Renal Apoptosis in Male Rats Induced by Extensive Dietary Exposure to Ochratoxins
Abstract
1. Introduction
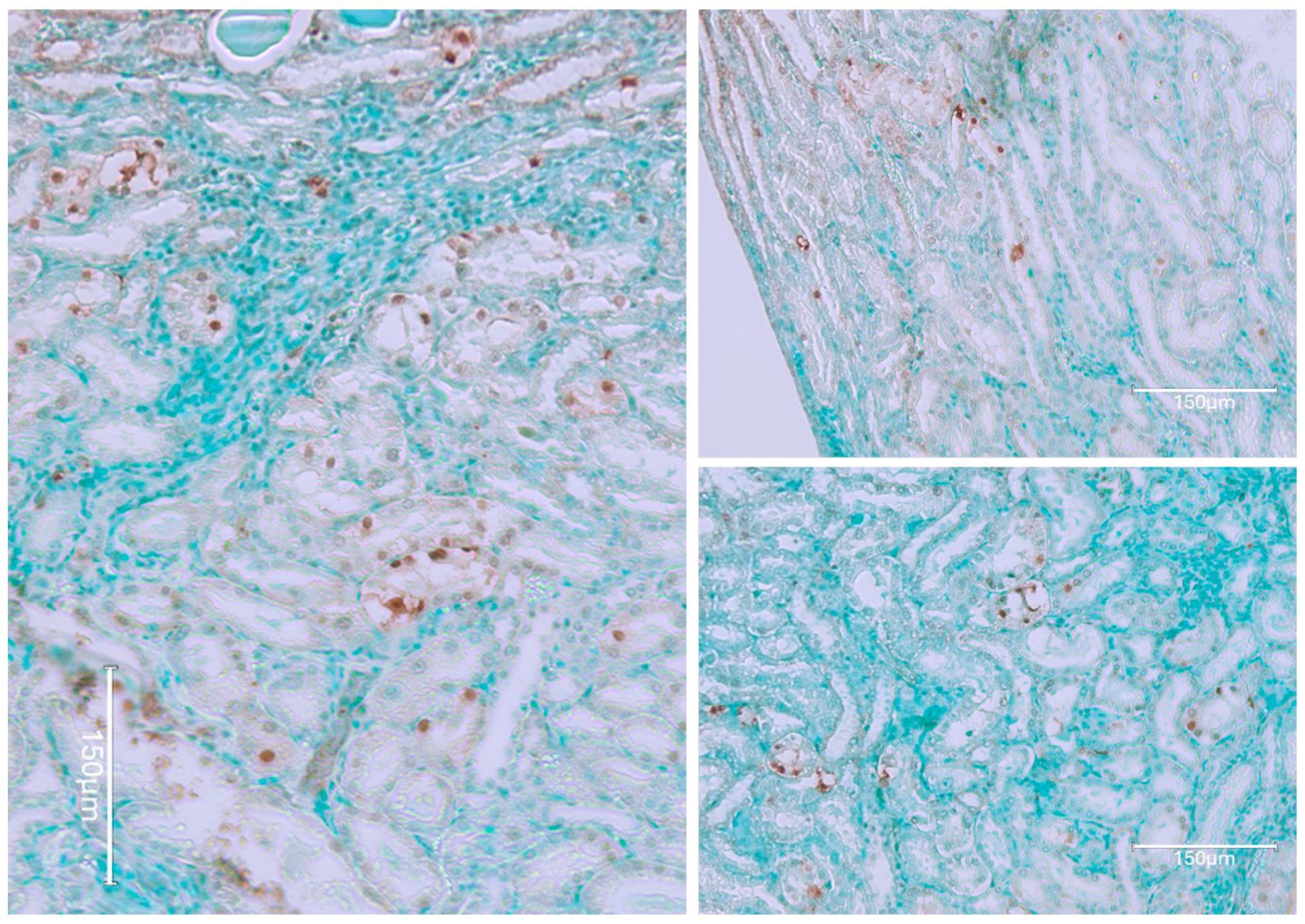
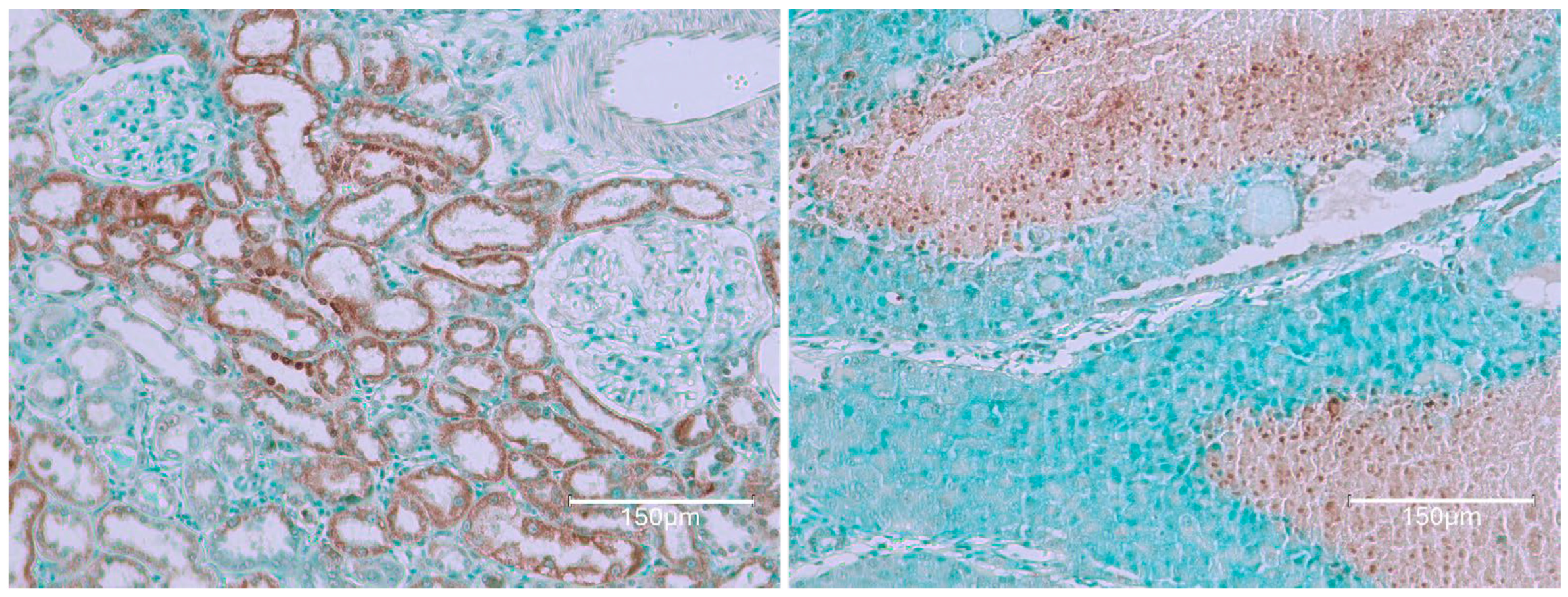
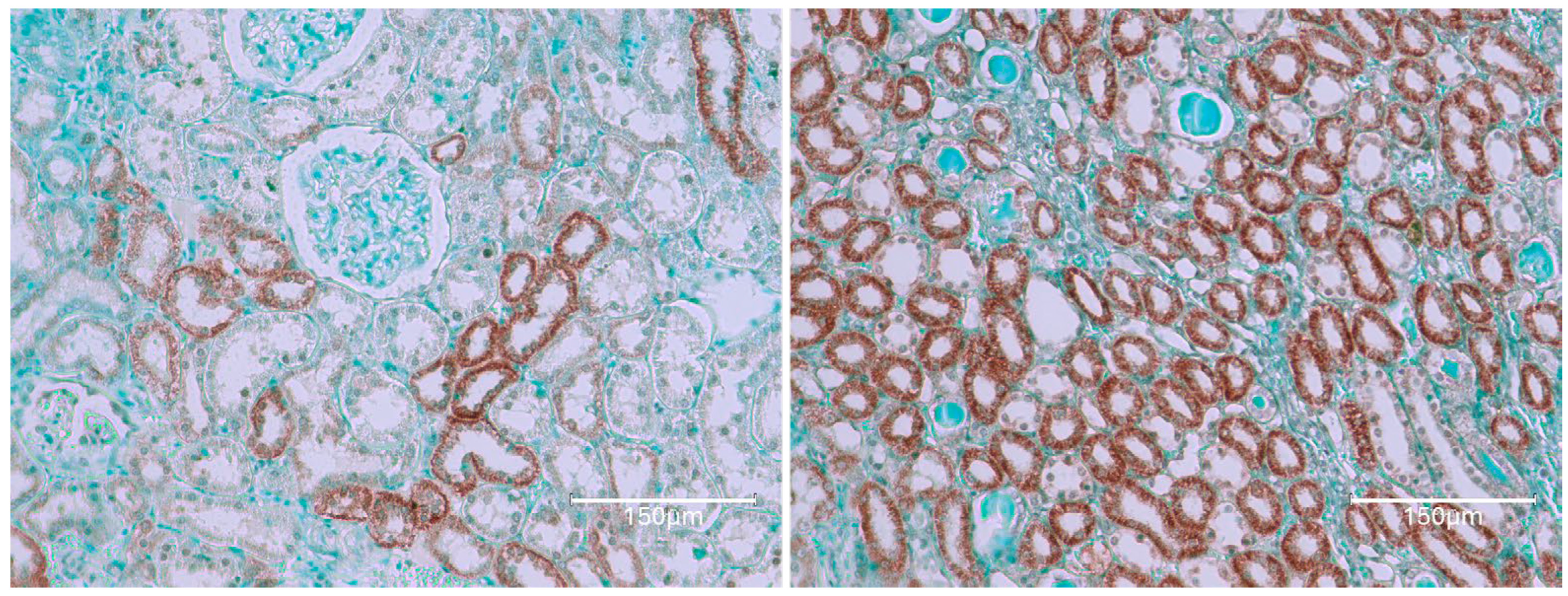
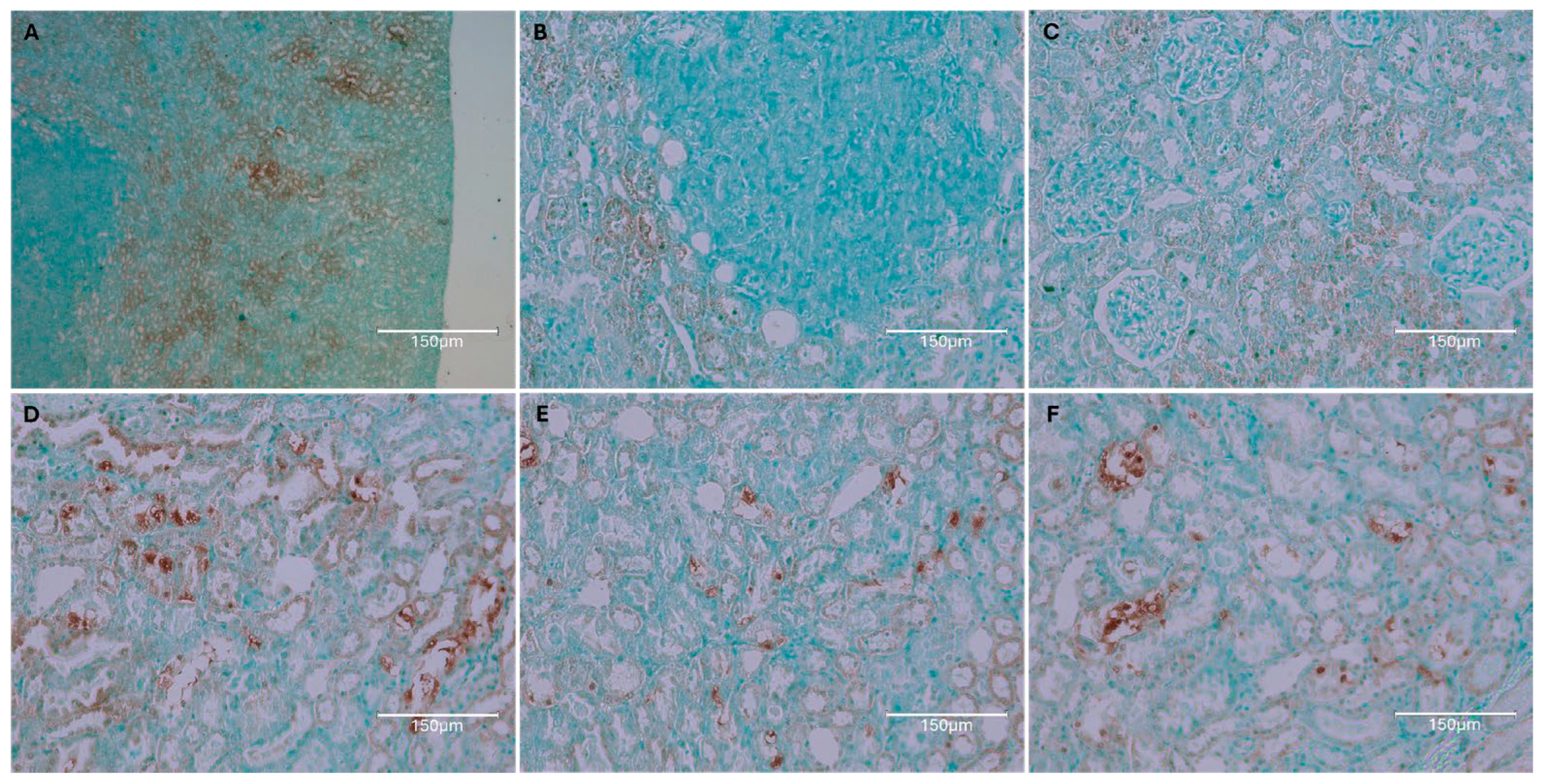
2. Results
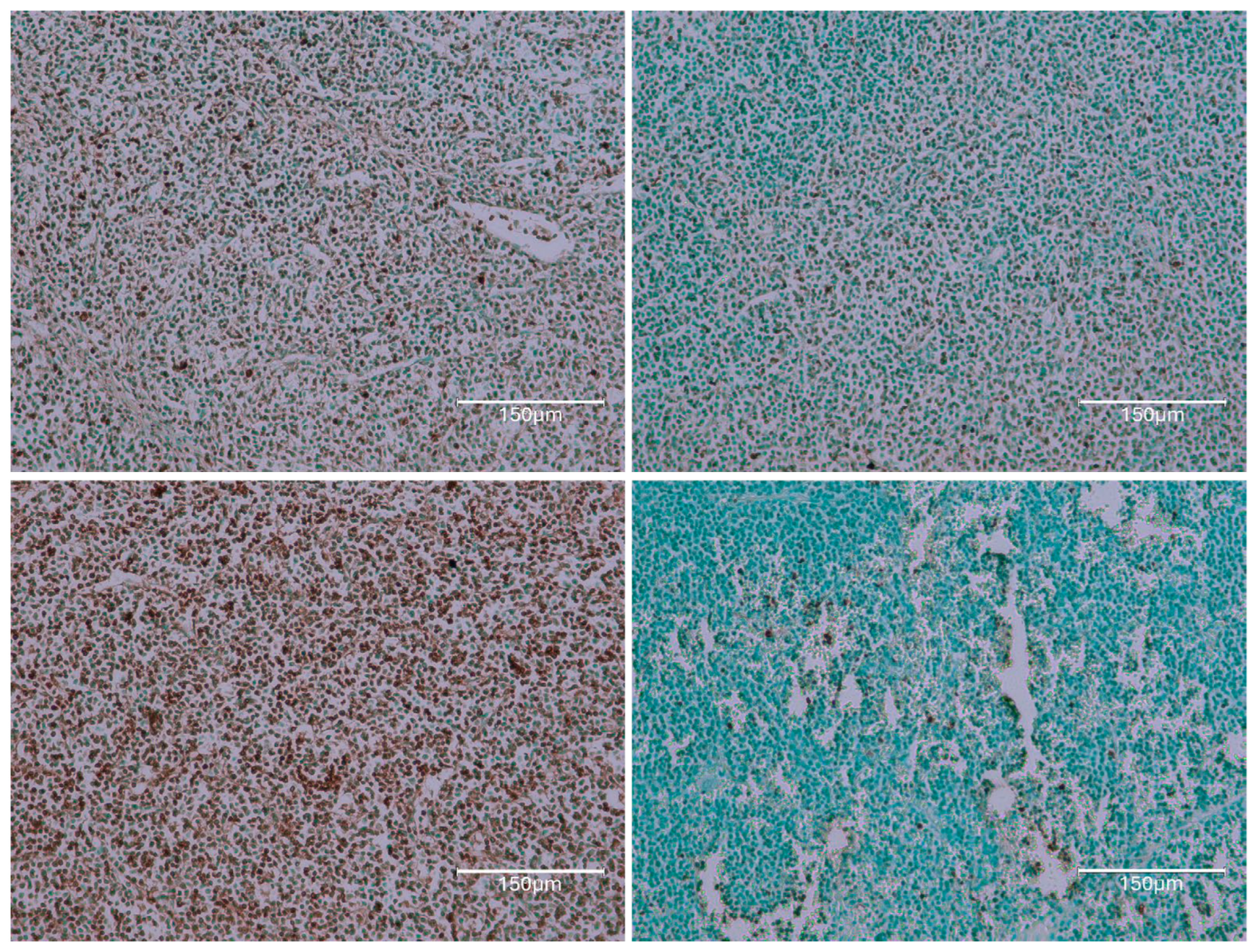
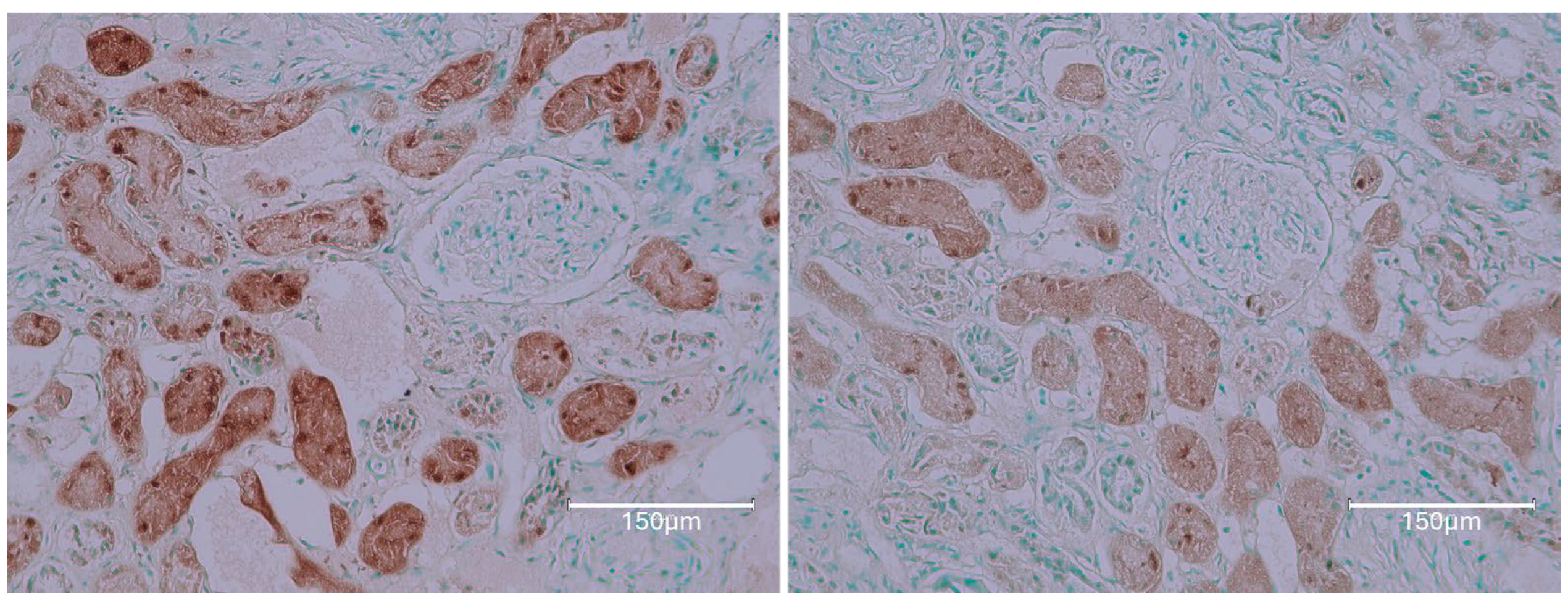
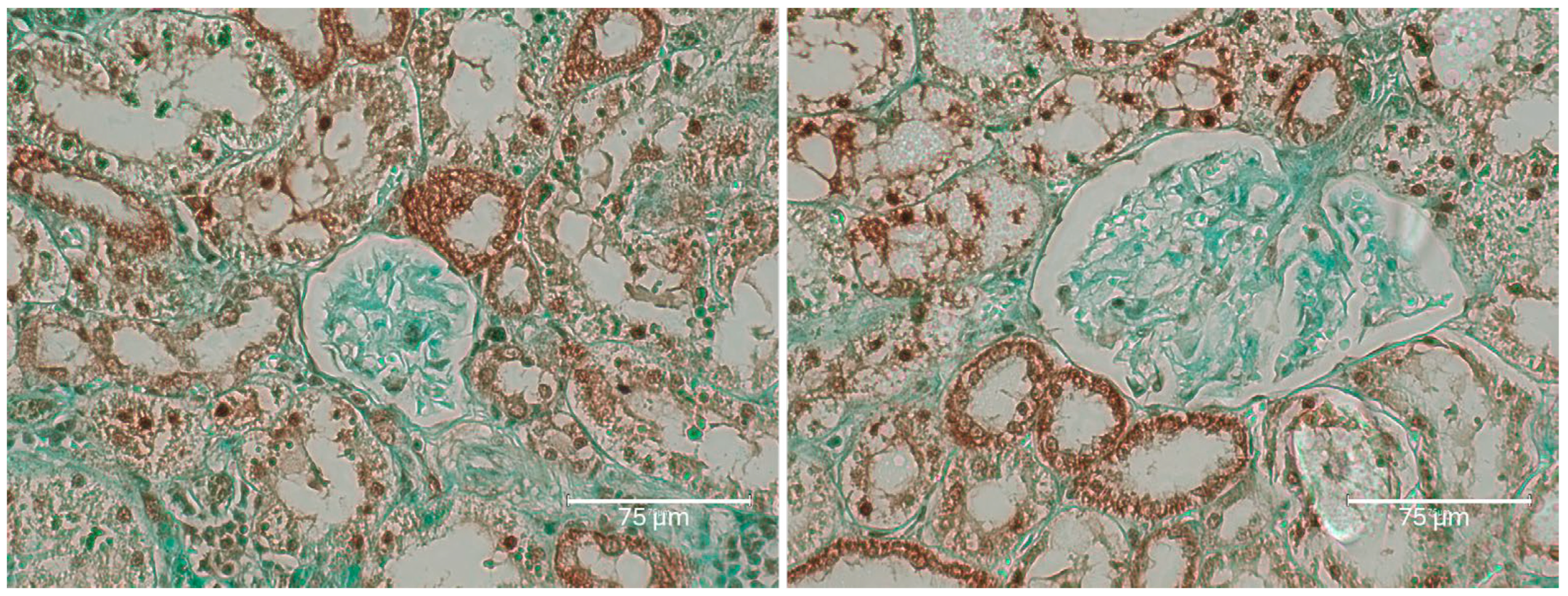
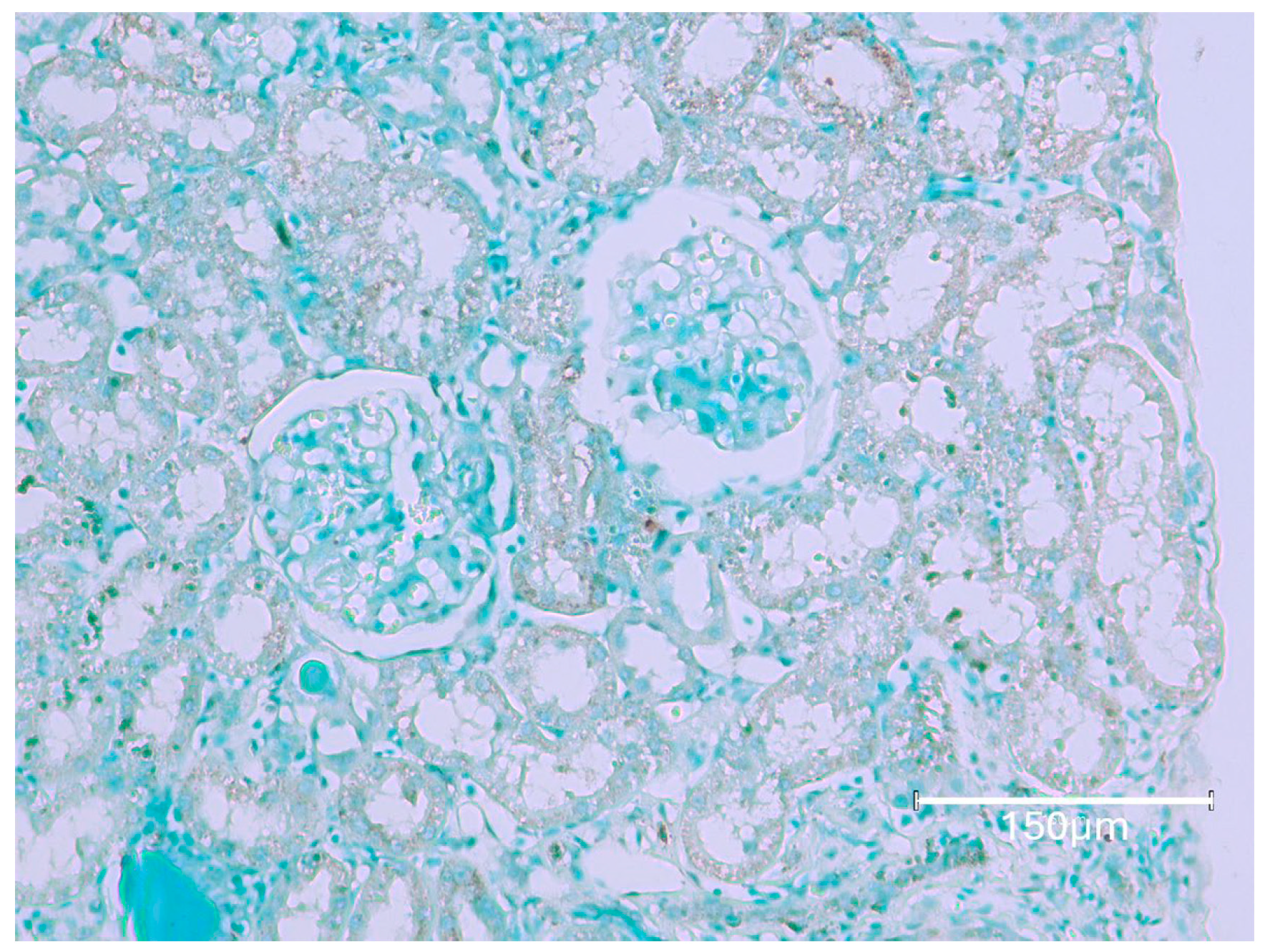
2.1. Carcinogenic Dose of Ochratoxin for Life for Three Fischer Rats
2.2. Low-Dose Ochratoxin for Life for Three Fischer Rats
2.3. Fischer Rat—Second Year OTA Only
2.4. Dark Agouti Rat—SINGLE RAT
3. Discussion
4. Materials and Methods
4.1. Tissue Section Preparation and Rehydration
4.2. Permeabilization of Specimen
4.3. Quenching: Inactivation of Endogenous Peroxidases
4.3.1. Labelling Reaction
4.3.2. Termination of Labelling Reaction
4.3.3. Detection
4.3.4. Development
4.3.5. Counterstain and Storage
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tata, C.A.; Orem, W.H.; Finkelman, R.B.; Feder, G.I. The aetiology of Balkan endemic nephropathy: Still more questions than answers. Environ. Health Perspect. 1998, 106, 189–700. [Google Scholar]
- Cosyns, J.P.; Dehoux, J.P.; Guiot, Y.; Goebbels, R.M.; Robert, A.; Bernard, A.M.; De Strihou, C.V.Y. Chronic aristolochic acid toxicity in rabbits: A model of Chinese herbs nephropathy? Kidney Int. 2001, 90, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, K.J.; Steyn, P.S.; Fourie, L. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature 1965, 205, 1112–1113. [Google Scholar] [CrossRef] [PubMed]
- Krogh, P. Porcine nephropathy associated with ochratoxin A. In Mycotoxins and Animal Foods; Smith, J.E., Henderson, R.S., Eds.; CRC Press: Boca Raton, FL, USA, 1991; Chapter 26; pp. 627–645. [Google Scholar]
- Boorman, G.A. Toxicology and Carcinogenesis Studies of Ochratoxin A. (CAS No 303-47-9) in F344/N Rats (Gavage Studies); Technical Report 358; National Toxicology Program: Research Triangle Park, NC, USA, 1989. [Google Scholar]
- Tanchev, Y.; Dorossiev, D. The first clinical description of Balkan endemic nephropathy (1956) and its vaiidity 35 years later. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumours; Castegnaro, M., Plestina, R., Dirheimer, G., Chernozemsky, I.N., Eds.; International Agency for Research on Cancer: Lyon, France, 1991; pp. 21–28. [Google Scholar]
- Pfohl-Leszkowicz, A.; Grosse, Y.; Castegnaro, M.; Nicolov, I.G.; Chernozemsky, I.N. Ochratoxin A-related DNA adducts in urinary tract tumours of Bulgarian subjects. IARC Sci. Publ. 1993, 124, 141–148. [Google Scholar]
- EFSA. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, 6113. [Google Scholar]
- Grollman, A.P.; Shibutani, S.; Moriya, M.; Miller, F.; Wu, L.; Moll, U.; Suzuki, N.; Fernandes, A.; Rosenquist, T.; Medverec, Z.; et al. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc. Natl. Acad. Sci. USA 2007, 104, 12129–12134. [Google Scholar] [CrossRef]
- Mantle, P.; Upadhyay, R.; Herman, D.; Batuman, V. Exploration of apoptosis in histopathologies of Balkan endemic nephropathies with both urothelial tumour and atrophied kidney. Ann. Urol. Oncol. 2024, 7, 61–69. [Google Scholar] [CrossRef]
- Mantle, P. Rat kidney cancers determined by dietary ochratoxin A in the first year of life. J. Kidney Cancer VHL 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Mantle, P.G. Minimum tolerable exposure period and maximum threshold dietary intake of ochratoxin A for causing renal cancer in male Dark Agouti rats. Food Chem. Toxicol. 2009, 47, 2419–2424. [Google Scholar] [CrossRef]
- Mantle, P.G.; Nolan, C.C. Pathological outcomes in kidney and brain in male Fischer rats given dietary ochratoxin A, commencing at one year of age. Toxins 2010, 2, 1100–1110. [Google Scholar] [CrossRef]
- Bendele, A.M.; Carlton, W.W.; Krogh, P.; Lillehoj, E.B. Ochratoxin A carcinogenesis in the (C57BL/6J x C3HB)F1 mouse. J. Natl. Cancer Inst. 1985, 75, 733–742. [Google Scholar] [PubMed]
- Herman, D.; Stanojevic, G.; Pavlovic, N.; Mantle, P. Histopathological review of renal pelvic tumours of Balkan nephropathy cases from Southern Serbia. Ann. Urol. Oncol. 2020, 3, 15–21. [Google Scholar]
- Herman, D.; Mantle, P. Rat tumour histopathology associated with experimental chronic dietary exposure to ochratoxin A in prediction of the mycotoxin’s risk for human cancers. Toxins 2021, 11, 205. [Google Scholar] [CrossRef]
- Herman, D.; Mantle, P. Immunohistochemical analysis of rat renal tumours caused by ochratoxin A. Toxins 2017, 9, 384. [Google Scholar] [CrossRef]
- Mor, F.; Kilic, M.A.; Ozmen, O.; Yilmaz, M.; Eker, I.; Uran, K. The effects of orchidectomy on toxicological responses to dietary ochratoxin A in Wistar rats. Exp. Toxicol. Pathol. 2014, 66, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.A.; Simpson, D.M.; Armstrong, S.D.; Davidson, A.J.; Robertson, D.H.; McLean, L.; Beynon, R.J.; Hurst, J.L. Darcin: A male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol. 2010, 8, 75. [Google Scholar] [CrossRef]
- Vettorazzi, A.; Wait, R.; Nagy, J.; Monreal, J.I.; Mantle, P. Changes in male rat urinary protein profile during puberty: A pilot study. BMC Res. Notes 2013, 6, 232. [Google Scholar] [CrossRef] [PubMed]
- Mantle, P.G.; Nagy, J.M. Binding of ochratoxin A to a urinary globulin: A new concept to account for gender difference in rat nephrocarcinogenic responses. Int. J. Med. Sci. 2008, 9, 719–735. [Google Scholar] [CrossRef]
- Mantle, P.; Kilic, M.A.; Mor, F.; Ozmen, O. Contribution of organ vasculature in rat renal analysis for ochratoxin A: Relevance to toxicology of nephrotoxins. Toxins 2015, 7, 1005–1017. [Google Scholar] [CrossRef]
- Mor, F.; Sengul, O.; Topsakal, S.T.; Kilic, M.A.; Ozman, O. Diabetogenic effects of ochratoxin A in female rats. Toxins 2017, 9, 144. [Google Scholar] [CrossRef]
- Bárta, F.; Dedíková, A.; Bebová, M.; Dušková, Š.; Mráz, J.; Schmeiser, H.H.; Arlt, V.M.; Hodek, P.; Stiborová, M. Co-exposure to aristolochic acids 1 and 11 increases DNA adduct formation responsible to aristolochic acid -mediated carcinogenicity in rats. IJMS 2021, 22, 10479. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Carter, R.; Peristianis, G.; Austwick, P.; Flynn, F.; Aldridge, W. Balkan (endemic) nephropathy and a toxin-producing strain of Penicillium verrucosum var. cyclopium: An experimental model in rats. Lancet 1977, 289, 671–675. [Google Scholar]
- Macgeorge, K.M.; Mantle, P.G. Nephrotoxic fungi in a Yugoslavian community in which Balkan nephropathy is hyperendemic. Mycol. Res. 1991, 95, 600–664. [Google Scholar] [CrossRef]
- Mantle, P.G. Interpretation of the pharmacokinetics of ochratoxin A in blood plasma of rats, during and after acute or chronic ingestion. Food Chem. Toxicol. 2008, 46, 1808–1816. [Google Scholar] [CrossRef]
- Mantle, P.G.; Nicholls, A.W.; Shockcor, J.P. H NMR spectroscopy-based metabolomic assessment of uremic toxicity, with toxicological outcomes, in male rats following an acute, mid-life insult from ochratoxin A. Toxins 2011, 3, 504–519. [Google Scholar] [CrossRef]
- Kova, A.D.; Barta, F.; Martinek, V.; Kotalic, K. In vivo metabolism of aristolochic acid 1 and 11 in rats is influenced by their co-exposure. Chem. Res. Toxicol. 2020, 33, 2804–2818. [Google Scholar]
- Zhang, J.; Chan, C.-K.; Pavlovic, N.M.; Chan, W. Effect of diet on aristolochic acid—DNA adduct formation: Implications for Balkan endemic nephropathy. Chem. Res. Toxicol. 2023, 36, 438–445. [Google Scholar] [CrossRef]
- Sgone, R.; Wick, G. Methods for the detection of apoptosis. Int. Arch. Allergy Immunol. 1994, 105, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, A.; Mantle, P. Renal apoptosis in the mycotoxicology of Penicillium polonicum and ochratoxin in rats. Life 2022, 12, 352. [Google Scholar] [CrossRef]
- Miljkovic, A.; Mantle, P. Chromatographic fractionation of Penicillium polonicum fermentation metabolites in search of the nephrotoxin(s} for rats. Life 2022, 12, 747. [Google Scholar] [CrossRef]
- Livingstone, T. (Producer). 1991. The Curse of Karash [TV Program]. British Broadcasting Corporation. Available online: https://www.youtube.com/watch?v=[yBL3-pC4dK8] (accessed on 29 April 2024).
- Mantle, P.; Herman, D.; Tatu, C. Is aristolochic acid really the cause of the Balkan Endemic Nephropathy? J. Controv. Biomed. Res. 2016, 2, 9–20. [Google Scholar] [CrossRef][Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantle, P.; Upadhyay, R.; Herman, D.; Batuman, V. Renal Apoptosis in Male Rats Induced by Extensive Dietary Exposure to Ochratoxins. Int. J. Mol. Sci. 2025, 26, 4553. https://doi.org/10.3390/ijms26104553
Mantle P, Upadhyay R, Herman D, Batuman V. Renal Apoptosis in Male Rats Induced by Extensive Dietary Exposure to Ochratoxins. International Journal of Molecular Sciences. 2025; 26(10):4553. https://doi.org/10.3390/ijms26104553
Chicago/Turabian StyleMantle, Peter, Rohit Upadhyay, Diana Herman, and Vecihi Batuman. 2025. "Renal Apoptosis in Male Rats Induced by Extensive Dietary Exposure to Ochratoxins" International Journal of Molecular Sciences 26, no. 10: 4553. https://doi.org/10.3390/ijms26104553
APA StyleMantle, P., Upadhyay, R., Herman, D., & Batuman, V. (2025). Renal Apoptosis in Male Rats Induced by Extensive Dietary Exposure to Ochratoxins. International Journal of Molecular Sciences, 26(10), 4553. https://doi.org/10.3390/ijms26104553




