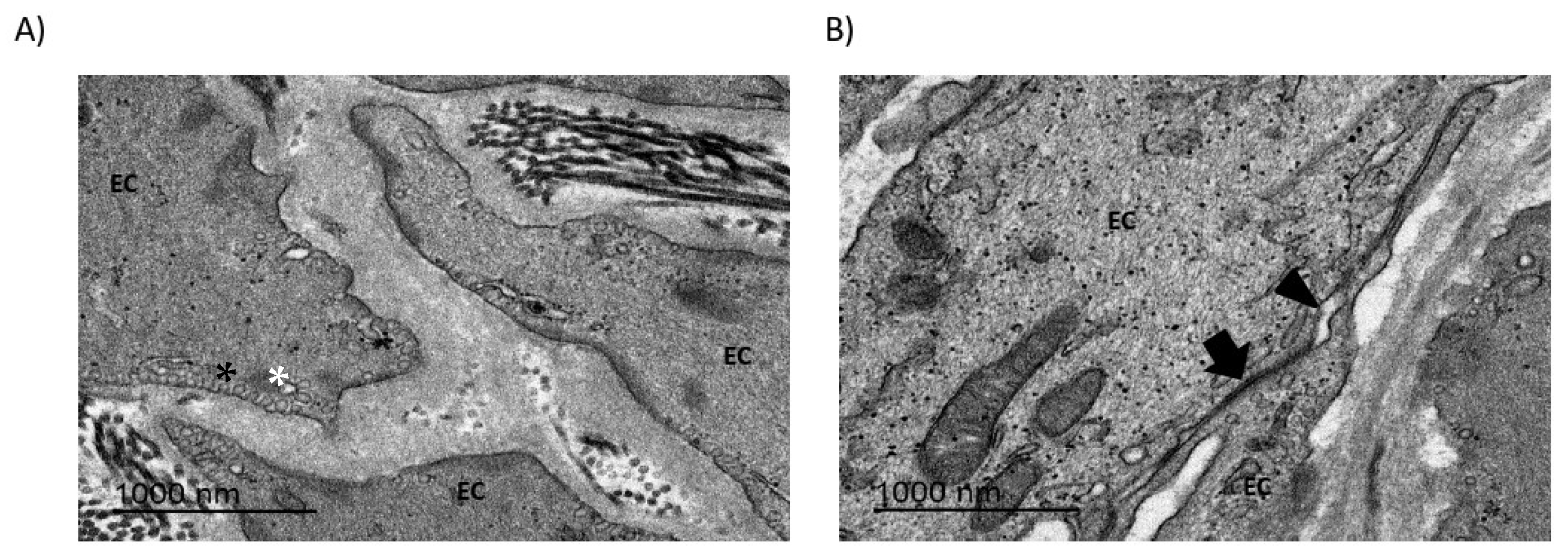
2.3. Endothelial Cell Morphology and Connections Are Disturbed in bAVMs
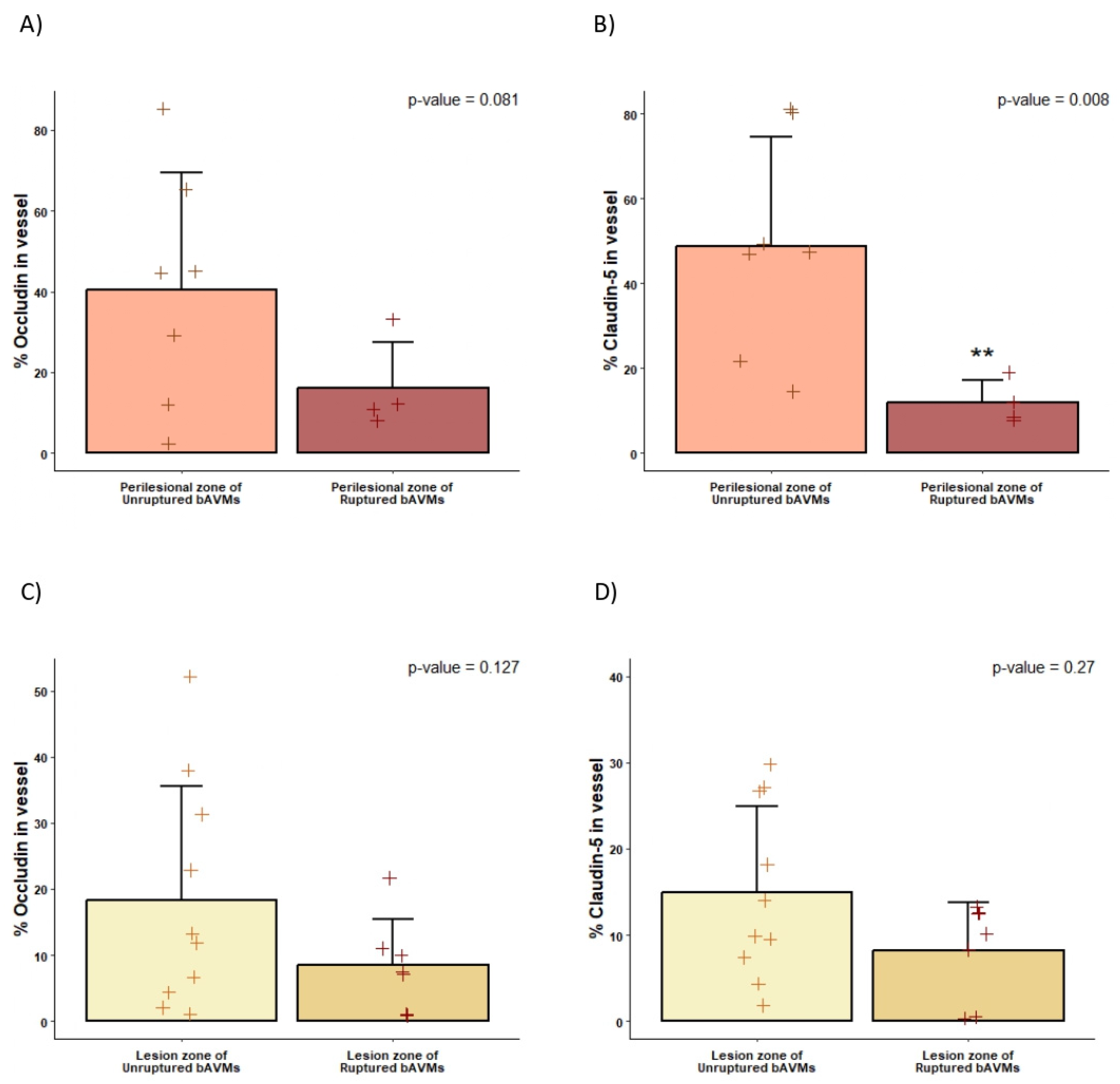
2.4. Differences in the Perinidal Tight Junctions of Ruptured vs. Unruptured bAVMs
3. Discussion
3.1. BBB Disruption in bAVMs
3.2. BBB Disruption Can Be Analyzed via TEM
3.3. Hemorrhage and BBB Disruption
3.4. BBB Disruption in CCMs
3.5. Limitations
4. Materials and Methods
4.1. Patient Selection
4.2. Clinical, Angiographic, and Neuropsychological Evaluation
4.3. Brain Samples
4.4. Immunofluorescence
4.5. Western Blotting
4.6. TEM
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| bAVMs | brain arteriovenous malformations |
| VEGF | vascular endothelial growth factor |
| BBB | blood–brain barrier |
| TJs | tight junctions |
| CCMs | cerebral cavernous malformations |
| SM | Spetzler–Martin |
| mRS | modified Rankin scale |
| ARI | AVM rupture index |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DTT | dithiothreitol |
| TEM | transmission electron microscopy |
| SEM | scanning electron microscopy |
| MMPs | matrix metalloproteinases |
| KO | knockout |
| EC | endothelial cell |
References
- Rutledge, C.; Cooke, D.L.; Hetts, S.W.; Abla, A.A. Brain arteriovenous malformations. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 176, pp. 171–178. [Google Scholar] [CrossRef]
- Mosteiro, A.; Pedrosa, L.; Torne, R.; Rodríguez-Hernández, A.; Amaro, S.; Reyes, L.A.; Hoyos, J.A.; Roman, L.S.; de Riva, N.; Domínguez, C.J.; et al. Venous tortuosity as a novel biomarker of rupture risk in arteriovenous malformations: ARI score. J. Neurointerv. Surg. 2022, 14, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Nornes, H.; Grip, A. Hemodynamic aspects of cerebral arteriovenous malformations. J. Neurosurg. 1980, 53, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Sahlein, D.H.; Mora, P.; Becske, T.; Huang, P.; Jafar, J.J.; Connolly, E.S.; Nelson, P.K. Features predictive of brain arteriovenous malformation hemorrhage: Extrapolation to a physiologic model. Stroke 2014, 45, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.T.; Rutledge, W.C.; Kim, H.; Stapf, C.; Whitehead, K.J.; Li, D.Y.; Krings, T.; Terbrugge, K.; Kondziolka, D.; Morgan, M.K.; et al. Brain arteriovenous malformations. Nat. Rev. Dis. Primers 2015, 1, 15008. [Google Scholar] [CrossRef]
- Cheng, P.; Ma, L.; Shaligram, S.; Walker, E.J.; Yang, S.-T.; Tang, C.; Zhu, W.; Zhan, L.; Li, Q.; Zhu, X.; et al. Effect of elevation of vascular endothelial growth factor level on exacerbation of hemorrhage in mouse brain arteriovenous malformation. J. Neurosurg. 2019, 132, 1566–1573. [Google Scholar] [CrossRef]
- Chen, W.; Guo, Y.; Walker, E.J.; Shen, F.; Jun, K.; Oh, S.P.; Degos, V.; Lawton, M.T.; Tihan, T.; Davalos, D.; et al. Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 305. [Google Scholar] [CrossRef]
- Argaw, A.T.; Gurfein, B.T.; Zhang, Y.; Zameer, A.; John, G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. USA 2009, 106, 1977–1982. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Rhea, E.M.; Banks, W.A. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front. Neurosci. 2019, 13, 521. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914. [Google Scholar] [CrossRef]
- Luissint, A.C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.O. Tight junctions at the blood brain barrier: Physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Keaney, J.; Campbell, M. The dynamic blood-brain barrier. FEBS J. 2015, 282, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Keep, R.F.; Hua, Y.; Xi, G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012, 11, 720–731. [Google Scholar] [CrossRef]
- Jiang, X.; Andjelkovic, A.V.; Zhu, L.; Yang, T.; Bennett, M.V.L.; Chen, J.; Keep, R.F.; Shi, Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018, 163–164, 144–171. [Google Scholar] [CrossRef]
- Keep, R.F.; Andjelkovic, A.V.; Xiang, J.; Stamatovic, S.M.; Antonetti, D.A.; Hua, Y.; Xi, G. Brain endothelial cell junctions after cerebral hemorrhage: Changes, mechanisms and therapeutic targets. J. Cereb. Blood Flow Metab. 2018, 38, 1255–1275. [Google Scholar] [CrossRef]
- Chen, S.P.; Chang, Y.A.; Chou, C.H.; Juan, C.; Lee, H.; Chen, L.; Wu, P.; Wang, Y.; Fuh, J.; Lirng, J.; et al. Circulating microRNAs Associated With Reversible Cerebral Vasoconstriction Syndrome. Ann. Neurol. 2021, 89, 459–473. [Google Scholar] [CrossRef]
- Wu, X.; Fu, S.; Liu, Y.; Luo, H.; Li, F.; Wang, Y.; Gao, M.; Cheng, Y.; Xie, Z. NDP-MSH binding melanocortin-1 receptor ameliorates neuroinflammation and BBB disruption through CREB/Nr4a1/NF-κB pathway after intracerebral hemorrhage in mice. J. Neuroinflamm. 2019, 16, 192. [Google Scholar] [CrossRef]
- Moxon-Emre, I.; Schlichter, L.C. Neutrophil depletion reduces blood-brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. J. Neuropathol. Exp. Neurol. 2011, 70, 218–235. [Google Scholar] [CrossRef]
- Knowland, D.; Arac, A.; Sekiguchi, K.J.; Hsu, M.; Lutz, S.E.; Perrino, J.; Steinberg, G.K.; Barres, B.A.; Nimmerjahn, A.; Agalliu, D. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 2014, 82, 603–617. [Google Scholar] [CrossRef]
- Dashti, S.R.; Spalding, A.; Kadner, R.J.; Yao, T.; Kumar, A.; Sun, D.A.; LaRocca, R. Targeted intraarterial anti-VEGF therapy for medically refractory radiation necrosis in the brain. J. Neurosurg. Pediatr. 2015, 15, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Revuelta, J.M.; Zamarrón, Á.; Fortes, J.; Rodríguez-Boto, G.; Vaquero, J.; Gutiérrez-González, R. Experimental rat model of chronic cerebral hypoperfusion–reperfusion mimicking normal perfusion pressure breakthrough phenomenon. Neurocirugia 2020, 31, 209–215. [Google Scholar] [CrossRef]
- Toyama, K.; Spin, J.M.; Mogi, M.; Tsao, P.S. Therapeutic perspective on vascular cognitive impairment. Pharmacol. Res. 2019, 146, 104266. [Google Scholar] [CrossRef]
- Jabbour, M.N.; Elder, J.B.; Samuelson, C.G.; Khashabi, S.; Hofman, F.M.; Giannotta, S.L.; Liu, C.Y. Aberrant angiogenic characteristics of human brain arteriovenous malformation endothelial cells. Neurosurgery 2009, 64, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Gasche, Y.; Soccal, P.M.; Kanemitsu, M.; Copin, J.C. Matrix metalloproteinases and diseases of the central nervous system with a special emphasis on ischemic brain. Front. Biosci. 2006, 11, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A.; Yang, Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg. Focus 2007, 22, 1–9. [Google Scholar] [CrossRef]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef]
- Hagen, S.J. Non-canonical functions of claudin proteins: Beyond the regulation of cell-cell adhesions. Tissue Barriers 2017, 5, e1327839. [Google Scholar] [CrossRef]
- Ronaldson, P.T.; Davis, T.P. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J. Cereb. Blood Flow Metab. 2020, 40 (Suppl. 1), S6–S24. [Google Scholar] [CrossRef]
- Baluk, P.; McDonald, D.M. Buttons and Zippers: Endothelial Junctions in Lymphatic Vessels. Cold Spring Harb. Perspect. Med. 2022, 12, a041178. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Tietgens, A.J.; Anderson, J.M. Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through ZO-1. Mol. Biol. Cell 2017, 28, 524–534. [Google Scholar] [CrossRef]
- Wong, J.H.; Awad, I.A.; Kim, J.H. Ultrastructural pathological features of cerebrovascular malformations: A preliminary report. Neurosurgery 2000, 46, 1454–1459. [Google Scholar] [CrossRef]
- Tu, J.; Karunanayaka, A.; Windsor, A.; Stoodley, M.A. Comparison of an animal model of arteriovenous malformation with human arteriovenous malformation. J. Clin. Neurosci. 2010, 17, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhu, X.; Tang, C.; Pan, P.; Yadav, A.; Liang, R.; Press, K.; Nelson, J.; Su, H. CNS resident macrophages enhance dysfunctional angiogenesis and circulating monocytes infiltration in brain arteriovenous malformation. Res. Sq. 2023. [Google Scholar] [CrossRef] [PubMed]
- Clatterbuck, R.E.; Eberhart, C.G.; Crain, B.J.; Rigamonti, D. Ultrastructural and immunocytochemical evidence that an incompetent blood-brain barrier is related to the pathophysiology of cavernous malformations. J. Neurol. Neurosurg. Psychiatry 2001, 71, 188–192. [Google Scholar] [CrossRef]
- Schneider, H.; Errede, M.; Ulrich, N.H.; Virgintino, D.; Frei, K.; Bertalanffy, H. Impairment of Tight Junctions and Glucose Transport in Endothelial Cells of Human Cerebral Cavernous Malformations. J. Neuropathol. Exp. Neurol. 2011, 70, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.M.; Sladojevic, N.; Keep, R.F.; Andjelkovic, A.V. PDCD10 (CCM3) regulates brain endothelial barrier integrity in cerebral cavernous malformation type 3: Role of CCM3-ERK1/2-cortactin cross-talk. Acta Neuropathol. 2015, 130, 731–750. [Google Scholar] [CrossRef]
- Burkhardt, J.K.; Schmidt, D.; Schoenauer, R.; Brokopp, C.; Agarkova, I.; Bozinov, O.; Bertalanffy, H.; Hoerstrup, S.P. Upregulation of transmembrane endothelial junction proteins in human cerebral cavernous malformations. Neurosurg. Focus 2010, 29, E3. [Google Scholar] [CrossRef]
- Mosteiro, A.; Pedrosa, L.; Amaro, S.; Menéndez-Girón, S.; Reyes, L.; de Riva, N.; Misis, M.; Blasco, J.; Vert, C.; Dominguez, C.J.; et al. Understanding the Importance of Blood-Brain Barrier Alterations in Brain Arteriovenous Malformations and Implications for Treatment: A Dynamic Contrast-Enhanced-MRI–Based Prospective Study. Neurosurgery 2024, 96, 811–823. [Google Scholar] [CrossRef]



| All bAVMs (n = 17) | Ruptured bAVMs (n = 6) | Unruptured bAVMs (n = 11) | p-Value | |
|---|---|---|---|---|
| Age | 43.47 (13.29) | 41.83 (18.14) | 44.36 (10.75) | 0.763 |
| Sex, female (%) | 9 (52.94%) | 2 (33.33%) | 7 (63.64%) | 0.330 |
| Clinical onset | 0.018 | |||
| Asymptomatic | 3 (17.65%) | 1 (16.67%) | 2 (18.18%) | |
| Headache | 1 (5.88%) | 0 (0.00%) | 1 (9.09%) | |
| Seizures | 5 (29.41%) | 0 (0.00%) | 5 (45.45%) | |
| Focal deficit | 2 (11.76%) | 0 (0.00%) | 2 (18.18%) | |
| Bleeding | 6 (35.29%) | 5 (83.33%) | 1 (9.09%) | |
| Location | 0.140 | |||
| Supratentorial superficial | 12 (70.59%) | 3 (50.00%) | 9 (81.82%) | |
| Supratentorial deep | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Paraventricular | 3 (17.65%) | 1 (16.67%) | 2 (18.18%) | |
| Infratentorial | 2 (11.76%) | 2 (33.33%) | 0 (0.00%) | |
| SM, n (%) | 1.000 | |||
| 1 | 4 (23.53%) | 1 (16.67%) | 3 (27.27%) | |
| 2 | 7 (41.18%) | 3 (50.00%) | 4 (36.36%) | |
| 3 | 6 (35.29%) | 2 (33.33%) | 4 (36.36%) | |
| Nidus diameter (mm) | 26.24 (9.28) | 26.45 (2.74) | 25.83 (4.28) | 0.900 |
| Intranidal aneurysms, n (%) | 3 (17.65%) | 2 (33.33%) | 1 (9.09%) | 0.510 |
| Venous drainage, n (%) | 0.322 | |||
| Only deep | 2 (11.76%) | 1 (16.67%) | 1 (9.09%) | |
| Not only deep | 3 (17.65%) | 2 (33.33%) | 1 (9.09%) | |
| Superficial | 12 (70.59%) | 3 (50.00%) | 9 (81.82%) | |
| Vein diameter (mm) | 3.57 (1.45) | 3.43 (1.61) | 3.64 (1.43) | 0.791 |
| N° curves 45–90° | 1.29 (1.05) | 1.33 (0.82) | 1.27 (1.19) | 0.903 |
| N° curves 90–180° | 1.35 (0.93) | 1.17 (0.75) | 1.45 (1.03) | 0.522 |
| N° curves > 180° | 1.23 (1.39) | 2.00 (1.67) | 0.82 (1.078) | 0.160 |
| Venous length (mm) | 72.77 (46.22) | 72.76 (51.49) | 72.77 (45.74) | 0.999 |
| ARI | 0.756 | |||
| 0–1 | 9 (52.94%) | 3 (50.00%) | 6 (54.55%) | |
| 2–3 | 8 (47.06%) | 3 (50.00%) | 5 (45.45%) | |
| Treatment type | 0.600 | |||
| Surgery | 6 (35.29%) | 3 (50.00%) | 3 (27.27%) | |
| Endovascular | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Combined | 11 (64.71%) | 3 (50.00%) | 8 (72.73%) |
| All CCMs (n = 16) | Ruptured CCMs (n = 9) | Unruptured CCMs (n = 7) | p-Value | |
|---|---|---|---|---|
| Age | 48.11 (15.66) | 50.43 (15.28) | 40.00 (19.79) | 0.5893 |
| Sex, female (%) | 7 (77.78%) | 6 (85.71%) | 1 (50.00%) | 0.416 |
| Clinical onset | 0.5556 | |||
| Asymptomatic | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Headache | 1 (11.11%) | 1 (14.29%) | 0 (0.00%) | |
| Seizures | 4 (44.44%) | 2 (28.57%) | 2 (100.00%) | |
| Focal deficit | 4 (44.44%) | 4 (57.14%) | 0 (0.00%) | |
| Location | 0 | 0.5556 | ||
| Supratentorial superficial | 3 (33.33%) | 2 (28.57%) | 1 (50.00%) | |
| Supratentorial deep | 1 (11.11%) | 0 (0.00%) | 1 (50.00%) | |
| TE | 2 (22.22%) | 2 (28.57%) | 0 (0.00%) | |
| Cerebellum | 2 (22.22%) | 2 (28.57%) | 0 (0.00%) | |
| Multiple | 1 (11.11%) | 1 (14.29%) | 0 (0.00%) | |
| Diameter (mm) | 25.67 (6.06) | 27.14 (6.07) | 20.50 (2.12) | |
| Multiple, n (%) | 4 (44.44%) | 3 (42.86%) | 1 (50.00%) | 1 |
| Treatment type, surgery, n (%) | 9 (100.00%) | 7 (100.00%) | 2 (100.00%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrosa, L.; Mosteiro, A.; Reyes, L.; Amaro, S.; Menéndez-Girón, S.; Rivera, M.C.; Domínguez, C.J.; Planas, A.M.; Torné, R.; Rodríguez-Hernández, A. Profiling Tight Junction Protein Expression in Brain Vascular Malformations. Int. J. Mol. Sci. 2025, 26, 4558. https://doi.org/10.3390/ijms26104558
Pedrosa L, Mosteiro A, Reyes L, Amaro S, Menéndez-Girón S, Rivera MC, Domínguez CJ, Planas AM, Torné R, Rodríguez-Hernández A. Profiling Tight Junction Protein Expression in Brain Vascular Malformations. International Journal of Molecular Sciences. 2025; 26(10):4558. https://doi.org/10.3390/ijms26104558
Chicago/Turabian StylePedrosa, Leire, Alejandra Mosteiro, Luis Reyes, Sergio Amaro, Sebastián Menéndez-Girón, Mateo Cortés Rivera, Carlos J. Domínguez, Anna M. Planas, Ramon Torné, and Ana Rodríguez-Hernández. 2025. "Profiling Tight Junction Protein Expression in Brain Vascular Malformations" International Journal of Molecular Sciences 26, no. 10: 4558. https://doi.org/10.3390/ijms26104558
APA StylePedrosa, L., Mosteiro, A., Reyes, L., Amaro, S., Menéndez-Girón, S., Rivera, M. C., Domínguez, C. J., Planas, A. M., Torné, R., & Rodríguez-Hernández, A. (2025). Profiling Tight Junction Protein Expression in Brain Vascular Malformations. International Journal of Molecular Sciences, 26(10), 4558. https://doi.org/10.3390/ijms26104558






