In Vitro Evaluation of Phytobiotic Mixture Antibacterial Potential against Enterococcus spp. Strains Isolated from Broiler Chicken
Abstract
1. Introduction
2. Results
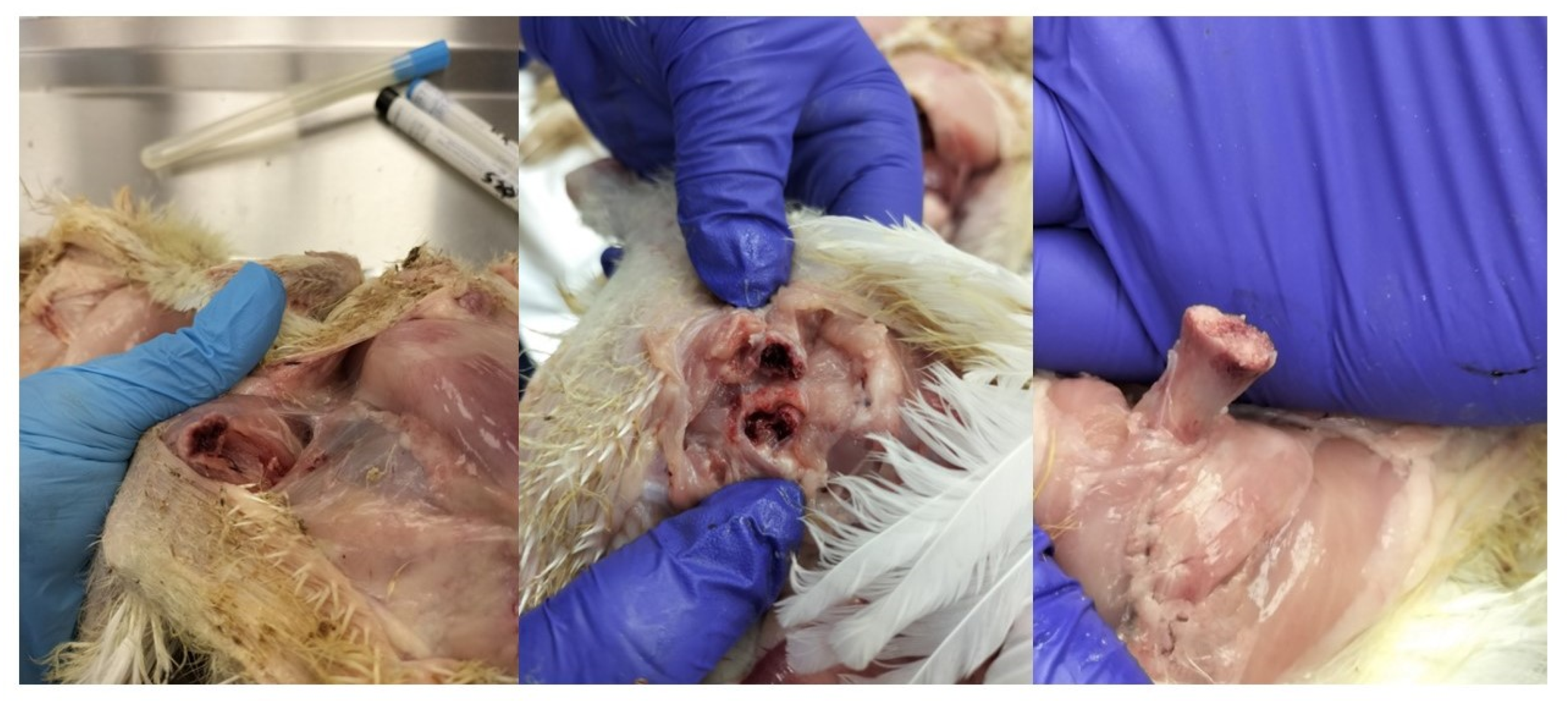
2.1. Post-Mortem Examination
- Arthritis;
- Femoral head necrosis (one or both sides);
- Necrosis within the vertebrae;
- Necrotic changes in the pelvic bones;
- Septicemia;
- Endocarditis;
- Peritonitis;
- Pericarditis/hydropericardium.
- -
- poor flock uniformity in terms of slaughter weight;
- -
- severe changes in the skin of the soles (pododermatitis) and ankle joints (hock burns);
- -
- brittle bones in the hip joints;
- -
- general decrease in the quality of the raw material.
2.2. Biochemical Features
2.3. Phytoncide Mixture Test
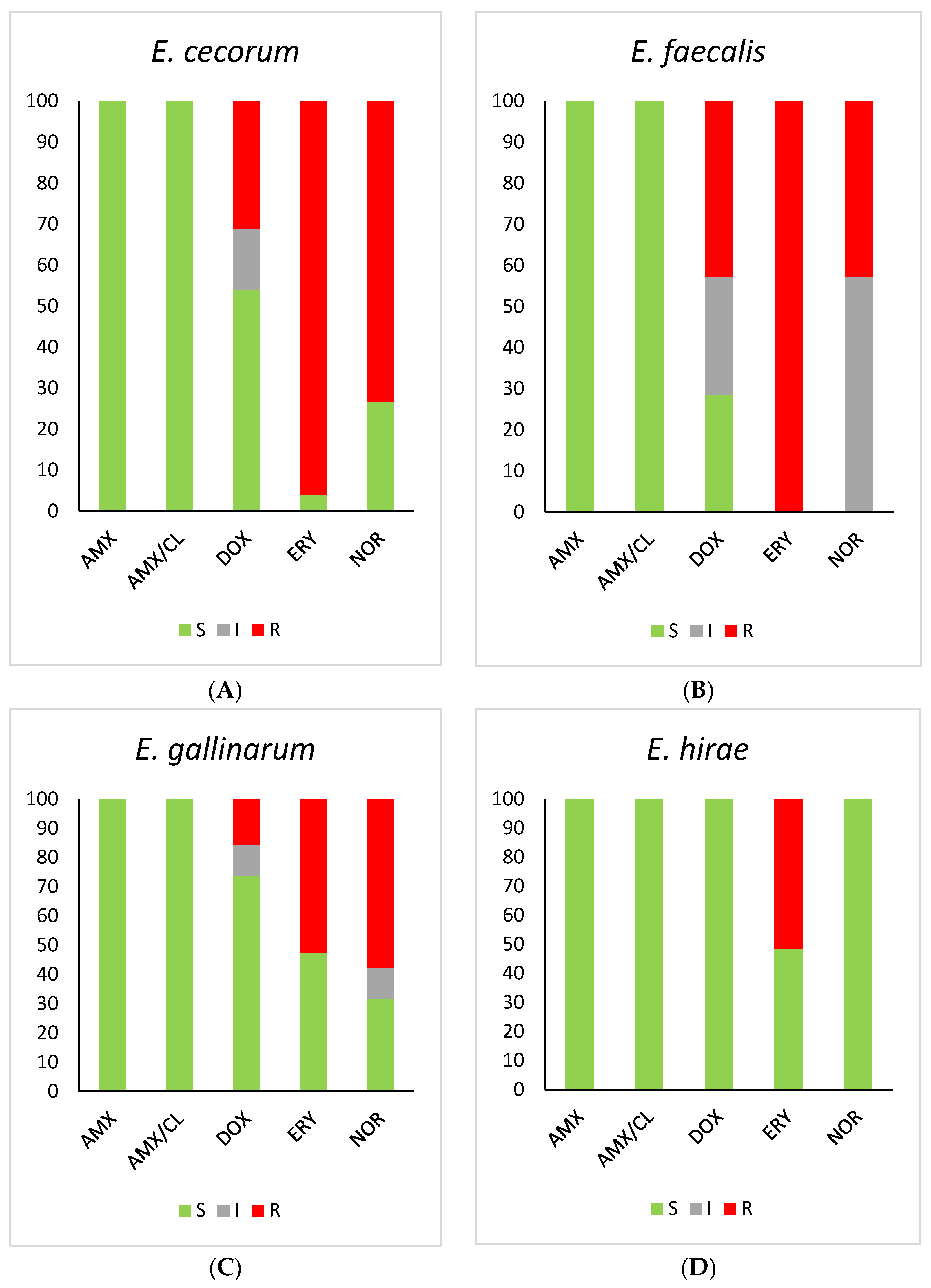
2.4. Antibiotic Susceptibility Testing
3. Discussion
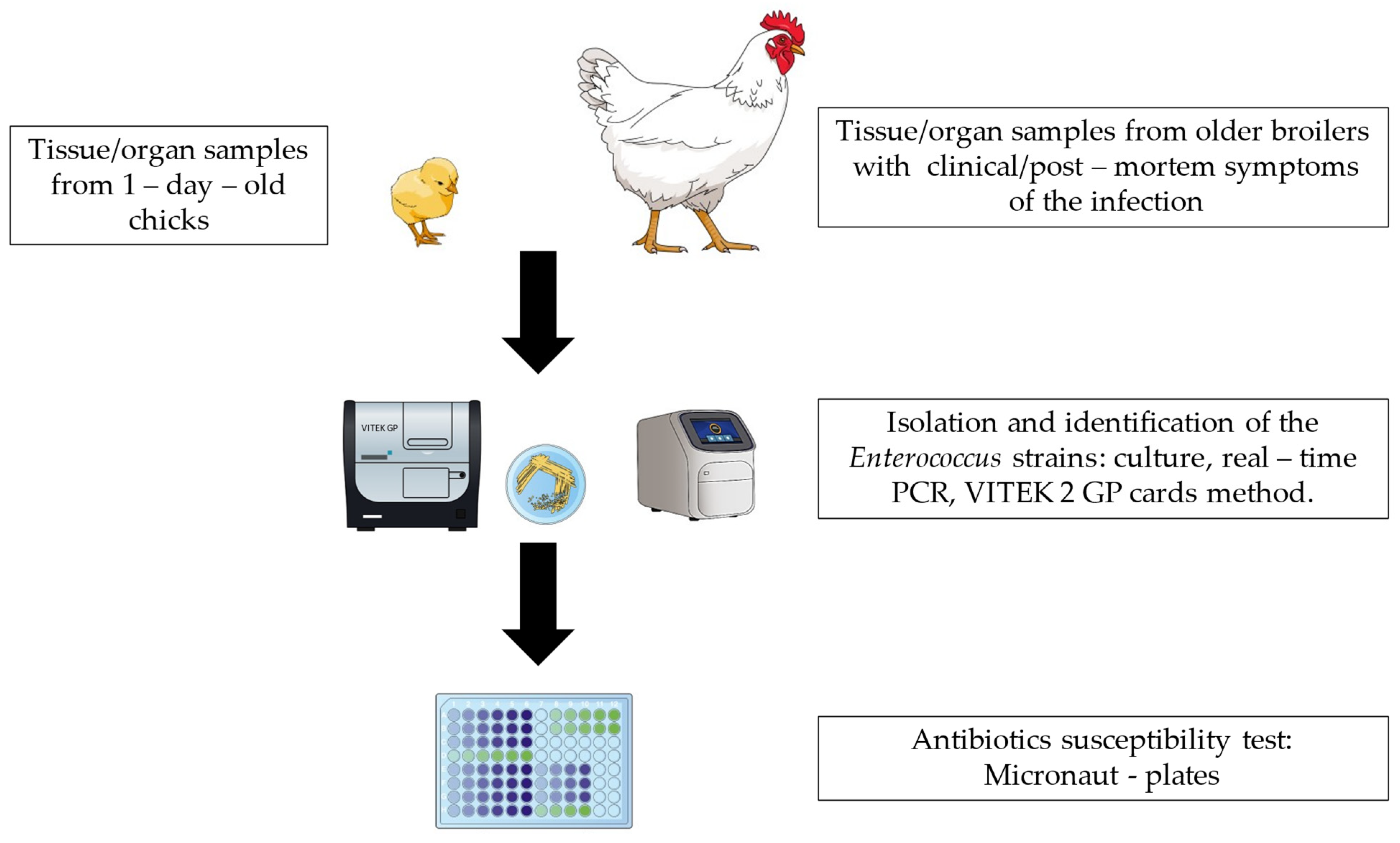
4. Materials and Methods
4.1. Broiler Sampling
4.2. Enterococcus spp. Isolation and Identification
4.3. Phytoncide Combination
4.4. Phytoncide Mixture Test by Broth Microdilution Method
4.5. Antibiotic Susceptibility Testing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehman, M.A.; Yin, X.; Zaheer, R.; Goji, N.; Amoako, K.K.; McAllister, T.; Pritchard, J.; Topp, E.; Diarra, M.S. Genotypes and Phenotypes of Enterococci Isolated from Broiler Chickens. Front. Sustain. Food Syst. 2018, 2, 83. [Google Scholar] [CrossRef]
- Dubin, K.; Pamer, E.G. Enterococci and Their Interactions with the Intestinal Microbiome. Microbiol. Spectr. 2014, 5. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2011; Reporting on 2009 surveillance data and 2010 epidemic intelligence data; ECDC: Stockholm, Sweden, 2011. [Google Scholar]
- Torres, C.; Alonso, C.A.; Ruiz-Ripa, L.; León-Sampedro, R.; Del Campo, R.; Coque, T.M. Antimicrobial Resistance in Enterococcus spp. of animal origin. Microbiol. Spectr. 2018, 6, 185–227. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, L.L.; Bisgaard, M.; Son, N.T.; Trung, N.V.; An, H.M.; Dalsgaard, A. Enterococcus faecalis clones in poultry and in humans with urinary tract infections, Vietnam. Emerg. Infect. Dis. 2012, 18, 1096–1100. [Google Scholar] [CrossRef]
- Bortolaia, V.; Guardabassi, L. Zoonotic Transmission of Antimicrobial Resistant Enterococci: A Threat to Public Health or an Overemphasised Risk? In Zoonoses—Infections Affecting Humans and Animals: Focus on Public Health Aspects; Sing, A., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 407–431. [Google Scholar]
- Abat, C.; Chaudet, H.; Rolain, J.M.; Colson, P.; Raoult, D. Traditional and syndromic surveillance of infectious diseases and pathogens. Int. J. Infect. Dis. 2016, 48, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar, C.; Herskin, M.; Michel, V.; et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): Antimicrobial-resistant Enterococcus faecalis in poultry. EFSA J. Eur. Food Saf. Auth. 2022, 20, e07127. [Google Scholar] [CrossRef] [PubMed]
- Gross, W.B.; Domermuth, C.H. Bacterial endocarditis of poultry. Am. J. Vet. Res. 1962, 23, 320–329. [Google Scholar] [PubMed]
- Jortner, B.S.; Helmboldt, C.F. Streptococcal bacterial endocarditis in chickens. Associated lesions of the central nervous system. Vet. Pathol. 1971, 8, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Souillard, R.; Laurentie, J.; Kempf, I.; Le Caër, V.; Le Bouquin, S.; Serror, P.; Allain, V. Increasing incidence of Enterococcus-associated diseases in poultry in France over the past 15 years. Vet. Microbiol. 2022, 269, 109426. [Google Scholar] [CrossRef]
- Hernandez, J.; Roberts, E.D.; Adams, L.G.; Vera, T. Pathogenesis of hepatic granulomas in turkeys infected with Streptococcus faecalis var. liquefaciens. Avian Dis. 1972, 16, 201–216. [Google Scholar]
- Landman, W.J.M.; Veldman, K.T.; Mevius, D.J.; van Eck, J.H.H. Investigations of Enterococcus lis-induced bacteraemia in brown layer pullets through different inoculation routes in relation to the production of arthritis. Avian Pathol. 2003, 32, 463–471. [Google Scholar] [CrossRef]
- Steentjes, A.; Veldman, K.; Mevius, D.; Landman, W. Molecular epidemiology of unilateral amyloid arthropathy in broiler breeders associated with Enterococcus faecalis. Avian Pathol. 2002, 31, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Landman, W.J.M.; Gruys, E. Amyloid arthropathy in an Indian peafowl. Vet. Rec. 1998, 142, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, R.H.; Petersen, A.; Christensen, H.; Bisgaard, M. Multilocus sequence typing of Enterococcus faecalis isolates demonstrating different lesion types in broiler breeders. Avian Pathol. 2010, 39, 435–440. [Google Scholar] [CrossRef]
- Karunarathna, R.; Ahmed, K.A.; Goonewardene, K.; Gunawardana, T.; Kurukulasuriya, S.; Liu, M.; Gupta, A.; Popowich, S.; Ayalew, L.; Chow-Lockerbie, B.; et al. Exposure of embryonating eggs to Enterococcus faecalis and Escherichia coli potentiates E. coli pathogenicity and increases mortality of neonatal chickens. Poult. Sci. 2022, 101, 101983. [Google Scholar] [CrossRef] [PubMed]
- Tankson, J.D.; Thaxton, J.P.; Vizzier-Thaxton, Y. Pulmonary hypertension syndrome in broilers caused by Enterococcus faecalis. Infect. Immun. 2001, 69, 6318–6322. [Google Scholar] [CrossRef] [PubMed]
- Falker-Gieske, C.; Paul, N.F.; Spourita, M.; Gilthorpe, J.D.; Gustmann, K.; Tetens, J. Resistance to chicken amyloid arthropathy is associated with a dysfunctional mutation in serum amyloid A. FASEB J. 2023, 37, e22700. [Google Scholar] [CrossRef]
- Sandhu, T.S. Fecal streptococcal infection of commercial white pekin ducklings. Avian Dis. 1988, 32, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Zommiti, M.; Chevalier, S.; Feuilloley, M.G.J.; Connil, N. Special Issue “Enterococci for Probiotic Use: Safety and Risk”: Editorial. Microorganisms 2022, 10, 604. [Google Scholar] [CrossRef]
- Kalia, V.C.; Shim, W.Y.; Patel, S.K.S.; Gong, C.; Lee, J.K. Recent developments in antimicrobial growth promoters in chicken health: Opportunities and challenges. Sci. Total Environ. 2022, 834, 155300. [Google Scholar] [CrossRef]
- Robbins, K.M.; Suyemoto, M.M.; Lyman, R.L.; Martin, M.P.; Barnes, H.J.; Borst, L.B. An outbreak and source investigation of enterococcal spondylitis in broilers caused by Enterococcus cecorum. Avian Dis. 2012, 56, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Islam, M.S.; Paul, A.; Ievy, S.; Talukder, M.; Sobur, M.A.; Ballah, F.M.; Khan, M.S.R.; Rahman, M.T. Molecular detection and antibiotyping of multi-drug resistant Enterococcus faecium from healthy broiler chickens in Bangladesh. Vet. Med. Sci. 2022, 8, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Bae, Y.; Jeon, E.; Kwon, Y.; Joh, S. Multiplex PCR analysis of virulence genes and their influence on antibiotic resistance in Enterococcus spp. isolated from broiler chicken. J. Vet. Sci. 2019, 20, e26. [Google Scholar] [CrossRef] [PubMed]
- Kolbjørnsen, Ø.; David, B.; Gilhuus, M. Bacterial osteomyelitis in a 3-week-old broiler chicken associated with Enterococcus hirae. Vet. Pathol. 2011, 48, 1134–1137. [Google Scholar] [CrossRef] [PubMed]
- Velkers, F.; Van De Graaf-Bloois, L.; Wagenaar, J.; Westendorp, S.; Van Bergen, M.; Dwars, R.; Landman, W. Enterococcus hirae-associated endocarditis outbreaks in broiler flocks: Clinical and pathological characteristics and molecular epidemiology. Vet. Q. 2011, 31, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Gallucci, M.N.; Oliva, M.; Casero, C.; Dambolena, J.; Luna, A.; Zygadlo, J.; Demo, M. Antimicrobial combined action of terpenes against the food-borne microorganisms Escherichia coli, Staphylococcus aureus and Bacillus cereus. Flavour Fragr. J. 2009, 24, 348–354. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. In sppspp inhibition of vancomycin-susceptible and vancomycin-resistant Enterococcus faecium and E. faecalis in the presence of citrus essential oils. Br. J. Biomed. Sci. 2009, 66, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kennes, Y.M.; Lepp, D.; Yin, X.; Wang, Q.; Yu, H.; Yang, C.; Gong, J.; Diarra, M.S. Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis. Poult. Sci. 2020, 99, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Stamilla, A.; Messina, A.; Sallemi, S.; Condorelli, L.; Antoci, F.; Puleio, R.; Loria, G.R.; Cascone, G.; Lanza, M. Effects of Microencapsulated Blends of Organics Acids (OA) and Essential Oils (EO) as a Feed Additive for Broiler Chicken. A Focus on Growth Performance, Gut Morphology and Microbiology. Animals 2020, 10, 442. [Google Scholar] [CrossRef]
- Chodkowska, K.A.; Iwiński, H.; Wódz, K.; Nowak, T.; Różański, H. In Vitro Assessment of Antimicrobial Activity of Phytobiotics Composition towards of Avian Pathogenic Escherichia coli (APEC) and Other E. coli Strains Isolated from Broiler Chickens. Antibiotics 2022, 11, 1818. [Google Scholar] [CrossRef]
- Iwiński, H.; Wódz, K.; Chodkowska, K.; Nowak, T.; Różański, H. In Vitro Evaluation of Antimicrobial Effect of Phytobiotics Mixture on Salmonella spp. Isolated from Chicken Broiler. Antibiotics 2022, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Chodkowska, K.A.; Abramowicz-Pindor, P.A.; Tuśnio, A.; Gawin, K.; Taciak, M.; Barszcz, M. Effect of Phytobiotic Composition on Production Parameters, Oxidative Stress Markers and Myokine Levels in Blood and Pectoral Muscle of Broiler Chickens. Animals 2022, 12, 2625. [Google Scholar] [CrossRef] [PubMed]
- Iwiński, H.; Chodkowska, K.A.; Drabik, K.; Batkowska, J.; Karwowska, M.; Kuropka, P.; Szumowski, A.; Szumny, A.; Różański, H. The Impact of a Phytobiotic Mixture on Broiler Chicken Health and Meat Safety. Animals 2023, 13, 2155. [Google Scholar] [CrossRef] [PubMed]
- Dolka, B.; Chrobak-Chmiel, D.; Makrai, L.; Szeleszczuk, P. Phenotypic and genotypic characterization of Enterococcus cecorum strains associated with infections in poultry. BMC Vet. Res. 2016, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Stępień-Pyśniak, D.; Marek, A.; Banach, T.; Adaszek, L.; Pyzik, E.; Wilczyński, J.; Winiarczyk, S. Prevalence and antibiotic resistance of strains of the genus Enterococcus isolated from poultry. Acta Vet. Hung. 2016, 64, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Bzdil, J.; Sladecek, V.; Senk, D.; Stolar, P.; Waicova, Z.; Kollertova, N.; Zouharova, M.; Matiaskova, K.; Linhart, P.; Nedbalcova, K. Enterococci Isolated from One-Day-Old Chickens and Their Phenotypic Susceptibility to Antimicrobials in the Czech Republic. Antibiotics 2023, 12, 1487. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, O.M.; Fayez, M.; Alswat, A.S.; Alkafafy, M.; Mahmoud, S.F.; Al-Marri, T.; Almuslem, A.; Ashfaq, H.; Yusuf, S. Antimicrobial Resistance, Biofilm Formation, and Virulence Genes in Enterococcus Species from Small Backyard Chicken Flocks. Antibiotics 2022, 11, 380. [Google Scholar] [CrossRef]
- Maasjost, J.; Muehldorfer, K.; Jäckel, S.; Hafez, H. Antimicrobial Susceptibility Patterns of Enterococcus faecalis and Enterococcus faecium Isolated from Poultry Flocks in Germany. Avian Dis. 2015, 59, 143–148. [Google Scholar] [CrossRef]
- Ruzauskas, M.; Siugzdiniene, R.; Spakauskas, V.; Povilonis, J.; Seputiene, V.; Suziedeliene, E.; Daugelavicius, R.; Pavilonis, A. Susceptibility of bacteria of the Enterococcus genus isolated on Lithuanian poultry farms. Vet. Med. 2010, 54, 583–588. [Google Scholar] [CrossRef]
- Bortolaia, V.; Mander, M.; Jensen, L.B.; Olsen, J.E.; Guardabassi, L. Persistence of vancomycin resistance in multiple clones of Enterococcus faecium isolated from Danish broilers 15 years after the ban of avoparcin. Antimicrob. Agents Chemother. 2015, 59, 2926–2929. [Google Scholar] [CrossRef]
- Ghosh, A.; Zurek, L. Chapter 9—Antibiotic Resistance in Enterococci: A Food Safety Perspective. In Antimicrobial Resistance and Food Safety; Chen, C.-Y., Yan, X., Jackson, C.R., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 155–180. [Google Scholar]
- Zhan, X.; Tan, Y.; Lv, Y.; Fang, J.; Zhou, Y.; Gao, X.; Zhu, H.; Shi, C. The Antimicrobial and Antibiofilm Activity of Oregano Essential Oil against Enterococcus faecalis and Its Application in Chicken Breast. Foods 2022, 11, 2296. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. The mechanism of action of a citrus oil blend against Enterococcus faecium and Enterococcus faecalis. J. Appl. Microbiol. 2009, 106, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Vlachojannis, C.; Chrubasik-Hausmann, S.; Hellwig, E.; Al-Ahmad, A. A Preliminary Investigation on the Antimicrobial Activity of Listerine®, Its Components, and of Mixtures Thereof. Phytother. Res. PTR 2015, 29, 1590–1594. [Google Scholar] [CrossRef] [PubMed]
- Pecarski, D.; Ketin, S.; Omerovic, I.; Mirkovic, M.; Jugovic, Z.; Biocanin, R. Chemical compositions and antimicrobial activities of oregano and thyme essential oils. Bulg. Chem. Commun. 2016, 48, 678–683. [Google Scholar]
- Božik, M.; Hovorková, P.; Kloucek, P. Antibacterial Effect of Carvacrol and Coconut Oil on Selected Pathogenic Bacteria. Sci. Agric. Bohem. 2018, 49, 46–52. [Google Scholar] [CrossRef][Green Version]
- Kense, M.J.; Landman, W.J.M. Enterococcus cecorum infections in broiler breeders and their offspring: Molecular epidemiology. Avian Pathol. 2011, 40, 603–612. [Google Scholar] [CrossRef]
- Makrai, L.; Nemes, C.; Simon, A.; Ivanics, E.; Dudás, Z.; Fodor, L.; Glávits, R. Association of Enterococcus cecorum with vertebral osteomyelitis and spondylolisthesis in broiler parent chicks. Acta Vet. Hung. 2011, 59, 11–21. [Google Scholar] [CrossRef]
- Tessin, J.; Jung, A.; Silberborth, A.; Rohn, K.; Schulz, J.; Visscher, C.; Kemper, N. Detection of Enterococcus cecorum to identify persistently contaminated locations using faecal and environmental samples in broiler houses of clinically healthy flocks. Avian Pathol. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dolka, B.; Czopowicz, M.; Dolka, I.; Szeleszczuk, P. Chicken embryo lethality assay for determining the lethal dose, tissue distribution and pathogenicity of clinical Enterococcus cecorum isolates from poultry. Sci. Rep. 2022, 12, 10675. [Google Scholar] [CrossRef]
- Jung, A.; Chen, L.; Suyemoto, M.; Barnes, H.; Borst, L. A Review of Enterococcus cecorum Infection in Poultry. Avian Dis. 2018, 62, 261–271. [Google Scholar] [CrossRef]
- Borst, L.; Suyemoto, M.; Keelara Veerappa, S.; Dunningan, S.; Guy, J.; Barnes, H. A Chicken Embryo Lethality Assay for Pathogenic Enterococcus cecorum. Avian Dis. 2014, 58, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Mudenda, S.; Matafwali, S.K.; Malama, S.; Munyeme, M.; Yamba, K.; Katemangwe, P.; Siluchali, G.; Mainda, G.; Mukuma, M.; Bumbangi, F.N.; et al. Prevalence and antimicrobial resistance patterns of Enterococcus species isolated from laying hens in Lusaka and Copperbelt provinces of Zambia: A call for AMR surveillance in the poultry sector. JAC-Antimicrob. Resist. 2022, 4. [Google Scholar] [CrossRef] [PubMed]
- Semedo-Lemsaddek, T.; Bettencourt Cota, J.; Ribeiro, T.; Pimentel, A.; Tavares, L.; Bernando, F.; Oliveira, M. Resistance and virulence distribution in enterococci isolated from broilers reared in two farming systems. Ir. Vet. J. 2021, 74, 22. [Google Scholar] [CrossRef] [PubMed]
- ISO 20776-1:2006; Clinical Laboratory Testing and In Vitro Diagnostic Test Systems. Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices. Part 1: Reference Method for Testing the In Vitro Activity of Antimicrobial Agents against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. International Organization for Standardization (ISO): Geneva, Switzerland, 2006.

| Test | E. cecorum | E. columbae | E. cecorum/E. columbae |
|---|---|---|---|
| D-xylose | − | + | + |
| beta-galactosidase | −/+ | + | + |
| cyclodextrin | + | −/+ | + |
| beta-glucuronidase | −/+ | − | − |
| alanine arylamidase | −/+ | − | − |
| D-ribose | −/+ | + | + |
| lactose | −/+ | + | + |
| bacitracin resistance | −/+ | + | + |
| growth in 6.5% NaCl | −/+ | − | + |
| mannitol | −/+ | + | − |
| Antibiotic | MIC Breakpoints µg/mL (CLSI, EUCAST) | ||||
|---|---|---|---|---|---|
| E. cecorum | E. faecalis | E. faecium | E. gallinarum | E. hirae | |
| Amoxicillin | S (154/154) | S (453/453) | S (1/1) | S (57/57) | S (29/29) |
| Amoxicillin–clavulanic acid | S (154/154) | S (453/453) | S (1/1) | S (57/57) | S (29/29) |
| Doxycycline | S (83/154) I (23/154) R (48/154) | S (129/453) I (130/453) R (194/154) | S (1/1) I (0/1) R (0/1) | S (42/57) I (6/57) R (9/57) | S (29/29) I (0/29) R (0/29) |
| Erythromycin | S (6/154) I (0/154) R (148/154) | S (0/453) I (0/453) R (453/453) | S (1/1) I (0/1) R (0/1) | S (27/57) I (0/57) R (30/57) | S (14/29) I (0/29) R (15/29) |
| Norfloxacin | S (41/154) I (0/154) R (113/154) | S (0/453) I (259/453) R (194/453) | S (1/1) I (0/1) R (0/1) | S (18/57) I (6/57) R (33/57) | S (29/29) I (0/29) R (0/29 |
| Antibiotics | Interpretative Category and MIC Breakpoints µg/mL | ||
|---|---|---|---|
| S | I | R | |
| Erythromycin | ≤0.5 | 1–4 | ≥1 |
| Doxycycline | ≤4 | 8 | ≥16 |
| Norfloxacin | ≤4 | 8 | ≥16 |
| Antibiotics | Interpretative Category and MIC Breakpoints mg/L | |
|---|---|---|
| S | R | |
| Amoxicillin | ≤4 | >8 |
| Amoxicillin–clavulanic acid | ≤4 | >8 |
| Antibiotic | Tentative MIC Breakpoints | ||
|---|---|---|---|
| E. faecalis | E. faecium | E. hirae | |
| Doxycycline | 1 | 0.5 | - |
| Erythromycin | 4 | 4 | 1 |
| Florfenicol | 8 | 8 | - |
| Gentamicin | 64 | 32 | 32 |
| Neomycin | 256 | 64 | 128 |
| Streptomycin | 512 | 128 | 128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wódz, K.; Chodkowska, K.A.; Iwiński, H.; Różański, H.; Wojciechowski, J. In Vitro Evaluation of Phytobiotic Mixture Antibacterial Potential against Enterococcus spp. Strains Isolated from Broiler Chicken. Int. J. Mol. Sci. 2024, 25, 4797. https://doi.org/10.3390/ijms25094797
Wódz K, Chodkowska KA, Iwiński H, Różański H, Wojciechowski J. In Vitro Evaluation of Phytobiotic Mixture Antibacterial Potential against Enterococcus spp. Strains Isolated from Broiler Chicken. International Journal of Molecular Sciences. 2024; 25(9):4797. https://doi.org/10.3390/ijms25094797
Chicago/Turabian StyleWódz, Karolina, Karolina A. Chodkowska, Hubert Iwiński, Henryk Różański, and Jakub Wojciechowski. 2024. "In Vitro Evaluation of Phytobiotic Mixture Antibacterial Potential against Enterococcus spp. Strains Isolated from Broiler Chicken" International Journal of Molecular Sciences 25, no. 9: 4797. https://doi.org/10.3390/ijms25094797
APA StyleWódz, K., Chodkowska, K. A., Iwiński, H., Różański, H., & Wojciechowski, J. (2024). In Vitro Evaluation of Phytobiotic Mixture Antibacterial Potential against Enterococcus spp. Strains Isolated from Broiler Chicken. International Journal of Molecular Sciences, 25(9), 4797. https://doi.org/10.3390/ijms25094797






