Towards Optical Biopsy in Glioma Surgery
Abstract
1. Introduction
2. Basic Principles of Methods Used for Optical Biopsy
3. Applications of Optical Biopsy in Glioma Surgery
3.1. Optical Biopsy for Intraoperative Histopathological Diagnosis
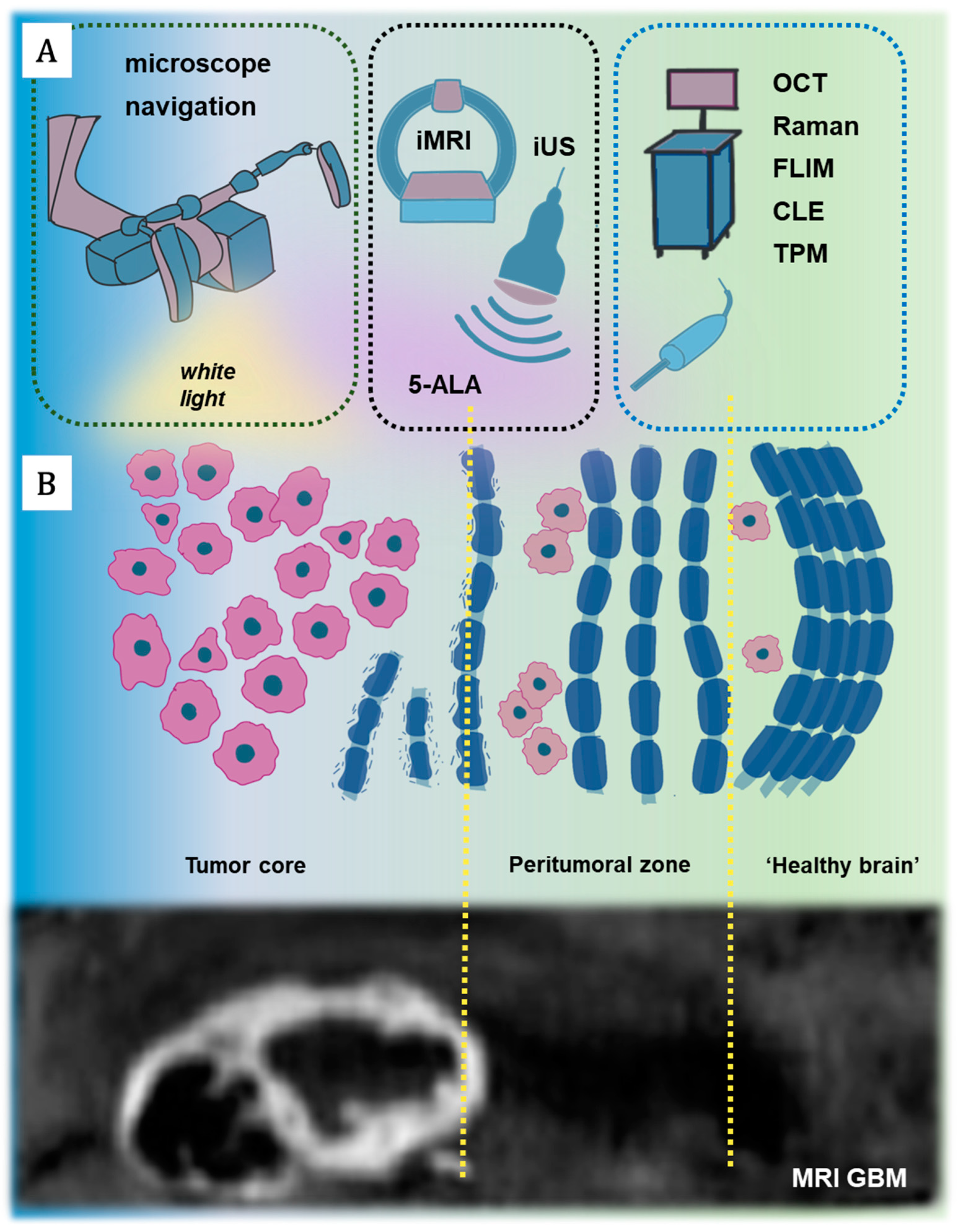
3.2. Optical Imaging for Neurosurgical Guidance
| Technology | iMRI | iUS | 5-ALA | Raman | OCT | FLIM | CLE | TPM |
|---|---|---|---|---|---|---|---|---|
| Type of energy measured | radio waves | high-frequency sound waves | visible light | visible or near-infrared light | near-infra-red light | visible or near-infrared light | visible or near- infrared light | near-infrared light |
| Penetration level | organ-tissue | organ-tissue | tissue | cellular-molecular | tissue-cellular | tissue-cellular | tissue-cellular | tissue-cellular |
| Imaging application | whole brain | tissue surface/subsurface of tissue | tissue surface | tissue surface | tissue surface/subsurface of tissue | tissue surface | tissue surface | tissue surface/subsurface |
| Spatial resolution | 20–100 um | 50–500 um | 0.03 mm | 300 nm–1 μm | 0.02 mm | 300 nm–15 μm | 300 nm–500 nm | 300 nm–1000 nm |
| Time resolution | minutes to hours | seconds to minutes | seconds | seconds | seconds | ps-ms | seconds | seconds |
| Contrast enhanced | label free or small molecules nanoparticle | label free or microbubble | labeled | label free | label free | label free | labeled | label free or labeled |
| Cost | very high | moderate | moderate | high | low | high | moderate | high |
| Intraoperative tools | - | probes | probes or contactless | probes | probes or contactless | probes | contactless | contactless |
| Type of information | structural | structural | metabolic | “optical fingerprint” | structural | metabolic | structural | structural |
| Sensitivity (%) | 41–96 for HGG | 46–80 for LGG and HGG | 91 for HGG | 84–96 for LGG/HGG | 85 for HGG 90 for LGG | 58 for HGG | 85–91 for HGG | 100 for HGG |
| Specificity (%) | 57–100 for HGG | 28–100 for LGG/HGG | 80–89 for HGG | 89–100 for LGG/HGG | 85 for HGG 90 for LGG | 72 for HGG | 81–94 for HGG | 50 for HGG |
| GTR achieving | 96–100% | 73.4% | ~76% | No data | No data | No data | No data | No data |
| References | [102,121,122,123] | [124,125,126,127] | [122,128,129,130] | [38,131,132,133] | [107,108,134,135,136] | [87] | [71,137] | [48,61] |
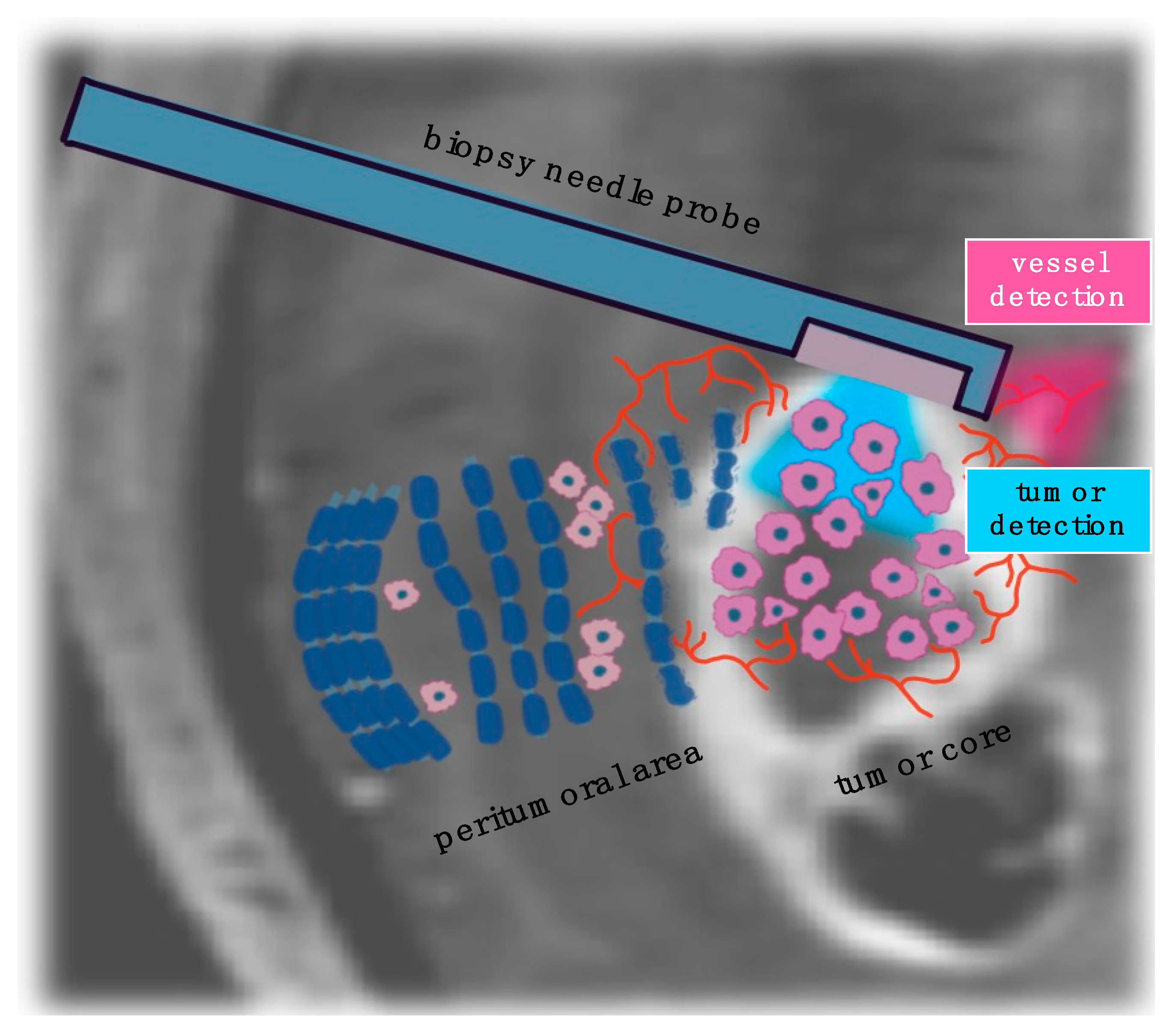
3.3. Optical Imaging for Stereotactic Biopsy Guidance
| Technology | iMRI | iUS | 5-ALA | Raman | OCT |
|---|---|---|---|---|---|
| Tumor detection | yes | yes | yes | yes | yes |
| Vessel detection | yes | yes | yes | no | yes |
| Sensitivity | - | - | 63–69 for biopsy acquisition | 80 for biopsy acquisition | 91.2 for blood vessel |
| Specificity | - | - | 100 for biopsy acquisition | 90 for biopsy acquisition | 97.7 for blood vessel |
| Diagnostic accuracy (%) | over 97 for biopsy acquisition | 88.4–91.5 for biopsy acquisition | 98 for biopsy acquisition | 84 for biopsy acquisition | - |
| References | [102] | [160] | [161,162] | [38] | [161,163] |
4. Real-Time Molecular Characterization Using Optical Technologies: Transforming Intraoperative Surgical Strategies
5. Challenges and Future Prospects of Optical Technologies in Glioma Surgery
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| 5-ALA | 5-aminolevulinic acid |
| CLE | confocal laser endomicroscopy |
| EOR | extent of resection |
| GBM | glioblastoma |
| GTR | gross total resection |
| FLIM | fluorescence lifetime imaging |
| ICG | indocyanine green |
| IDH | isocitrate dehydrogenase |
| iMRI | intraoperative magnetic resonance imaging |
| iUS | intraoperative ultrasound |
| SRH | stimulated Raman histology |
| OCT | optical coherence tomography |
| TPM | two-photon microscopy |
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Verhaak, R.G.; Canoll, P. The cellular origin for malignant glioma and prospects for clinical advancements. Expert Rev. Mol. Diagn. 2012, 12, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.; von Felten, S.; Frank, S.; Vassella, E.; Vajtai, I.; Taylor, E.; Schulz, M.; Hutter, G.; Hench, J.; Schucht, P.; et al. IDH/MGMT-driven molecular classification of low-grade glioma is a strong predictor for long-term survival. Neuro-Oncology 2013, 15, 469–479. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Brandes, A.A.; Taphoorn, M.J.; Kros, J.M.; Kouwenhoven, M.C.; Delattre, J.Y.; Bernsen, H.J.; Frenay, M.; Tijssen, C.C.; Grisold, W.; et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: Long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013, 31, 344–350. [Google Scholar] [CrossRef]
- Cahill, D.P. Extent of Resection of Glioblastoma: A Critical Evaluation in the Molecular Era. Neurosurg. Clin. N. Am. 2021, 32, 23–29. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21, v1–v100. [Google Scholar] [CrossRef]
- Wallner, K.E.; Galicich, J.H.; Krol, G.; Arbit, E.; Malkin, M.G. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 1405–1409. [Google Scholar] [CrossRef]
- Sherriff, J.; Tamangani, J.; Senthil, L.; Cruickshank, G.; Spooner, D.; Jones, B.; Brookes, C.; Sanghera, P. Patterns of relapse in glioblastoma multiforme following concomitant chemoradiotherapy with temozolomide. Br. J. Radiol. 2013, 86, 20120414. [Google Scholar] [CrossRef]
- Tripathi, S.; Vivas-Buitrago, T.; Domingo, R.A.; Biase, G.; Brown, D.; Akinduro, O.O.; Ramos-Fresnedo, A.; Sherman, W.; Gupta, V.; Middlebrooks, E.H.; et al. IDH-wild-type glioblastoma cell density and infiltration distribution influence on supramarginal resection and its impact on overall survival: A mathematical model. J. Neurosurg. 2022, 136, 1567–1575. [Google Scholar] [CrossRef]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef]
- Kotrotsou, A.; Elakkad, A.; Sun, J.; Thomas, G.A.; Yang, D.; Abrol, S.; Wei, W.; Weinberg, J.S.; Bakhtiari, A.S.; Kircher, M.F.; et al. Multi-center study finds postoperative residual non-enhancing component of glioblastoma as a new determinant of patient outcome. J. Neurooncol. 2018, 139, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, F.; Roca, E.; Spena, G. Supramarginal Resection for Glioblastoma: It Is Time to Set Boundaries! A Critical Review on a Hot Topic. Brain Sci. 2022, 12, 652. [Google Scholar] [CrossRef] [PubMed]
- Wach, J.; Vychopen, M.; Kuhnapfel, A.; Seidel, C.; Guresir, E. A Systematic Review and Meta-Analysis of Supramarginal Resection versus Gross Total Resection in Glioblastoma: Can We Enhance Progression-Free Survival Time and Preserve Postoperative Safety? Cancers 2023, 15, 1772. [Google Scholar] [CrossRef] [PubMed]
- Bonosi, L.; Marrone, S.; Benigno, U.E.; Buscemi, F.; Musso, S.; Porzio, M.; Silven, M.P.; Torregrossa, F.; Grasso, G. Maximal Safe Resection in Glioblastoma Surgery: A Systematic Review of Advanced Intraoperative Image-Guided Techniques. Brain Sci. 2023, 13, 216. [Google Scholar] [CrossRef]
- Duffau, H.; Mandonnet, E. The “onco-functional balance” in surgery for diffuse low-grade glioma: Integrating the extent of resection with quality of life. Acta Neurochir. 2013, 155, 951–957. [Google Scholar] [CrossRef]
- Hamer, R.P.; Jain, S.; Teo, C.; Loh, W.N.; Chan, H.M.; Yeo, T.T.; Teo, K. Optimizing the onco-functional balance in supratentorial brain tumour surgery: A single institution’s initial experience with intraoperative cortico-subcortical mapping and monitoring in Singapore. J. Clin. Neurosci. 2020, 79, 224–230. [Google Scholar] [CrossRef]
- Schupper, A.J.; Yong, R.L.; Hadjipanayis, C.G. The Neurosurgeon’s Armamentarium for Gliomas: An Update on Intraoperative Technologies to Improve Extent of Resection. J. Clin. Med. 2021, 10, 236. [Google Scholar] [CrossRef]
- Zolotova, A.S.; Evstigneyev, M.S.; Yashin, K.S.; Ermolayev, A.Y.; Ostapyuk, M.V.; Al-Madhadjy, V.M.A.; Zagrekov, V.I.; Antonova, N.Y.; Shibanova, M.V.; Kravets, L.Y.; et al. Combination of Multimodal MRI, Neuronavigation, and Awake Craniotomy in Removing Tumors of Eloquent Areas. Sovrem. Tehnol. Med. 2022, 14, 59–65. [Google Scholar] [CrossRef]
- Dadario, N.B.; Brahimaj, B.; Yeung, J.; Sughrue, M.E. Reducing the Cognitive Footprint of Brain Tumor Surgery. Front. Neurol. 2021, 12, 711646. [Google Scholar] [CrossRef]
- Samuel, N.; Vetkas, A.; Pancholi, A.; Sarica, C.; Loh, A.; Germann, J.; Harmsen, I.E.; Tasserie, J.; Milano, V.; Yamamoto, K.; et al. A Network-Based Approach to Glioma Surgery: Insights from Functional Neurosurgery. Cancers 2021, 13, 6127. [Google Scholar] [CrossRef]
- Dimertsev, A.V.; Zuev, A.A.; Podgurskaya, M.G. Surgical treatment of gliomas in motor zone under control of neurophysiological monitoring. Russ. J. Neurosurg. 2023, 25, 10–20. [Google Scholar] [CrossRef]
- Whiting, B.B.; Lee, B.S.; Mahadev, V.; Borghei-Razavi, H.; Ahuja, S.; Jia, X.; Mohammadi, A.M.; Barnett, G.H.; Angelov, L.; Rajan, S.; et al. Combined use of minimal access craniotomy, intraoperative magnetic resonance imaging, and awake functional mapping for the resection of gliomas in 61 patients. J. Neurosurg. 2020, 132, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, A.Y.; Dashyan, V.G. Intraoperative sonography in cranial neurosurgery: New possibilities and integration with neuronavigation. Review. Russ. J. Neurosurg. 2021, 23, 104–112. [Google Scholar] [CrossRef]
- Goriaǐnov, S.A.; Potapov, A.A.; Pitskhelauri, D.I.; Kobiakov, G.L.; Okhlopkov, V.A.; Gavrilov, A.G.; Shurkhaǐ, V.A.; Zhukov, V.I.; Shishkina, L.V.; Loshchenov, V.B.; et al. Intraoperative fluorescence diagnostics upon recurrent operations for brain gliomas. Zhurnal Vopr. Neirokhirurgii Im. N. N. Burdenko 2014, 78, 21–30. [Google Scholar]
- Potapov, A.A.; Gavrilov, A.G.; Goriainov, S.A.; Gol’bin, D.A.; Zelenkov, P.V.; Kobiakov, G.L.; Okhlopkov, V.A.; Zhukov, V.; Shishkina, L.V.; Shukhrai, V.A.; et al. Intraoperative fluorescent visualization and laser spectrosopy in intrinsic brain tumor surgery. Zhurnal Vopr. Neirokhirurgii Im. N. N. Burdenko 2012, 76, 3–11, discussion 12. [Google Scholar]
- Coburger, J.; Wirtz, C.R. Fluorescence guided surgery by 5-ALA and intraoperative MRI in high grade glioma: A systematic review. J. Neuro-Oncol. 2019, 141, 533–546. [Google Scholar] [CrossRef]
- Haddad, A.F.; Aghi, M.K.; Butowski, N. Novel intraoperative strategies for enhancing tumor control: Future directions. Neuro-Oncology 2022, 24, S25–S32. [Google Scholar] [CrossRef]
- Bin-Alamer, O.; Abou-Al-Shaar, H.; Gersey, Z.C.; Huq, S.; Kallos, J.A.; McCarthy, D.J.; Head, J.R.; Andrews, E.; Zhang, X.; Hadjipanayis, C.G. Intraoperative Imaging and Optical Visualization Techniques for Brain Tumor Resection: A Narrative Review. Cancers 2023, 15, 4890. [Google Scholar] [CrossRef]
- Valdes, P.A.; Roberts, D.W.; Lu, F.K.; Golby, A. Optical technologies for intraoperative neurosurgical guidance. Neurosurg. Focus 2016, 40, E8. [Google Scholar] [CrossRef]
- Lagarto, J.L.; Shcheslavskiy, V.; Saverio Pavone, F.; Cicchi, R. Simultaneous fluorescence lifetime and Raman fiber-based mapping of tissues. Opt. Lett. 2020, 45, 2247–2250. [Google Scholar] [CrossRef]
- Alfano, R.; Pu, Y. Optical biopsy for cancer detection. In Lasers for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2013; pp. 325–367. [Google Scholar]
- Nishio, N.; van den Berg, N.S.; van Keulen, S.; Martin, B.A.; Fakurnejad, S.; Teraphongphom, N.; Chirita, S.U.; Oberhelman, N.J.; Lu, G.; Horton, C.E.; et al. Optical molecular imaging can differentiate metastatic from benign lymph nodes in head and neck cancer. Nat. Commun. 2019, 10, 5044. [Google Scholar] [CrossRef] [PubMed]
- Alfano, R.; Tata, D.; Cordero, J.; Tomashefsky, P.; Longo, F.; Alfano, M. Laser induced fluorescence spectroscopy from native cancerous and normal tissue. IEEE J. Quantum Electron. 1984, 20, 1507–1511. [Google Scholar] [CrossRef]
- Restelli, F.; Pollo, B.; Vetrano, I.G.; Cabras, S.; Broggi, M.; Schiariti, M.; Falco, J.; de Laurentis, C.; Raccuia, G.; Ferroli, P.; et al. Confocal Laser Microscopy in Neurosurgery: State of the Art of Actual Clinical Applications. J. Clin. Med. 2021, 10, 2035. [Google Scholar] [CrossRef]
- Belykh, E.; Patel, A.A.; Miller, E.J.; Bozkurt, B.; Yagmurlu, K.; Woolf, E.C.; Scheck, A.C.; Eschbacher, J.M.; Nakaji, P.; Preul, M.C. Probe-based three-dimensional confocal laser endomicroscopy of brain tumors: Technical note. Cancer Manag. Res. 2018, 10, 3109–3123. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Georges, J.; Eschbacher, J.M.; Cavalcanti, D.D.; Elhadi, A.M.; Abdelwahab, M.G.; Scheck, A.C.; Nakaji, P.; Spetzler, R.F.; Preul, M.C. Potential application of a handheld confocal endomicroscope imaging system using a variety of fluorophores in experimental gliomas and normal brain. Neurosurg. Focus 2014, 36, E16. [Google Scholar] [CrossRef]
- Pekmezci, M.; Morshed, R.A.; Chunduru, P.; Pandian, B.; Young, J.; Villanueva-Meyer, J.E.; Tihan, T.; Sloan, E.A.; Aghi, M.K.; Molinaro, A.M.; et al. Detection of glioma infiltration at the tumor margin using quantitative stimulated Raman scattering histology. Sci. Rep. 2021, 11, 12162. [Google Scholar] [CrossRef]
- Desroches, J.; Jermyn, M.; Pinto, M.; Picot, F.; Tremblay, M.A.; Obaid, S.; Marple, E.; Urmey, K.; Trudel, D.; Soulez, G.; et al. A new method using Raman spectroscopy for in vivo targeted brain cancer tissue biopsy. Sci. Rep. 2018, 8, 1792. [Google Scholar] [CrossRef]
- Alfonso-Garcia, A.; Zhou, X.; Bec, J.; Anbunesan, S.N.; Fereidouni, F.; Jin, L.W.; Lee, H.S.; Bloch, O.; Marcu, L. First in patient assessment of brain tumor infiltrative margins using simultaneous time-resolved measurements of 5-ALA-induced PpIX fluorescence and tissue autofluorescence. J. Biomed. Opt. 2022, 27, 020501. [Google Scholar] [CrossRef]
- Noble Anbunesan, S.; Alfonso-Garcia, A.; Zhou, X.; Bec, J.; Lee, H.S.; Jin, L.W.; Bloch, O.; Marcu, L. Intraoperative detection of IDH-mutant glioma using fluorescence lifetime imaging. J. Biophotonics 2023, 16, e202200291. [Google Scholar] [CrossRef]
- Lukina, M.; Yashin, K.; Kiseleva, E.E.; Alekseeva, A.; Dudenkova, V.; Zagaynova, E.V.; Bederina, E.; Medyanic, I.; Becker, W.; Mishra, D.; et al. Label-Free Macroscopic Fluorescence Lifetime Imaging of Brain Tumors. Front. Oncol. 2021, 11, 666059. [Google Scholar] [CrossRef]
- Yashin, K.; Bonsanto, M.M.; Achkasova, K.; Zolotova, A.; Wael, A.M.; Kiseleva, E.; Moiseev, A.; Medyanik, I.; Kravets, L.; Huber, R.; et al. OCT-Guided Surgery for Gliomas: Current Concept and Future Perspectives. Diagnostics 2022, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, N.L.; Eschbacher, J.M.; Kalani, M.Y.; Turner, J.D.; Belykh, E.; Spetzler, R.F.; Nakaji, P.; Preul, M.C. Prospective evaluation of the utility of intraoperative confocal laser endomicroscopy in patients with brain neoplasms using fluorescein sodium: Experience with 74 cases. Neurosurg. Focus 2016, 40, E11. [Google Scholar] [CrossRef] [PubMed]
- Hohne, J.; Schebesch, K.M.; Zoubaa, S.; Proescholdt, M.; Riemenschneider, M.J.; Schmidt, N.O. Intraoperative imaging of brain tumors with fluorescein: Confocal laser endomicroscopy in neurosurgery. Clinical and user experience. Neurosurg. Focus 2021, 50, E19. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.; Meyronet, D.; Meyer-Bisch, V.; Armoiry, X.; Pikul, B.; Dumot, C.; Beuriat, P.A.; Signorelli, F.; Guyotat, J. Intraoperative Probe-Based Confocal Laser Endomicroscopy in Surgery and Stereotactic Biopsy of Low-Grade and High-Grade Gliomas: A Feasibility Study in Humans. Neurosurgery 2016, 79, 604–612. [Google Scholar] [CrossRef]
- Sanai, N.; Snyder, L.A.; Honea, N.J.; Coons, S.W.; Eschbacher, J.M.; Smith, K.A.; Spetzler, R.F. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J. Neurosurg. 2011, 115, 740–748. [Google Scholar] [CrossRef]
- Dudenkova, V.V.; Yashin, K.S.; Kiseleva, E.B.; Kuznetsov, S.S.; Timofeeva, L.B.; Khalansky, A.S.; Elagin, V.V.; Gubarkova, E.V.; Karabut, M.M.; Pavlova, N.P.; et al. Multiphoton Tomography and Cross-Polarization Optical Coherence Tomography for Diagnosing Brain Gliomas: Pilot Study. Sovrem. Tehnol. Med. 2016, 8, 64–75. [Google Scholar] [CrossRef]
- Poulon, F.; Pallud, J.; Varlet, P.; Zanello, M.; Chretien, F.; Dezamis, E.; Abi-Lahoud, G.; Nataf, F.; Turak, B.; Devaux, B.; et al. Real-time Brain Tumor imaging with endogenous fluorophores: A diagnosis proof-of-concept study on fresh human samples. Sci. Rep. 2018, 8, 14888. [Google Scholar] [CrossRef]
- Mehidine, H. A Multimodal Two-Photon Fluorescence Endomicroscope and Its Associated Tissue Database to Discriminate Brain Tumors Intraoperatively [Endomicroscope de Fluorescence Multimodale Sous Excitation Biphotonique et Sa Base de Données Tissulaire Associée Pour la Discrimination Per-opératoire des Tumeurs Cérébrales]. Ph.D. Dissertation, Université Paris Cité, Paris, France, 2020. [Google Scholar]
- Shcheslavskiy, V.I.; Shirmanova, M.V.; Yashin, K.S.; Ruck, A.C.; Skala, M.C.; Becker, W. Fluorescence Lifetime Imaging Techniques-A Review on Principles, Applications and Clinical Relevance. J. Biophotonics 2025, 247, e202400450. [Google Scholar] [CrossRef]
- Bi, J.; Chowdhry, S.; Wu, S.; Zhang, W.; Masui, K.; Mischel, P.S. Altered cellular metabolism in gliomas—An emerging landscape of actionable co-dependency targets. Nat. Rev. Cancer 2020, 20, 57–70. [Google Scholar] [CrossRef]
- Sulkowski, P.L.; Corso, C.D.; Robinson, N.D.; Scanlon, S.E.; Purshouse, K.R.; Bai, H.; Liu, Y.; Sundaram, R.K.; Hegan, D.C.; Fons, N.R.; et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci. Transl. Med. 2017, 9, eaal2463. [Google Scholar] [CrossRef]
- Zhou, W.; Wahl, D.R. Metabolic Abnormalities in Glioblastoma and Metabolic Strategies to Overcome Treatment Resistance. Cancers 2019, 11, 1231. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Shcheslavskiy, V.I.; Shirmanova, M.V.; Dudenkova, V.V.; Lukyanov, K.A.; Gavrina, A.I.; Shumilova, A.V.; Zagaynova, E.; Becker, W. Fluorescence time-resolved macroimaging. Opt. Lett. 2018, 43, 3152–3155. [Google Scholar] [CrossRef] [PubMed]
- Shcheslavskiy, V.I.; Yuzhakova, D.V.; Sachkova, D.A.; Shirmanova, M.V.; Becker, W. Macroscopic temporally and spectrally resolved fluorescence imaging enhanced by laser-wavelength multiplexing. Opt. Lett. 2023, 48, 5309–5312. [Google Scholar] [CrossRef]
- Erkkila, M.T.; Reichert, D.; Gesperger, J.; Kiesel, B.; Roetzer, T.; Mercea, P.A.; Drexler, W.; Unterhuber, A.; Leitgeb, R.A.; Woehrer, A.; et al. Macroscopic fluorescence-lifetime imaging of NADH and protoporphyrin IX improves the detection and grading of 5-aminolevulinic acid-stained brain tumors. Sci. Rep. 2020, 10, 20492. [Google Scholar] [CrossRef]
- Lagarto, J.L.; Shcheslavskiy, V.; Pavone, F.S.; Cicchi, R. Real-time fiber-based fluorescence lifetime imaging with synchronous external illumination: A new path for clinical translation. J. Biophotonics 2019, 13, e201960119. [Google Scholar] [CrossRef]
- Marcu, L.; Hartl, B.A. Fluorescence Lifetime Spectroscopy and Imaging in Neurosurgery. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1465–1477. [Google Scholar] [CrossRef]
- Zanello, M.; Poulon, F.; Pallud, J.; Varlet, P.; Hamzeh, H.; Abi Lahoud, G.; Andreiuolo, F.; Ibrahim, A.; Pages, M.; Chretien, F.; et al. Multimodal optical analysis discriminates freshly extracted human sample of gliomas, metastases and meningiomas from their appropriate controls. Sci. Rep. 2017, 7, 41724. [Google Scholar] [CrossRef]
- Kantelhardt, S.R.; Kalasauskas, D.; König, K.; Kim, E.; Weinigel, M.; Uchugonova, A.; Giese, A. In vivo multiphoton tomography and fluorescence lifetime imaging of human brain tumor tissue. J. Neuro-Oncol. 2016, 127, 473–482. [Google Scholar] [CrossRef]
- Stupak, E.V.; Glotov, V.M.; Askandaryan, A.S.; Clancy, S.E.; Hiana, J.C.; Cherkasova, O.P.; Stupak, V.V. Raman Spectroscopy in the Diagnosis of Brain Gliomas: A Literature Review. Cureus 2025, 17, e79165. [Google Scholar] [CrossRef]
- DePaoli, D.; Lemoine, E.; Ember, K.; Parent, M.; Prud’homme, M.; Cantin, L.; Petrecca, K.; Leblond, F.; Cote, D.C. Rise of Raman spectroscopy in neurosurgery: A review. J. Biomed. Opt. 2020, 25, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Haka, A.S.; Volynskaya, Z.; Gardecki, J.A.; Nazemi, J.; Lyons, J.; Hicks, D.; Fitzmaurice, M.; Dasari, R.R.; Crowe, J.P.; Feld, M.S. In vivo Margin Assessment during Partial Mastectomy Breast Surgery Using Raman Spectroscopy. Cancer Res. 2006, 66, 3317–3322. [Google Scholar] [CrossRef] [PubMed]
- Puppels, G.J.; de Mul, F.F.M.; Otto, C.; Greve, J.; Robert-Nicoud, M.; Arndt-Jovin, D.J.; Jovin, T.M. Studying single living cells and chromosomes by confocal Raman microspectroscopy. Nature 1990, 347, 301–303. [Google Scholar] [CrossRef]
- Gebhart, S.C.; Lin, W.C.; Mahadevan-Jansen, A. In vitrodetermination of normal and neoplastic human brain tissue optical properties using inverse adding-doubling. Phys. Med. Biol. 2006, 51, 2011–2027. [Google Scholar] [CrossRef]
- Bevilacqua, F.; Piguet, D.; Marquet, P.; Gross, J.D.; Tromberg, B.J.; Depeursinge, C. In vivo local determination of tissue optical properties: Applications to human brain. Appl. Opt. 1999, 38, 4939–4950. [Google Scholar] [CrossRef]
- Liewald, D.; Miller, R.; Logothetis, N.; Wagner, H.-J.; Schüz, A. Distribution of axon diameters in cortical white matter: An electron-microscopic study on three human brains and a macaque. Biol. Cybern. 2014, 108, 541–557. [Google Scholar] [CrossRef]
- Tuchin, V.V. Tissue Optics and Photonics: Light-Tissue Interaction. J. Biomed. Photonics Eng. 2015, 1, 98–134. [Google Scholar] [CrossRef]
- Eichberg, D.G.; Shah, A.H.; Di, L.; Semonche, A.M.; Jimsheleishvili, G.; Luther, E.M.; Sarkiss, C.A.; Levi, A.D.; Gultekin, S.H.; Komotar, R.J.; et al. Stimulated Raman histology for rapid and accurate intraoperative diagnosis of CNS tumors: Prospective blinded study. J. Neurosurg. 2021, 134, 137–143. [Google Scholar] [CrossRef]
- Restelli, F.; Mathis, A.M.; Hohne, J.; Mazzapicchi, E.; Acerbi, F.; Pollo, B.; Quint, K. Confocal laser imaging in neurosurgery: A comprehensive review of sodium fluorescein-based CONVIVO preclinical and clinical applications. Front. Oncol. 2022, 12, 998384. [Google Scholar] [CrossRef]
- Mat Zin, A.A.; Zulkarnain, S. Diagnostic Accuracy of Cytology Smear and Frozen Section in Glioma. Asian Pac. J. Cancer Prev. 2019, 20, 321–325. [Google Scholar] [CrossRef]
- Fuller, C. A little piece of mind: Best practices for brain tumor intraoperative consultation. Mod. Pathol. 2019, 32, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, S.S.S.; Hollon, T.C.; Adapa, A.; Urias, E.; Srinivasan, S.; Jairath, N.; Szczepanski, J.; Ouillette, P.; Camelo-Piragua, S.; Orringer, D.A. Automated histologic diagnosis of CNS tumors with machine learning. CNS Oncol. 2020, 9, CNS56. [Google Scholar] [CrossRef] [PubMed]
- Ermolaev, A.Y.; Kravets, L.Y.; Smetanina, S.V.; Kolpakova, A.A.; Yashin, K.S.; Morev, A.V.; Smetatina, O.V.; Klyuev, E.A.; Medyanik, I.A. Cytologic control of the resection margins of hemispheric gliomas and metastases. Zhurnal Vopr. Neirokhirurgii Im. N. N. Burdenko 2020, 84, 33–42. [Google Scholar] [CrossRef]
- Robboy, S.J.; Weintraub, S.; Horvath, A.E.; Jensen, B.W.; Alexander, C.B.; Fody, E.P.; Crawford, J.M.; Clark, J.R.; Cantor-Weinberg, J.; Joshi, M.G.; et al. Pathologist workforce in the United States: I. Development of a predictive model to examine factors influencing supply. Arch. Pathol. Lab. Med. 2013, 137, 1723–1732. [Google Scholar] [CrossRef]
- Fortin Ensign, S.; Hrachova, M.; Chang, S.; Mrugala, M.M. Assessing the utility and attitudes toward molecular testing in neuro-oncology: A survey of the Society for Neuro-Oncology members. Neurooncol. Pract. 2021, 8, 310–316. [Google Scholar] [CrossRef]
- Nasrallah, M.P.; Zhao, J.; Tsai, C.C.; Meredith, D.; Marostica, E.; Ligon, K.L.; Golden, J.A.; Yu, K.H. Machine learning for cryosection pathology predicts the 2021 WHO classification of glioma. Medicine 2023, 4, 526–540.e524. [Google Scholar] [CrossRef]
- Assayag, O.; Grieve, K.; Devaux, B.; Harms, F.; Pallud, J.; Chretien, F.; Boccara, C.; Varlet, P. Imaging of non-tumorous and tumorous human brain tissues with full-field optical coherence tomography. Neuroimage Clin. 2013, 2, 549–557. [Google Scholar] [CrossRef]
- Hollon, T.; Jiang, C.; Chowdury, A.; Nasir-Moin, M.; Kondepudi, A.; Aabedi, A.; Adapa, A.; Al-Holou, W.; Heth, J.; Sagher, O.; et al. Artificial-intelligence-based molecular classification of diffuse gliomas using rapid, label-free optical imaging. Nat. Med. 2023, 29, 828–832. [Google Scholar] [CrossRef]
- Acerbi, F.; Pollo, B.; De Laurentis, C.; Restelli, F.; Falco, J.; Vetrano, I.G.; Broggi, M.; Schiariti, M.; Tramacere, I.; Ferroli, P.; et al. Ex Vivo Fluorescein-Assisted Confocal Laser Endomicroscopy (CONVIVO(R) System) in Patients with Glioblastoma: Results from a Prospective Study. Front. Oncol. 2020, 10, 606574. [Google Scholar] [CrossRef]
- Eschbacher, J.; Martirosyan, N.L.; Nakaji, P.; Sanai, N.; Preul, M.C.; Smith, K.A.; Coons, S.W.; Spetzler, R.F. In vivo intraoperative confocal microscopy for real-time histopathological imaging of brain tumors. J. Neurosurg. 2012, 116, 854–860. [Google Scholar] [CrossRef]
- Di, L.; Eichberg, D.G.; Huang, K.; Shah, A.H.; Jamshidi, A.M.; Luther, E.M.; Lu, V.M.; Komotar, R.J.; Ivan, M.E.; Gultekin, S.H. Stimulated Raman Histology for Rapid Intraoperative Diagnosis of Gliomas. World Neurosurg. 2021, 150, e135–e143. [Google Scholar] [CrossRef] [PubMed]
- Radtke, K.; Schulz-Schaeffer, W.J.; Oertel, J. Confocal laser endomicroscopy in glial tumors—A histomorphological analysis. Neurosurg. Rev. 2024, 47, 65. [Google Scholar] [CrossRef] [PubMed]
- Orringer, D.A.; Pandian, B.; Niknafs, Y.S.; Hollon, T.C.; Boyle, J.; Lewis, S.; Garrard, M.; Hervey-Jumper, S.L.; Garton, H.J.L.; Maher, C.O.; et al. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nat. Biomed. Eng. 2017, 1, 0027. [Google Scholar] [CrossRef]
- Li, Y.; Charalampaki, P.; Liu, Y.; Yang, G.Z.; Giannarou, S. Context aware decision support in neurosurgical oncology based on an efficient classification of endomicroscopic data. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1187–1199. [Google Scholar] [CrossRef]
- Alfonso-Garcia, A.; Anbunesan, S.N.; Bec, J.; Lee, H.S.; Jin, L.W.; Bloch, O.; Marcu, L. In vivo characterization of the human glioblastoma infiltrative edge with label-free intraoperative fluorescence lifetime imaging. Biomed. Opt. Express 2023, 14, 2196–2208. [Google Scholar] [CrossRef]
- Yong, W.H.; Butte, P.V.; Pikul, B.K.; Jo, J.A.; Fang, Q.; Papaioannou, T.; Black, K.; Marcu, L. Distinction of brain tissue, low grade and high grade glioma with time-resolved fluorescence spectroscopy. Front. Biosci. 2006, 11, 1255–1263. [Google Scholar] [CrossRef]
- Marcu, L.; Jo, J.A.; Butte, P.V.; Yong, W.H.; Pikul, B.K.; Black, K.L.; Thompson, R.C. Fluorescence lifetime spectroscopy of glioblastoma multiforme. Photochem. Photobiol. 2004, 80, 98–103. [Google Scholar] [CrossRef]
- Shirmanova, M.V.; Lukina, M.; Kisileva, E.B.; Fedoseeva, V.V.; Dudenkova, V.V.; Zagaynova, E.V.; Becker, W.; Shcheslavskiy, V.I. Interrogation of glioma metabolism on macroscale by FLIM. In Proceedings of the SPIE 10882, Multiphoton Microscopy in the Biomedical Sciences XIX, San Francisco, CA, USA, 22 February 2019; p. 1088209. [Google Scholar] [CrossRef]
- Grishin, A.S.; Achkasova, K.A.; Kukhnina, L.S.; Sharova, V.A.; Ostapyuk, M.V.; Yashin, K.S. Peritumoral Brain Zone in Astrocytoma: Morphology, Molecular Aspects, and Clinical Manifestations (Review). Sovrem Tekhnologii Med. 2024, 16, 79–88. [Google Scholar] [CrossRef]
- Ballestin, A.; Armocida, D.; Ribecco, V.; Seano, G. Peritumoral brain zone in glioblastoma: Biological, clinical and mechanical features. Front. Immunol. 2024, 15, 1347877. [Google Scholar] [CrossRef]
- Giambra, M.; Di Cristofori, A.; Valtorta, S.; Manfrellotti, R.; Bigiogera, V.; Basso, G.; Moresco, R.M.; Giussani, C.; Bentivegna, A. The peritumoral brain zone in glioblastoma: Where we are and where we are going. J. Neurosci. Res. 2023, 101, 199–216. [Google Scholar] [CrossRef]
- Lemee, J.M.; Clavreul, A.; Menei, P. Intratumoral heterogeneity in glioblastoma: Don’t forget the peritumoral brain zone. Neuro Oncol. 2015, 17, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xu, S.; Zhong, Y.; Tu, T.; Xu, Y.; Li, X.; Wang, B.; Yang, F. High gene expression levels of VEGFA and CXCL8 in the peritumoral brain zone are associated with the recurrence of glioblastoma: A bioinformatics analysis. Oncol. Lett. 2019, 18, 6171–6179. [Google Scholar] [CrossRef] [PubMed]
- Trusheim, J.; Dunbar, E.; Battiste, J.; Iwamoto, F.; Mohile, N.; Damek, D.; Bota, D.A.; Connelly, J. A state-of-the-art review and guidelines for tumor treating fields treatment planning and patient follow-up in glioblastoma. CNS Oncol. 2017, 6, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Eyupoglu, I.Y.; Buchfelder, M.; Savaskan, N.E. Surgical resection of malignant gliomas-role in optimizing patient outcome. Nat. Rev. Neurol. 2013, 9, 141–151. [Google Scholar] [CrossRef]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–576. [Google Scholar] [CrossRef]
- McGirt, M.J.; Chaichana, K.L.; Gathinji, M.; Attenello, F.J.; Than, K.; Olivi, A.; Weingart, J.D.; Brem, H.; Quinones-Hinojosa, A.R. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J. Neurosurg. 2009, 110, 156–162. [Google Scholar] [CrossRef]
- Gerard, I.J.; Kersten-Oertel, M.; Petrecca, K.; Sirhan, D.; Hall, J.A.; Collins, D.L. Brain shift in neuronavigation of brain tumors: A review. Med. Image Anal. 2017, 35, 403–420. [Google Scholar] [CrossRef]
- Stepp, H.; Stummer, W. 5-ALA in the management of malignant glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef]
- Rogers, C.M.; Jones, P.S.; Weinberg, J.S. Intraoperative MRI for Brain Tumors. J. Neurooncol. 2021, 151, 479–490. [Google Scholar] [CrossRef]
- Dixon, L.; Lim, A.; Grech-Sollars, M.; Nandi, D.; Camp, S. Intraoperative ultrasound in brain tumor surgery: A review and implementation guide. Neurosurg. Rev. 2022, 45, 2503–2515. [Google Scholar] [CrossRef]
- Goryaynov, S.A.; Widhalm, G.; Goldberg, M.F.; Chelushkin, D.; Spallone, A.; Chernyshov, K.A.; Ryzhova, M.; Pavlova, G.; Revischin, A.; Shishkina, L.; et al. The Role of 5-ALA in Low-Grade Gliomas and the Influence of Antiepileptic Drugs on Intraoperative Fluorescence. Front. Oncol. 2019, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Kiesel, B.; Freund, J.; Reichert, D.; Wadiura, L.; Erkkilae, M.T.; Woehrer, A.; Hervey-Jumper, S.; Berger, M.S.; Widhalm, G. 5-ALA in Suspected Low-Grade Gliomas: Current Role, Limitations, and New Approaches. Front. Oncol. 2021, 11, 699301. [Google Scholar] [CrossRef]
- Martinez-Moreno, M.; Kiesel, B.; Woehrer, A.; Mischkulnig, M.; Furtner, J.; Timelthaler, G.; Berger, W.; Knosp, E.; Hainfellner, J.A.; Wolfsberger, S.; et al. Ex-vivo analysis of quantitative 5-ALA fluorescence intensity in diffusely infiltrating gliomas using a handheld spectroscopic probe: Correlation with histopathology, proliferation and microvascular density. Photodiagn. Photodyn. Ther. 2019, 27, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Yashin, K.S.; Kiseleva, E.B.; Gubarkova, E.V.; Moiseev, A.A.; Kuznetsov, S.S.; Shilyagin, P.A.; Gelikonov, G.V.; Medyanik, I.A.; Kravets, L.Y.; Potapov, A.A.; et al. Cross-Polarization Optical Coherence Tomography for Brain Tumor Imaging. Front. Oncol. 2019, 9, 201. [Google Scholar] [CrossRef]
- Juarez-Chambi, R.M.; Kut, C.; Rico-Jimenez, J.J.; Chaichana, K.L.; Xi, J.; Campos-Delgado, D.U.; Rodriguez, F.J.; Quinones-Hinojosa, A.; Li, X.; Jo, J.A. AI-Assisted In Situ Detection of Human Glioma Infiltration Using a Novel Computational Method for Optical Coherence Tomography. Clin. Cancer Res. 2019, 25, 6329–6338. [Google Scholar] [CrossRef]
- Abramov, I.; Mathis, A.M.; Xu, Y.; On, T.J.; Belykh, E.; Mignucci-Jimenez, G.; Hartke, J.N.; Restelli, F.; Pollo, B.; Acerbi, F.; et al. Intraoperative confocal laser endomicroscopy during 5-aminolevulinic acid–guided glioma surgery: Significant considerations for resection at the tumor margin. J. Neurosurg. 2025, 142, 429–442. [Google Scholar] [CrossRef]
- Abramov, I.; Xu, Y.; Mignucci-Jiménez, G.; Hartke, J.N.; Belykh, E.; Porter, R.; Sanai, N.; Smith, K.A.; Eschbacher, J.; Preul, M.C. 292 Intraoperative Confocal Laser Endomicroscopy During 5-ALA Guided Glioma Surgery: Significant Considerations for Resection at the Tumor Margins. Neurosurgery 2024, 70, 83–84. [Google Scholar] [CrossRef]
- Lakomkin, N.; Hadjipanayis, C.G. The Use of Spectroscopy Handheld Tools in Brain Tumor Surgery: Current Evidence and Techniques. Front. Surg. 2019, 6, 30. [Google Scholar] [CrossRef]
- Bohringer, H.J.; Lankenau, E.; Stellmacher, F.; Reusche, E.; Huttmann, G.; Giese, A. Imaging of human brain tumor tissue by near-infrared laser coherence tomography. Acta Neurochir. 2009, 151, 507–517, discussion 517. [Google Scholar] [CrossRef]
- Lankenau, E.; Klinger, D.; Winter, C.; Malik, A.; Müller, H.H.; Oelckers, S.; Pau, H.-W.; Just, T.; Hüttmann, G. Combining Optical Coherence Tomography (OCT) with an Operating Microscope. In Advances in Medical Engineering; Springer Proceedings in Physics; Springer: Berlin/Heidelberg, Germany, 2007; pp. 343–348. [Google Scholar]
- Orillac, C.; Stummer, W.; Orringer, D.A. Fluorescence Guidance and Intraoperative Adjuvants to Maximize Extent of Resection. Neurosurgery 2021, 89, 727–736. [Google Scholar] [CrossRef]
- Gautheron, A.; Bernstock, J.D.; Picart, T.; Guyotat, J.; Valdes, P.A.; Montcel, B. 5-ALA induced PpIX fluorescence spectroscopy in neurosurgery: A review. Front. Neurosci. 2024, 18, 1310282. [Google Scholar] [CrossRef] [PubMed]
- Potapov, A.A.; Goriainov, S.A.; Loshchenov, V.B.; Savel’eva, T.A.; Gavrilov, A.G.; Okhlopkov, V.A.; Zhukov, V.; Zelenkov, P.V.; Gol’bin, D.A.; Shurkhai, V.A.; et al. Intraoperative combined spectroscopy (optical biopsy) of cerebral gliomas. Zhurnal Vopr. Neirokhirurgii Im. N. N. Burdenko 2013, 77, 3–10. [Google Scholar]
- Widhalm, G.; Olson, J.; Weller, J.; Bravo, J.; Han, S.J.; Phillips, J.; Hervey-Jumper, S.L.; Chang, S.M.; Roberts, D.W.; Berger, M.S. The value of visible 5-ALA fluorescence and quantitative protoporphyrin IX analysis for improved surgery of suspected low-grade gliomas. J. Neurosurg. 2020, 133, 79–88. [Google Scholar] [CrossRef]
- Valdes, P.A.; Jacobs, V.; Harris, B.T.; Wilson, B.C.; Leblond, F.; Paulsen, K.D.; Roberts, D.W. Quantitative fluorescence using 5-aminolevulinic acid-induced protoporphyrin IX biomarker as a surgical adjunct in low-grade glioma surgery. J. Neurosurg. 2015, 123, 771–780. [Google Scholar] [CrossRef]
- Stummer, W.; Tonn, J.C.; Goetz, C.; Ullrich, W.; Stepp, H.; Bink, A.; Pietsch, T.; Pichlmeier, U. 5-Aminolevulinic acid-derived tumor fluorescence: The diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery 2014, 74, 310–319, discussion 319–320. [Google Scholar] [CrossRef]
- Herta, J.; Cho, A.; Roetzer-Pejrimovsky, T.; Hoftberger, R.; Marik, W.; Kronreif, G.; Peilnsteiner, T.; Rossler, K.; Wolfsberger, S. Optimizing maximum resection of glioblastoma: Raman spectroscopy versus 5-aminolevulinic acid. J. Neurosurg. 2023, 139, 334–343. [Google Scholar] [CrossRef]
- Coburger, J.; Scheuerle, A.; Thal, D.R.; Engelke, J.; Hlavac, M.; Wirtz, C.R.; Konig, R. Linear array ultrasound in low-grade glioma surgery: Histology-based assessment of accuracy in comparison to conventional intraoperative ultrasound and intraoperative MRI. Acta Neurochir. 2015, 157, 195–206. [Google Scholar] [CrossRef]
- Coburger, J.; Engelke, J.; Scheuerle, A.; Thal, D.R.; Hlavac, M.; Wirtz, C.R.; Konig, R. Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: A prospective study based on histopathological assessment. Neurosurg. Focus 2014, 36, E3. [Google Scholar] [CrossRef]
- Kubben, P.L.; Wesseling, P.; Lammens, M.; Schijns, O.E.; Ter Laak-Poort, M.P.; van Overbeeke, J.J.; van Santbrink, H. Correlation between contrast enhancement on intraoperative magnetic resonance imaging and histopathology in glioblastoma. Surg. Neurol. Int. 2012, 3, 158. [Google Scholar] [CrossRef]
- Munkvold, B.K.R.; Jakola, A.S.; Reinertsen, I.; Sagberg, L.M.; Unsgard, G.; Solheim, O. The Diagnostic Properties of Intraoperative Ultrasound in Glioma Surgery and Factors Associated with Gross Total Tumor Resection. World Neurosurg. 2018, 115, e129–e136. [Google Scholar] [CrossRef]
- Sweeney, J.F.; Smith, H.; Taplin, A.; Perloff, E.; Adamo, M.A. Efficacy of intraoperative ultrasonography in neurosurgical tumor resection. J. Neurosurg. Pediatr. 2018, 21, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Arlt, F.; Chalopin, C.; Muns, A.; Meixensberger, J.; Lindner, D. Intraoperative 3D contrast-enhanced ultrasound (CEUS): A prospective study of 50 patients with brain tumours. Acta Neurochir. 2016, 158, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Gerganov, V.M.; Samii, A.; Giordano, M.; Samii, M.; Fahlbusch, R. Two-dimensional high-end ultrasound imaging compared to intraoperative MRI during resection of low-grade gliomas. J. Clin. Neurosci. 2011, 18, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Van Hese, L.; De Vleeschouwer, S.; Theys, T.; Rex, S.; Heeren, R.M.A.; Cuypers, E. The diagnostic accuracy of intraoperative differentiation and delineation techniques in brain tumours. Discov. Oncol. 2022, 13, 123. [Google Scholar] [CrossRef]
- Panciani, P.P.; Fontanella, M.; Schatlo, B.; Garbossa, D.; Agnoletti, A.; Ducati, A.; Lanotte, M. Fluorescence and image guided resection in high grade glioma. Clin. Neurol. Neurosurg. 2012, 114, 37–41. [Google Scholar] [CrossRef]
- Valdes, P.A.; Leblond, F.; Kim, A.; Harris, B.T.; Wilson, B.C.; Fan, X.; Tosteson, T.D.; Hartov, A.; Ji, S.; Erkmen, K.; et al. Quantitative fluorescence in intracranial tumor: Implications for ALA-induced PpIX as an intraoperative biomarker. J. Neurosurg. 2011, 115, 11–17. [Google Scholar] [CrossRef]
- Jermyn, M.; Mok, K.; Mercier, J.; Desroches, J.; Pichette, J.; Saint-Arnaud, K.; Bernstein, L.; Guiot, M.-C.; Petrecca, K.; Leblond, F. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci. Transl. Med. 2015, 7, 274ra19. [Google Scholar] [CrossRef]
- Livermore, L.J.; Isabelle, M.; Bell, I.M.; Edgar, O.; Voets, N.L.; Stacey, R.; Ansorge, O.; Vallance, C.; Plaha, P. Raman spectroscopy to differentiate between fresh tissue samples of glioma and normal brain: A comparison with 5-ALA-induced fluorescence-guided surgery. J. Neurosurg. 2021, 135, 469–479. [Google Scholar] [CrossRef]
- Kalkanis, S.N.; Kast, R.E.; Rosenblum, M.L.; Mikkelsen, T.; Yurgelevic, S.M.; Nelson, K.M.; Raghunathan, A.; Poisson, L.M.; Auner, G.W. Raman spectroscopy to distinguish grey matter, necrosis, and glioblastoma multiforme in frozen tissue sections. J. Neurooncol. 2014, 116, 477–485. [Google Scholar] [CrossRef]
- Kut, C.; Chaichana, K.L.; Xi, J.; Raza, S.M.; Ye, X.; McVeigh, E.R.; Rodriguez, F.J.; Quiñones-Hinojosa, A.; Li, X. Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci. Transl. Med. 2015, 7, 292ra100. [Google Scholar] [CrossRef]
- Almasian, M.; Wilk, L.S.; Bloemen, P.R.; van Leeuwen, T.G.; ter Laan, M.; Aalders, M.C.G. Pilot feasibility study of in vivo intraoperative quantitative optical coherence tomography of human brain tissue during glioma resection. J. Biophotonics 2019, 12, e201900037. [Google Scholar] [CrossRef] [PubMed]
- Yashin, K.S.; Kiseleva, E.B.; Moiseev, A.A.; Kuznetsov, S.S.; Timofeeva, L.B.; Pavlova, N.P.; Gelikonov, G.V.; Medyanik, I.A.; Kravets, L.Y.; Zagaynova, E.V.; et al. Quantitative nontumorous and tumorous human brain tissue assessment using microstructural co- and cross-polarized optical coherence tomography. Sci. Rep. 2019, 9, 2024. [Google Scholar] [CrossRef] [PubMed]
- Belykh, E.; Zhao, X.; Ngo, B.; Farhadi, D.S.; Byvaltsev, V.A.; Eschbacher, J.M.; Nakaji, P.; Preul, M.C. Intraoperative Confocal Laser Endomicroscopy Ex Vivo Examination of Tissue Microstructure During Fluorescence-Guided Brain Tumor Surgery. Front. Oncol. 2020, 10, 599250. [Google Scholar] [CrossRef] [PubMed]
- Do, T.H.; Howard, M.A.; Palzer, E.F.; Huling, J.D.; Alvi, M.A.; Cramer, S.W.; Zhu, P.; Johnson, R.A.; Jean, J.; Lu, J.; et al. Readmission risk of malignant brain tumor patients undergoing laser interstitial thermal therapy (LITT) and stereotactic needle biopsy (SNB): A covariate balancing weights analysis of the National Readmissions Database (NRD). J. Neurooncol. 2022, 159, 553–561. [Google Scholar] [CrossRef]
- Zoeller, G.K.; Benveniste, R.J.; Landy, H.; Morcos, J.J.; Jagid, J. Outcomes and Management Strategies after Nondiagnostic Stereotactic Biopsies of Brain Lesions. Stereotact. Funct. Neurosurg. 2009, 87, 174–181. [Google Scholar] [CrossRef]
- Dammers, R.; Haitsma, I.K.; Schouten, J.W.; Kros, J.M.; Avezaat, C.J.J.; Vincent, A.J.P.E. Safety and efficacy of frameless and frame-based intracranial biopsy techniques. Acta Neurochir. 2008, 150, 23–29. [Google Scholar] [CrossRef]
- Dammers, R.; Schouten, J.W.; Haitsma, I.K.; Vincent, A.J.P.E.; Kros, J.M.; Dirven, C.M.F. Towards improving the safety and diagnostic yield of stereotactic biopsy in a single centre. Acta Neurochir. 2010, 152, 1915–1921. [Google Scholar] [CrossRef]
- Tilgner, J.; Herr, M.; Ostertag, C.; Volk, B. Validation of intraoperative diagnoses using smear preparations from stereotactic brain biopsies: Intraoperative versus final diagnosis--influence of clinical factors. Neurosurgery 2005, 56, 257–265. [Google Scholar] [CrossRef]
- Woodworth, G.; McGirt, M.J.; Samdani, A.; Garonzik, I.; Olivi, A.; Weingart, J.D. Accuracy of frameless and frame-based image-guided stereotactic brain biopsy in the diagnosis of glioma: Comparison of biopsy and open resection specimen. Neurol. Res. 2013, 27, 358–362. [Google Scholar] [CrossRef]
- Heper, A.O.; Erden, E.; Savas, A.; Ceyhan, K.; Erden, I.; Akyar, S.; Kanpolat, Y. An analysis of stereotactic biopsy of brain tumors and nonneoplastic lesions: A prospective clinicopathologic study. Surg. Neurol. 2005, 64, S82–S88. [Google Scholar] [CrossRef]
- Gralla, J.; Nimsky, C.; Buchfelder, M.; Fahlbusch, R.; Ganslandt, O. Frameless Stereotactic Brain Biopsy Procedures Using the Stealth Station: Indications, Accuracy and Results. Zentralblatt Für Neurochir. 2003, 64, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Shooman, D.; Belli, A.; Grundy, P.L. Image-guided frameless stereotactic biopsy without intraoperative neuropathological examination. J. Neurosurg. 2010, 113, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Field, M.; Witham, T.F.; Flickinger, J.C.; Kondziolka, D.; Lunsford, L.D. Comprehensive assessment of hemorrhage risks and outcomes after stereotactic brain biopsy. J. Neurosurg. 2001, 94, 545–551. [Google Scholar] [CrossRef]
- Grossman, R.; Sadetzki, S.; Spiegelmann, R.; Ram, Z. Haemorrhagic complications and the incidence of asymptomatic bleeding associated with stereotactic brain biopsies. Acta Neurochir. 2005, 147, 627–631. [Google Scholar] [CrossRef]
- Dorward, N.L.; Paleologos, T.S.; Alberti, O.; Thomas, D.G. The advantages of frameless stereotactic biopsy over frame-based biopsy. Br. J. Neurosurg. 2002, 16, 110–118. [Google Scholar] [CrossRef]
- Lunsford, L.D.; Niranjan, A.; Khan, A.A.; Kondziolka, D. Establishing a Benchmark for Complications Using Frame-Based Stereotactic Surgery. Stereotact. Funct. Neurosurg. 2008, 86, 278–287. [Google Scholar] [CrossRef]
- Bex, A.; Mathon, B. Advances, technological innovations, and future prospects in stereotactic brain biopsies. Neurosurg. Rev. 2022, 46, 5. [Google Scholar] [CrossRef]
- Markwardt, N.A.; Haj-Hosseini, N.; Hollnburger, B.; Stepp, H.; Zelenkov, P.; Ruhm, A. 405 nm versus 633 nm for protoporphyrin IX excitation in fluorescence-guided stereotactic biopsy of brain tumors. J. Biophotonics 2016, 9, 901–912. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ishikawa, E.; Miki, S.; Sakamoto, N.; Zaboronok, A.; Matsuda, M.; Akutsu, H.; Nakai, K.; Tsuruta, W.; Matsumura, A. Photodynamic Diagnosis Using 5-Aminolevulinic Acid in 41 Biopsies for Primary Central Nervous System Lymphoma. Photochem. Photobiol. 2015, 91, 1452–1457. [Google Scholar] [CrossRef]
- von Campe, G.; Moschopulos, M.; Hefti, M. 5-Aminolevulinic acid-induced protoporphyrin IX fluorescence as immediate intraoperative indicator to improve the safety of malignant or high-grade brain tumor diagnosis in frameless stereotactic biopsies. Acta Neurochir. 2012, 154, 585–588, discussion 588. [Google Scholar] [CrossRef]
- Widhalm, G.; Minchev, G.; Woehrer, A.; Preusser, M.; Kiesel, B.; Furtner, J.; Mert, A.; Di Ieva, A.; Tomanek, B.; Prayer, D.; et al. Strong 5-aminolevulinic acid-induced fluorescence is a novel intraoperative marker for representative tissue samples in stereotactic brain tumor biopsies. Neurosurg. Rev. 2012, 35, 381–391, discussion 391. [Google Scholar] [CrossRef] [PubMed]
- Millesi, M.; Kiesel, B.; Wohrer, A.; Mercea, P.A.; Bissolo, M.; Roetzer, T.; Wolfsberger, S.; Furtner, J.; Knosp, E.; Widhalm, G. Is Intraoperative Pathology Needed if 5-Aminolevulinic-Acid-Induced Tissue Fluorescence Is Found in Stereotactic Brain Tumor Biopsy? Neurosurgery 2020, 86, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.P.; Wierwille, J.; Moreira, T.; Schwartzbauer, G.; Jafri, M.S.; Tang, C.M.; Chen, Y. A forward-imaging needle-type OCT probe for image guided stereotactic procedures. Opt. Express 2011, 19, 26283–26294. [Google Scholar] [CrossRef]
- Scolaro, L.; Lorenser, D.; Madore, W.J.; Kirk, R.W.; Kramer, A.S.; Yeoh, G.C.; Godbout, N.; Sampson, D.D.; Boudoux, C.; McLaughlin, R.A. Molecular imaging needles: Dual-modality optical coherence tomography and fluorescence imaging of labeled antibodies deep in tissue. Biomed. Opt. Express 2015, 6, 1767–1781. [Google Scholar] [CrossRef]
- Akshulakov, S.K.; Kerimbayev, T.T.; Biryuchkov, M.Y.; Urunbayev, Y.A.; Farhadi, D.S.; Byvaltsev, V.A. Current Trends for Improving Safety of Stereotactic Brain Biopsies: Advanced Optical Methods for Vessel Avoidance and Tumor Detection. Front. Oncol. 2019, 9, 947. [Google Scholar] [CrossRef]
- Benediktsson, H.; Andersson, T.; Sjolander, U.; Hartman, M.; Lindgren, P.G. Ultrasound guided needle biopsy of brain tumors using an automatic sampling instrument. Acta Radiol. 1992, 33, 512–517. [Google Scholar] [CrossRef]
- Pichette, J.; Goyette, A.; Picot, F.; Tremblay, M.A.; Soulez, G.; Wilson, B.C.; Leblond, F. Sensitivity analysis aimed at blood vessels detection using interstitial optical tomography during brain needle biopsy procedures. Biomed. Opt. Express 2015, 6, 4238–4254. [Google Scholar] [CrossRef]
- Kiesel, B.; Millesi, M.; Woehrer, A.; Furtner, J.; Bavand, A.; Roetzer, T.; Mischkulnig, M.; Wolfsberger, S.; Preusser, M.; Knosp, E.; et al. 5-ALA-induced fluorescence as a marker for diagnostic tissue in stereotactic biopsies of intracranial lymphomas: Experience in 41 patients. Neurosurg. Focus 2018, 44, E7. [Google Scholar] [CrossRef]
- Ramakonar, H.; Quirk, B.C.; Kirk, R.W.; Li, J.; Jacques, A.; Lind, C.R.P.; McLaughlin, R.A. Intraoperative detection of blood vessels with an imaging needle during neurosurgery in humans. Sci. Adv. 2018, 4, eaav4992. [Google Scholar] [CrossRef]
- Gobel, W.; Brucker, D.; Kienast, Y.; Johansson, A.; Kniebuhler, G.; Ruhm, A.; Eigenbrod, S.; Fischer, S.; Goetz, M.; Kreth, F.W.; et al. Optical needle endoscope for safe and precise stereotactically guided biopsy sampling in neurosurgery. Opt. Express 2012, 20, 26117–26126. [Google Scholar] [CrossRef]
- Richter, J.; Haj-Hosseini, N.; Milos, P.; Hallbeck, M.; Wardell, K. Optical Brain Biopsy with a Fluorescence and Vessel Tracing Probe. Oper. Neurosurg. 2021, 21, 217–224. [Google Scholar] [CrossRef]
- McLaughlin, R.A.; Quirk, B.C.; Curatolo, A.; Kirk, R.W.; Scolaro, L.; Lorenser, D.; Robbins, P.D.; Wood, B.A.; Saunders, C.M.; Sampson, D.D. Imaging of Breast Cancer with Optical Coherence Tomography Needle Probes: Feasibility and Initial Results. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1184–1191. [Google Scholar] [CrossRef]
- Scolaro, L.; Lorenser, D.; McLaughlin, R.A.; Quirk, B.C.; Kirk, R.W.; Sampson, D.D. High-sensitivity anastigmatic imaging needle for optical coherence tomography. Opt. Lett. 2012, 37, 5247–5249. [Google Scholar] [CrossRef]
- Lorenser, D.; Yang, X.; Kirk, R.W.; Quirk, B.C.; McLaughlin, R.A.; Sampson, D.D. Ultrathin side-viewing needle probe for optical coherence tomography. Opt. Lett. 2011, 36, 3894–3896. [Google Scholar] [CrossRef]
- Kiseleva, E.B.; Shilyagin, P.A.; Romashov, V.N.; Korzhimanova, Y.V.; Sirotkina, M.A.; Yashin, K.S.; Zagaynova, E.V.; Gelikonov, G.V.; Gladkova, N.D. Cross-polarization OCT needle probe for combined blood vessels detection and tissue differentiation during stereotactic biopsy of brain tumors. In Proceedings of the European Conference on Biomedical Optics 2019, Munich Germany, 23–25 June 2019. [Google Scholar]
- Galli, R.; Meinhardt, M.; Koch, E.; Schackert, G.; Steiner, G.; Kirsch, M.; Uckermann, O. Rapid Label-Free Analysis of Brain Tumor Biopsies by Near Infrared Raman and Fluorescence Spectroscopy-A Study of 209 Patients. Front. Oncol. 2019, 9, 1165. [Google Scholar] [CrossRef]
- Uckermann, O.; Yao, W.; Juratli, T.A.; Galli, R.; Leipnitz, E.; Meinhardt, M.; Koch, E.; Schackert, G.; Steiner, G.; Kirsch, M. IDH1 mutation in human glioma induces chemical alterations that are amenable to optical Raman spectroscopy. J. Neuro-Oncol. 2018, 139, 261–268. [Google Scholar] [CrossRef]
- Livermore, L.J.; Isabelle, M.; Bell, I.M.; Scott, C.; Walsby-Tickle, J.; Gannon, J.; Plaha, P.; Vallance, C.; Ansorge, O. Rapid intraoperative molecular genetic classification of gliomas using Raman spectroscopy. Neurooncol. Adv. 2019, 1, vdz008. [Google Scholar] [CrossRef]
- Sciortino, T.; Secoli, R.; d’Amico, E.; Moccia, S.; Conti Nibali, M.; Gay, L.; Rossi, M.; Pecco, N.; Castellano, A.; De Momi, E.; et al. Raman Spectroscopy and Machine Learning for IDH Genotyping of Unprocessed Glioma Biopsies. Cancers 2021, 13, 4196. [Google Scholar] [CrossRef]
- Tom, M.C.; Cahill, D.P.; Buckner, J.C.; Dietrich, J.; Parsons, M.W.; Yu, J.S. Management for Different Glioma Subtypes: Are All Low-Grade Gliomas Created Equal? American Society of Clinical Oncology Educational Book: Chicago, IL, USA, 2019; pp. 133–145. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, K.; Liu, X.; Fang, S.; Li, L.; Wang, Y.; Jiang, T. Molecular subtype impacts surgical resection in low-grade gliomas: A Chinese Glioma Genome Atlas database analysis. Cancer Lett. 2021, 522, 14–21. [Google Scholar] [CrossRef]
- Dono, A.; Zhu, P.; Takayasu, T.; Arevalo, O.; Riascos, R.; Tandon, N.; Ballester, L.Y.; Esquenazi, Y. Extent of Resection Thresholds in Molecular Subgroups of Newly Diagnosed Isocitrate Dehydrogenase–Wildtype Glioblastoma. Neurosurgery 2024, 95, 932–940. [Google Scholar] [CrossRef]
- Ding, X.; Wang, Z.; Chen, D.; Wang, Y.; Zhao, Z.; Sun, C.; Chen, D.; Tang, C.; Xiong, J.; Chen, L.; et al. The prognostic value of maximal surgical resection is attenuated in oligodendroglioma subgroups of adult diffuse glioma: A multicenter retrospective study. J. Neuro-Oncol. 2018, 140, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, S.; Akhtar, J.; Schartner, E.; Ebendorff-Heidepriem, H.; Mahadevan-Jansen, A.; Li, J. Multimodal Raman spectroscopy and optical coherence tomography for biomedical analysis. J. Biophotonics 2022, 16, e202200231. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zheng, X.; Wang, S.; Qi, Q.; Li, Z. Goblet cells segmentation from confocal laser endomicroscopy with an improved U-Net. Biomed. Phys. Eng. Express 2023, 9, 055013. [Google Scholar] [CrossRef] [PubMed]

| Frozen Section | Stimulated Raman Histology | Full-Field OCT | Macroscopic FLIM | Confocal Microscopy | Two-Photon Microscopy | |
|---|---|---|---|---|---|---|
| Label free | label free | label free | label free | label free | labeled | label free or labeled |
| FOV | 10–20 mm | 100 μm–1 mm | 10 mm | 20 mm | 100 μm–1 mm | 100 μm–1 mm |
| Lateral spatial resolution | 300 nm | 10 μm | 15 μm | 300 nm | 500 nm | |
| Time of diagnosis | ~30–40 min | ~2–10 min | no data | no data | no data | no data |
| Cost | High | High | Low | High | Moderate | High |
| Type of information | Morphology | Morphology, “optical fingerprint” | Morphology | Metabolism | Morphology and metabolism | Morphology and metabolism |
| Identify malignant cells | yes | yes | yes | no | yes | yes |
| Molecular information | no | yes | no | no data | no | no |
| Diagnostic accuracy for tumor identification | ~78.4% to 95% | ~90–100% | no data | no data | ~80% | no data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yashin, K.S.; Shcheslavskiy, V.I.; Medyanik, I.A.; Kravets, L.Y.; Shirmanova, M.V. Towards Optical Biopsy in Glioma Surgery. Int. J. Mol. Sci. 2025, 26, 4554. https://doi.org/10.3390/ijms26104554
Yashin KS, Shcheslavskiy VI, Medyanik IA, Kravets LY, Shirmanova MV. Towards Optical Biopsy in Glioma Surgery. International Journal of Molecular Sciences. 2025; 26(10):4554. https://doi.org/10.3390/ijms26104554
Chicago/Turabian StyleYashin, Konstantin S., Vladislav I. Shcheslavskiy, Igor A. Medyanik, Leonid Ya. Kravets, and Marina V. Shirmanova. 2025. "Towards Optical Biopsy in Glioma Surgery" International Journal of Molecular Sciences 26, no. 10: 4554. https://doi.org/10.3390/ijms26104554
APA StyleYashin, K. S., Shcheslavskiy, V. I., Medyanik, I. A., Kravets, L. Y., & Shirmanova, M. V. (2025). Towards Optical Biopsy in Glioma Surgery. International Journal of Molecular Sciences, 26(10), 4554. https://doi.org/10.3390/ijms26104554





