The Role of Glutathione Transferase Omega-Class Variant Alleles in Individual Susceptibility to Ovarian Cancer
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- PDQ Adult Treatment Editorial Board Ovarian Epithelial. Fallopian Tube, and Primary Peritoneal Cancer Treatment (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Sambasivan, S. Epithelial Ovarian Cancer: Review Article. Cancer Treat. Res. Commun. 2022, 33, 100629. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Chen, L.; Hou, J.Y.; Burke, W.M.; Tergas, A.I.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L. Association of Hospital Volume and Quality of Care with Survival for Ovarian Cancer. Obs. Gynecol. 2017, 130, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian Cancer. Nat. Rev. Dis. Prim. 2016, 2, 16061. [Google Scholar] [CrossRef]
- Gilbert, L.; Basso, O.; Sampalis, J.; Karp, I.; Martins, C.; Feng, J.; Piedimonte, S.; Quintal, L.; Ramanakumar, A.V.; Takefman, J.; et al. Assessment of Symptomatic Women for Early Diagnosis of Ovarian Cancer: Results from the Prospective DOvE Pilot Project. Lancet Oncol. 2012, 13, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Nebgen, D.R.; Lu, K.H.; Bast, R.C. Novel Approaches to Ovarian Cancer Screening. Curr. Oncol. Rep. 2019, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Achimas-Cadariu, P.; Kubelac, P.; Irimie, A.; Berindan-Neagoe, I.; Rühli, F. Evolutionary Perspectives, Heterogeneity and Ovarian Cancer: A Complicated Tale from Past to Present. J. Ovarian Res. 2022, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.M.; Cardenas, C.; Tedja, R. The Role of Intra-Tumoral Heterogeneity and Its Clinical Relevance in Epithelial Ovarian Cancer Recurrence and Metastasis. Cancers 2019, 11, 1083. [Google Scholar] [CrossRef] [PubMed]
- Veneziani, A.C.; Gonzalez-Ochoa, E.; Alqaisi, H.; Madariaga, A.; Bhat, G.; Rouzbahman, M.; Sneha, S.; Oza, A.M. Heterogeneity and Treatment Landscape of Ovarian Carcinoma. Nat. Rev. Clin. Oncol. 2023, 20, 820–842. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Yang, J.; Zhao, X.; Wei, X. Tumor Microenvironment in Ovarian Cancer: Function and Therapeutic Strategy. Front. Cell Dev. Biol. 2020, 8, 758. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Mijatović, S.; Savić-Radojević, A.; Plješa-Ercegovac, M.; Simić, T.; Nicoletti, F.; Maksimović-Ivanić, D. The Double-Faced Role of Nitric Oxide and Reactive Oxygen Species in Solid Tumors. Antioxidants 2020, 9, 374. [Google Scholar] [CrossRef]
- Tew, K.D.; Townsend, D.M. Glutathione-S-Transferases as Determinants of Cell Survival and Death. Antioxid. Redox Signal. 2012, 17, 1728–1737. [Google Scholar] [CrossRef]
- Pljesa-Ercegovac, M.; Savic-Radojevic, A.; Matic, M.; Coric, V.; Djukic, T.; Radic, T.; Simic, T. Glutathione Transferases: Potential Targets to Overcome Chemoresistance in Solid Tumors. Int. J. Mol. Sci. 2018, 19, 3785. [Google Scholar] [CrossRef]
- Wu, B.; Dong, D. Human Cytosolic Glutathione Transferases: Structure, Function, and Drug Discovery. Trends Pharmacol. Sci. 2012, 33, 656–668. [Google Scholar] [CrossRef]
- Board, P.G.; Menon, D. Glutathione Transferases, Regulators of Cellular Metabolism and Physiology. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3267–3288. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, H.S.; Suh, D.H.; Kim, M.-K.; Chung, H.H.; Song, Y.-S. Ovarian Cancer Biomarker Discovery Based on Genomic Approaches. J. Cancer Prev. 2013, 18, 298–312. [Google Scholar] [CrossRef]
- Bast, R.C.; Hennessy, B.; Mills, G.B. The Biology of Ovarian Cancer: New Opportunities for Translation. Nat. Rev. Cancer 2009, 9, 415–428. [Google Scholar] [CrossRef]
- Simic, P.; Pljesa, I.; Nejkovic, L.; Jerotic, D.; Coric, V.; Stulic, J.; Kokosar, N.; Popov, D.; Savic-Radojevic, A.; Pazin, V.; et al. Glutathione Transferase P1: Potential Therapeutic Target in Ovarian Cancer. Medicina 2022, 58, 1660. [Google Scholar] [CrossRef]
- Sawers, L.; Ferguson, M.J.; Ihrig, B.R.; Young, H.C.; Chakravarty, P.; Wolf, C.R.; Smith, G. Glutathione S-Transferase P1 (GSTP1) Directly Influences Platinum Drug Chemosensitivity in Ovarian Tumour Cell Lines. Br. J. Cancer 2014, 111, 1150–1158. [Google Scholar] [CrossRef]
- Ferracini, A.C.; Lopes-Aguiar, L.; Lourenço, G.J.; Yoshida, A.; Lima, C.S.P.; Sarian, L.O.; Derchain, S.; Kroetz, D.L.; Mazzola, P.G. GSTP1 and ABCB1 Polymorphisms Predicting Toxicities and Clinical Management on Carboplatin and Paclitaxel-Based Chemotherapy in Ovarian Cancer. Clin. Transl. Sci. 2021, 14, 720–728. [Google Scholar] [CrossRef]
- Pljesa, I.; Berisavac, M.; Simic, T.; Pekmezovic, T.; Coric, V.; Suvakov, S.; Stamatovic, L.; Matic, M.; Gutic, B.; Milenkovic, S.; et al. Polymorphic Expression of Glutathione Transferases A1, M1, P1 and T1 in Epithelial Ovarian Cancer: A Serbian Case-Control Study. J. BUON 2017, 22, 72–79. [Google Scholar] [PubMed]
- Zhang, Z.; Xie, Z.; Sun, G.; Yang, P.; Li, J.; Yang, H.; Xiao, S.; Liu, Y.; Qiu, H.; Qin, L.; et al. Reversing Drug Resistance of Cisplatin by Hsp90 Inhibitors in Human Ovarian Cancer Cells. Int. J. Clin. Exp. Med. 2015, 8, 6687–6701. [Google Scholar] [PubMed]
- Fontana, F.; Carollo, E.; Melling, G.E.; Carter, D.R.F. Extracellular Vesicles: Emerging Modulators of Cancer Drug Resistance. Cancers 2021, 13, 749. [Google Scholar] [CrossRef] [PubMed]
- Whitbread, A.K.; Masoumi, A.; Tetlow, N.; Schmuck, E.; Coggan, M.; Board, P.G. Characterization of the Omega Class of Glutathione Transferases. Methods Enzym. 2005, 401, 78–99. [Google Scholar] [CrossRef] [PubMed]
- Board, P.G.; Menon, D. Structure, Function and Disease Relevance of Omega-Class Glutathione Transferases. Arch. Toxicol. 2016, 90, 1049–1067. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.; Board, P.G. A Role for Glutathione Transferase Omega 1 (GSTO1-1) in the Glutathionylation Cycle. J. Biol. Chem. 2013, 288, 25769–25779. [Google Scholar] [CrossRef]
- Piaggi, S.; Raggi, C.; Corti, A.; Pitzalis, E.; Mascherpa, M.C.; Saviozzi, M.; Pompella, A.; Casini, A.F. Glutathione Transferase Omega 1-1 (GSTO1-1) Plays an Anti-Apoptotic Role in Cell Resistance to Cisplatin Toxicity. Carcinogenesis 2010, 31, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Pan, L.; Yuan, Y.; Lang, J.; Mao, N. Identification of Platinum-Resistance Associated Proteins through Proteomic Analysis of Human Ovarian Cancer Cells and Their Platinum-Resistant Sublines. J. Proteome Res. 2007, 6, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Shigeto, T.; Miura, R.; Kobayashi, A.; Mizunuma, M.; Yamauchi, A.; Futagami, M.; Mizunuma, H. Differences in the Sensitivity of Ovarian Cancer to Photodynamic Therapy and the Mechanisms for Those Differences. Oncol. Lett. 2017, 13, 4933–4938. [Google Scholar] [CrossRef]
- Zhou, H.; Brock, J.; Liu, D.; Board, P.G.; Oakley, A.J. Structural Insights into the Dehydroascorbate Reductase Activity of Human Omega-Class Glutathione Transferases. J. Mol. Biol. 2012, 420, 190–203. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-T.; Wang, J.; Yin, R.; Qiu, M.-T.; Xu, L.; Wang, J.; Xu, L. Genetic Polymorphisms in Glutathione S-Transferase Omega (GSTO) and Cancer Risk: A Meta-Analysis of 20 Studies. Sci. Rep. 2014, 4, 6578. [Google Scholar] [CrossRef] [PubMed]
- Marahatta, S.B.; Punyarit, P.; Bhudisawasdi, V.; Paupairoj, A.; Wongkham, S.; Petmitr, S. Polymorphism of Glutathione S-Transferase Omega Gene and Risk of Cancer. Cancer Lett. 2006, 236, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Whitbread, A.K.; Tetlow, N.; Eyre, H.J.; Sutherland, G.R.; Board, P.G. Characterization of the Human Omega Class Glutathione Transferase Genes and Associated Polymorphisms. Pharmacogenetics 2003, 13, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Salavaggione, O.E.; Pelleymounter, L.L.; Moon, I.; Eckloff, B.W.; Schaid, D.J.; Wieben, E.D.; Weinshilboum, R.M. Glutathione S-transferase omega 1 and omega 2 pharmacogenomics. Drug Metab. Dispos. 2006, 34, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Pongstaporn, W.; Rochanawutanon, M.; Wilailak, S.; Linasamita, V.; Weerakiat, S.; Petmitr, S. Genetic Alterations in Chromosome 10q24.3 and Glutathione S-Transferase Omega 2 Gene Polymorphism in Ovarian Cancer. J. Exp. Clin. Cancer Res. 2006, 25, 107–114. [Google Scholar]
- Bumbasirevic, U.; Bojanic, N.; Pljesa-Ercegovac, M.; Zivkovic, M.; Djukic, T.; Zekovic, M.; Milojevic, B.; Kajmakovic, B.; Janicic, A.; Simic, T.; et al. The Polymorphisms of Genes Encoding Catalytic Antioxidant Proteins Modulate the Susceptibility and Progression of Testicular Germ Cell Tumor. Cancers 2022, 14, 1068. [Google Scholar] [CrossRef]
- Hughes, M.M.; Hooftman, A.; Angiari, S.; Tummala, P.; Zaslona, Z.; Runtsch, M.C.; McGettrick, A.F.; Sutton, C.E.; Diskin, C.; Rooke, M.; et al. Glutathione Transferase Omega-1 Regulates NLRP3 Inflammasome Activation through NEK7 Deglutathionylation. Cell Rep. 2019, 29, 151–161.e5. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Simic, T.; Djukic, T.; Radic, T.; Savic-Radojevic, A.; Zekovic, M.; Durutovic, O.; Janicic, A.; Milojevic, B.; Kajmakovic, B.; et al. The Polymorphisms in GSTO Genes (GSTO1 Rs4925, GSTO2 Rs156697, and GSTO2 Rs2297235) Affect the Risk for Testicular Germ Cell Tumor Development: A Pilot Study. Life 2023, 13, 1269. [Google Scholar] [CrossRef]
- Djukic, T.; Simic, T.; Radic, T.; Matic, M.; Pljesa-Ercegovac, M.; Suvakov, S.; Coric, V.; Pekmezovic, T.; Novakovic, I.; Dragicevic, D.; et al. GSTO1*C/GSTO2*G Haplotype Is Associated with Risk of Transitional Cell Carcinoma of Urinary Bladder. Int. Urol. Nephrol. 2015, 47, 625–630. [Google Scholar] [CrossRef]
- Radic, T.M.; Coric, V.M.; Pljesa-Ercegovac, M.S.; Basta-Jovanovic, G.M.; Radojevic-Skodric, S.M.; Dragicevic, D.P.; Matic, M.G.; Bogdanovic, L.M.; Dzamic, Z.M.; Simic, T.P.; et al. Concomitance of Polymorphisms in Glutathione Transferase Omega Genes Is Associated with Risk of Clear Cell Renal Cell Carcinoma. Tohoku J. Exp. Med. 2018, 246, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Bhardwaj, M.; Kang, S.C. GSTO1 Confers Drug Resistance in HCT-116 Colon Cancer Cells through an Interaction with TNFαIP3/A20. Int. J. Oncol. 2022, 61, 136. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, L.; Liu, M.; Luo, J. Protein Post-Translational Modifications in the Regulation of Cancer Hallmarks. Cancer Gene Ther. 2023, 30, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Uys, J.D.; Tew, K.D.; Townsend, D.M. S-Glutathionylation: From Molecular Mechanisms to Health Outcomes. Antioxid. Redox Signal 2011, 15, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Laliberte, R.E.; Perregaux, D.G.; Hoth, L.R.; Rosner, P.J.; Jordan, C.K.; Peese, K.M.; Eggler, J.F.; Dombroski, M.A.; Geoghegan, K.F.; Gabel, C.A. Glutathione S-Transferase Omega 1-1 Is a Target of Cytokine Release Inhibitory Drugs and May Be Responsible for Their Effect on Interleukin-1beta Posttranslational Processing. J. Biol. Chem. 2003, 278, 16567–16578. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, S.; Monaci, P.M.; Polimanti, R.; Manfellotto, D.; Fuciarelli, M. GSTO2*N142D Gene Polymorphism Associated with Hypothyroidism in Italian Patients. Mol. Biol. Rep. 2013, 40, 1967–1971. [Google Scholar] [CrossRef]
- Tian, W.; Wang, Y.; Xu, Y.; Guo, X.; Wang, B.; Sun, L.; Liu, L.; Cui, F.; Zhuang, Q.; Bao, X.; et al. The Hypoxia-Inducible Factor Renders Cancer Cells More Sensitive to Vitamin C-Induced Toxicity. J. Biol. Chem. 2014, 289, 3339–3351. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial Effects from a Mechanistic Perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Yeh, S.-D.; Shen, K.-H.; Shen, C.-H.; Juang, G.-D.; Hsu, L.-I.; Chiou, H.-Y.; Chen, C.-J. A Significantly Joint Effect between Arsenic and Occupational Exposures and Risk Genotypes/Diplotypes of CYP2E1, GSTO1 and GSTO2 on Risk of Urothelial Carcinoma. Toxicol. Appl. Pharmacol. 2009, 241, 111–118. [Google Scholar] [CrossRef]
- Ding, D.-N.; Xie, L.-Z.; Shen, Y.; Li, J.; Guo, Y.; Fu, Y.; Liu, F.-Y.; Han, F.-J. Insights into the Role of Oxidative Stress in Ovarian Cancer. Oxid. Med. Cell Longev. 2021, 2021, 8388258. [Google Scholar] [CrossRef]
- Schmuck, E.M.; Board, P.G.; Whitbread, A.K.; Tetlow, N.; Cavanaugh, J.A.; Blackburn, A.C.; Masoumi, A. Characterization of the Monomethylarsonate Reductase and Dehydroascorbate Reductase Activities of Omega Class Glutathione Transferase Variants: Implications for Arsenic Metabolism and the Age-at-Onset of Alzheimer’s and Parkinson’s Diseases. Pharmacogenet Genom. 2005, 15, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, M. Linkage Disequilibrium—Understanding the Evolutionary Past and Mapping the Medical Future. Nat. Rev. Genet. 2008, 9, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Zou, F.; Chai, H.S.; Younkin, C.S.; Miles, R.; Nair, A.A.; Crook, J.E.; Pankratz, V.S.; Carrasquillo, M.M.; Rowley, C.N.; et al. Glutathione S-Transferase Omega Genes in Alzheimer and Parkinson Disease Risk, Age-at-Diagnosis and Brain Gene Expression: An Association Study with Mechanistic Implications. Mol. Neurodegener. 2012, 7, 13. [Google Scholar] [CrossRef]
- Ranganathan, S.; Gribskov, M.R.; Nakai, K.; Schönbach, C. (Eds.) Encyclopedia of Bioinformatics and Computational Biology; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-811432-2. [Google Scholar]
- Corrigan, C. ‘Allergy (4th Edition)’ Edited by HolgateST, ChurchMK, BroideDH, MartinezFD. Clin. Exp. Allergy 2012, 42, 1299. [Google Scholar] [CrossRef]
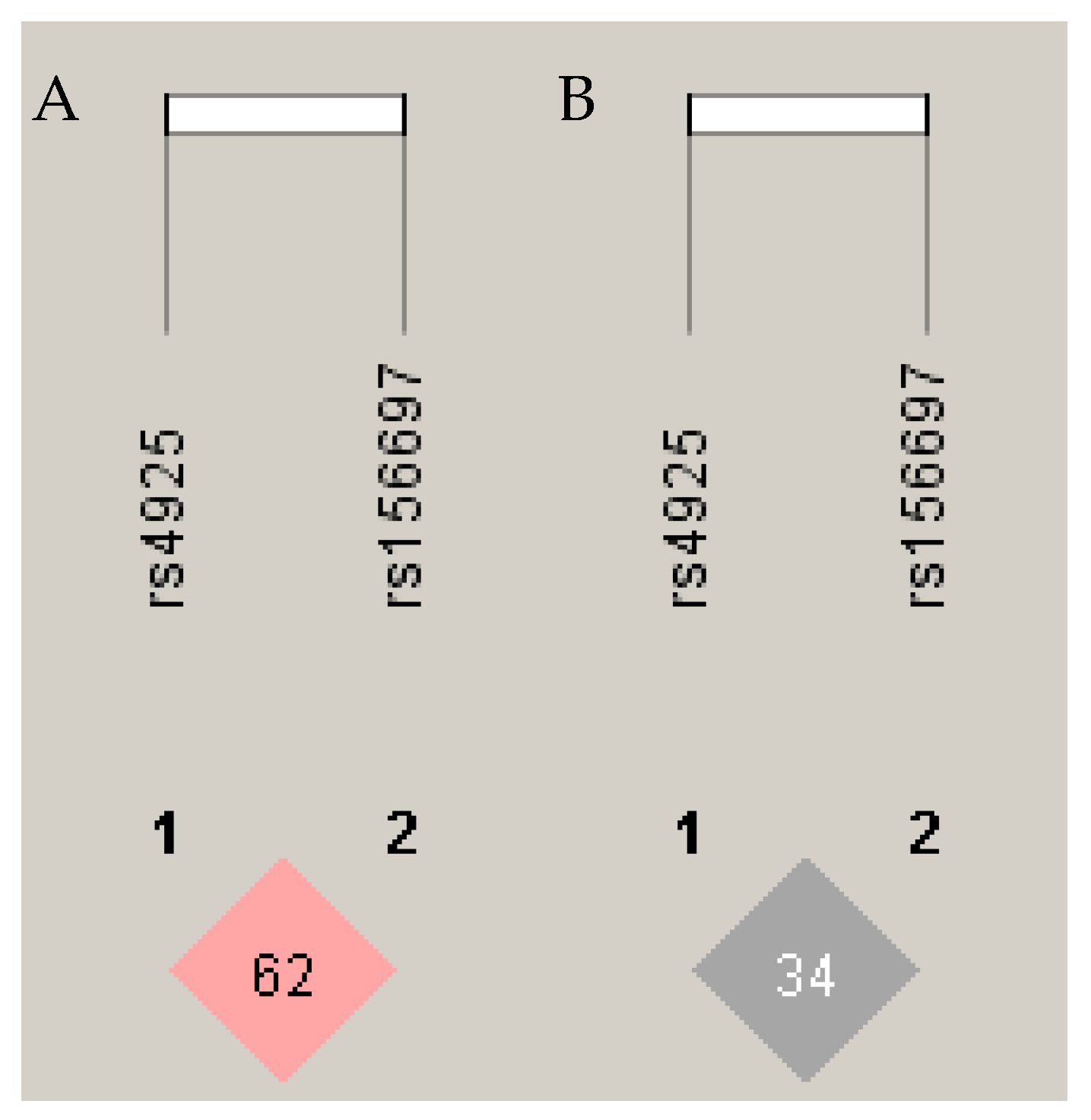
| Parameters 1 | Patients, n (%) | Control, n (%) | p-Value |
|---|---|---|---|
| Age (years) | 58.14 ± 9.8 2 | 57.05 ± 8.10 | 0.361 |
| Obesity | |||
| BMI < 25 | 53 (49) | 48 (42) | 0.297 |
| BMI > 25 | 55 (51) | 66 (58) | |
| BMI (kg/m2) | 25.79 ± 4.59 2 | 26.50 ± 4.71 | 0.253 |
| Smoking 3 | |||
| Never | 50 (47) | 70 (54) | 0.249 |
| Ever | 57 (53) | 59 (46) | |
| Hypertension | |||
| Yes | 34 (32) | 49 (38) | 0.296 |
| No | 74 (68) | 80 (62) |
| Parameters 1 | Patients, n (%) |
|---|---|
| Parity | |
| 0 | 12 (11) |
| 1 | 27 (25) |
| 2 | 60 (55) |
| >3 | 11 (9) |
| Family history of ovarian cancer | |
| Yes | 14 (13) |
| No | 96 (87) |
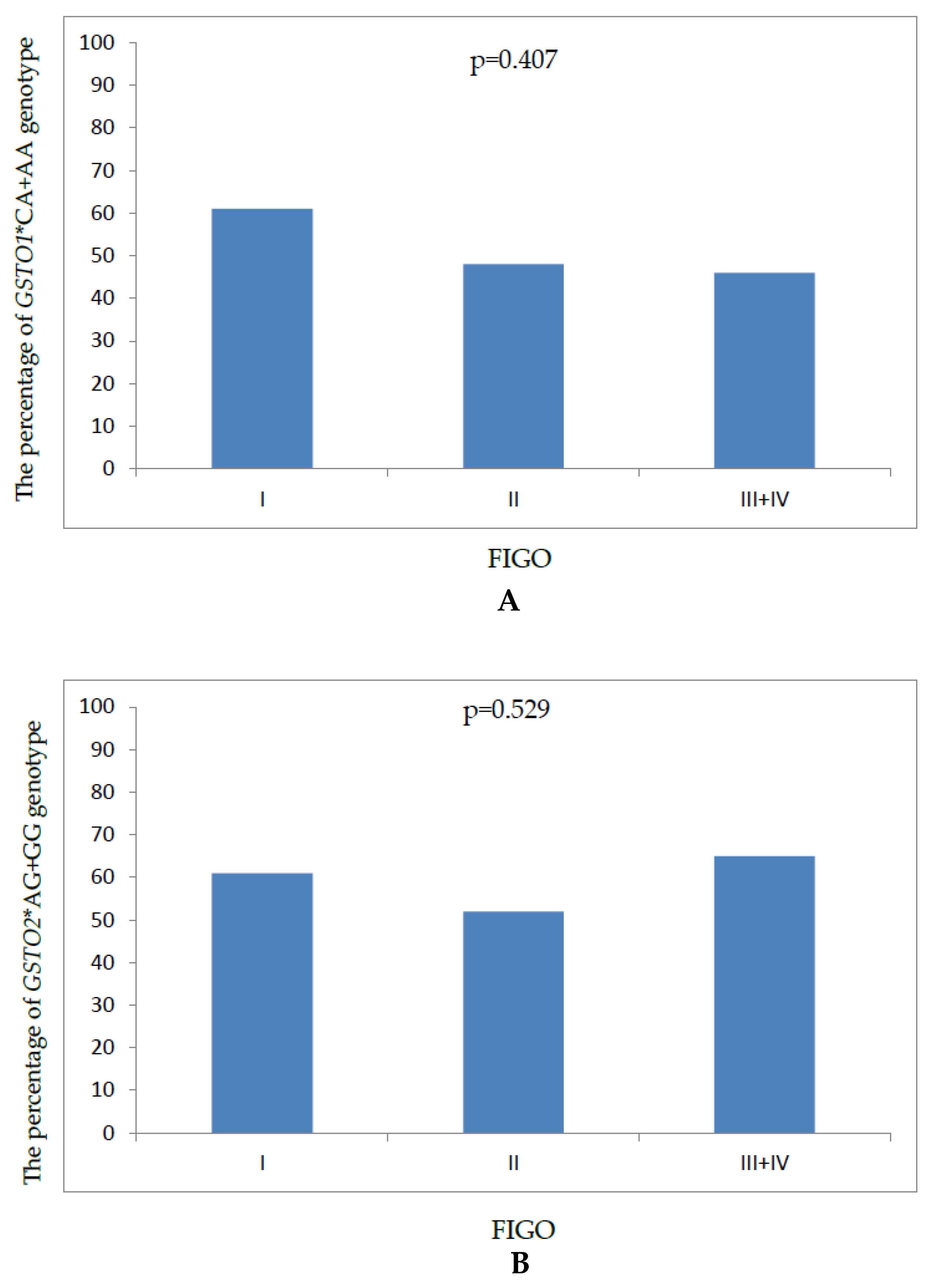
| FIGO stage | |
| I | 33 (30) |
| II | 25 (23) |
| III | 50 (46) |
| IV | 2 (1) |
| Grade of tumor | |
| I | 11 (11) |
| II | 55 (52) |
| III | 39 (37) |
| GST Genotype | Patients n (%) | Controls n (%) | OR1 (95% CI) | p Value | OR2 (95% CI) | p Value |
|---|---|---|---|---|---|---|
| GSTO1 | ||||||
| *CC | 54 (49) | 59 (46) | 1.00 * | 1.00 * | ||
| *CA | 42 (38) | 63 (49) | 0.72 (0.42–1.24) | 0.25 | 0.72 (0.40–1.27) | 0.25 |
| *AA | 14 (13) | 7 (5) | 2.18 (0.82–5.81) | 0.11 | 2.09 (0.72–6.05) | 0.17 |
| *CC | 54 (49) | 59 (46) | 1.00 * | 1.00 * | ||
| *CA-AA | 56 (51) | 70 (54) | 0.87 (0.52–1.45) | 0.60 | 0.84 (0.49–1.46) | 0.55 |
| GSTO2 | ||||||
| *AA | 43 (39) | 62 (48) | 1.00 * | 1.00 * | ||
| *AG | 52 (47) | 57 (44) | 1.31 (0.76–2.25) | 0.32 | 1.43 (0.80–2.55) | 0.21 |
| *GG | 15 (14) | 10 (8) | 2.16 (0.88–5.26) | 0.08 | 2.49 (0.93–6.61) | 0.06 |
| *AA | 43 (39) | 62 (48) | 1.00 * | 1.00 * | ||
| *AG-GG | 67 (61) | 67 (52) | 1.44 (0.86–2.41) | 0.16 | 1.57 (0.90–2.73) | 0.10 |
| GSTO1 | GSTO2 | Count (Frequency) | OR1 (95% CI) | p-Value | OR2 (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| C | A | 129 (0.58) | 1.00 * | 1.00 * | ||
| A | G | 51 (0.22) | 1.47 (0.93–2.33) | 0.10 | 1.54 (0.93–2.56) | 0.095 |
| C | G | 23 (0.10) | 0.76 (0.39–1.48) | 0.57 | 0.82 (0.41–1.63) | 0.57 |
| A | A | 18 (0.08) | 0.29 (0.12–0.70) | 0.007 | 0.27 (0.11–0.67) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simic, P.; Coric, V.; Pljesa, I.; Savic-Radojevic, A.; Zecevic, N.; Kocic, J.; Simic, T.; Pazin, V.; Pljesa-Ercegovac, M. The Role of Glutathione Transferase Omega-Class Variant Alleles in Individual Susceptibility to Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 4986. https://doi.org/10.3390/ijms25094986
Simic P, Coric V, Pljesa I, Savic-Radojevic A, Zecevic N, Kocic J, Simic T, Pazin V, Pljesa-Ercegovac M. The Role of Glutathione Transferase Omega-Class Variant Alleles in Individual Susceptibility to Ovarian Cancer. International Journal of Molecular Sciences. 2024; 25(9):4986. https://doi.org/10.3390/ijms25094986
Chicago/Turabian StyleSimic, Petar, Vesna Coric, Igor Pljesa, Ana Savic-Radojevic, Nebojsa Zecevic, Jovana Kocic, Tatjana Simic, Vladimir Pazin, and Marija Pljesa-Ercegovac. 2024. "The Role of Glutathione Transferase Omega-Class Variant Alleles in Individual Susceptibility to Ovarian Cancer" International Journal of Molecular Sciences 25, no. 9: 4986. https://doi.org/10.3390/ijms25094986
APA StyleSimic, P., Coric, V., Pljesa, I., Savic-Radojevic, A., Zecevic, N., Kocic, J., Simic, T., Pazin, V., & Pljesa-Ercegovac, M. (2024). The Role of Glutathione Transferase Omega-Class Variant Alleles in Individual Susceptibility to Ovarian Cancer. International Journal of Molecular Sciences, 25(9), 4986. https://doi.org/10.3390/ijms25094986









