A View on the Chemical and Biological Attributes of Five Edible Fruits after Finishing Their Shelf Life: Studies on Caco-2 Cells
Abstract
1. Introduction
2. Results
2.1. Chemical Analytical Results
2.2. Antioxidant Activity Results
2.3. In Vitro Pharmacological Activity Results
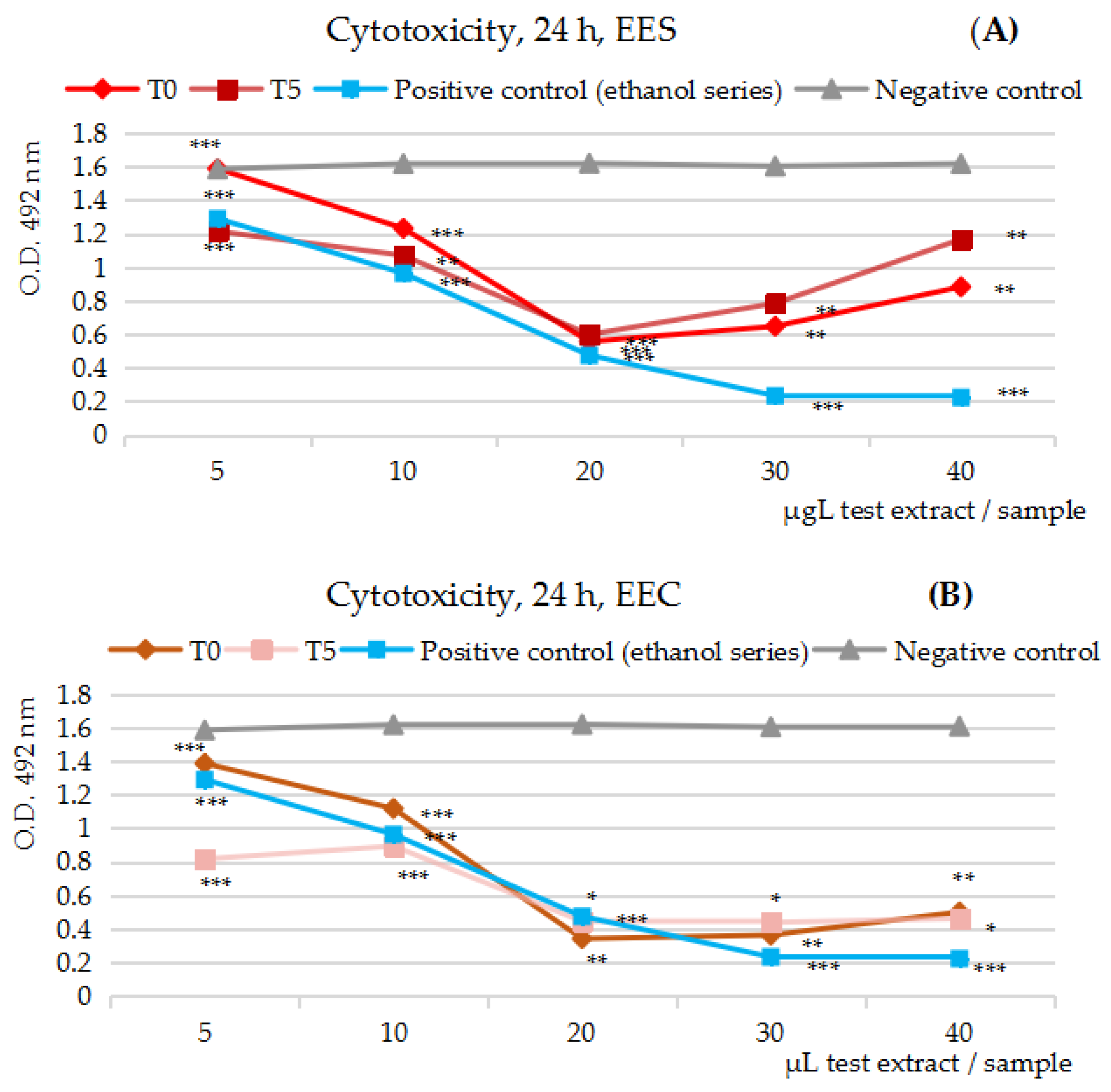
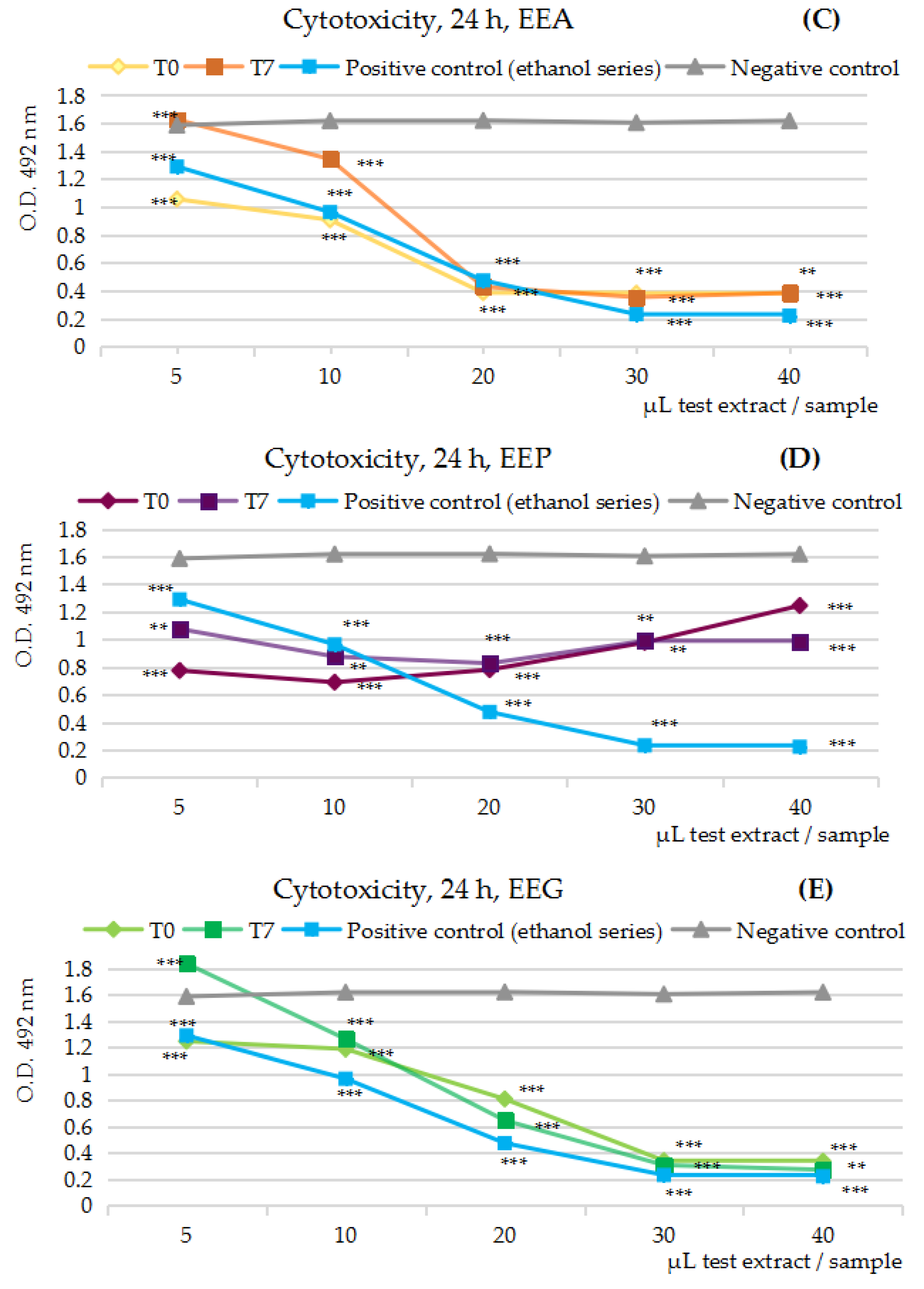
2.3.1. Cytotoxicity MTS Assay Results on the Five Series of Fruits Extracts, at t = 0 and t = 5/7
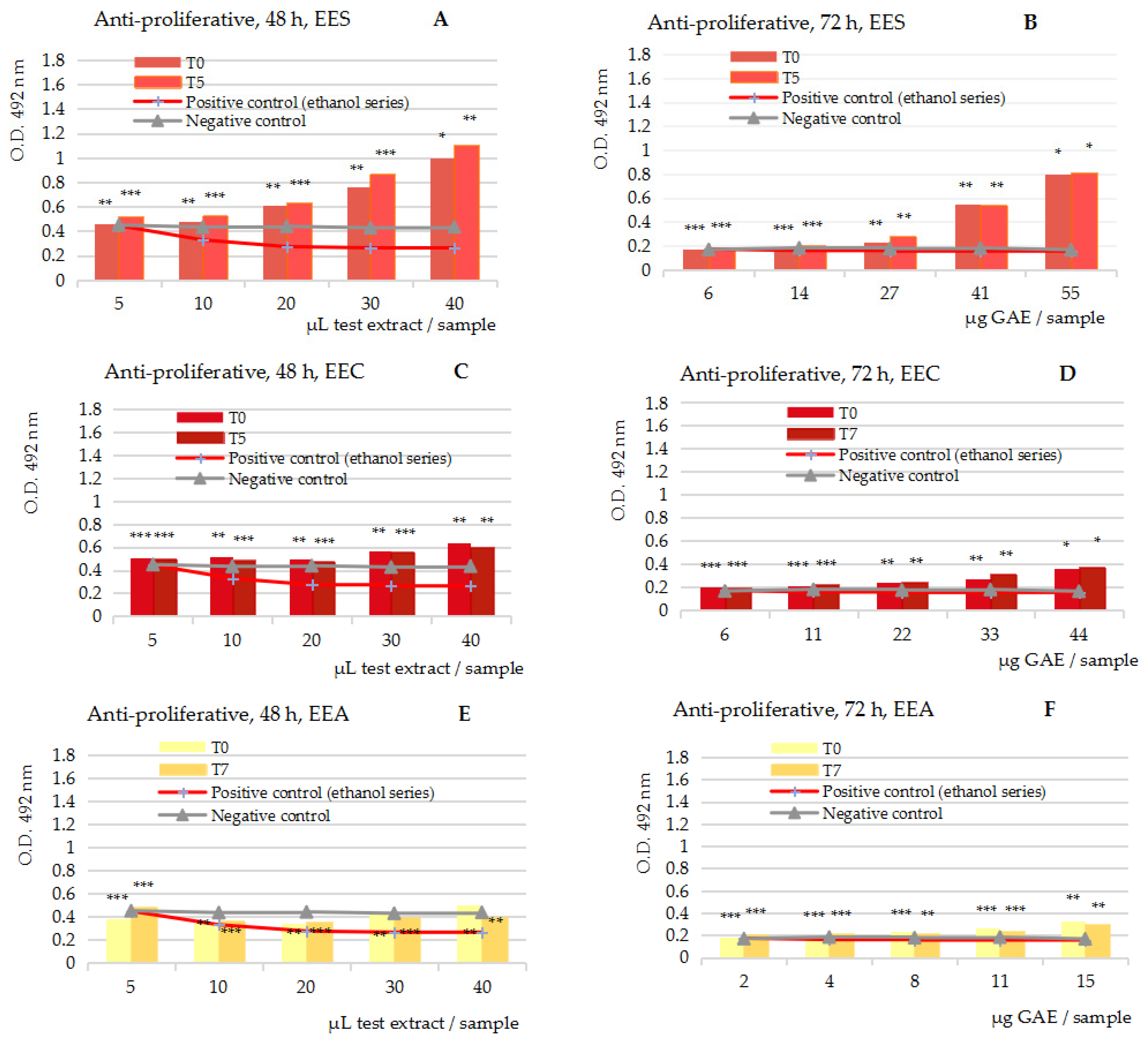
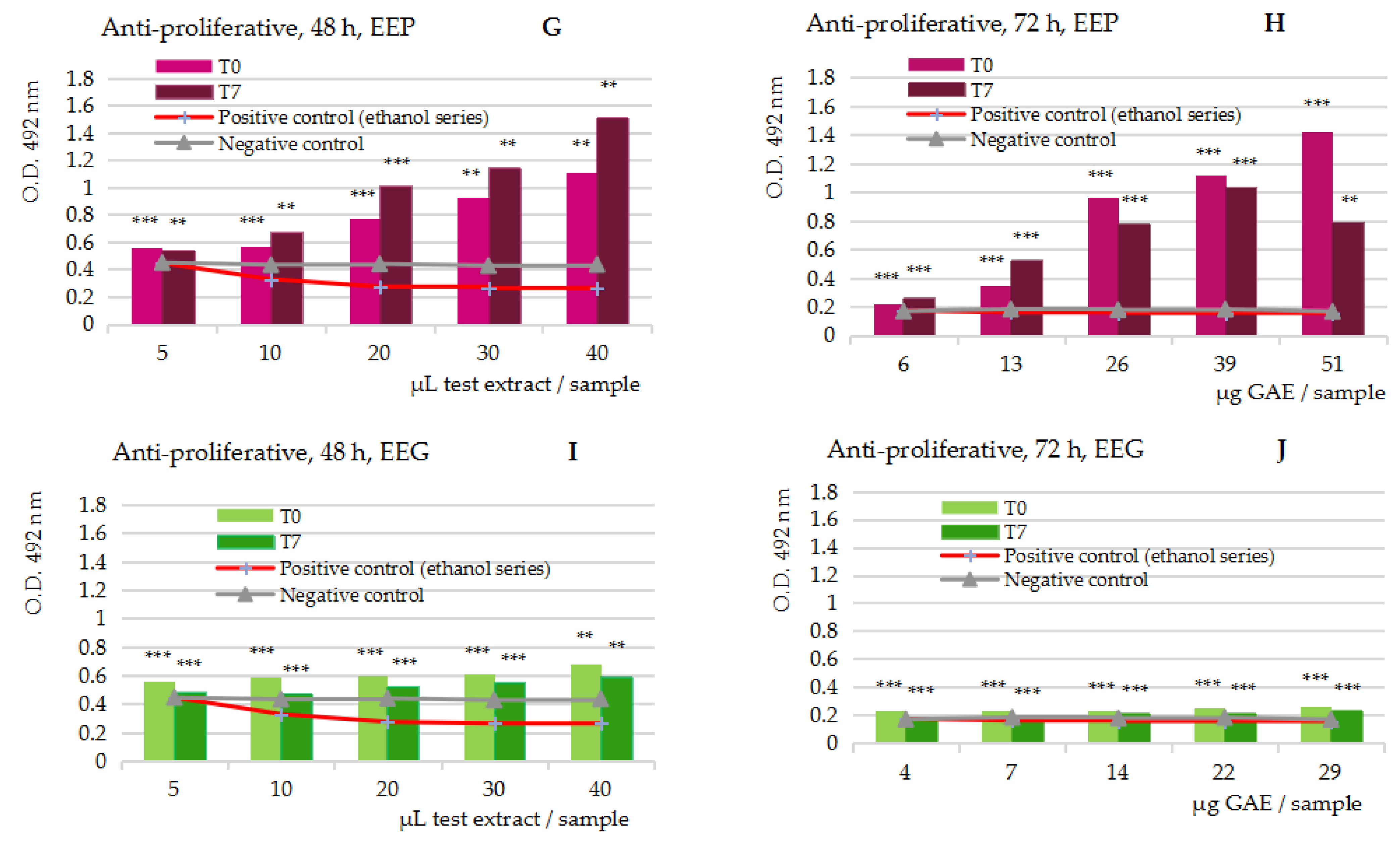
2.3.2. Anti-Proliferative Assay on the Five Series of 50% Ethanolic Extracts, at t = 0 and t = 5/7
3. Discussion
4. Materials and Methods
4.1. Fruits’ Derived Products Preparation
4.2. Fruit-Derived Products’ (EE and AP) Analytical Characterization
4.3. Antioxidant Activity and ROS Efficacy of EE Products
4.4. In Vitro Pharmacological Studies on the EE Products
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of Food and Agriculture 2023—Revealing the True Cost of Food to Transform Agrifood Systems; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Vegetal Waste—Final Consumption by Country, Energy Statistics Database|United Nations Statistics Division, Viewed 11 April 2017. Available online: http://data.un.org/Data.aspx?d=EDATA&f=cmID:VW (accessed on 25 February 2024).
- Lord, S. Hidden Costs of Agrifood Systems and Recent Trends from 2016 to 2023; FAO Agricultural Development Economics Technical Study, No. 31; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Food Waste in America in 2024: Statistics & Facts—RTS. Available online: https://www.rts.com/resources/guides/food-waste-america (accessed on 26 February 2024).
- Kirci, M.; Isaksson, O.; Seifert, R. Managing Perishability in the Fruit and Vegetable Supply Chains. Sustainability 2022, 14, 5378. [Google Scholar] [CrossRef]
- Magnin, C. How Big Data Will Revolutionize the Global Food Chain; McKinsey & Company: Brussels, Belgium, 2016; Available online: https://www.mckinsey.com/capabilities/mckinsey-digital/our-insights/how-big-data-will-revolutionize-the-global-food-chain#/ (accessed on 19 March 2024).
- Gustavsson, J.; Cederberg, C.; Sonesson, U. Global Food Losses and Food Waste; Save Food Congress: Dusseldor, Germany, 2011. [Google Scholar]
- Filho, W.L.; Kovaleva, M. Food Waste and Sustainable Food Waste Management in the Baltic Sea Region; Springer: Charm, Switzerland, 2015. [Google Scholar] [CrossRef]
- Vegetal Waste-Final Consumption by Country, ChartsBin.com, Viewed 26 February 2024. Available online: https://chartsbin.com/view/44477 (accessed on 26 February 2024).
- Shen, G.; Li, Z.; Hong, T.; Ru, X.; Wang, K.; Gu, Y.; Han, J.; Guo, Y. The status of the global food waste mitigation policies: Experience and inspiration for China. Environ. Dev. Sustain. 2023, 26, 8329–8357. [Google Scholar] [CrossRef]
- Doria, E.; Boncompagni, E.; Marra, A.; Dossena, M.; Verri, M.; Buonocore, D. Polyphenols Extraction From Vegetable Wastes Using a Green and Sustainable Method. Front. Sustain. Food Syst. 2021, 5, 690399. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Fakayode, O.A.; Li, H. Green Extraction of Polyphenols via Deep Eutectic Solvents and Assisted Technologies from Agri-Food By-Products. Molecules 2023, 28, 6852. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Riaz, S.; Sameen, A.; Naumovski, N.; Iqbal, M.W.; Rehman, A.; Mehany, T.; Zeng, X.-A.; Manzoor, M.F. The Disposition of Bioactive Compounds from Fruit Waste, Their Extraction, and Analysis Using Novel Technologies: A Review. Processes 2022, 10, 2014. [Google Scholar] [CrossRef]
- Shankar Prasad, S.; Debabrata, M.; Sourav, S.; Sudipta Kumar, R.; Sumit Prasad, S.; Kriti, G.; Soumen, B. Fruit waste: A current perspective for the sustainable production of pharmacological, nutraceutical, and bioactive resources. Front. Microbiol. 2023, 14, 1260071. [Google Scholar] [CrossRef]
- Shukla, S.; Sondhi, A.; Tripathi, A.D.; Lee, J.-K.; Patel, S.K.S.; Agarwal, A. Valorisation of fruit waste for harnessing the bioactive compounds and its therapeutic application. Trends Food Sci. Technol. 2024, 114, 104302. [Google Scholar] [CrossRef]
- Mishraa, A.K.; Singhb, R.; Rawatb, H.; Kumarb, V.; Jagtapb, C.; Jainc, A. The influence of food matrix on the stability and bioavailability of phytochemicals: A comprehensive review. Food Humanit. 2024, 2, 100202. [Google Scholar] [CrossRef]
- Nicolescu, A.; Babota, M.; Barros, L.; Rocchetti, G.; Lucini, L.; Tanase, C.; Mocan, A.; Bunea, C.I.; Crisan, G. Bioaccessibility and bioactive potential of different phytochemical classes from nutraceuticals and functional foods. Front. Nutr. 2023, 10, 1184535. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Schneider, R.G. Quinoa flavonoids and their bioaccessibility during in vitro gastrointestinal digestion. J. Cereal. Sci. 2020, 95, 103070. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Borompichaichartkul, C.; Iwamoto, S. Bioaccessibility andantioxidant activity of phenolic acids, flavonoids, and anthocyanins of encapsulated Thai rice bran extracts during in vitro gastrointestinal digestion. Food Chem. 2021, 361, 130161. [Google Scholar] [CrossRef] [PubMed]
- Scrob, T.; Covaci, E.; Hosu, A.; Tanaselia, C.; Casoni, D.; Torok, A.I.; Frentiu, T.; Cimpoiu, C. Effect of in vitro simulated gastrointestinal digestion on some nutritional characteristics of several dried fruits. Food Chem. 2022, 385, 132713. [Google Scholar] [CrossRef] [PubMed]
- Cubero-Leon, E.; Peñalver, R.; Maquet, A. Review on metabolomics for food authentication. Food Res. Int. 2014, 60, 95–107. [Google Scholar] [CrossRef]
- Selamat, J.; Rozani, N.A.A.; Murugesu, S. Application of the Metabolomics Approach in Food Authentication. Molecules 2021, 26, 7565. [Google Scholar] [CrossRef] [PubMed]
- USDA. U.S. Department of Agriculture, Agricultural Research Service. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169949/nutrients (accessed on 26 February 2024).
- The European Market Potential for Strawberries. Available online: https://www.cbi.eu/market-information/fresh-fruit-vegetables/strawberries/market-potential (accessed on 26 February 2024).
- Forecast and Data for the 2023/2024 EU Season from the USDAS’s Stone Fruit Report. Available online: https://cherrytimes.it/en/news/Forecasts-and-data-for-the-2023-2024-EU-season-from-the-USDAs-stone-fruit-report (accessed on 28 February 2024).
- Eurostat Statistics Explaine. Agricultural Production—Orchards. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_orchards#General_overview (accessed on 29 February 2024).
- Plum Production by Country 2024. Available online: https://worldpopulationreview.com/country-rankings/plum-production-by-country (accessed on 1 March 2024).
- The European Market Potential for Fresh Plums and Other Stone Fruit. Available online: https://www.cbi.eu/sites/default/files/pdf/research/1210.pdf (accessed on 1 March 2024).
- The European Market Potential for Table Grapes. Available online: https://www.cbi.eu/market-information/fresh-fruit-vegetables/table-grapes/market-potential (accessed on 1 March 2024).
- Gampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive Compounds of Strawberry and Blueberry and their Potential Health Effects Based on Human Intervention Studies: A Brief Overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef]
- Heinonen, M.I.; Meyer, A.S.; Frankel, E.N. Antioxidant Activity of Berry Phenolics on Human Low-Density Lipoprotein and Liposome Oxidation. J. Agric. Food Chem. 1998, 46, 4107–4112. [Google Scholar] [CrossRef]
- Maraei, R.W.; Elsawy, K.M. Chemical quality and nutrient composition of strawberry fruits treated by γ-irradiation. J. Radiat. Res. Appl. Sci. 2017, 10, 80–87. [Google Scholar] [CrossRef]
- Erkan, M.; Wang, S.Y.; Wang, C.Y. Effect of UV treatment on antioxidant capacity, antioxidant enzyme activity and decay in strawberry fruits. Postharvest Biol. Technol. 2008, 48, 163–171. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry Antioxidants: From Farm to Table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef] [PubMed]
- Serano, M.; Guillen, F.; Martinez-Romero, D.; Castillo, S.; Valero, D. Chemical Constituents and Antioxidant Activity of Sweet Cherry at Different Ripening Stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar] [CrossRef] [PubMed]
- Mozetic, B.; Trebse, P.; Simcis, M.; Hribar, J. Changes of anthocyanins and hydroxycinnamic acids affecting the skin colour during maturation of sweet cherries (Prunus avium L.). Lebensm. Wiss. Technol. 2004, 37, 123–128. [Google Scholar] [CrossRef]
- Chaovanalikit, A.; Wrolstad, R.E. Anthocyanin and polyphenolic composition of fresh and processed cherries. J. Food Sci. 2004, 69, FCT73–FCT83. [Google Scholar] [CrossRef]
- Gomez-Martínez, H.; Bermejo, A.; Zuriaga, E.; Badenes, M.L. Polyphenol content in apricot fruits. Sci. Hortic. 2021, 277, 109828. [Google Scholar] [CrossRef]
- Dragovic-Uzelac, V.; Delonga, K.; Levaj, B.; Djakovic, S.; Pospisil, J. Phenolic profiles of raw apricots pumpkins and their purees in the evaluation of apricot nectars and jams authenticity. J. Agric. Food Chem. 2005, 53, 4836–4842. [Google Scholar] [CrossRef] [PubMed]
- Campbell, O.E.; Merwin, I.A.; Padilla-Zakour, O.I. Characterization and the Effect of Maturity at Harvest on the Phenolic and Carotenoid Content of Northeast USA Apricot (Prunus armeniaca) Varieties. J. Agric. Food Chem. 2013, 61, 12700–12710. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; Gundogdu, M.; Ercisli, S.; Celik, F.; Gecer, M.K.; Kodad, O.; Zia-Ul-Haq, M. Phenolic compounds and vitamins in wild and cultivated apricot (Prunus armeniaca L.) fruits grown in irrigated and dry farming conditions. Biol. Res. 2014, 47, 46. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, H.J. Effects of Apricot and Apricot Kernels on Human Health and Nutrition: A Review of Recent Human Research. Tech. Biochem. 2021, 2, 139–162. [Google Scholar] [CrossRef]
- Al-Soufi, M.H.; Alshwyeh, H.A.; Alqahtani, H.; Al-Zuwaid, S.K.; Al-Ahmed, F.O.; Al-Abdulaziz, F.T.; Raed, D.; Hellal, K.; Mohd Nani, N.H.; Zubaidi, S.N.; et al. A Review with Updated Perspectives on Nutritional and Therapeutic Benefits of Apricot and the Industrial Application of Its Underutilized Parts. Molecules 2022, 27, 5016. [Google Scholar] [CrossRef]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, A.; Swinny, E.E.; Ching, S.Y.L.; Jacob, S.R.; Lacey, K.; Hodgson, J.M.; Croft, K.D.; Considine, M.J. Polyphenol composition of plum selections in relation to total antioxidant capacity. J Agric Food Chem. 2012, 60, 10256–10262. [Google Scholar] [CrossRef] [PubMed]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chem. X 2021, 12, 100149. [Google Scholar] [CrossRef] [PubMed]
- Phenol-Explorer. Database on Polyphenol Content in Food. Available online: http://phenol-explorer.eu/contents/food/143 (accessed on 1 March 2024).
- Lenntech. Mineral Content of Fruit and Vegetables. Available online: https://www.lenntech.com/fruit-vegetable-mineral-content.htm (accessed on 14 February 2024).
- Mawari, G.; Kumar, N.; Sarkar, S.; Daga, M.K.; Singh, M.M.; Joshi, T.K.; Khan, N.A. Heavy Metal Accumulation in Fruits and Vegetables and Human Health Risk Assessment: Findings from Maharashtra, India. Environ. Health Insights 2022, 16, 11786302221119151. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Gong, W.; Li, B.; Zuo, J.; Pan, L.; Liu, X. Analysis of heavy metals in foodstuffs and an assessment of the health risks to the general public via consumption in Beijing, China. Int. J. Environ. Res. Public Health 2019, 16, 909. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Pan, X.D.; Wu, P.G.; Han, J.L.; Chen, Q. Heavy metals in vegetables and the health risk to population in Zhejiang, China. Food Control 2014, 36, 248–252. [Google Scholar] [CrossRef]
- Kim, Y.; Ha, E.H.; Park, H.; HA, M.; Kim, Y.; Hong, Y.-C.; Kim, E.-J.; Kim, B.-N. Prenatal lead and cadmium co-exposure and infant neurodevelopment at 6 months of age: The Mothers and Children’s Environmental Health (MOCEH) study. NeuroToxicology 2013, 35, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Guallar, E.; Silbergeld, E.K.; Rothenberg, S.J. Lead exposure and cardiovascular disease–a systematic review. Environ. Health Perspect. 2007, 115, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Nieboer, E.; Tsuji, L.J.; Martin, I.D.; Liberda, E.N. Human biomonitoring issues related to lead exposure. Environ. Sci. Process Impacts. 2013, 15, 1824–1829. [Google Scholar] [CrossRef]
- Medina-Pizzali, M.; Robles, P.; Mendoza, M.; Torres, C. [Arsenic intake: Impact in Human Nutrition and health]. Rev. Peru. Med. Exp. Salud Publica 2018, 35, 93–102. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/fileadmin/user_upload/livestockgov/documents/1_CXS_193e.pdf (accessed on 4 March 2024).
- Stüber, M.; Reemtsma, T. Evaluation of three calibration methods to compensate matrix effects in environmental analysis with LC-ESI-MS. Anal. Bioanal. Chem. 2004, 378, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.; Gigliobianco, M.R.; Magnoni, F.; Censi, R.; Di Martino, P. Compensate for or Minimize Matrix Effects? Strategies for Overcoming Matrix Effects in Liquid Chromatography-Mass Spectrometry Technique: A Tutorial Review. Molecules 2020, 25, 3047. [Google Scholar] [CrossRef]
- Kamiguchi, H.; Yamaguchi, M.; Murabayashi, M.; Mori, I.; Horinouchi, A. Method development and validation for simultaneous quantitation of endogenous hippuric acid and phenylacetylglycine in rat urine using liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1035, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Ye, D.-Q.; Zhu, B.-Q.; Wu, G.-F.; Duan, C.-Q. Rapid HPLC analysis of amino acids and biogenic amines in wines during fermentation and evaluation of matrix effect. Food Chem. 2014, 163, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, B.K. Standard line slopes as a measure of a relative matrix effect in quantitative HPLC–MS bioanalysis. J. Chromatogr. B 2006, 830, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Raterink, R.-J.; Lindenburg, P.W.; Vreeken, R.J.; Ramautar, R.; Hankemeier, T. Recent developments in sample-pretreatment techniques for mass spectrometry-based metabolomics. Trac Trends Anal. Chem. 2014, 61, 157–167. [Google Scholar] [CrossRef]
- Moein, M.M.; El Beqqali, A.; Abdel-Rehim, M. Bioanalytical method development and validation: Critical concepts and strategies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1043, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Pirvu, L.; Sha’at, F.; Miclea, L.C.; Savopol, T.; Neagu, G.; Udeanu, D.I.; Moisescu, M.G. Polygonum bistorta L. herba et flores. Polyphenols profile, antioxidant properties and cytotoxic effect on murine fibroblast cell line (NIH 3T3). Rev. Farm. 2017, 65, 571–576. [Google Scholar]
- Pirvu, L.; Stefaniu, A.; Neagu, G.; Pintilie, L. Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies. Open Chem. 2022, 20, 299–312. [Google Scholar] [CrossRef]
- Protocols & Applications Guide. Available online: https://www.promega.com (accessed on 20 September 2023).
- Solnier, J.; Chang, C.; Pizzorno, J. Consideration for Flavonoid-Containing Dietary Supplements to Tackle Deficiency and Optimize Health. Int. J. Mol. Sci. 2023, 24, 8663. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.-M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Braune, A.; Blaut, M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Yang, L.; Gao, Y.; Farag, M.F.; Gong, J.; Su, Q.; Cao, H.; Zhang, W.; Zhao, Y.; Wang, H. Dietary flavonoids and gut microbiota interaction: A focus on animal and human studies to maximize their health benefits. Food Front. 2023, 4, 1794–1809. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martinez-Florez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; Sanchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free. Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. J. 2017, 8, 4644–4656. [Google Scholar] [CrossRef]
- Sun, W.L.; Li, X.Y.; Dou, H.Y.; Wang, X.D.; Li, J.D.; Shen, L.; Li, H.F. Myricetin supplementation decreases hepatic lipid synthesis and inflammation by modulating gut microbiota. Cell Rep. 2021, 36, 109641. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonne, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. Apolyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary polyphenols promote growth of the gut bacterium Akkermansiamuciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Istas, G.; Wood, E.; Le Sayec, M.; Rawlings, C.; Yoon, J.; Dandavate, V.; Rodriguez-Mateos, A. Effects of aronia berry (poly)phenols on vascular function and gut microbiota: A double-blind randomized controlled trial in adult men. Am. J. Clin. Nutr. 2019, 110, 316–329. [Google Scholar] [CrossRef]
- Molan, A.L.; Lila, M.A.; Mawson, J.; De, S. In vitro and in vivo evaluation of the prebiotic activity of water-soluble blueberry extracts. World J. Microbiol. Biotechnol. 2009, 25, 1243–1249. [Google Scholar] [CrossRef]
- Li, Z.; Ren, Z.; Zhao, L.; Chen, L.; Yu, Y.; Wang, D.; Mao, X.; Cao, G.; Zhao, Z.; Yang, H. Unique roles in health promotion of dietary flavonoids through gut microbiota regulation: Current understanding and future perspectives. Food Chem. 2023, 399, 133959. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef] [PubMed]
- van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Elzen, E.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Dore, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Cong, Y. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef]
- Fang, W.; Xue, H.; Chen, X.; Chen, K.; Ling, W. Supplementation with sodium butyrate modulates the composition of the gut microbiota and ameliorates high-fat diet-induced obesity in mice. J. Nutr. 2019, 149, 747–754. [Google Scholar] [CrossRef]
- Bai, D.; Sun, T.; Zhao, J.; Du, J.; Bu, X.; Cao, W.; Lu, N. Oroxylin A maintains the colonic mucus barrier to reduce disease susceptibility by reconstituting a dietary fiber-deprived gut microbiota. Cancer Lett. 2021, 515, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.Y.; Liu, H.M.; Leu, Y.L.; Hsu, C.H.; Lee, T.Y. Modulation of gut microbiota combined with upregulation of intestinal tight junction explains anti-inflammatory effect of corylin on colitis-associated cancer in mice. Int. J. Mol. Sci. 2022, 23, 2667. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Daza, M.C.; Daoust, L.; Boutkrabt, L.; Pilon, G.; Varin, T.; Dudonne, S.; Desjardins, Y. Wild blueberry proanthocyanidins shape distinct gut microbiota profile and influence glucose homeostasis and intestinal phenotypes in high-fat high-sucrose fed mice. Sci. Rep. 2020, 10, 2217. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, W.; Wu, Z.; Tian, X.; Xiang, J.; Li, L.; Zhao, Y. Baicalin and the liver-gut system: Pharmacological bases explaining its therapeutic effects. Pharmacol. Res. 2021, 165, 105444. [Google Scholar] [CrossRef] [PubMed]
- Uyanga, V.A.; Amevor, F.K.; Liu, M.; Cui, Z.; Zhao, X.; Lin, H. Potential implications of citrulline and quercetin on gut functioning of monogastric animals and humans: A comprehensive review. Nutrients 2021, 13, 3782. [Google Scholar] [CrossRef]
- Estruel-Amades, S.; Massot-Cladera, M.; Perez-Cano, F.J.; Franch, A.; Castell, M.; Camps-Bossacoma, M. Hesperidin effects on gut microbiota and gut-associated lymphoid tissue in healthy rats. Nutrients 2019, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Romandini, S.; Bompadre, S.; Diamanti, J.; Capocasa, F.; Mezzetti, B.; Quiles, J.L.; Ferreiro, M.S.; et al. The potential impact of strawberry on human health. Nat. Prod. Res. 2013, 27, 448–455. [Google Scholar] [CrossRef]
- Diamanti, J.; Mezzetti, B.; Giampieri, F.; Alvarez-Suarez, J.M.; Quiles, J.L.; Gonzalez-Alonso, A.; Ramirez-Tortosa, M.C.; Granados-Principal, S.; Gonzales-Panamas, A.M.; Santos-Buelga, C.; et al. Doxorubicin-induced oxidative stress in rats is efficiently counteracted by dietary anthocyanin differently enriched strawberry (Fragariaananassa). J. Agric. Food Chem. 2014, 62, 3935–3943. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and human health: Effects beyond antioxidant activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; Di Stefano, G.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef]
- Tulipani, S.; Armeni, T.; Giampieri, F.; Alvarez-Suarez, J.M.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Busco, F.; Principato, G.; Bompadre, S.; Quiles, J.L.; et al. Strawberry intake increases blood fluid, erythrocyte and mononuclear cell defenses against oxidative challenge. Food Chem. 2014, 156, 87–93. [Google Scholar] [CrossRef]
- Tulipani, S.; Marzban, G.; Herndl, A.; Laimer, M.; Mezzetti, B.; Battino, M. Influence of environmental and genetic factors on health-related compounds in strawberry. Food Chem. 2011, 124, 906–913. [Google Scholar] [CrossRef]
- Wang, S.Y.; Jiao, H. Scavenging Capacity of Berry Crops on Superoxide Radicals, Hydrogen Peroxide, Hydroxyl Radicals, and Singlet Oxygen. J. Agric. Food Chem. 2000, 48, 5677–5684. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Shiow, Y.W.; Wei, Z.; Gene, G.J. Cultural System Affects Fruit Quality and Antioxidant Capacity in Strawberries. J. Agric. Food Chem. 2002, 50, 6534–6542. [Google Scholar] [CrossRef]
- Heo, H.J.; Lee, C.Y. Strawberry and its anthocyanins reduce oxidative stress-induced apoptosis in PC12 cells. J. Agric. Food Chem. 2005, 53, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernandeza, T.Y.; Gasparrinia, M.; Afrina, S.; Mazzonic, L.; Reboredod, P.; Giampieria, F. A comparative study on cytotoxic effects of strawberry extract on different cellular models. J. Berry Res. 2016, 6, 263–275. [Google Scholar] [CrossRef]
- Olsson, M.E.; Andersson, C.S.; Oredsson, S.; Berglund, R.H.; Gustavsson, K.E. Antioxidant levels and inhibition of cancer cell proliferationin vitro by extracts from organically and conventionally cultivated strawberries. J. Agric. Food Chem. 2006, 54, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- McCune, L.M.; Kubota, C.; Stendell-Hollis, N.R.; Thomson, C.A. Cherries and health: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.; Espino, J.; Toribio-Delgado, A.F.; Cubero, J.; Maynar-Marino, J.I.; Barriga, C.; Paredes, S.D.; Rodriguez, A.B. A jerte valley cherry-based product as a supply of tryptophan. Int. J. Tryptophan Res. 2012, 5, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A review of the multiple actions of melatonin on the immune system. Endocrine 2005, 27, 189–200. [Google Scholar] [CrossRef]
- Garrido, M.; Espino, J.; Gonzalez-Gomej, D.; Lozano, M.; Cubero, J.; Toribio-Delgado, A.F.; Maynar-Marino, J.I.; Terron, M.P.; Munoz, J.L.; Pariente, J.A.; et al. A Nutraceutical Product Based on Jerte Valley Cherries Improves Sleep and Augments the Antioxidant Status in Humans. e-SPEN Eur. e-J. Clin. Nutr. Metab. 2009, 4, e321–e323. [Google Scholar] [CrossRef]
- Garrido, M.; Gonzalez-Gomez, D.; Lozano, M.; Barriga, C.; Paredes, S.D.; Rodriguez, A.B. A Jerte valley cherry product provides beneficial effects on sleep quality. Influence on aging. J. Nutr. Health Aging 2013, 17, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, W.R.; Carr, M.; Gorman, C.; Perlis, M.L. Effects of a tart cherry juice beverage on the sleep of older adults with insomnia: A pilot study. J. Med. Food 2010, 13, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.; Espino, J.; Gonzalez-Gomez, D.; Lozano, M.; Barriga, C.; Paredes, S.D.; Rodriguez, A.B. The consumption of a Jerte Valley cherry product in humans enhances mood, and increases 5-hydroxyindoleacetic acid but reduces cortisol levels in urine. Exp. Gerontol. 2012, 47, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Varadinova, M.; Docheva-Drenska, D.; Boyadjieva, N. Effects of anthocyanins on active avoidance test of rats exposed to disruption of diurnal rhythm. Am. J. Ther. 2013, 20, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; Haskell-Ramsay, C.F.; Veasey, R.C.; Howatson, G. Montmorency Tart cherries (Prunus cerasus L.) modulate vascular function acutely, in the absence of improvement in cognitive performance. Br. J. Nutr. 2016, 116, 1935–1944. [Google Scholar] [CrossRef]
- Lamport, D.J.; Saunders, C.; Butler, L.T.; Spencer, J.P. Fruits, vegetables, 100% juices, and cognitive function. Nutr. Rev. 2014, 72, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yang, H.P.; Pang, W.; Lu, H.; Hu, Y.D.; Li, J.; Lu, S.J.; Zhang, W.Q.; Jiang, Y.G. Cyanidin-3-O-galactoside and blueberry extracts supplementation improves spatial memory and regulates hippocampal ERK expression in senescence-accelerated mice. Biomed. Environ. Sci. 2014, 27, 186–196. [Google Scholar] [PubMed]
- Thangthaeng, N.; Poulose, S.M.; Gomes, S.M.; Miller, M.G.; Bielinski, D.F.; Shukitt-Hale, B. Tart cherry supplementation improves working memory, hippocampal inflammation, and autophagy in aged rats. Age 2016, 38, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.N.; Zhang, J.L.; Qin, M.L. Protective effect of cyanidin3-O-glucoside on beta-amyloid peptide-induced cognitive impairment in rats. Neurosci. Lett. 2013, 534, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Uchino, K.; Watanabe, Y.; Adachi, T.; Nakanishi, M.; Ichino, H.; Hongo, K.; Mizobata, T.; Kobayashi, S.; Nakashima, K.; et al. Anthocyanin suppresses the toxicity of AB deposits through diversion of molecular forms in in vitro and in vivo models of Alzheimer’s diesease. Nutr. Neurosci. 2016, 19, 32–42. [Google Scholar] [CrossRef]
- Connolly, D.A.; McHugh, M.P.; Padilla-Zakour, O.I.; Carlson, L.; Sayers, S.P. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br. J. Sports Med. 2006, 40, 679–683. [Google Scholar] [CrossRef]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Sumners, D.P.; Dyer, A.; Fox, P.; Mileva, K.N. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med. Sci. Sports Exerc. 2011, 43, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.; Howatson, G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Levers, K.; Dalton, R.; Galvan, E.; Goodenough, C.; O’Connor, A.; Simbo, S.; Barringer, N.; Mertens-Talcott, S.U.; Rasmussen, C.; Greenwood, M.; et al. Effects of powdered Montmorency tart cherry supplementation on an acute bout of intense lower body strength exercise in resistance trained males. J. Int. Soc. Sports Nutr. 2015, 12, 41. [Google Scholar] [CrossRef]
- Dimitriou, L.; Hill, J.A.; Jehnali, A.; Dunbar, J.; Brouner, J.; McHugh, M.P.; Howatson, G. Influence of a montmorency cherry juice blend on indices of exercise-induced stress and upper respiratory tract symptoms following marathon running–a pilot investigation. J. Int. Soc. Sports Nutr. 2015, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Levers, K.; Dalton, R.; Galvan, E.; O’Connor, A.; Goodenough, C.; Simbo, S.; Mertens-Talcott, S.U.; Rasmussen, C.; Greenwood, M.; Riechman, S.; et al. Effects of powdered Montmorency tart cherry supplementation on acute endurance exercise performance in aerobically trained individuals. J. Int. Soc. Sports Nutr. 2016, 13, 22. [Google Scholar] [CrossRef]
- McCormick, R.; Peeling, P.; Binnie, M.; Dawson, B.; Sim, M. Effect of tart cherry juice on recovery and next day performance in well-trained Water Polo players. J. Int. Soc. Sports Nutr. 2016, 13, 41. [Google Scholar] [CrossRef]
- Bell, P.G.; Stevenson, E.; Davison, G.W.; Howatson, G. The Effects of Montmorency Tart Cherry Concentrate Supplementation on Recovery Following Prolonged, Intermittent Exercise. Nutrients 2016, 8, 441. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Costa, A.R.; Flores-Félix, J.D.; Falcão, A.; Alves, G.; Silva, L.R. Anti-Inflammatory and Antiproliferative Properties of Sweet Cherry Phenolic-Rich Extracts. Molecules 2022, 27, 268. [Google Scholar] [CrossRef]
- Afsar, T.; Trembley, J.H.; Salomon, C.E.; Razak, S.; Khan, M.R.; Ahmed, K. Growth inhibition and apoptosis in cancer cells induced by polyphenolic compounds of Acacia hydaspica: Involvement of multiple signal transduction pathways. Sci. Rep. 2016, 6, 23077. [Google Scholar] [CrossRef]
- Xiao, T.; Luo, Z.; Guo, Z.; Wang, X.; Ding, M.; Wang, W.; Shen, X.; Zhao, Y. Multiple roles of black raspberry anthocyanins protecting against alcoholic liver disease. Molecules 2021, 26, 2313. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.C.; Flores-Felix, J.D.; Costa, A.R.; Falcao, A.; Alves, G.; Silva, L.R. Hepatoprotective Effects of Sweet Cherry Extracts (cv. Saco). Foods 2021, 10, 2623. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Rodrigues, M.; Santos, A.O.; Alves, G.; Silva, L.R. Antioxidant status, antidiabetic properties and effects on Caco-2 cells of colored and non-colored enriched extracts of sweet cherry fruits. Nutrients 2018, 10, 1688. [Google Scholar] [CrossRef] [PubMed]
- Manogna, C.; Bhaumik, A.; Haritha, T.; Nasreen, S.K.; Sucharitha, M.; Uttara, M. Evaluation of cytotoxic activity of various extracts of sweet cherry (Prunus avium) against human colorectal adenocarcinoma HT-29 cell line. Int. J. Chem. Stud. 2016, 4, 17–21. [Google Scholar]
- Canadanovic-Brunet, J.M.; Vulic, J.; Cetkovic, G.S.; Djilas, S.M.; Saponjac, T.V. Bioactive compounds and antioxidant properties of dried apricot. Acta Period. Technol. 2013, 44, 193–205. [Google Scholar] [CrossRef]
- Gong, X.P.; Tang, Y.; Song, Y.Y.; Du, G.; Li, J. Comprehensive Review of Phytochemical Constituents, Pharmacological Properties, and Clinical Applications of Prunus Mume. Front. Pharmacol. 2021, 12, 679378. [Google Scholar] [CrossRef]
- Bousselma, A.; Tahraoui, H.; Abdessemed, D.; Amrane, A.; Kebir, M.; Moula, N.; Saadoudi, M.; Temagoult, A. Antioxidant and Biological Activities of Fresh and Dried Apricot Extracts Obtained by Cold Soaking and Ultrasonic Extraction. Kem. Ind. 2023, 72, 193–200. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The Effect of Lutein on Eye and Extra-Eye Health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Lee, J.C.; Leung, H.H.; Lam, W.C.; Fu, Z.; Lo, A.C.Y. Lutein Supplementation for Eye Diseases. Nutrients 2020, 12, 1721. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, S.; Yanaoka, K.; Utsunomiya, H.; Niwa, T.; Inada, K.; Deguchi, H.; Ueda, K.; Mukoubayashi, C.; Inoue, I.; Maekita, T.; et al. Inhibitory Effects of Japanese Apricot (Prunus Mume Siebold et Zucc.; UME) on Helicobacter Pylori-Related Chronic Gastritis. Nat. News. 2010, 64, 714–719. [Google Scholar] [CrossRef]
- Wang, X.; Du, J.; Zhou, J. Antibiotic activities of extracts from Prunus mume fruit against food-borne bacterial pathogens and its active components. Ind. Crops Prod. 2019, 133, 409–413. [Google Scholar] [CrossRef]
- Lima, E.M.F.; Matsumura, C.H.S.; da Silva, G.L.; Patrocínio, I.C.S.; Santos, C.A.; Pereira, P.A.P.; Hassimotto, N.M.A.; Pinto, U.M.; da Cunha, L.R. Antimicrobial and Antioxidant Activity of Apricot (Mimusopsis comersonii) Phenolic-Rich Extract and Its Application as an Edible Coating for Fresh-Cut Vegetable Preservation. Biomed Res Int. 2022, 2022, 8440304. [Google Scholar] [CrossRef]
- Tareen, A.K.; Panezai, M.A.; Sajjad, A.; Achakzai, J.K.; Kakar, A.M.; Khan, K.Y. Comparative analysis of antioxidant activity, toxicity, and mineral composition of kernel and pomace of apricot (Prunus armeniaca L.) grown in Balochistan, Pakistan. Saudi J. Biol. Sci. 2021, 28, 2830–2839. [Google Scholar] [CrossRef]
- Wani, S.M.; Masoodi, F.A.; Ahmad, M.; Mir, S.A. Processing and storage of apricots: Effect on physicochemical and antioxidant properties. J. Food Sci. Technol. 2018, 55, 4505–4514. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.; Kamel, G.; Awad, N.E.; Shokry, A.A.; Fayed, H.M. The pharmacological effect of apricot seeds extracts and amygdalin in experimentally induced liver damage and hepatocellular carcinoma. J. Herbmed Pharmacol. 2020, 9, 400–407. [Google Scholar] [CrossRef]
- Kitic, D.; Miladinovic, B.; Randjelovic, M.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Seidel, V. Anticancer Potential and Other Pharmacological Properties of Prunus armeniaca L.: An Updated Overview. Plants 2022, 11, 1885. [Google Scholar] [CrossRef]
- Karabulut, A.B.; Karadag, N.; Gurocak, S.; Kiran, T.; Tuzcu, M.; Sahin, K. Apricot attenuates oxidative stress and modulates of Bax, Bcl-2, caspases, NFκ-B, AP-1, CREB expression of rats bearing DMBA-induced liver damage and treated with a combination of radiotherapy. Food Chem. Toxicol. 2014, 70, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-J.; Kim, S.-H.; Jun, M.-S.; Oh, H.-T.; Choi, H.-J.; Ham, S.-S. Antioxidative, antimutagenic and cytotoxic effects of Prunus armeniaca extracts. Korean J. Food Preserv. 2007, 14, 220–225. [Google Scholar]
- Tinker, L.F.; Schneeman, B.O.; Davis, P.A.; Gallaher, D.D.; Waggoner, C.R. Consumption of prunes as a source of dietary fiber in men with mild hypercholesterolemia. Am. J. Clin. Nutr. 1991, 53, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Igwe, E.; Charlton, K. A Systematic Review on the Health Effects of Plums (Prunus domestica and Prunus salicina). Phytother. Res. 2016, 30, 701–731. [Google Scholar] [CrossRef] [PubMed]
- Stacewicz-Sapuntzakis, M. Dried plums and their products: Composition and health effects–an updated review. Crit. Rev. Food Sci. Nutr. 2013, 53, 1277–1302. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekar, C.; Rasool, M. Morin, a Dietary Bioflavonol Suppresses Monosodium Urate Crystal-Induced Inflammation in an Animal Model of Acute Gouty Arthritis with Reference to Nlrp3 Inflammasome, Hypo-Xanthine Phospho-Ribosyl Transferase, And Inflammatory Mediators. Eur. J. Pharmacol. 2016, 786, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, W.; Wu, L.; Chen, S.; Zheng, Z.; Song, H. Anti-Inflammatory Effects of Polyphenols from Plum (Prunus salicina Lindl) on RAW264.7 Macrophages Induced by Monosodium Urate and Potential Mechanisms. Foods 2023, 12, 254. [Google Scholar] [CrossRef]
- Kuo, P.-H.; Lin, C.-I.; Chen, Y.-H.; Chiu, W.-C.; Lin, S.-H. A high-cholesterol diet enriched with polyphenols from Oriental plums (Prunus salicina) improves cognitive function and lowers braincholesterol levels and neurodegenerative-related proteinexpression in mice. Br. J. Nutr. 2015, 113, 1550–1557. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Kalt, W.; Carey, A.N.; Vinqvist-Tymchuk, M.; Mcdonald, J.; Joseph, J.A. Plum juice, but not dried plum powder, iseffective in mitigating cognitive deficits in aged rats. Nutrition 2009, 25, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Rammal, H.; Dicko, A.; Younos, C.; Soulimani, R. The antioxidant effect of plums and polyphenolic compounds against H(2)O(2)-induced oxidative stress in mouse blood granulocytes. J. Med. Food 2009, 12, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sisodia, R. Modulation of radiation induced oxidative stress in swiss albino mice brain by Prunus domestica. Int. J. Pharma Bio. Sci. 2013, 4, B79–B88. [Google Scholar]
- Pawlowski, J.W.; Martin, B.R.; Mccabe, G.P.; Ferruzzi, M.G.; Weaver, C.M. Plum and soy aglycon extracts superior at increasing bone calcium retention in ovariectomized sprague dawley rats. J. Agric. Food Chem. 2014, 62, 6108–6611. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Bahrin, A.A.; Moshawih, S.; Dhaliwal, J.S.; Kanakal, M.M.; Khan, A.; Lee, K.S.; Goh, B.H.; Goh, H.P.; Kifli, N.; Ming, L.C. Cancer protective effects of plums: A systematic review. Biomed. Pharmacother. 2022, 146, 112568. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Ikami, J.W.; Xu, K. Ikeda. Prune extract (Prunus domestica L.) suppresses the proliferation and induces the apoptosis of human colon carcinoma Caco-2. J. Nutr. Sci. Vitam. 2006, 52, 389–391. [Google Scholar] [CrossRef]
- St Leger, A.S.; Cochrane, A.L.; Moore, F. Factors associated with cardiac mortality in developed countries with particular reference to the consumption of wine. Lancet 1979, 1, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Dohadwala, M.M.; Vita, J.A. Grapes and Cardiovascular Disease. J. Nutr. 2009, 139, 1788S–1793S. [Google Scholar] [CrossRef] [PubMed]
- Rivera, K.; Salas-Pérez, F.; Echeverría, G.; Urquiaga, I.; Dicenta, S.; Pérez, D.; de la Cerda, P.; González, L.; Andia, M.E.; Uribe, S.; et al. Red Wine Grape Pomace Attenuates Atherosclerosis and Myocardial Damage and Increases Survival in Association with Improved Plasma Antioxidant Activity in a Murine Model of Lethal Ischemic Heart Disease. Nutrients 2019, 11, 2135. [Google Scholar] [CrossRef]
- Guler, A.; Sahin, M.A.; Yucel, O.; Yokusoglu, M.; Gamsizkan, M.; Ozal, E.; Demirkilic, U.; Arslan, M. Proanthocyanidin prevents myocardial ischemic injury in adult rats. Med. Sci. Monit. 2011, 17, BR326–BR331. [Google Scholar] [CrossRef]
- Bocsan, I.C.; Magureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei, S.O.; Patrasca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases. Biomedicines 2022, 10, 2337. [Google Scholar] [CrossRef]
- Shen, M.; Wu, R.-X.; Zhao, L.; Li, J.; Guo, H.-T.; Fan, R.; Cui, Y.; Wang, Y.-M.; Yue, S.-Q.; Pei, J.-M. Resveratrol Attenuates Ischemia/Reperfusion Injury in Neonatal Cardiomyocytes and Its Underlying Mechanism. PLoS ONE 2012, 7, e51223. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Gao, R.Y.; Mukhopadhyay, P.; Mohanraj, R.; Wang, H.; Horvath, B.; Yin, S. Resveratrol attenuates azidothymidine-induced cardiotoxicity by decreasing mitochondrial reactive oxygen species generation in human cardiomyocytes. Mol. Med. Rep. 2010, 4, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Lang, F.; Chen, Y.; Zhang, H.; Cong, X.; Shen, X.; Su, G. Resveratrol Alleviates Endotoxin-Induced Myocardial Toxicity via the Nrf2 Transcription Factor. PLoS ONE 2013, 8, e69452. [Google Scholar] [CrossRef] [PubMed]
- Grujić-Milanović, J.; Jaćević, V.; Miloradović, Z.; Jovović, D.; Milosavljević, I.; Milanović, S.D.; Mihailović-Stanojević, N. Resveratrol protects cardiac tissue in experimental malignant hypertension due to antioxidant, anti-inflammatory, and anti-apoptotic properties. Int. J. Mol. Sci. 2021, 22, 5006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Wong, C.W.; Tan, Y.H.; Foo, J.P.Y.; Wong, S.K.; Chan, H.T. Resveratrol and pterostilbene: A comparative overview of their chemistry, biosynthesis, plant sources and pharmacological properties. J. Appl. Pharm. Sci. 2019, 9, 124–129. [Google Scholar] [CrossRef]
- Pallauf, K.; Rimbach, G.; Rupp, P.M.; Chin, D.; Wolf, I.M.A. Resveratrol and Lifespan in Model Organisms. Curr. Med. Chem. 2016, 23, 4639–4680. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- José, M.; Pérez-Ortiz, L.F. Alguacil, Elisabet Salas, Isidro Hermosín-Gutiérrez, Sergio Gómez-Alonso, Carmen González-Martín. Antiproliferative and cytotoxic effects of grape pomace and grape seed extracts on colorectal cancer cell lines. Food Sci. Nutr. 2019, 7, 2948–2957. [Google Scholar] [CrossRef]
- Miskovic Spoljaric, K.; Selo, G.; Pesut, E.; Martinovic, J.; Planinic, M.; Tisma, M.; Bucic-Kojic, A. Antioxidant and antiproliferative potentials of phenolic-rich extracts from biotransformed grape pomace in colorectal Cancer. BMC Complement. Med. Ther. 2023, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Collective Work. Romanian Pharmacopoeia, Xth ed.; Medicala: Bucharest, Romania, 1993. [Google Scholar]
- Rusu, N.; Meghea, A. Bioacumulation of toxic metals in fish oils capsules. UPB Sci. Bull. Series B 2015, 77, 131–140. [Google Scholar]
| Strawberry (Fragaria ananassa) | Sweet Cherry (Prunus avium) | Apricot (Prunus armenica) | Plum (Prunus domestica) | Green Grape (Vitis vinifera) |
|---|---|---|---|---|
| pelargonidin-3-O-glucoside cyanidin-3-O-glucoside pelargonidin-3-O-rutinoside catechin epicatechin chlorogenic acid caffeic acid cinnamic acid 3,4,5-methoxycinnamic acid protocatechuic acid p-coumaric acid ellagic acid salicylic acid vanillic acid ferulic acid iso-ferulic acid pyrogallol catechol coumarin [31,32,33,34,35] | cyanidin-3-O-rutinoside cyanidin-3-O-glucoside peonidin-3-O-glucoside peonidin-3-O-rutinoside pelargonidin-3-O-rutinoside catechin epicatechin epigallocatechin epicatechin gallate epigallocatechin gallate hydroxycinnamic acid quercetin apigenin luteolin chrysin eriodyctiol hesperitin naringenin genistein daidzein glycitein formononectin [36,37,38,39] | caffeic acid chlorogenic acid neochlorogenic acid cyanidin-3-O-glucoside procyanidins B1, B2, B3 catechin epicatechin p-coumaric acid gallic acid ferulic acids kaempferol myricetin quercetin-3-O-rutinoside lutein zeaxanthin violaxanthin β-cryptoxanthin α-, β-, γ-carotene [40,41,42,43,44,45] | chlorogenic acid caffeic acid cyanidin peonidin catechin epicatechin quercetin derivatives [46,47] | procyanidins B1,B2,B3,B4, procyanidins C1 procyanidins EEC catechin epicatechin epigallocatechin gallocatechin epicatechin-3-O-gallate isorhamnetin-3-O-glucoside kaempferol-3-O–galactoside kaempferol-3-O–glucoside quercetin-3-O-glucuronide quercetin-3-O-rutinoside caffeoyl tartaric acid cis-caffeoyl tartaric acid cis-p-coumaroyl tartaric acid p-coumaroyl tartaric acid trans-caffeoyl tartaric acid trans-p-coumaroyl tartaric acid resveratrol, resveratrol-3-O-glucoside, trans-resveratrol [48,49] |
| Test Batches along the Shelf Life (days) | EES | EEC | EEA | EEP | EEG |
|---|---|---|---|---|---|
| mg GAE/100 g Fresh Fruit (Mean Values, mg%, FW) | |||||
| t = 0 | 129 ± 3 | 118 ± 4 | 33 ± 1 | 123 ± 2 | 74 ± 2 |
| t = 5/7 | 134 ± 4 | 110 ± 3 | 37 ± 1 | 131 ± 3 | 72 ± 2 |
| Total phenolics’ dynamic (%) | +4.40% | −7.74% | +11.04% | +6.15% | −1.72% |
| Dehydration process’ dynamic (%) | −3.0% | −10.1% | −11.70% | −2.7% | −1.3% |
| Minerals and Microelements Content in Batches | APS | APC | APA | PP | APG |
|---|---|---|---|---|---|
| mg/μg * per 100 g Fresh Fruit (Mean Values, mg%, FW) | |||||
| K | 41 ± 1.5 | 59 ± 2.1 | 59 ± 2.3 | 34 ± 2.5 | 62 ± 2.8 |
| Ca | 11 ± 0.52 | 9 ± 0.69 | 7.5 ± 0.43 | 4 ± 0.68 | 5.3 ± 0.61 |
| Mg | 6 ± 0.24 | 5.5 ± 0.41 | 4 ± 0.35 | 2.6 ± 0.32 | 2.3 ± 0.38 |
| Fe | 0.41 ± 0.03 | 0.24 ± 0.01 | 0.13 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 |
| Cu * | 15 ± 0.15 | 44 ± 3.08 | 32 ± 2.24 | 32 ± 1.89 | 18 ± 1.26 |
| Zn * | 66 ± 4.62 | 92 ± 6.44 | 77 ± 5.39 | 63 ± 4.41 | 84 ± 5.88 |
| As * | 3.3 ± 0.10 | 5.28 ± 0.16 | 2.87 ± 0.09 | 2.32 ± 0.07 | 2.28 ± 0.07 |
| Pb * | 4.75 ± 0.14 | 5.76 ± 0.17 | 5.49 ± 0.16 | 3.29 ± 0.10 | 3.60 ± 0.11 |
| Total elements in AP series | 58 | 74 | 71 | 40 | 70 |
| Test Element | Location | Fresh Fruits Content vs. Vegetal Waste Content in the Study (AP); mg/100 g Fresh Fruit and the Percent (%) in the Vegetal Waste | ||||
|---|---|---|---|---|---|---|
| Strawberries | Cherries | Apricots | Plums | Green Grapes | ||
| K | fresh fruit/literature data waste/acetone powder (AP) | 183 41 (22%) | 248 59 (24%) | 283 59 (21%) | 197 34 (17%) | 203 62 (30%) |
| Ca | fresh fruit/literature data waste/acetone powder (AP) | 15 11 (73%) | 20 9 (45%) | 20 7.5 (38%) | 8 4 (50%) | 14 5.3 (38%) |
| Mg | fresh fruit/literature data waste/acetone powder (AP) | 13 6 (46%) | 10 5.5 (55%) | 12 4 (33%) | 7 2.6 (37%) | 8 2.3 (29%) |
| Fe | fresh fruit/literature data waste/acetone powder (AP) | 0.5 0.41 (82%) | 0.5 0.24 (48%) | 0.8 0.13 (16%) | 0.3 0.16 (53%) | 0.3 0.16 (53%) |
| The percent of test elements in the AP series | APS-28% | APC-27% | APA-22% | APP-19% | APG-31% | |
| Test Extract | AA (%) | IC50 (μg GAE/μL Sample) | IC50 Variation (%) | ||
|---|---|---|---|---|---|
| T = 0 | T = 5/7 | T = 0 | T = 5/7 | ||
| EES | 99 | 99 | 3.22 | 3.35 | −4% |
| EEC | 99 | 99 | 5.93 | 5.50 | +7% |
| EEA | 99 | 99 | 1.67 | 1.86 | −11% |
| EEP | 99 | 99 | 3.08 | 3.27 | −6% |
| EEG | 99 | 99 | 3.70 | 3.64 | +2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirvu, L.C.; Rusu, N.; Bazdoaca, C.; Androne, E.; Neagu, G.; Albulescu, A. A View on the Chemical and Biological Attributes of Five Edible Fruits after Finishing Their Shelf Life: Studies on Caco-2 Cells. Int. J. Mol. Sci. 2024, 25, 4848. https://doi.org/10.3390/ijms25094848
Pirvu LC, Rusu N, Bazdoaca C, Androne E, Neagu G, Albulescu A. A View on the Chemical and Biological Attributes of Five Edible Fruits after Finishing Their Shelf Life: Studies on Caco-2 Cells. International Journal of Molecular Sciences. 2024; 25(9):4848. https://doi.org/10.3390/ijms25094848
Chicago/Turabian StylePirvu, Lucia Camelia, Nicoleta Rusu, Cristina Bazdoaca, Elena Androne, Georgeta Neagu, and Adrian Albulescu. 2024. "A View on the Chemical and Biological Attributes of Five Edible Fruits after Finishing Their Shelf Life: Studies on Caco-2 Cells" International Journal of Molecular Sciences 25, no. 9: 4848. https://doi.org/10.3390/ijms25094848
APA StylePirvu, L. C., Rusu, N., Bazdoaca, C., Androne, E., Neagu, G., & Albulescu, A. (2024). A View on the Chemical and Biological Attributes of Five Edible Fruits after Finishing Their Shelf Life: Studies on Caco-2 Cells. International Journal of Molecular Sciences, 25(9), 4848. https://doi.org/10.3390/ijms25094848






