Role of Sclerostin in Cardiovascular System
Abstract
1. Introduction
2. Methods
3. Pre-Marketing and Post-Marketing Cardiovascular Safety Evaluation of Therapeutic Sclerostin Antibody
3.1. Pre-Marketing Cardiovascular Safety Evaluation
3.2. Post-Marketing Cardiovascular Safety Evaluation
4. The Association Between SOST Variants and Cardiovascular Diseases
5. The Association Between Sclerostin and Cardiovascular Diseases
5.1. The Association Between Sclerostin and the Presence of Cardiovascular Diseases
5.1.1. Serum Sclerostin Levels in Patients with Vascular and Valve Calcification
5.1.2. Serum Sclerostin Levels in Patients with Arterial Stiffness
5.1.3. Serum Sclerostin Levels in Patients with Atherosclerosis
5.1.4. Serum Sclerostin Levels in Patients with Peripheral Arterial Disease
5.1.5. Serum Sclerostin Levels in Patients with Acute Ischemic Stroke
| Patient Population | Cardiovascular-Related Diseases | Association (Serum Sclerostin Levels and Cardiovascular Diseases) | p-Value | References | |
|---|---|---|---|---|---|
| ND-CKD patients (N = 154) | vascular calcification | aortic calcification | Positive (Univariate analysis) | 0.0003 | Claes et al. [33] |
| Negative (Multivariate analysis) | 0.04 | ||||
| ND-CKD patients (N = 241) | coronary artery calcification | Positive | <0.001 | Morena et al. [36] | |
| CKD patients (N = 162) | coronary artery calcification | Positive | 0.001 | Ma et al. [38] | |
| CKD patients (N = 97) | vascular calcification | Positive | <0.05 | Lv et al. [39] | |
| Males undergoing CABG (N = 61) | coronary artery calcification | Positive | 0.005 | Kim et al. [41] | |
| CKD patients (N = 80) | abdominal aortic calcification | Positive | <0.05 | Elarbagy et al. [37] | |
| valve calcification | aortic valve calcification | Positive | <0.05 | ||
| Patients with aortic valve calcification (N = 115) | aortic valve calcification | Positive | <0.001 | Koos et al. [34] | |
| CKD patients (N = 110) | heart valve calcification | Positive | <0.05 | Ji et al. [40] | |
| Renal transplant recipients (N = 82) | arterial stiffness | Positive | 0.001 | Hsu et al. [42] | |
| Postmenopausal women (N = 149) | Positive | 0.03 | Hampson et al. [43] | ||
| Hypertensive patients (N = 105) | Positive | <0.001 | Chang et al. [44] | ||
| End-stage renal disease patients (N = 194) | Positive | 0.0001 | Wu et al. [45] | ||
| T2DM patients (N = 125) | Positive | <0.001 | Yang et al. [46] | ||
| Adult healthy outpatient subjects (N = 67) | Negative | <0.05 | Gaudio et al. [47] | ||
| Prevalent hemodialysis patients (N = 122) | atherosclerosis | Positive | 0.016 | Nephrology et al. [48] | |
| T2DM patients (N = 78) | Positive | 0.006 | Morales-Santana et al. [49] | ||
| African American men/women with T2DM (N = 450) | No Association (in women) | - | Register et al. [51] | ||
| Negative (in men) | 0.03 | ||||
| T2DM patients and controls (N = 70) | subclinical atherosclerosis | Positive | <0.001 | Shalash et al. [50] | |
| Postmenopausal women (N = 40) | Negative | 0.006 | Gaudio et al. [52] | ||
| Elderly persons (>65 years) (N = 68) | peripheral arterial disease | Positive | <0.001 | Teng et al. [53] | |
| Hypertensive patients (N = 92) | Positive | <0.001 | Wang et al. [54] | ||
| Patients with acute ischemic stroke (N = 122) | large-artery atherosclerotic stroke | Positive | <0.001 | He et al. [55] | |
| small-artery occlusion stroke | |||||
5.2. The Association Between Sclerostin and Severity/Outcomes of Cardiovascular Diseases
5.3. The Tissue Levels of Sclerostin in Cardiovascular Diseases
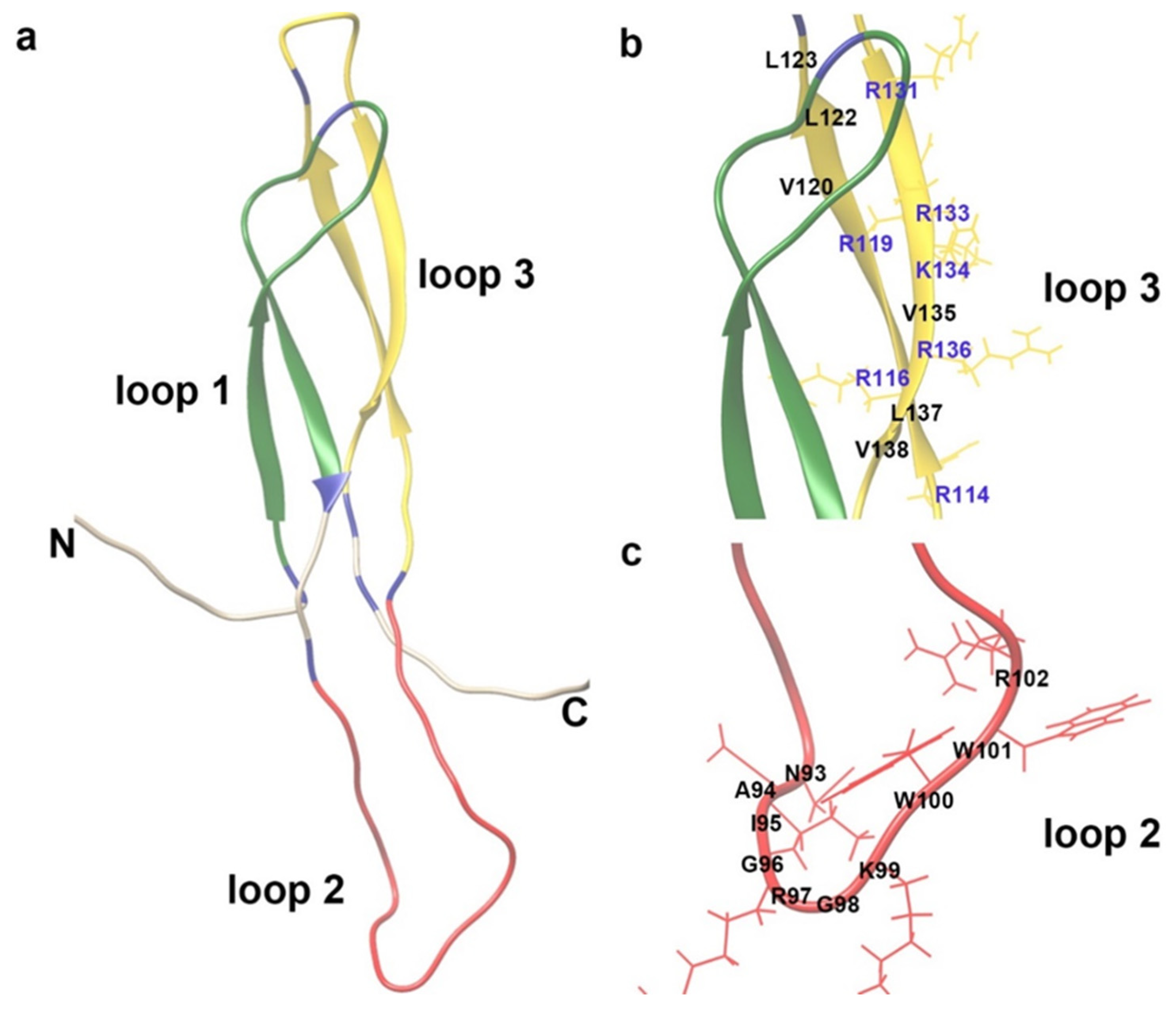
6. Molecular Understanding of the Role of Sclerostin in the Cardiovascular System
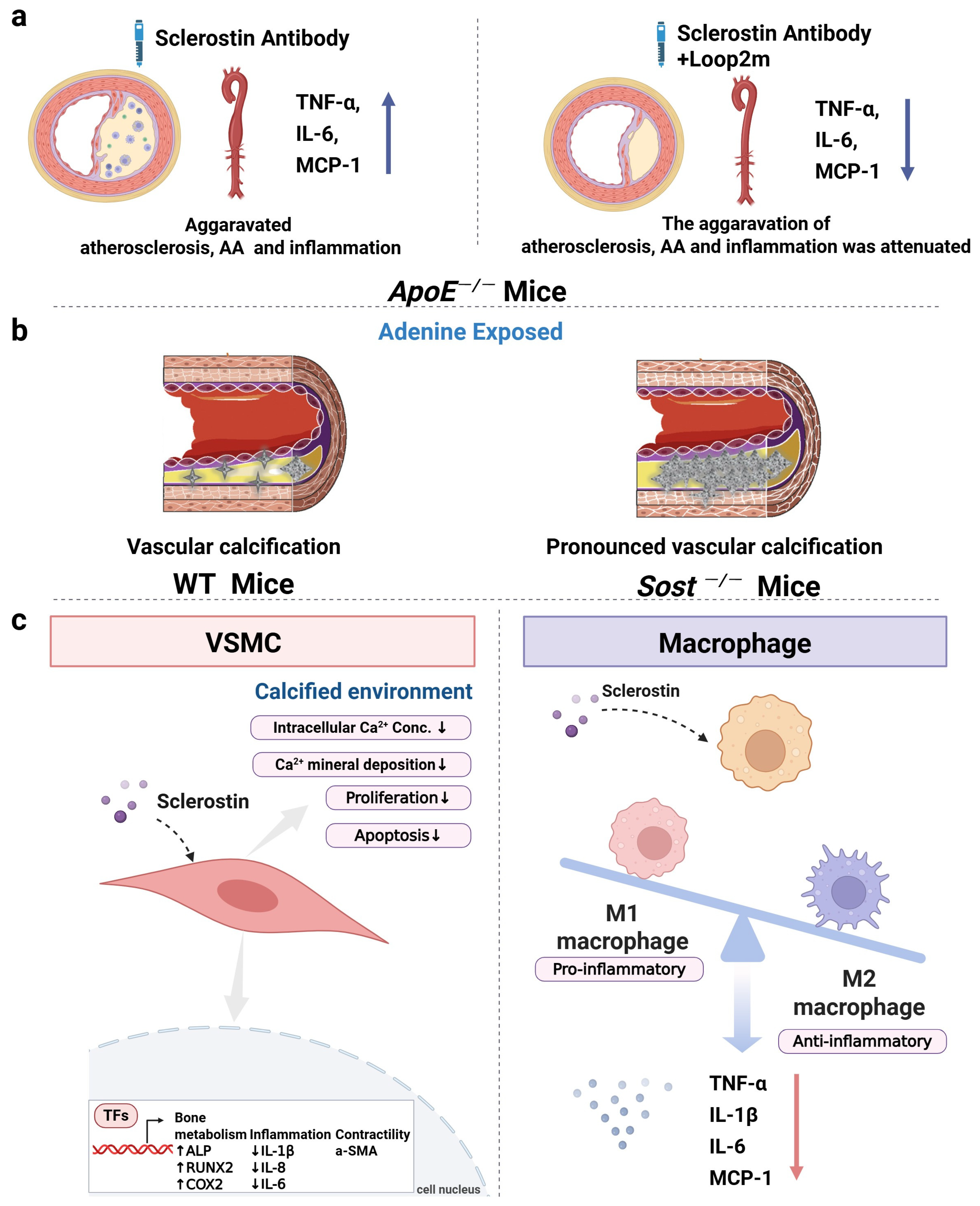
6.1. The Role of Sclerostin in Inflammatory Responses, Aortic Aneurysm, and Atherosclerosis of ApoE−/− Mice
6.2. The Role of Sclerostin in Vascular Calcification of Mice
6.3. The Role of Sclerostin-Related Pathway in the Cardiovascular System
6.4. The Role of Sclerostin and ApoE in Cardiovascular System
7. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | aortic aneurysm |
| ABI | ankle brachial index |
| AngII | angiotensin II |
| ALN | alendronate |
| ApoER2 | apolipoprotein E receptor 2 |
| ApoE−/− | apolipoprotein E-deficient |
| ARCH | active-controlled fracture study in postmenopausal women with osteoporosis at high risk |
| baPWV | brachial-ankle PWV |
| BMD | Bone Mineral Density |
| BRIDGE | placebo-controlled study evaluating the efficacy and safety of romosozumab in treating men with osteoporosis |
| CABG | coronary artery bypass graft |
| CAC | coronary artery calcification |
| CAD | coronary artery disease |
| cfPWV | carotid–femoral PWV |
| CIMT | carotid artery media thickness |
| CKD | chronic kidney disease |
| FDA | Food and Drug Administration |
| FRAME | The fracture study in postmenopausal women with osteoporosis |
| FAERS | Food and Drug Administration Adverse Event Reporting System |
| GWAS | genome-wide association study |
| HAoSMC | human primary aortic smooth muscle cell |
| hSOSTki | full-length sclerostin knock-in |
| ICAM1 | intercellular adhesion molecule 1 |
| IL-6 | interleukin-6 |
| JADER | Japanese Adverse Drug Event Report |
| LDLR | low-density lipoprotein receptor |
| LRP5/6 | low-density lipoprotein receptor-related protein 5/6 |
| LRP8 | low-density lipoprotein receptor-related protein 8 |
| MACCEs | main adverse cardiovascular and cerebrovascular events |
| MACEs | major cardiovascular events |
| MCP-1 | monocyte chemoattractant protein-1 |
| MI | myocardial infarction |
| NCT | national clinical trial |
| ND-CKD | non-dialysis chronic kidney disease |
| OVX | ovariectomized |
| PWV | pulse wave velocity |
| SNP | single-nucleotide polymorphism |
| TNFα | tumor necrosis factor alpha |
| T2DM | type 2 diabetes mellitus |
| VSMC | vascular smooth muscle cell |
References
- McClung, M.R.; Grauer, A.; Boonen, S.; Bolognese, M.A.; Brown, J.P.; Diez-Perez, A.; Langdahl, B.L.; Reginster, J.-Y.; Zanchetta, J.R.; Wasserman, S.M. Romosozumab in postmenopausal women with low bone mineral density. New Engl. J. Med. 2014, 370, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A. Romosozumab treatment in postmenopausal women with osteoporosis. New Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or alendronate for fracture prevention in women with osteoporosis. New Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Blicharski, T.; Goemaere, S.; Lippuner, K.; Meisner, P.D.; Miller, P.D.; Miyauchi, A.; Maddox, J.; Chen, L.; Horlait, S. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J. Clin. Endocrinol. Metab. 2018, 103, 3183–3193. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H. Serious adverse events with romosozumab use in Japanese patients: Need for clear formulation of contraindications worldwide. J. Bone Miner. Res. 2020, 35, 994–995. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard Kvist, A.; Faruque, J.; Vallejo-Yagüe, E.; Weiler, S.; Winter, E.M.; Burden, A.M. Cardiovascular Safety Profile of Romosozumab: A Pharmacovigilance Analysis of the US Food and Drug Administration Adverse Event Reporting System (FAERS). J. Clin. Med. 2021, 10, 1660. [Google Scholar] [CrossRef] [PubMed]
- Stokar, J.; Szalat, A. Cardiovascular Safety of Romosozumab vs. PTH Analogs for Osteoporosis Treatment: A Propensity Score Matched Cohort Study. J. Clin. Endocrinol. Metab. 2024, 110, e861–e867. [Google Scholar] [CrossRef]
- Krishna, S.M.; Seto, S.-W.; Jose, R.J.; Li, J.; Morton, S.K.; Biros, E.; Wang, Y.; Nsengiyumva, V.; Lindeman, J.H.; Loots, G.G. Wnt signaling pathway inhibitor sclerostin inhibits angiotensin II–induced aortic aneurysm and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 553–566. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, L.; Ni, S.; Li, D.; Liu, J.; Chu, H.Y.; Zhang, N.; Sun, M.; Li, N.; Ren, Q.; et al. Targeting loop3 of sclerostin preserves its cardiovascular protective action and promotes bone formation. Nat. Commun. 2022, 13, 4241. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Y.; Ni, S.; Li, D.; Liu, J.; Xie, D.; Chu, H.Y.; Ren, Q.; Zhong, C.; Zhang, N.; et al. Therapeutic aptamer targeting sclerostin loop3 for promoting bone formation without increasing cardiovascular risk in osteogenesis imperfecta mice. Theranostics 2022, 12, 5645–5674. [Google Scholar] [CrossRef]
- González-Salvatierra, S.; García-Fontana, C.; Lacal, J.; Andújar-Vera, F.; Martínez-Heredia, L.; Sanabria-de la Torre, R.; Ferrer-Millán, M.; Moratalla-Aranda, E.; Muñoz-Torres, M.; García-Fontana, B. Cardioprotective function of sclerostin by reducing calcium deposition, proliferation, and apoptosis in human vascular smooth muscle cells. Cardiovasc. Diabetol. 2023, 22, 301. [Google Scholar] [CrossRef]
- Cummings, S.; McCulloch, C. Explanations for the difference in rates of cardiovascular events in a trial of alendronate and romosozumab. Osteoporos. Int. 2020, 31, 1019–1021. [Google Scholar] [CrossRef]
- Khosla, S. Bone diseases: Romosozumab—On track or derailed? Nat. Rev. Endocrinol. 2017, 13, 697–698. [Google Scholar] [CrossRef]
- Kim, D.H.; Rogers, J.R.; Fulchino, L.A.; Kim, C.A.; Solomon, D.H.; Kim, S.C. Bisphosphonates and risk of cardiovascular events: A meta-analysis. PLoS ONE 2015, 10, e0122646. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.; Zhang, S.; Zhang, W.; Zhang, B.; Tang, Q.; Li, Z.; Wu, J. Romosozumab treatment in postmenopausal women with osteoporosis: A meta-analysis of randomized controlled trials. Climacteric 2018, 21, 189–195. [Google Scholar] [CrossRef]
- Schemitsch, E.H.; Miclau, T.; Karachalios, T.; Nowak, L.L.; Sancheti, P.; Poolman, R.W.; Caminis, J.; Daizadeh, N.; Dent-Acosta, R.E.; Egbuna, O.; et al. A Randomized, Placebo-Controlled Study of Romosozumab for the Treatment of Hip Fractures. JBJS 2020, 102, 693–702. [Google Scholar] [CrossRef]
- Baek, K.H.; Chung, Y.S.; Koh, J.M.; Kim, I.J.; Kim, K.M.; Min, Y.K.; Park, K.D.; Dinavahi, R.; Maddox, J.; Yang, W.; et al. Romosozumab in Postmenopausal Korean Women with Osteoporosis: A Randomized, Double-Blind, Placebo-Controlled Efficacy and Safety Study. Endocrinol. Metab. 2021, 36, 60–69. [Google Scholar] [CrossRef]
- Mochizuki, T.; Yano, K.; Ikari, K.; Hiroshima, R.; Okazaki, K. Comparison of romosozumab versus denosumab treatment on bone mineral density after 1 year in rheumatoid arthritis patients with severe osteoporosis: A randomized clinical pilot study. Mod. Rheumatol. 2023, 33, 490–495. [Google Scholar] [CrossRef]
- Bovijn, J.; Krebs, K.; Chen, C.Y.; Boxall, R.; Censin, J.C.; Ferreira, T.; Pulit, S.L.; Glastonbury, C.A.; Laber, S.; Millwood, I.Y.; et al. Evaluating the cardiovascular safety of sclerostin inhibition using evidence from meta-analysis of clinical trials and human genetics. Sci. Transl. Med. 2020, 12, eaay6570. [Google Scholar] [CrossRef]
- Golledge, J.; Thanigaimani, S. Role of Sclerostin in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e187–e202. [Google Scholar] [CrossRef]
- Langdahl, B.L.; Libanati, C.; Crittenden, D.B.; Bolognese, M.A.; Brown, J.P.; Daizadeh, N.S.; Dokoupilova, E.; Engelke, K.; Finkelstein, J.S.; Genant, H.K.; et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: A randomised, open-label, phase 3 trial. Lancet 2017, 390, 1585–1594. [Google Scholar] [CrossRef]
- McClung, M.R.; Brown, J.P.; Diez-Perez, A.; Resch, H.; Caminis, J.; Meisner, P.; Bolognese, M.A.; Goemaere, S.; Bone, H.G.; Zanchetta, J.R.; et al. Effects of 24 Months of Treatment With Romosozumab Followed by 12 Months of Denosumab or Placebo in Postmenopausal Women with Low Bone Mineral Density: A Randomized, Double-Blind, Phase 2, Parallel Group Study. J. Bone Miner. Res. 2018, 33, 1397–1406. [Google Scholar] [CrossRef]
- Ishibashi, H.; Crittenden, D.B.; Miyauchi, A.; Libanati, C.; Maddox, J.; Fan, M.; Chen, L.; Grauer, A. Romosozumab increases bone mineral density in postmenopausal Japanese women with osteoporosis: A phase 2 study. Bone 2017, 103, 209–215. [Google Scholar] [CrossRef]
- Kotake, K.; Mitsuboshi, S.; Omori, Y.; Kawakami, Y.; Kawakami, Y. Evaluation of risk of cardiac or cerebrovascular events in romosozumab users focusing on comorbidities: Analysis of the Japanese Adverse Drug Event Report Database. J. Pharm. Technol. 2023, 39, 23–28. [Google Scholar] [CrossRef]
- Hólm, H.; Sulem, P.; Tragante, V.; Thorsteinsdottir, U.; Gudbjartsson, D.F.; Stefansson, K. Comment on “Evaluating the cardiovascular safety of sclerostin inhibition using evidence from meta-analysis of clinical trials and human genetics”. Sci. Transl. Med. 2021, 13, eabe8497. [Google Scholar] [CrossRef]
- Bovijn, J.; Krebs, K.; Chen, C.-Y.; Boxall, R.; Censin, J.C.; Ferreira, T.; Pulit, S.L.; Glastonbury, C.A.; Laber, S.; Millwood, I.Y.; et al. Response to comment on “Evaluating the cardiovascular safety of sclerostin inhibition using evidence from meta-analysis of clinical trials and human genetics”. Sci. Transl. Med. 2021, 13, eabf4530. [Google Scholar] [CrossRef]
- Holdsworth, G.; Staley, J.R.; Hall, P.; van Koeverden, I.; Vangjeli, C.; Okoye, R.; Boyce, R.W.; Turk, J.R.; Armstrong, M.; Wolfreys, A. Sclerostin downregulation globally by naturally occurring genetic variants, or locally in atherosclerotic plaques, does not associate with cardiovascular events in humans. J. Bone Miner. Res. 2021, 36, 1326–1339. [Google Scholar] [CrossRef]
- Zheng, J.; Wheeler, E.; Pietzner, M.; Andlauer, T.F.; Yau, M.S.; Hartley, A.E.; Brumpton, B.M.; Rasheed, H.; Kemp, J.P.; Frysz, M. Lowering of circulating sclerostin may increase risk of atherosclerosis and its risk factors: Evidence from a genome-wide association meta-analysis followed by Mendelian randomization. Arthritis Rheumatol. 2023, 75, 1781–1792. [Google Scholar] [CrossRef]
- Staley, J.R.; Giannakopoulou, O.; Holdsworth, G.; Armstrong, M. Genetic data do not provide evidence that lower sclerostin is associated with increased risk of atherosclerosis: Comment on the article by Zheng et al. Arthritis Rheumatol. 2023. [Google Scholar] [CrossRef]
- Zheng, J.; Davey Smith, G.; Tobias, J.H. Reply. Arthritis Rheumatol. 2023. [Google Scholar] [CrossRef]
- Alcalde-Herraiz, M.; Xie, J.; Newby, D.; Prats, C.; Gill, D.; Gordillo-Marañón, M.; Prieto-Alhambra, D.; Català, M.; Prats-Uribe, A. Effect of genetically predicted sclerostin on cardiovascular biomarkers, risk factors, and disease outcomes. Nat. Commun. 2024, 15, 9832. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, N.; Liu, J.; Yang, X.; Yu, Y.; Li, D.; Jiang, H.; Sun, M.; Li, N.; Ma, D.; et al. Macrophagic Sclerostin Loop2-ApoER2 Interaction Required by Sclerostin for Suppressing Inflammatory Responses. Metab.-Clin. Exp. 2023, 142, 155427. [Google Scholar] [CrossRef]
- Claes, K.J.; Viaene, L.; Heye, S.; Meijers, B.; d’Haese, P.; Evenepoel, P. Sclerostin: Another vascular calcification inhibitor? J. Clin. Endocrinol. Metab. 2013, 98, 3221–3228. [Google Scholar] [CrossRef]
- Koos, R.; Brandenburg, V.; Mahnken, A.H.; Schneider, R.; Dohmen, G.; Autschbach, R.; Marx, N.; Kramann, R. Sclerostin as a potential novel biomarker for aortic valve calcification: An in-vivo and ex-vivo study. J. Heart Valve Dis. 2013, 22, 317–325. [Google Scholar]
- Wang, X.R.; Yuan, L.; Zhang, J.J.; Hao, L.; Wang, D.G. Serum sclerostin values are associated with abdominal aortic calcification and predict cardiovascular events in patients with chronic kidney disease stages 3–5D. Nephrology 2017, 22, 286–292. [Google Scholar] [CrossRef]
- Morena, M.; Jaussent, I.; Dupuy, A.-M.; Bargnoux, A.-S.; Kuster, N.; Chenine, L.; Leray-Moragues, H.; Klouche, K.; Vernhet, H.; Canaud, B. Osteoprotegerin and sclerostin in chronic kidney disease prior to dialysis: Potential partners in vascular calcifications. Nephrol. Dial. Transplant. 2015, 30, 1345–1356. [Google Scholar] [CrossRef]
- Elarbagy, A.R.; Yassein, Y.S.; Emara, M.M.; Sonbol, A.A.; Zorkaney, K.M.; El Deen, A.A.S. Study of serum sclerostin levels and its role in vascular calcification in patients with chronic kidney disease. Egypt. J. Intern. Med. 2019, 31, 813–821. [Google Scholar] [CrossRef]
- Ma, X.; Sheng, Y.; Yang, X.; Wang, N.; Zhang, H.; Xu, H.; Sun, F. Expression of mir-29a-5p, sclerostin and fetuin-A in patients with chronic kidney disease and their correlation with vascular calcification. Cell. Mol. Biol. 2022, 68, 70–74. [Google Scholar]
- Lv, W.; Guan, L.; Zhang, Y.; Yu, S.; Cao, B.; Ji, Y. Sclerostin as a new key factor in vascular calcification in chronic kidney disease stages 3 and 4. Int. Urol. Nephrol. 2016, 48, 2043–2050. [Google Scholar] [CrossRef]
- Ji, Y.-Q.; Guan, L.-N.; Yu, S.-X.; Yin, P.-Y.; Shen, X.-Q.; Sun, Z.-W.; Liu, J.; Lv, W.; Yu, G.-P.; Ren, C. Serum sclerostin as a potential novel biomarker for heart valve calcification in patients with chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8822–8829. [Google Scholar]
- Kim, K.M.; Lim, S.; Moon, J.H.; Jin, H.; Jung, K.Y.; Shin, C.S.; Park, K.S.; Jang, H.C.; Choi, S.H. Lower uncarboxylated osteocalcin and higher sclerostin levels are significantly associated with coronary artery disease. Bone 2016, 83, 178–183. [Google Scholar] [CrossRef]
- Hsu, B.-G.; Liou, H.-H.; Lee, C.-J.; Chen, Y.-C.; Ho, G.-J.; Lee, M.-C. Serum sclerostin as an independent marker of peripheral arterial stiffness in renal transplantation recipients: A cross-sectional study. Medicine 2016, 95, e3300. [Google Scholar] [CrossRef]
- Hampson, G.; Edwards, S.; Conroy, S.; Blake, G.M.; Fogelman, I.; Frost, M.L. The relationship between inhibitors of the Wnt signalling pathway (Dickkopf-1 (DKK1) and sclerostin), bone mineral density, vascular calcification and arterial stiffness in post-menopausal women. Bone 2013, 56, 42–47. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Hsu, B.-G.; Liou, H.-H.; Lee, C.-J.; Wang, J.-H. Serum levels of sclerostin as a potential biomarker in central arterial stiffness among hypertensive patients. BMC Cardiovasc. Disord. 2018, 18, 214. [Google Scholar] [CrossRef]
- Wu, C.-F.; Hou, J.-S.; Wang, C.-H.; Lin, Y.-L.; Lai, Y.-H.; Kuo, C.-H.; Liou, H.-H.; Tsai, J.-P.; Hsu, B.-G. Serum sclerostin but not DKK-1 correlated with central arterial stiffness in end stage renal disease patients. Int. J. Environ. Res. Public Health 2020, 17, 1230. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Wu, D.-A.; Chen, M.-C.; Hsu, B.-G. Correlation between sclerostin and Dickkopf-1 with aortic arterial stiffness in patients with type 2 diabetes: A prospective, cross-sectional study. Diabetes Vasc. Dis. Res. 2019, 16, 281–288. [Google Scholar] [CrossRef]
- Gaudio, A.; Fiore, V.; Rapisarda, R.; Sidoti, M.H.; Xourafa, A.; Catalano, A.; Tringali, G.; Zanoli, L.; Signorelli, S.S.; Fiore, C.E. Sclerostin is a possible candidate marker of arterial stiffness: Results from a cohort study in Catania. Mol. Med. Rep. 2017, 15, 3420–3424. [Google Scholar] [CrossRef]
- Kirkpantur, A.; Balci, M.; Turkvatan, A.; Afsar, B. Independent association between serum sclerostin levels and carotid artery atherosclerosis in prevalent haemodialysis patients. Clin. Kidney journal 2015, 8, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Morales-Santana, S.; García-Fontana, B.; GARCia-MARTin, A.; Rozas-Moreno, P.; García-Salcedo, J.A.; Reyes-García, R.; Muñoz-Torres, M. Atherosclerotic disease in type 2 diabetes is associated with an increase in sclerostin levels. Diabetes Care 2013, 36, 1667–1674. [Google Scholar] [CrossRef]
- Shalash, M.A.M.; Rohoma, K.H.; Kandil, N.S.; Mohsen, M.A.A.; Taha, A.A.F. Serum sclerostin level and its relation to subclinical atherosclerosis in subjects with type 2 diabetes. J. Diabetes Its Complicat. 2019, 33, 592–597. [Google Scholar] [CrossRef]
- Register, T.C.; Hruska, K.A.; Divers, J.; Bowden, D.W.; Palmer, N.D.; Carr, J.J.; Wagenknecht, L.E.; Hightower, R.C.; Xu, J.; Smith, S.C. Sclerostin is positively associated with bone mineral density in men and women and negatively associated with carotid calcified atherosclerotic plaque in men from the African American-Diabetes Heart Study. J. Clin. Endocrinol. Metab. 2014, 99, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Gaudio, A.; Privitera, F.; Pulvirenti, I.; Canzonieri, E.; Rapisarda, R.; Fiore, C.E. The relationship between inhibitors of the Wnt signalling pathway (sclerostin and Dickkopf-1) and carotid intima-media thickness in postmenopausal women with type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 2014, 11, 48–52. [Google Scholar] [CrossRef]
- Teng, I.-C.; Wang, J.-H.; Lee, C.-J.; Hou, J.-S.; Hsu, B.-G. Serum sclerostin as an independent marker of peripheral artery disease in elderly persons. Int. J. Clin. Exp. Pathol. 2018, 11, 2816. [Google Scholar] [PubMed]
- Wang, H.-S.; Hsiao, C.-H.; Wang, J.-H.; Hsu, B.-G. Increased serum sclerostin level is a risk factor for peripheral artery occlusive disease in patients with hypertension. J. Hypertens. 2023, 41, e174. [Google Scholar] [CrossRef]
- He, X.-W.; Wang, E.; Bao, Y.-Y.; Wang, F.; Zhu, M.; Hu, X.-F.; Jin, X.-P. High serum levels of sclerostin and Dickkopf-1 are associated with acute ischaemic stroke. Atherosclerosis 2016, 253, 22–28. [Google Scholar] [CrossRef]
- Evenepoel, P.; Goffin, E.; Meijers, B.; Kanaan, N.; Bammens, B.; Coche, E.; Claes, K.; Jadoul, M. Sclerostin serum levels and vascular calcification progression in prevalent renal transplant recipients. J. Clin. Endocrinol. Metab. 2015, 100, 4669–4676. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Chang, Z.-F.; Chau, Y.-P.; Chen, A.; Yang, W.-C.; Yang, A.-H.; Lee, O.K.-S. Circulating Wnt/β-catenin signalling inhibitors and uraemic vascular calcifications. Nephrol. Dial. Transplant. 2015, 30, 1356–1363. [Google Scholar] [CrossRef]
- Lin, Y.; Mao, L.; Chen, S.; Zhou, C. Serum sclerostin in vascular calcification in CKD: A meta-analysis. Ren. Fail. 2023, 45, 2186151. [Google Scholar] [CrossRef]
- Drechsler, C.; Evenepoel, P.; Vervloet, M.G.; Wanner, C.; Ketteler, M.; Marx, N.; Floege, J.; Dekker, F.W.; Brandenburg, V.M. High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: Results from the NECOSAD study. Nephrol. Dial. Transplant. 2015, 30, 288–293. [Google Scholar] [CrossRef]
- He, W.; Li, C.; Chen, Q.; Xiang, T.; Wang, P.; Pang, J. Serum sclerostin and adverse outcomes in elderly patients with stable coronary artery disease undergoing percutaneous coronary intervention. Aging Clin. Exp. Res. 2020, 32, 2065–2072. [Google Scholar] [CrossRef]
- Qureshi, A.R.; Olauson, H.; Witasp, A.; Haarhaus, M.; Brandenburg, V.; Wernerson, A.; Lindholm, B.; Söderberg, M.; Wennberg, L.; Nordfors, L. Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification. Kidney Int. 2015, 88, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, H.; Yang, M.; Xing, C. Relationship between serum sclerostin, vascular sclerostin expression and vascular calcification assessed by different methods in ESRD patients eligible for renal transplantation: A cross-sectional study. Int. Urol. Nephrol. 2019, 51, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, A.L.; Miljkovic, I.; Carr, J.J.; Terry, J.G.; Nestlerode, C.S.; Ge, Y.; Bunker, C.H.; Patrick, A.L.; Zmuda, J.M. Association of circulating sclerostin with vascular calcification in Afro-Caribbean men. Atherosclerosis 2015, 239, 218–223. [Google Scholar] [CrossRef]
- Frysz, M.; Gergei, I.; Scharnagl, H.; Smith, G.D.; Zheng, J.; Lawlor, D.A.; Herrmann, M.; Maerz, W.; Tobias, J.H. Circulating sclerostin levels are positively related to coronary artery disease severity and related risk factors. J. Bone Miner. Res. 2020, 37, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.L.C.; Elias, R.M.; Dos Reis, L.M.; Graciolli, F.G.; Zampieri, F.G.; Oliveira, R.B.; Jorgetti, V.; Moysés, R.M. Serum sclerostin is an independent predictor of mortality in hemodialysis patients. BMC Nephrol. 2014, 15, 190. [Google Scholar] [CrossRef]
- Kalousová, M.; Dusilová-Sulková, S.; Kuběna, A.A.; Zakiyanov, O.; Tesař, V.; Zima, T. Sclerostin levels predict cardiovascular mortality in long-term hemodialysis patients: A prospective observational cohort study. Physiol. Res. 2019, 68, 547–558. [Google Scholar] [CrossRef]
- Stavrinou, E.; Sarafidis, P.A.; Loutradis, C.; Memmos, E.; Faitatzidou, D.; Giamalis, P.; Koumaras, C.; Karagiannis, A.; Papagianni, A. Associations of serum sclerostin and Dickkopf-related protein-1 proteins with future cardiovascular events and mortality in haemodialysis patients: A prospective cohort study. Clin. Kidney J. 2021, 14, 1165–1172. [Google Scholar] [CrossRef]
- Gong, L.; Zheng, D.; Yuan, J.; Cao, L.; Ni, Z.; Fang, W. Elevated levels of serum sclerostin are linked to adverse cardiovascular outcomes in peritoneal dialysis patients. Int. Urol. Nephrol. 2018, 50, 955–961. [Google Scholar] [CrossRef]
- Novo-Rodríguez, C.; García-Fontana, B.; Luna-Del Castillo, J.D.D.; Andujar-Vera, F.; Avila-Rubio, V.; Garcia-Fontana, C.; Morales-Santana, S.; Rozas-Moreno, P.; Munoz-Torres, M. Circulating levels of sclerostin are associated with cardiovascular mortality. PLoS ONE 2018, 13, e0199504. [Google Scholar] [CrossRef]
- Kanbay, M.; Siriopol, D.; Saglam, M.; Kurt, Y.G.; Gok, M.; Cetinkaya, H.; Karaman, M.; Unal, H.U.; Oguz, Y.; Sari, S. Serum sclerostin and adverse outcomes in nondialyzed chronic kidney disease patients. J. Clin. Endocrinol. Metab. 2014, 99, E1854–E1861. [Google Scholar] [CrossRef]
- Miteva, K.; Burger, F.; Roth, A.; Thouverey, C.; Ferrari, S.; Mach, F. Ischemic stroke is associated with reduced sclerostin expression in human atherosclerotic plaques. Eur. Heart J. 2023, 44 (Suppl. S2), ehad655-3265. [Google Scholar] [CrossRef]
- Turk, J.R.; Deaton, A.M.; Yin, J.; Stolina, M.; Felx, M.; Boyd, G.; Bienvenu, J.-G.; Varela, A.; Guillot, M.; Holdsworth, G. Nonclinical cardiovascular safety evaluation of romosozumab, an inhibitor of sclerostin for the treatment of osteoporosis in postmenopausal women at high risk of fracture. Regul. Toxicol. Pharmacol. 2020, 115, 104697. [Google Scholar] [CrossRef]
- Veverka, V.; Henry, A.J.; Slocombe, P.M.; Ventom, A.; Mulloy, B.; Muskett, F.W.; Muzylak, M.; Greenslade, K.; Moore, A.; Zhang, L.; et al. Characterization of the structural features and interactions of sclerostin: Molecular insight into a key regulator of Wnt-mediated bone formation. J. Biol. Chem. 2009, 284, 10890–10900. [Google Scholar] [CrossRef]
- Mineo, C. Lipoprotein receptor signalling in atherosclerosis. Cardiovasc. Res. 2020, 116, 1254–1274. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, V.; Konaniah, E.S.; Herz, J.; Gerard, R.D.; Jung, E.; Yuhanna, I.S.; Ahmed, M.; Hui, D.Y.; Mineo, C.; Shaul, P.W. Genetic variants of ApoE and ApoER2 differentially modulate endothelial function. Proc. Natl. Acad. Sci. USA 2014, 111, 13493–13498. [Google Scholar] [CrossRef] [PubMed]
- Baitsch, D.; Bock, H.H.; Engel, T.; Telgmann, R.; Müller-Tidow, C.; Varga, G.; Bot, M.; Herz, J.; Robenek, H.; Von Eckardstein, A. Apolipoprotein E induces antiinflammatory phenotype in macrophages. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1160–1168. [Google Scholar] [CrossRef]
- Waltmann, M.D.; Basford, J.E.; Konaniah, E.S.; Weintraub, N.L.; Hui, D.Y. Apolipoprotein E receptor-2 deficiency enhances macrophage susceptibility to lipid accumulation and cell death to augment atherosclerotic plaque progression and necrosis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 1395–1405. [Google Scholar] [CrossRef]
- Lagrange, J.; Finger, S.; Kossmann, S.; Garlapati, V.; Ruf, W.; Wenzel, P. Angiotensin II infusion leads to aortic dissection in LRP8 deficient mice. Int. J. Mol. Sci. 2020, 21, 4916. [Google Scholar] [CrossRef]
- Rong, S.; Zhao, X.; Jin, X.; Zhang, Z.; Chen, L.; Zhu, Y.; Yuan, W. Vascular calcification in chronic kidney disease is induced by bone morphogenetic protein-2 via a mechanism involving the Wnt/β-catenin pathway. Cell Physiol. Biochem. 2014, 34, 2049–2060. [Google Scholar] [CrossRef]
- Mikhaylova, L.; Malmquist, J.; Nurminskaya, M. Regulation of in vitro vascular calcification by BMP4, VEGF and Wnt3a. Calcif. Tissue Int. 2007, 81, 372–381. [Google Scholar] [CrossRef]
- Cai, T.; Sun, D.; Duan, Y.; Wen, P.; Dai, C.; Yang, J.; He, W. WNT/β-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp. Cell Res. 2016, 345, 206–217. [Google Scholar] [CrossRef] [PubMed]
- De Maré, A.; Opdebeeck, B.; Neven, E.; D’Haese, P.C.; Verhulst, A. Sclerostin Protects Against Vascular Calcification Development in Mice. J. Bone Miner. Res. 2022, 37, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yang, Y.; Cheng, A.; Xu, G.; He, F. Metabolism of vascular smooth muscle cells in vascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H613–H631. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, J.; Choi, H.J.; Chung, K.Y. Conformational Dynamics of Sclerostin-LRP6 Complex Analyzed by HDX-MS. Biomol. Ther. 2021, 29, 527–535. [Google Scholar] [CrossRef]
- Guo, Y.F.; Xiong, D.H.; Shen, H.; Zhao, L.J.; Xiao, P.; Guo, Y.; Wang, W.; Yang, T.L.; Recker, R.R.; Deng, H.W. Polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J. Med. Genet. 2006, 43, 798–803. [Google Scholar] [CrossRef]
- Suwazono, Y.; Kobayashi, E.; Uetani, M.; Miura, K.; Morikawa, Y.; Ishizaki, M.; Kido, T.; Nakagawa, H.; Nogawa, K. G-protein beta 3 subunit polymorphism C1429T and low-density lipoprotein receptor-related protein 5 polymorphism A1330V are risk factors for hypercholesterolemia in Japanese males--a prospective study over 5 years. Metabolism 2006, 55, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Suwazono, Y.; Kobayashi, E.; Uetani, M.; Miura, K.; Morikawa, Y.; Ishizaki, M.; Kido, T.; Nakagawa, H.; Nogawa, K. Low-density lipoprotein receptor-related protein 5 variant Q89R is associated with hypertension in Japanese females. Blood Press. 2006, 15, 80–87. [Google Scholar] [CrossRef]
- Mani, A.; Radhakrishnan, J.; Wang, H.; Mani, A.; Mani, M.-A.; Nelson-Williams, C.; Carew, K.S.; Mane, S.; Najmabadi, H.; Wu, D. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 2007, 315, 1278–1282. [Google Scholar] [CrossRef]
- Khan, K.; Yu, B.; Tardif, J.-C.; Rhéaume, E.; Al-Kindi, H.; Filimon, S.; Pop, C.; Genest, J.; Cecere, R.; Schwertani, A. Significance of the Wnt signaling pathway in coronary artery atherosclerosis. Front. Cardiovasc. Med. 2024, 11, 1360380. [Google Scholar] [CrossRef]
- Mahley, R.W. Apolipoprotein E: From cardiovascular disease to neurodegenerative disorders. J. Mol. Med. 2016, 94, 739–746. [Google Scholar] [CrossRef]
- Schaefer, E.; Gregg, R.; Ghiselli, G.; Forte, T.; Ordovas, J.; Zech, L.; Brewer, H. Familial apolipoprotein E deficiency. J. Clin. Investig. 1986, 78, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Hofker, M.H.; van Vlijmen, B.J.; Havekes, L.M. Transgenic mouse models to study the role of APOE in hyperlipidemia and atherosclerosis. Atherosclerosis 1998, 137, 1–11. [Google Scholar] [CrossRef] [PubMed]
| Adverse Event | 12-Month FRAME Trial [2] | 12-Month ARCH Trial [3] | 12-Month BRIDGE Trial [4] | |||
|---|---|---|---|---|---|---|
| Placebo (N = 3576) | Romosozumab (N = 3581) | Alendronate (N = 2014) | Romosozumab (N = 2040) | Placebo (N = 81) | Romosozumab (N = 163) | |
| Number of Patients (Percent) | ||||||
| Serious adverse event | 312(8.7) | 344(9.6) | 278(13.8) | 262(12.8) | 10(12.3) | 21(12.9) |
| Events leading to discontinuation of trial regimen | 94(2.6) | 103(2.9) | 64(3.2) | 70(3.4) | 1(1.2) | 5(3.1) |
| Adjudicated serious cardiovascular event a | 15(0.4) | 17(0.5) | 38(1.9) | 50(2.5) | 8(4.9) | 2(2.5) |
| Cardiac ischemic event | NA | NA | 6(0.3) | 16(0.8) | 0 | 3(1.8) |
| Cerebrovascular event | NA | NA | 7(0.3) | 16(0.8) | 1(1.2) | 3(1.8) |
| Arthralgia | 429(12.0) | 467(13.0) | NA | NA | NA | NA |
| Nasopharyngitis | 438(12.2) | 459(12.8) | 218(10.8) | 213(10.4) | NA | NA |
| Back pain | 378(10.6) | 375(10.5) | 228(11.3) | 186(9.1) | NA | NA |
| Injection-site reaction b | 104(2.9) | 187(5.2) | 53(2.6) | 90(4.4) | 3(3.7) | 9(5.5) |
| Osteonecrosis of the jaw | 0 | 1(<0.1) | 0 | 0 | 0 | 0 |
| Atypical femoral fracture | 0 | 1(<0.1) | 0 | 0 | 0 | 0 |
| Adverse Events | Total [6] | Japan | United States |
|---|---|---|---|
| Romosozumab | Romosozumab | Romosozumab | |
| (N = 1995) | (N = 1188) | (N = 787) | |
| Number of Patients (Percent) | |||
| Major cardiovascular event Myocardial infarction | 206(10.3) | 164(13.8) | 41(5.2) |
| 42(2.1) | 28(2.4) | 13(1.7) | |
| Stroke Cardiovascular death Other cardiovascular event General cardiac events | 84(4.2) | 57(4.8) | 27(3.4) |
| 86(4.3) | 83(7.0) | <5 | |
| 58(2.9) | 42(3.5) | 16(2.0) | |
| 16(0.8) | 10(0.8) | 6(0.8) | |
| Bleeding Thrombosis | 19(1.0) | 19(1.6) | - |
| 23(1.2) | 13(1.1) | 10(1.3) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Wang, L.; Li, X.; Yang, X.; Tao, X.; Jiang, H.; Yu, Y.; Liu, J.; Yu, S.; Ma, Y.; et al. Role of Sclerostin in Cardiovascular System. Int. J. Mol. Sci. 2025, 26, 4552. https://doi.org/10.3390/ijms26104552
Zhang N, Wang L, Li X, Yang X, Tao X, Jiang H, Yu Y, Liu J, Yu S, Ma Y, et al. Role of Sclerostin in Cardiovascular System. International Journal of Molecular Sciences. 2025; 26(10):4552. https://doi.org/10.3390/ijms26104552
Chicago/Turabian StyleZhang, Ning, Luyao Wang, Xiaofei Li, Xin Yang, Xiaohui Tao, Hewen Jiang, Yuanyuan Yu, Jin Liu, Sifan Yu, Yuan Ma, and et al. 2025. "Role of Sclerostin in Cardiovascular System" International Journal of Molecular Sciences 26, no. 10: 4552. https://doi.org/10.3390/ijms26104552
APA StyleZhang, N., Wang, L., Li, X., Yang, X., Tao, X., Jiang, H., Yu, Y., Liu, J., Yu, S., Ma, Y., Zhang, B., & Zhang, G. (2025). Role of Sclerostin in Cardiovascular System. International Journal of Molecular Sciences, 26(10), 4552. https://doi.org/10.3390/ijms26104552






