Antioxidant Potential of Lactoferrin and Its Protective Effect on Health: An Overview
Abstract
1. Introduction
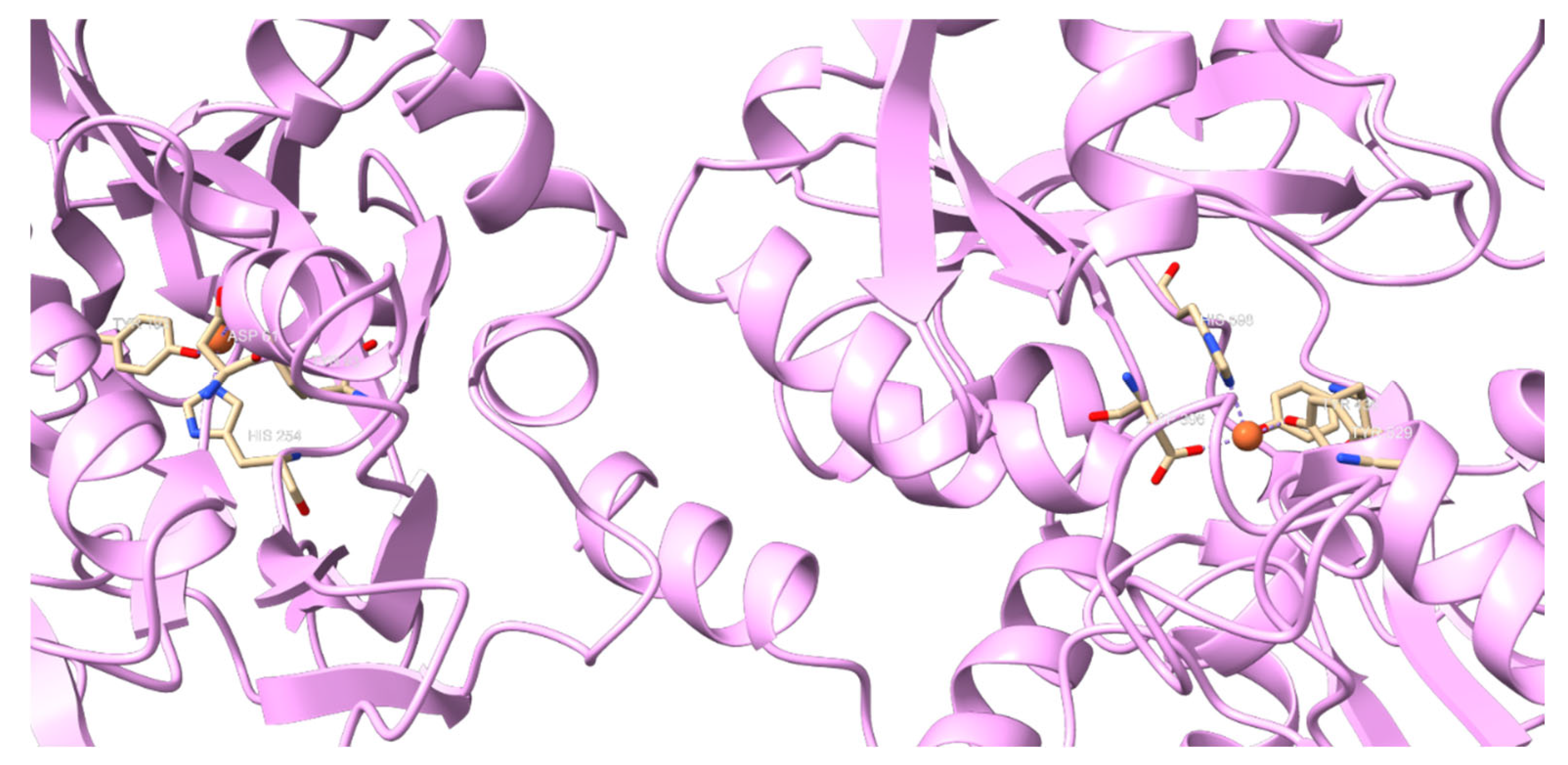
2. Lactoferrin Generalities and Structure
3. Lf Implication on Iron Metabolism
4. Lf Effect in Oxidative Stress
4.1. Reduction of Reactive Oxygen Species (ROS) Levels
4.2. Enhancement of Antioxidant Defense Mechanisms
5. Neuroprotective Effects Through Oxidative Stress Reduction
5.1. Neuroprotector Role of Lf in Parkinson’s Disease
5.2. Neuroprotector Role of Lf in Alzheimer’s Disease
6. Lf Antioxidant Impact on Other Systems
6.1. Lf in Obesity
6.2. Lf in the Immunological System
6.3. Lf in Anemia
6.4. Lf in Respiratory Disease
6.5. Lf in Hepatitis
6.6. Lf in Dry Eyeness Disease
6.7. Lf in Cardiovascular Disease
6.8. Lf in Angiogenesis
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mathers, C.D. History of global burden of disease assessment at the World Health Organization. Arch. Public Health 2020, 78, 77. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Statistics 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart disease and stroke statistics—2023 update: A report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [PubMed]
- Tahami Monfared, A.A.; Byrnes, M.J.; White, L.A.; Zhang, Q. Alzheimer’s disease: Epidemiology and clinical progression. Neurol. Ther. 2022, 11, 553–569. [Google Scholar] [CrossRef]
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The trends projection analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The role of oxidative stress in cardiac disease: From physiological response to injury factor. Oxidative Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Iglesias-Figueroa, B.F.; Espinoza-Sánchez, E.A.; Siqueiros-Cendón, T.S.; Rascón-Cruz, Q. Lactoferrin as a nutraceutical protein from milk, an overview. Int. Dairy J. 2019, 89, 37–41. [Google Scholar] [CrossRef]
- Niaz, B.; Saeed, F.; Ahmed, A.; Imran, M.; Maan, A.A.; Khan, M.K.I.; Tufail, T.; Anjum, F.M.; Hussain, S.; Suleria, H.A.R. Lactoferrin (LF): A natural antimicrobial protein. Int. J. Food Prop. 2019, 22, 1626–1641. [Google Scholar] [CrossRef]
- Wong, S.H.; Francis, N.; Chahal, H.; Raza, K.; Salmon, M.; Scheel-Toellner, D.; Lord, J.M. Lactoferrin is a survival factor for neutrophils in rheumatoid synovial fluid. Rheumatology 2009, 48, 39–44. [Google Scholar] [CrossRef]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301.e1–301.e8. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef] [PubMed]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a multiple bioactive protein: An overview. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Karav, S.; German, J.B.; Rouquié, C.; Le Parc, A.; Barile, D. Studying lactoferrin N-glycosylation. Int. J. Mol. Sci. 2017, 18, 870. [Google Scholar] [CrossRef]
- Legrand, D. Overview of lactoferrin as a natural immune modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; De la Garza, M. Lactoferrin: Balancing ups and downs of inflammation due to microbial infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef]
- Baker, E.N.; Baker, H.M.; Kidd, R.D. Lactoferrin and transferrin: Functional variations on a common structural framework. Biochem. Cell Biol. 2002, 80, 27–34. [Google Scholar] [CrossRef]
- Giansanti, F.; Panella, G.; Leboffe, L.; Antonini, G. Lactoferrin from milk: Nutraceutical and pharmacological properties. Pharmaceuticals 2016, 9, 61. [Google Scholar] [CrossRef]
- Roemhild, K.; von Maltzahn, F.; Weiskirchen, R.; Knüchel, R.; von Stillfried, S.; Lammers, T. Iron metabolism: Pathophysiology and pharmacology. Trends Pharmacol. Sci. 2021, 42, 640–656. [Google Scholar] [CrossRef]
- Gozzelino, R.; Arosio, P. Iron homeostasis in health and disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Shan, Q.; Wei, J.; Ma, F.; Sun, P. Science, Lactoferrin: Major physiological functions and applications. Curr. Protein Pept. Sci. 2019, 20, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Mazurier, J.; Spik, G. Comparative study of the iron-binding properties of human transferrins: I. Complete and sequential iron saturation and desaturation of the lactotransferrin. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1980, 629, 399–408. [Google Scholar] [CrossRef]
- Schlottmann, F.; Vera-Aviles, M.; Latunde-Dada, G.O. Latunde-Dada, Duodenal cytochrome b (Cybrd1) ferric reductase functional studies in cells. Metallomics 2017, 9, 1389–1393. [Google Scholar] [CrossRef]
- Kawakami, H.; Lonnerdal, B. Isolation and function of a receptor for human lactoferrin in human fetal intestinal brush-border membranes. Am. J. Physiol.-Gastrointest. Liver Physiol. 1991, 261, G841–G846. [Google Scholar] [CrossRef]
- Fu, J.; Yang, L.; Tan, D.; Liu, L. Iron transport mechanism of lactoferrin and its application in food processing. Food Sci. Technol. 2023, 43, e121122. [Google Scholar] [CrossRef]
- Sienes Bailo, P.; Llorente Martín, E.; Calmarza, P.; Montolio Breva, S.; Bravo Gómez, A.; Pozo Giráldez, A.; Sánchez-Pascuala Callau, J.J.; Vaquer Santamaría, J.M.; Dayaldasani Khialani, A.; Cerdá Micó, C.; et al. The role of oxidative stress in neurodegenerative diseases and potential antioxidant therapies. Adv. Lab. Med. Av. Med. Lab. 2022, 3, 342–350. [Google Scholar] [CrossRef]
- Guan, S.; Zhang, S.; Liu, M.; Guo, J.; Chen, Y.; Shen, X.; Deng, X.; Lu, J. Preventive effects of lactoferrin on acute alcohol-induced liver injury via iron chelation and regulation of iron metabolism. J. Dairy Sci. 2024, 107, 5316–5329. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Chen, C.; Tong, X.; Li, Y.; Yang, K.; Lv, H.; Xu, J.; Qin, L. Holo-lactoferrin: The link between ferroptosis and radiotherapy in triple-negative breast cancer. Theranostics 2021, 11, 3167. [Google Scholar] [CrossRef]
- Ashraf, M.F.; Zubair, D.; Bashir, M.N.; Alagawany, M.; Ahmed, S.; Shah, Q.A.; Buzdar, J.A. Nutraceutical and Health-Promoting Potential of Lactoferrin, an Iron-Binding Protein in Human and Animal: Current Knowledge. Biol. Trace Element Res. 2023, 202, 56–72. [Google Scholar] [CrossRef]
- Ojima, Y.; Nunogami, S.; Taya, M. Antibiofilm effect of warfarin on biofilm formation of Escherichia coli promoted by antimicrobial treatment. J. Glob. Antimicrob. Resist. 2016, 7, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Rosa, L.; di Patti, M.C.B.; Valenti, P.; Musci, G.; Cutone, A. Lactoferrin: From the structure to the functional orchestration of iron homeostasis. BioMetals 2022, 36, 391–416. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Guo, C. A Review on Lactoferrin and Central Nervous System Diseases. Cells 2021, 10, 1810. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Jin, S.; Kang, P.M. A Systematic Review on Advances in Management of Oxidative Stress-Associated Cardiovascular Diseases. Antioxidants 2024, 13, 923. [Google Scholar] [CrossRef]
- Park, S.Y.; Jeong, A.J.; Kim, G.Y.; Jo, A.; Lee, J.E.; Leem, S.H.; Yoon, J.H.; Ye, S.K.; Chung, J.W. Lactoferrin protects human mesenchymal stem cells from oxidative stress-induced senescence and apoptosis. J. Microbiol. Biotechnol. 2017, 27, 1877–1884. [Google Scholar] [CrossRef]
- Al Zharani, M.M.; Almuqri, E.A.; Ahmed, M.M.; Aljarba, N.H.; Rudayni, H.A.; Yaseen, K.N.; Alkahtani, S.H.; Nasr, F.A.; Al Doaiss, A.A. Use of Lactoferrin Supplement as an Efficient Antioxidant to Ameliorate the Effects of Mercury-induced Oxidative Stress in Male Wistar Rats. Biomed. Biotechnol. Res. J. 2024, 8, 45–52. [Google Scholar] [CrossRef]
- Safaeian, L.; Zabolian, H. Antioxidant Effects of Bovine Lactoferrin on Dexamethasone-Induced Hypertension in Rat. Int. Sch. Res. Not. 2014, 2014, 943523. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, H.; Zhu, N.; Xu, Z.; Wang, Y.; Qu, Y.; Wang, J. Lactoferrin protects against iron dysregulation, oxidative stress, and apoptosis in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced Parkinson’s disease in mice. J. Neurochem. 2020, 152, 397–415. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Lönnerdal, B. Characterization of mammalian receptors for lactoferrin. Biochem. Cell Biol. 2002, 80, 75–80. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Lactoferrin: Mammalian lactoferrin receptors: Structure and function. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef]
- Baveye, S.; Elass, E.; Fernig, D.G.; Blanquart, C.; Mazurier, J.; Legrand, D. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect. Immun. 2000, 68, 6519–6525. [Google Scholar] [CrossRef]
- Takayama, Y.; Aoki, R.; Uchida, R.; Tajima, A.; Aoki-Yoshida, A. Role of CXC chemokine receptor type 4 as a lactoferrin receptor. Biochem. Cell Biol. 2017, 95, 57–63. [Google Scholar] [CrossRef]
- Ando, K.; Hasegawa, K.; Shindo, K.I.; Furusawa, T.; Fujino, T.; Kikugawa, K.; Nakano, H.; Takeuchi, O.; Akira, S.; Akiyama, T.; et al. Human lactoferrin activates NF-κB through the Toll-like receptor 4 pathway while it interferes with the lipopolysaccharide-stimulated TLR4 signaling. FEBS J. 2010, 277, 2051–2066. [Google Scholar] [CrossRef]
- Grey, A.; Zhu, Q.; Watson, M.; Callon, K.; Cornish, J. Lactoferrin potently inhibits osteoblast apoptosis, via an LRP1-independent pathway. Mol. Cell. Endocrinol. 2006, 251, 96–102. [Google Scholar] [CrossRef]
- Takayama, Y.; Takahashi, H.; Mizumachi, K.; Takezawa, T. Low Density Lipoprotein Receptor-related Protein (LRP) Is Required for Lactoferrin-enhanced Collagen Gel Contractile Activity of Human Fibroblasts. J. Biol. Chem. 2003, 278, 22112–22118. [Google Scholar] [CrossRef]
- Yong, S.J.; Veerakumarasivam, A.; Lim, W.L.; Chew, J. Neuroprotective Effects of Lactoferrin in Alzheimer’s and Parkinson’s Diseases: A Narrative Review. ACS Chem. Neurosci. 2023, 14, 1342–1355. [Google Scholar] [CrossRef] [PubMed]
- Ginet, V.; van de Looij, Y.; Petrenko, V.; Toulotte, A.; Kiss, J.; Hüppi, P.S.; Sizonenko, S.V. Lactoferrin during lactation reduces lipopolysaccharide-induced brain injury. BioFactors 2016, 42, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Mulder, A.M.; Connellan, P.A.; Oliver, C.J.; Morris, C.A.; Stevenson, L.M. Bovine lactoferrin supplementation supports immune and antioxidant status in healthy human males. Nutr. Res. 2008, 28, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Burrow, H.; Kanwar, R.K.; Mahidhara, G.; Kanwar, J.R. Effect of selenium-saturated bovine lactoferrin (Se-bLF) on antioxidant enzyme activities in human gut epithelial cells under oxidative stress. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2011, 11, 762–771. [Google Scholar] [CrossRef]
- Al-Sharif, I.A. Protective effect of lactoferrin administration against brain tissue damage in diabetic rats. J. Umm Al-Qura Univ. Appl. Sci. 2023, 9, 115–122. [Google Scholar] [CrossRef]
- Sanches, E.; van de Looij, Y.; Sow, S.; Toulotte, A.; da Silva, A.; Modernell, L.; Sizonenko, S. Dose-Dependent Neuroprotective Effects of Bovine Lactoferrin Following Neonatal Hypoxia–Ischemia in the Immature Rat Brain. Nutrients 2021, 13, 3880. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Yang, C.; Shi, Y.; Dong, Z.; Troy, F.A. Functional Correlates and Impact of Dietary Lactoferrin Intervention and its Concentration-dependence on Neurodevelopment and Cognition in Neonatal Piglets. Mol. Nutr. Food Res. 2021, 65, 2001099. [Google Scholar] [CrossRef]
- Sokolov, A.V.; Miliukhina, I.V.; Belsky, Y.P.; Belska, N.V.; Vasilyev, V.B. Potential role of lactoferrin in early diagnostics and treatment of Parkinson disease. Med. Acad. J. 2020, 20, 37–44. [Google Scholar] [CrossRef]
- Li, X.; Feng, X.; Jiang, Z.; Jiang, Z. Association of small intestinal bacterial overgrowth with Parkinson’s disease: A systematic review and meta-analysis. Gut Pathog. 2021, 13, 1–10. [Google Scholar] [CrossRef]
- Mahoney-Sánchez, L.; Bouchaoui, H.; Ayton, S.; Devos, D.; Duce, J.A.; Devedjian, J.-C. Ferroptosis and its potential role in the physiopathology of Parkinson’s Disease. Prog. Neurobiol. 2020, 196, 101890. [Google Scholar] [CrossRef]
- Zeng, W.; Cai, J.; Zhang, L.; Peng, Q. Iron Deposition in Parkinson’s Disease: A Mini-Review. Cell. Mol. Neurobiol. 2024, 44, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bi, M.; Liu, H.; Song, N.; Xie, J. The protective effect of lactoferrin on ventral mesencephalon neurons against MPP+ is not connected with its iron binding ability. Sci. Rep. 2015, 5, 10729. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Suk, K. Emerging roles of protein kinases in microglia-mediated neuroinflammation. Biochem. Pharmacol. 2017, 146, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; López-Cuenca, I.; Rojas, P.; Triviño, A.; Ramírez, J.M. The role of microglia in retinal neurodegeneration: Alzheimer’s disease, Parkinson, and glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef]
- Grau, A.J.; Willig, V.; Fogel, W.; Werle, E. Assessment of plasma lactoferrin in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2001, 16, 131–134. [Google Scholar] [CrossRef]
- Xu, S.-F.; Zhang, Y.-H.; Wang, S.; Pang, Z.-Q.; Fan, Y.-G.; Li, J.-Y.; Wang, Z.-Y.; Guo, C. Lactoferrin ameliorates dopaminergic neurodegeneration and motor deficits in MPTP-treated mice. Redox Biol. 2018, 21, 101090. [Google Scholar] [CrossRef]
- Cao, X.; Ren, Y.; Lu, Q.; Wang, K.; Wu, Y.; Wang, Y.; Zhang, Y.; Cui, X.-S.; Yang, Z.; Chen, Z. Lactoferrin: A glycoprotein that plays an active role in human health. Front. Nutr. 2023, 9, 1018336. [Google Scholar] [CrossRef]
- Eker, F.; Bolat, E.; Pekdemir, B.; Duman, H.; Karav, S. Lactoferrin: Neuroprotection against Parkinson’s disease and secondary molecule for potential treatment. Front. Aging Neurosci. 2023, 15, 1204149. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Arturo, C.M.; María Antonia, M.S.; Liana Yanet, R.R. La Disfunción Mitocondrial y el Estrés Oxidativo en la Enfermedad de Alzheimer. In CIBAMANZ-2023. 2023. Available online: https://cibamanz.sld.cu/index.php/cibamanz/2023/paper/viewPaper/568 (accessed on 25 October 2024).
- Seo, E.H.; Lim, H.J.; Yoon, H.-J.; Choi, K.Y.; Lee, J.J.; Park, J.Y.; Choi, S.H.; Kim, H.; Kim, B.C.; Lee, K.H. Visuospatial memory impairment as a potential neurocognitive marker to predict tau pathology in Alzheimer’s continuum. Alzheimer’s Res. Ther. 2021, 13, 167. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Carvalho, C.; Zhu, X.; Smith, M.A.; Perry, G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2010, 1802, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; Martinez de Toda, I.; Garrido, A.; Carro, E.; Molina, J.A.; De la Fuente, M. Impairment of several immune functions and redox state in blood cells of Alzheimer’s disease patients. Relevant role of neutrophils in oxidative stress. Front. Immunol. 2018, 8, 1974. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Actor, J.K.; Zimecki, M.; Wise, J.; Płoszaj, P.; Mirza, S.; Hwang, S.-A.; Ba, X.; Boldogh, I. Novel recombinant human lactoferrin: Differential activation of oxidative stress related gene expression. J. Biotechnol. 2013, 168, 666–675. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Roy, K.; Patel, Y.; Zhou, S.-F.; Singh, M.R.; Singh, D.; Nasir, M.; Sehgal, R.; Sehgal, A.; Singh, R.S.; et al. Multifunctional Iron Bound Lactoferrin and Nanomedicinal Approaches to Enhance Its Bioactive Functions. Molecules 2015, 20, 9703–9731. [Google Scholar] [CrossRef]
- Wu, K.; El Zowalaty, A.E.; Sayin, V.I.; Papagiannakopoulos, T. The pleiotropic functions of reactive oxygen species in cancer. Nat. Cancer 2024, 5, 384–399. [Google Scholar] [CrossRef]
- Riley, P. Free Radicals in Biology: Oxidative Stress and the Effects of Ionizing Radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance Between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Clamp, J.R.; Creeth, J.M. Some non-mucin components of mucus and their possible biological roles. In Ciba Foundation Symposium 109—Mucus and Mucosa; Wiley Online Library: Hoboken, NJ, USA, 1984. [Google Scholar]
- Mohamed, W.A.; Schaalan, M.F. Antidiabetic efficacy of lactoferrin in type 2 diabetic pediatrics; controlling impact on PPAR-γ, SIRT-1, and TLR4 downstream signaling pathway. Diabetol. Metab. Syndr. 2018, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yan, Y.; Wu, Y.; Lu, M.; Xing, Y.; Bai, Y.; Zhao, H.; Ding, L.; Wu, Y.; Xu, J.; et al. Lactoferrin ameliorated obesity-induced endothelial dysfunction by inhibiting the Tak1/IL-18/eNOS pathway between PVAT and vascular endothelium. Free Radic. Biol. Med. 2024, 212, 309–321. [Google Scholar] [CrossRef]
- Stobiecka, M.; Król, J.; Brodziak, A. Antioxidant Activity of Milk and Dairy Products. Animals 2022, 12, 245. [Google Scholar] [CrossRef]
- Imoto, I.; Yasuma, T.; D’Alessandro-Gabazza, C.N.; Oka, S.; Misaki, M.; Horiki, N.; Gabazza, E.C. Antimicrobial Effects of Lactoferrin against Helicobacter pylori Infection. Pathogens 2023, 12, 599. [Google Scholar] [CrossRef]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef]
- Paesano, R.; Torcia, F.; Berlutti, F.; Pacifici, E.; Ebano, V.; Moscarini, M.; Valenti, P. Oral administration of lactoferrin increases hemoglobin and total serum iron in pregnant women. Biochem. Cell Biol. 2006, 84, 377–380. [Google Scholar] [CrossRef]
- Paesano, R.; Berlutti, F.; Pietropaoli, M.; Pantanella, F.; Pacifici, E.; Goolsbee, W.; Valenti, P. Lactoferrin efficacy versus ferrous sulfate in curing iron deficiency and iron deficiency anemia in pregnant women. BioMetals 2010, 23, 411–417. [Google Scholar] [CrossRef]
- Lönnerdal, B. Nutritional roles of lactoferrin. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 293–297. [Google Scholar] [CrossRef]
- Koikawa, N.; Nagaoka, I.; Yamaguchi, M.; Hamano, H.; Yamauchi, K.; Sawaki, K. Preventive effect of lactoferrin intake on anemia in female long distance runners. Biosci. Biotechnol. Biochem. 2008, 72, 931–935. [Google Scholar] [CrossRef]
- Holgate, S.T. The epidemic of allergy and asthma. Nature 1999, 402, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Boldogh, I.; Bacsi, A.; Choudhury, B.K.; Dharajiya, N.; Alam, R.; Hazra, T.K.; Mitra, S.; Goldblum, R.M.; Sur, S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J. Clin. Investig. 2005, 115, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Bacsi, A.; Choudhury, B.; Sur, S.; Boldogh, I. Lactoferrin decreases pollen antigen-induced allergic airway inflammation in a murine model of asthma. Immunology 2006, 119, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Zimecki, M.; Artym, J.; Kocięba, M.; Kaleta-Kuratewicz, K.; Kruzel, M.L. Lactoferrin restrains allergen-induced pleurisy in mice. Inflamm. Res. 2012, 61, 1247–1255. [Google Scholar] [CrossRef]
- Cutone, A.; Lepanto, M.S.; Rosa, L.; Scotti, M.J.; Rossi, A.; Ranucci, S.; De Fino, I.; Bragonzi, A.; Valenti, P.; Musci, G.; et al. Aerosolized bovine lactoferrin counteracts infection, inflammation and iron dysbalance in a cystic fibrosis mouse model of Pseudomonas aeruginosa chronic lung infection. Int. J. Mol. Sci. 2019, 20, 2128. [Google Scholar] [CrossRef]
- Kuhara, T.; Tanaka, A.; Yamauchi, K.; Iwatsuki, K. Bovine lactoferrin ingestion protects against inflammation via IL-11 induction in the small intestine of mice with hepatitis. Br. J. Nutr. 2014, 111, 1801–1810. [Google Scholar] [CrossRef]
- Tsubota, A.; Yoshikawa, T.; Nariai, K.; Mitsunaga, M.; Yumoto, Y.; Fukushima, K.; Hoshina, S.; Fujise, K. Bovine lactoferrin potently inhibits liver mitochondrial 8-OHdG levels and retrieves hepatic OGG1 activities in Long-Evans Cinnamon rats. J. Hepatol. 2008, 48, 486–493. [Google Scholar] [CrossRef]
- Konishi, M.; Iwasa, M.; Yamauchi, K.; Sugimoto, R.; Fujita, N.; Kobayashi, Y.; Watanabe, S.; Teraguchi, S.; Adachi, Y.; Kaito, M. Lactoferrin inhibits lipid peroxidation in patients with chronic hepatitis C. Hepatol. Res. 2006, 36, 27–32. [Google Scholar] [CrossRef]
- Pattamatta, U.; Willcox, M.; Stapleton, F.; Garrett, Q. Bovine Lactoferrin Promotes Corneal Wound Healing and Suppresses IL-1 Expression in Alkali Wounded Mouse Cornea. Curr. Eye Res. 2013, 38, 1110–1117. [Google Scholar] [CrossRef]
- Roda, M.; Corazza, I.; Reggiani, M.L.B.; Pellegrini, M.; Taroni, L.; Giannaccare, G.; Versura, P. Dry Eye Disease and Tear Cytokine Levels—A Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3111. [Google Scholar] [CrossRef]
- Sonobe, H.; Ogawa, Y.; Yamada, K.; Shimizu, E.; Uchino, Y.; Kamoi, M.; Saijo, Y.; Yamane, M.; Citterio, D.; Suzuki, K.; et al. A novel and innovative paper-based analytical device for assessing tear lactoferrin of dry eye patients. Ocul. Surf. 2019, 17, 160–166. [Google Scholar] [CrossRef]
- Lim, S.Y.; Raftery, M.J.; Goyette, J.; Hsu, K.; Geczy, C.L. Oxidative modifications of S100 proteins: Functional regulation by redox. J. Leukoc. Biol. 2009, 86, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Amico, C.; Tornetta, T.; Scifo, C.; Blanco, A.R. Antioxidant Effect of 0.2% Xanthan Gum in Ocular Surface Corneal Epithelial Cells. Curr. Eye Res. 2014, 40, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Pastori, V.; Tavazzi, S.; Lecchi, M. Lactoferrin-loaded contact lenses: Eye protection against oxidative stress. Cornea 2015, 34, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sheng, M.; Li, J.; Yan, G.; Lin, A.; Li, M.; Wang, W.; Chen, Y. Tear proteomic analysis of Sjögren syndrome patients with dry eye syndrome by two-dimensional-nano-liquid chromatography coupled with tandem mass spectrometry. Sci. Rep. 2014, 4, 5772. [Google Scholar] [CrossRef]
- Tong, L.; Zhou, L.; Beuerman, R.W.; Zhao, S.Z.; Li, X.R. Association of tear proteins with Meibomian gland disease and dry eye symptoms. Br. J. Ophthalmol. 2010, 95, 848–852. [Google Scholar] [CrossRef]
- Zhou, L.; Beuerman, R.W.; Chan, C.M.; Zhao, S.Z.; Li, X.R.; Yang, H.; Tong, L.; Liu, S.; Stern, M.E.; Tan, D. Identification of Tear Fluid Biomarkers in Dry Eye Syndrome Using iTRAQ Quantitative Proteomics. J. Proteome Res. 2009, 8, 4889–4905. [Google Scholar] [CrossRef]
- Maneva, A.; Taleva, B.; Maneva, L. Lactoferrin-Protector against Oxidative Stress and Regulator of Glycolysis in Human Erythrocytes. Z. Fur Naturforschung Sect. C-A J. Biosci. 2003, 58, 256–262. [Google Scholar] [CrossRef]
- Kim, C.W.; Son, K.-N.; Choi, S.-Y.; Kim, J. Human lactoferrin upregulates expression of KDR/Flk-1 and stimulates VEGF-A-mediated endothelial cell proliferation and migration. FEBS Lett. 2006, 580, 4332–4336. [Google Scholar] [CrossRef]
- Norrby, K.; Mattsby-Baltzer, I.; Innocenti, M.; Tuneberg, S. Orally administered bovine lactoferrin systemically inhibits VEGF165-mediated angiogenesis in the rat. Int. J. Cancer 2001, 91, 236–240. [Google Scholar] [CrossRef]
- Mader, J.S.; Smyth, D.; Marshall, J.; Hoskin, D.W. Bovine Lactoferricin Inhibits Basic Fibroblast Growth Factor- and Vascular Endothelial Growth Factor165-Induced Angiogenesis by Competing for Heparin-Like Binding Sites on Endothelial Cells. Am. J. Pathol. 2006, 169, 1753–1766. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rascón-Cruz, Q.; Siqueiros-Cendón, T.S.; Siañez-Estrada, L.I.; Villaseñor-Rivera, C.M.; Ángel-Lerma, L.E.; Olivas-Espino, J.A.; León-Flores, D.B.; Espinoza-Sánchez, E.A.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F. Antioxidant Potential of Lactoferrin and Its Protective Effect on Health: An Overview. Int. J. Mol. Sci. 2025, 26, 125. https://doi.org/10.3390/ijms26010125
Rascón-Cruz Q, Siqueiros-Cendón TS, Siañez-Estrada LI, Villaseñor-Rivera CM, Ángel-Lerma LE, Olivas-Espino JA, León-Flores DB, Espinoza-Sánchez EA, Arévalo-Gallegos S, Iglesias-Figueroa BF. Antioxidant Potential of Lactoferrin and Its Protective Effect on Health: An Overview. International Journal of Molecular Sciences. 2025; 26(1):125. https://doi.org/10.3390/ijms26010125
Chicago/Turabian StyleRascón-Cruz, Quintín, Tania Samanta Siqueiros-Cendón, Luis Ignacio Siañez-Estrada, Celina María Villaseñor-Rivera, Lidia Esmeralda Ángel-Lerma, Joel Arturo Olivas-Espino, Dyada Blanca León-Flores, Edward Alexander Espinoza-Sánchez, Sigifredo Arévalo-Gallegos, and Blanca Flor Iglesias-Figueroa. 2025. "Antioxidant Potential of Lactoferrin and Its Protective Effect on Health: An Overview" International Journal of Molecular Sciences 26, no. 1: 125. https://doi.org/10.3390/ijms26010125
APA StyleRascón-Cruz, Q., Siqueiros-Cendón, T. S., Siañez-Estrada, L. I., Villaseñor-Rivera, C. M., Ángel-Lerma, L. E., Olivas-Espino, J. A., León-Flores, D. B., Espinoza-Sánchez, E. A., Arévalo-Gallegos, S., & Iglesias-Figueroa, B. F. (2025). Antioxidant Potential of Lactoferrin and Its Protective Effect on Health: An Overview. International Journal of Molecular Sciences, 26(1), 125. https://doi.org/10.3390/ijms26010125






