A Molecular Perspective and Role of NAD+ in Ovarian Aging
Abstract
1. Introduction
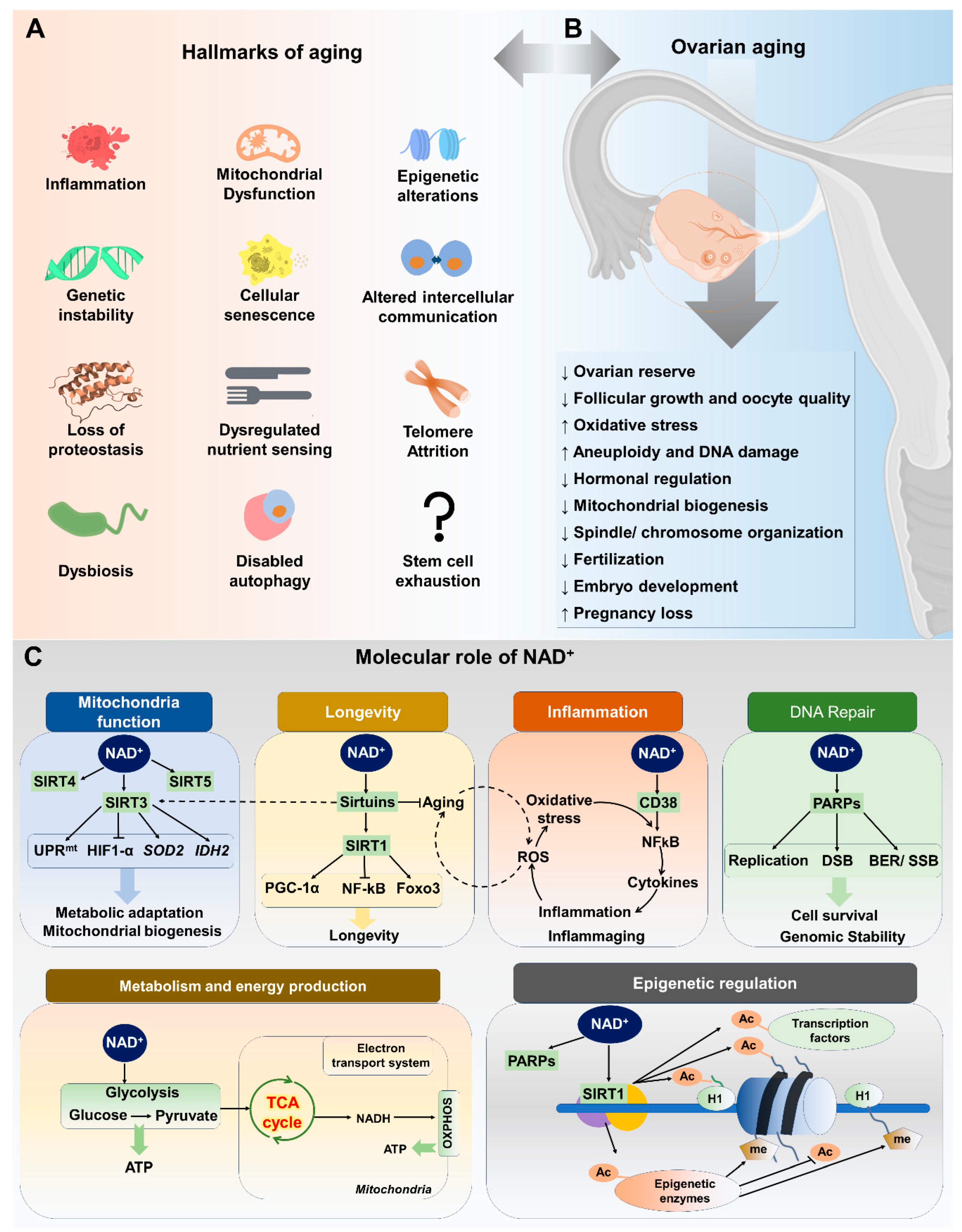
2. Factors Contributing to Ovarian Aging
3. Hallmarks of Aging and Ovarian Function
4. NAD+ and Its Metabolism
4.1. Overview of NAD+ and Its Importance in Cellular Functions
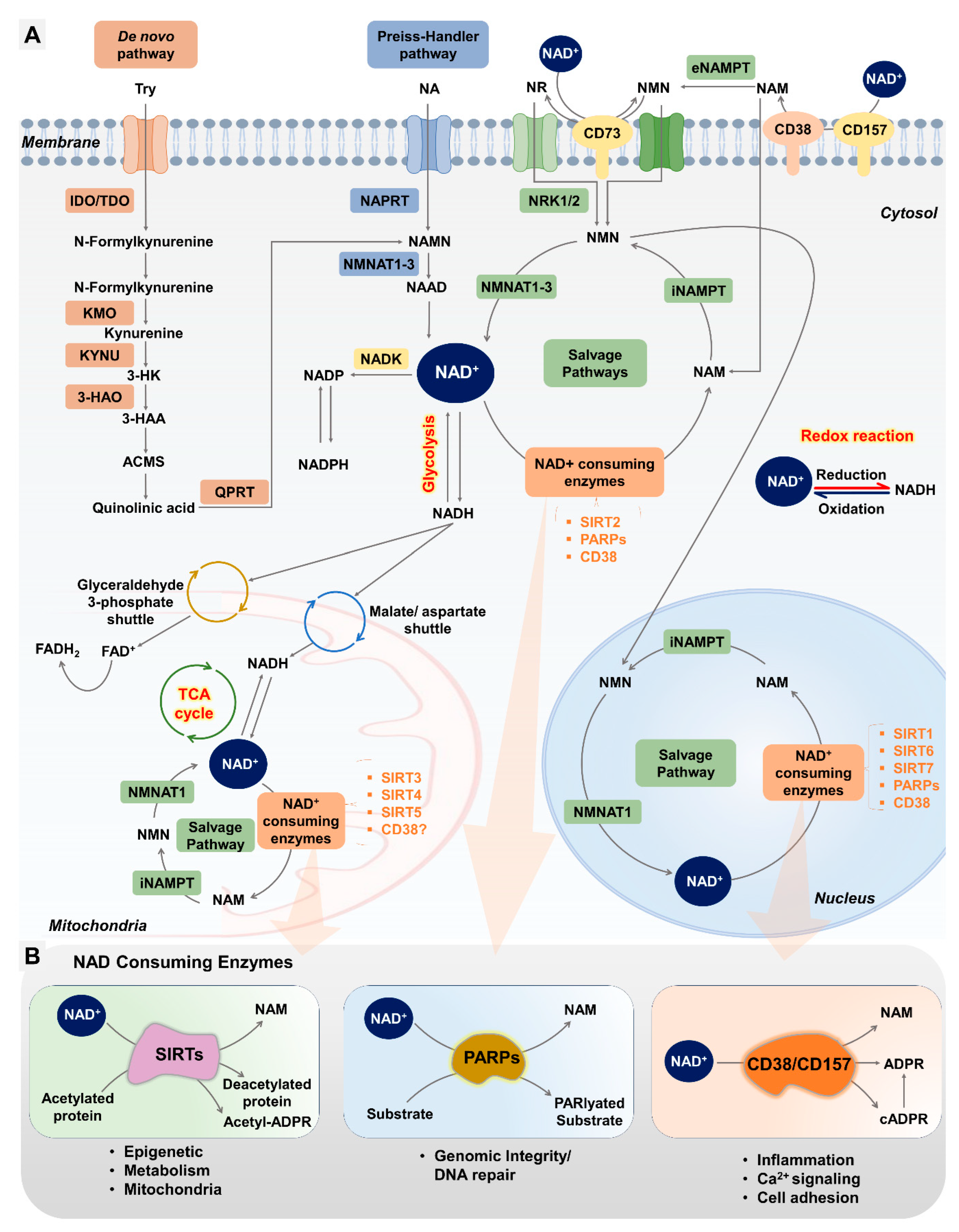
4.2. NAD+ Biosynthesis Pathways
4.2.1. De Novo Biosynthesis Pathway
4.2.2. Preiss–Handler Pathway (PHP)
4.2.3. Salvage Pathway
4.3. NAD+-Consuming Pathways
4.3.1. NAD+ Consumption by Sirtuins and Their Role in Female Reproduction
4.3.2. NAD+ Consumption by PARPs and Their Role in Female Reproduction
4.3.3. NAD+ Consumption by CD38 and Its Role in Female Reproduction
| Area | Role of NAD+ | Genes | References |
|---|---|---|---|
| Oocyte development and maturation | Energy production through mitochondria | NAMPT, SIRT1, SIRT3 | [82,167,168] |
| DNA repair and maintenance | PARPs (specifically PARP1) | [143,169,170] | |
| Cell signaling and gene expression | SIRT1, SIRT2, SIRT3 | [77,79,171] | |
| Maintenance of cellular redox balance | SIRT3, SIRT4 | [75,102,172,173,174] | |
| Follicle development and selection | Regulation of granulosa cell proliferation and differentiation | SIRT1, SIRT3, SIRT6 | [175,176,177,178,179] |
| Follicle-stimulating hormone (FSH) signaling | SIRT1 | [180,181] | |
| Estrogen biosynthesis | SIRT1, SIRT2 | [182,183] | |
| Embryo development and implantation | Mitochondrial function and ATP production in preimplantation embryos | SIRT3 | [80,107] |
| DNA repair and epigenetic modifications | PARP1, PARP2, SIRT1 | [184,185,186] | |
| Regulation of cell cycle progression and differentiation | SIRT2, SIRT4, NMNAT2, CD38 | [95,187,188,189] | |
| Maternal health and pregnancy outcomes | Regulation of insulin sensitivity and glucose metabolism | SIRT1, SIRT4, NMNAT3, NAMPT, CD38 | [111,148,190,191,192,193,194] |
| Immune modulation and inflammation control | CD38, NAMPT | [189,195,196] |
5. Boosting NAD+ as a Therapeutic Strategy for Ovarian Function
5.1. Supplementation of NAD+ Precursors
5.2. CD38 Inhibitors
5.3. NAD+ Biosynthesis through Nutrition
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADPR | Adenosine diphosphoribose |
| ANM | Age at natural menopause |
| cADPR | Cyclic ADP-ribose |
| CD38 | Cluster of Differentiation 38 |
| DDR | DNA damage response |
| FDA | Food and Drug Administration |
| FOXOs | Forkhead Box O Transcription Factors |
| FSH | Follicle-stimulating hormone |
| GCs | Granulosa cells |
| HDACs | Histone deacetylases |
| IVM | In vitro maturation |
| KO | Knockout |
| KP | Kynurenine pathway |
| LPS | Lipopolysaccharide |
| LXR | Liver X receptor |
| NA | Nicotinic acid |
| NAADP | Nicotinic acid adenine dinucleotide phosphate |
| NAD+ | Nicotinamide adenine dinucleotide |
| NADS | NAD synthase |
| NAM | Nicotinamide |
| NaMN | Nicotinate mononucleotide |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| NAPRT | Nicotinic acid phosphoribosyltransferase |
| NFκB | Nuclear Factor Kappa B |
| NMN | Nicotinamide mononucleotide |
| NMNAT | Nicotinamide mononucleotide adenylyltransferase |
| NR | Nicotinamide riboside |
| NRKs | Nicotinamide riboside kinases |
| OR | Ovarian reserve |
| PARP | Poly(ADP-ribose) polymerases |
| PARPi | PARP inhibitors |
| PCOS | Polycystic ovary syndrome |
| PDH | Pyruvate dehydrogenase |
| PGC1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha |
| POI | Premature ovarian insufficiency |
| QA | Quinolinic acid |
| QPRT | Quinolinate phosphoribosyltransferase |
| ROS | Reactive oxygen species |
| SIRTs | Sirtuins |
| STAT | Signal Transducer and Activator of Transcription |
| TDO | Tryptophan 2,3-dioxygenase |
References
- Amanvermez, R.; Tosun, M. An Update on Ovarian Aging and Ovarian Reserve Tests. Int. J. Fertil. Steril. 2016, 9, 411–415. [Google Scholar] [PubMed]
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: The challenges ahead. Lancet 2009, 374, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Inter, L.S.T. Variations in reproductive events across life: A pooled analysis of data from 505,147 women across 10 countries. Hum. Reprod. 2019, 34, 881–893. [Google Scholar]
- Wu, Y.; Li, M.; Zhang, J.; Wang, S. Unveiling uterine aging: Much more to learn. Ageing Res. Rev. 2023, 86, 101879. [Google Scholar] [CrossRef] [PubMed]
- McGee, E.A.; Hsueh, A.J. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [PubMed]
- Park, S.U.; Walsh, L.; Berkowitz, K.M. Mechanisms of ovarian aging. Reproduction 2021, 162, R19–R33. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Seifer, D.B. Ovarian reserve testing: A user’s guide. Am. J. Obstet. Gynecol. 2017, 217, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Broekmans, F.J.; Knauff, E.A.; te Velde, E.R.; Macklon, N.S.; Fauser, B.C. Female reproductive ageing: Current knowledge and future trends. Trends Endocrinol. Metab. 2007, 18, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Takahashi, T.; Nagase, S. Oocyte aging underlies female reproductive aging: Biological mechanisms and therapeutic strategies. Reprod. Med. Biol. 2015, 14, 159–169. [Google Scholar] [CrossRef]
- Ansere, V.A.; Ali-Mondal, S.; Sathiaseelan, R.; Garcia, D.N.; Isola, J.V.V.; Henseb, J.D.; Saccon, T.D.; Ocanas, S.R.; Tooley, K.B.; Stout, M.B.; et al. Cellular hallmarks of aging emerge in the ovary prior to primordial follicle depletion. Mech. Ageing Dev. 2021, 194, 111425. [Google Scholar] [CrossRef]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic potential of NAD-boosting molecules: The in vivo evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Aman, Y.; Qiu, Y.; Tao, J.; Fang, E.F. Therapeutic potential of boosting NAD+ in aging and age-related diseases. Transl. Med. Aging 2018, 2, 30–37. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, C.; Wang, G. Targeting NAD Metabolism for the Therapy of Age-Related Neurodegenerative Diseases. Neurosci. Bull. 2023, 40, 218–240. [Google Scholar] [CrossRef] [PubMed]
- Belenky, P.; Bogan, K.L.; Brenner, C. NAD+ metabolism in health and disease. Trends Biochem. Sci. 2007, 32, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012, 15, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Chini, C.C.; Tarragó, M.G.; Chini, E.N. NAD and the aging process: Role in life, death and everything in between. Mol. Cell. Endocrinol. 2017, 455, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef]
- Grive, K.J. Pathways coordinating oocyte attrition and abundance during mammalian ovarian reserve establishment. Mol. Reprod. Dev. 2020, 87, 843–856. [Google Scholar] [CrossRef]
- Richardson, M.C.; Guo, M.; Fauser, B.C.; Macklon, N.S. Environmental and developmental origins of ovarian reserve. Hum. Reprod. Update 2014, 20, 353–369. [Google Scholar] [CrossRef]
- Hansen, K.R.; Knowlton, N.S.; Thyer, A.C.; Charleston, J.S.; Soules, M.R.; Klein, N.A. A new model of reproductive aging: The decline in ovarian non-growing follicle number from birth to menopause. Hum. Reprod. 2008, 23, 699–708. [Google Scholar] [CrossRef]
- Picton, H.M. Activation of follicle development: The primordial follicle. Theriogenology 2001, 55, 1193–1210. [Google Scholar] [CrossRef]
- Pelosi, E.; Forabosco, A.; Schlessinger, D. Genetics of the ovarian reserve. Front. Genet. 2015, 6, 308. [Google Scholar] [CrossRef]
- Monniaux, D. Factors influencing establishment of the ovarian reserve and their effects on fertility. Anim. Reprod. 2018, 15 (Suppl. 1), 635–647. [Google Scholar] [CrossRef]
- Cavalcante, M.B.; Sampaio, O.G.M.; Camara, F.E.A.; Schneider, A.; de Avila, B.M.; Prosczek, J.; Masternak, M.M.; Campos, A.R. Ovarian aging in humans: Potential strategies for extending reproductive lifespan. Geroscience 2023, 45, 2121–2133. [Google Scholar] [CrossRef]
- Faddy, M.J.; Gosden, R.G.; Gougeon, A.; Richardson, S.J.; Nelson, J.F. Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum. Reprod. 1992, 7, 1342–1346. [Google Scholar] [CrossRef]
- te Velde, E.R.; Pearson, P.L. The variability of female reproductive ageing. Hum. Reprod. Update 2002, 8, 141–154. [Google Scholar] [CrossRef]
- Baerwald, A.R.; Adams, G.P.; Pierson, R.A. Ovarian antral folliculogenesis during the human menstrual cycle: A review. Hum. Reprod. Update 2012, 18, 73–91. [Google Scholar] [CrossRef]
- Crawford, N.M.; Steiner, A.Z. Age-related infertility. Obstet. Gynecol. Clin. N. Am. 2015, 42, 15–25. [Google Scholar] [CrossRef]
- Faubion, S.S.; Kuhle, C.L.; Shuster, L.T.; Rocca, W.A. Long-term health consequences of premature or early menopause and considerations for management. Climacteric 2015, 18, 483–491. [Google Scholar] [CrossRef]
- Shuster, L.T.; Rhodes, D.J.; Gostout, B.S.; Grossardt, B.R.; Rocca, W.A. Premature menopause or early menopause: Long-term health consequences. Maturitas 2010, 65, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Horstman, A.M.; Dillon, E.L.; Urban, R.J.; Sheffield-Moore, M. The role of androgens and estrogens on healthy aging and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Lu, A.T.; Chen, B.H.; Hernandez, D.G.; Singleton, A.B.; Ferrucci, L.; Bandinelli, S.; Salfati, E.; Manson, J.E.; Quach, A.; et al. Menopause accelerates biological aging. Proc. Natl. Acad. Sci. USA 2016, 113, 9327–9332. [Google Scholar] [CrossRef] [PubMed]
- Yureneva, S.; Averkova, V.; Silachev, D.; Donnikov, A.; Gavisova, A.; Serov, V.; Sukhikh, G. Searching for female reproductive aging and longevity biomarkers. Aging 2021, 13, 16873–16894. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Iwata, H.; Goto, H.; Shiratuki, S.; Tanaka, H.; Monji, Y.; Kuwayama, T. Effect of maternal age on the developmental competence and progression of nuclear maturation in bovine oocytes. Mol. Reprod. Dev. 2010, 77, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian aging: Mechanisms and clinical consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef]
- Lobo, R.A.; Davis, S.R.; De Villiers, T.J.; Gompel, A.; Henderson, V.W.; Hodis, H.N.; Lumsden, M.A.; Mack, W.J.; Shapiro, S.; Baber, R.J. Prevention of diseases after menopause. Climacteric 2014, 17, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Guo, Y.; Wei, S.; Xue, L.; Tang, W.; Chen, D.; Xiong, J.; Huang, Y.; Fu, F.; Wu, C.; et al. Biomaterials and advanced technologies for the evaluation and treatment of ovarian aging. J. Nanobiotechnol. 2022, 20, 374. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Wang, X.; Pepling, M.E. Regulation of meiotic prophase one in mammalian oocytes. Front. Cell Dev. Biol. 2021, 9, 667306. [Google Scholar] [CrossRef]
- Toupance, S.; Fattet, A.-J.; Thornton, S.N.; Benetos, A.; Guéant, J.-L.; Koscinski, I. Ovarian telomerase and female fertility. Biomedicines 2021, 9, 842. [Google Scholar] [CrossRef] [PubMed]
- Duncan, F.E.; Jasti, S.; Paulson, A.; Kelsh, J.M.; Fegley, B.; Gerton, J.L. Age-associated dysregulation of protein metabolism in the mammalian oocyte. Aging Cell 2017, 16, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Babayev, E.; Jiang, Z.; Li, G.; Zhang, M.; Esencan, E.; Horvath, T.; Seli, E. Mitochondrial unfolded protein response gene Clpp is required to maintain ovarian follicular reserve during aging, for oocyte competence, and development of pre-implantation embryos. Aging Cell 2018, 17, e12784. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hu, C.; Ye, H.; Luo, R.; Fu, X.; Li, X.; Huang, J.; Chen, W.; Zheng, Y. Inflamm-aging: A new mechanism affecting premature ovarian insufficiency. J. Immunol. Res. 2019, 2019, 8069898. [Google Scholar] [CrossRef] [PubMed]
- Lliberos, C.; Liew, S.H.; Mansell, A.; Hutt, K.J. The inflammasome contributes to depletion of the ovarian reserve during aging in mice. Front. Cell Dev. Biol. 2021, 8, 628473. [Google Scholar] [CrossRef] [PubMed]
- Lliberos, C.; Liew, S.H.; Zareie, P.; La Gruta, N.L.; Mansell, A.; Hutt, K. Evaluation of inflammation and follicle depletion during ovarian ageing in mice. Sci. Rep. 2021, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-H.; Kim, Y.J.; Cho, M.J.; Jang, J.; Koo, Y.D.; Kim, S.H.; Lee, J.H. Mitigating Age-Related Ovarian Dysfunction with the Anti-Inflammatory Agent MIT-001. Int. J. Mol. Sci. 2023, 24, 15158. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, X.; Yang, Z.; Li, S.; Wang, Y.; Ren, R.; Liu, Z.; Yu, D. Epigenetic regulation in premature ovarian failure: A literature review. Front. Physiol. 2023, 13, 998424. [Google Scholar] [CrossRef] [PubMed]
- Chamani, I.J.; Keefe, D.L. Epigenetics and female reproductive aging. Front. Endocrinol. 2019, 10, 473. [Google Scholar] [CrossRef]
- Sgueglia, G.; Longobardi, S.; Valerio, D.; Campitiello, M.R.; Colacurci, N.; Di Pietro, C.; Battaglia, R.; D’Hooghe, T.; Altucci, L.; Dell’Aversana, C. The impact of epigenetic landscape on ovarian cells in infertile older women undergoing IVF procedures. Clin. Epigenet. 2023, 15, 76. [Google Scholar] [CrossRef]
- Xie, J.; Xu, X.; Liu, S. Intercellular communication in the cumulus–oocyte complex during folliculogenesis: A review. Front. Cell Dev. Biol. 2023, 11, 1087612. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xu, W.; Liu, L. Ovarian aging: Mechanisms and intervention strategies. Med. Rev. 2022, 2, 590–610. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, S.; Ye, C.; Zhao, W. Gut microbiota dysbiosis in polycystic ovary syndrome: Mechanisms of progression and clinical applications. Front. Cell. Infect. Microbiol. 2023, 13, 1142041. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Shuai, P.; Shen, S.; Zheng, H.; Sun, P.; Zhang, R.; Lan, S.; Lan, Z.; Jayawardana, T.; Yang, Y. Perturbations in gut microbiota composition in patients with polycystic ovary syndrome: A systematic review and meta-analysis. BMC Med. 2023, 21, 302. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.G.; Thackray, V.G. Intersection of polycystic ovary syndrome and the gut microbiome. J. Endocr. Soc. 2021, 5, bvaa177. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD(+) metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Mo, F.; Zhang, Z.; Huang, M.; Wei, X. Nicotinamide Mononucleotide: A Promising Molecule for Therapy of Diverse Diseases by Targeting NAD+ Metabolism. Front. Cell Dev. Biol. 2020, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Kovac, V.; Milisav, I. Current Uncertainties and Future Challenges Regarding NAD+ Boosting Strategies. Antioxidants 2022, 11, 1637. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E. NAD(+) in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef]
- Yoshino, J.; Baur, J.A.; Imai, S.I. NAD(+) Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef]
- Bertoldo, M.J.; Listijono, D.R.; Ho, W.J.; Riepsamen, A.H.; Goss, D.M.; Richani, D.; Jin, X.L.; Mahbub, S.; Campbell, J.M.; Habibalahi, A.; et al. NAD(+) Repletion Rescues Female Fertility during Reproductive Aging. Cell Rep. 2020, 30, 1670–1681.e7. [Google Scholar] [CrossRef] [PubMed]
- Perrone, R.; Ashok Kumaar, P.V.; Haky, L.; Hahn, C.; Riley, R.; Balough, J.; Zaza, G.; Soygur, B.; Hung, K.; Prado, L.; et al. CD38 regulates ovarian function and fecundity via NAD(+) metabolism. iScience 2023, 26, 107949. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, A.; Kulikova, V.; Ziegler, M. The human NAD metabolome: Functions, metabolism and compartmentalization. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Cantó, C.; Wanders, R.J.; Auwerx, J. The secret life of NAD+: An old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 2010, 31, 194–223. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Imai, S.I. NAD+ biosynthesis, aging, and disease. F1000Research 2018, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Cambronne, X.A.; Kraus, W.L. Location, location, location: Compartmentalization of NAD+ synthesis and functions in mammalian cells. Trends Biochem. Sci. 2020, 45, 858–873. [Google Scholar] [CrossRef]
- van Zundert, S.K.; Broekhuizen, M.; Smit, A.J.; van Rossem, L.; Mirzaian, M.; Willemsen, S.P.; Danser, A.J.; De Rijke, Y.B.; Reiss, I.K.; Merkus, D. The Role of the Kynurenine Pathway in the (Patho) physiology of Maternal Pregnancy and Fetal Outcomes: A Systematic Review. Int. J. Tryptophan Res. 2022, 15, 11786469221135545. [Google Scholar] [CrossRef]
- Broekhuizen, M.; Danser, A.J.; Reiss, I.K.; Merkus, D. The function of the kynurenine pathway in the placenta: A novel pharmacotherapeutic target? Int. J. Environ. Res. Public Health 2021, 18, 11545. [Google Scholar] [CrossRef]
- Wang, S.; Mu, L.; Zhang, C.; Long, X.; Zhang, Y.; Li, R.; Zhao, Y.; Qiao, J. Abnormal Activation of Tryptophan-Kynurenine Pathway in Women With Polycystic Ovary Syndrome. Front. Endocrinol. 2022, 13, 877807. [Google Scholar] [CrossRef]
- Guarente, L.; Mostoslavsky, R.; Kazantsev, A. Introductory Review on Sirtuins in Biology, Aging, and Disease; Elsevie: Amsterdam, The Netherlands, 2018. [Google Scholar]
- North, B.J.; Verdin, E. Interphase nucleo-cytoplasmic shuttling and localization of SIRT2 during mitosis. PLoS ONE 2007, 2, e784. [Google Scholar] [CrossRef]
- Blander, G.; Guarente, L. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 2004, 73, 417–435. [Google Scholar] [CrossRef]
- Bodis, J.; Sulyok, E.; Koszegi, T.; Godony, K.; Premusz, V.; Varnagy, A. Serum and follicular fluid levels of sirtuin 1, sirtuin 6, and resveratrol in women undergoing in vitro fertilization: An observational, clinical study. J. Int. Med. Res. 2019, 47, 772–782. [Google Scholar] [CrossRef]
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD(+) in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Tatone, C.; Di Emidio, G.; Barbonetti, A.; Carta, G.; Luciano, A.M.; Falone, S.; Amicarelli, F. Sirtuins in gamete biology and reproductive physiology: Emerging roles and therapeutic potential in female and male infertility. Hum. Reprod. Update 2018, 24, 267–289. [Google Scholar] [CrossRef]
- Vazquez, B.N.; Vaquero, A.; Schindler, K. Sirtuins in female meiosis and in reproductive longevity. Mol. Reprod. Dev. 2020, 87, 1175–1187. [Google Scholar] [CrossRef]
- Okamoto, N.; Kawamura, K.; Kawamura, N.; Nishijima, C.; Ishizuka, B.; Suzuki, N.; Hirata, K. Effects of maternal aging on expression of sirtuin genes in ovulated oocyte and cumulus cells. J. Mamm. Ova Res. 2013, 30, 24–29. [Google Scholar] [CrossRef]
- Tatone, C.; Di Emidio, G.; Vitti, M.; Di Carlo, M.; Santini, S., Jr.; D’Alessandro, A.M.; Falone, S.; Amicarelli, F. Sirtuin Functions in Female Fertility: Possible Role in Oxidative Stress and Aging. Oxid. Med. Cell. Longev. 2015, 2015, 659687. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Uchijima, Y.; Horike, N.; Tonami, K.; Nishiyama, K.; Amano, T.; Asano, T.; Kurihara, Y.; Kurihara, H. Sirt3 protects in vitro-fertilized mouse preimplantation embryos against oxidative stress-induced p53-mediated developmental arrest. J. Clin. Investig. 2010, 120, 2817–2828. [Google Scholar] [CrossRef]
- Kwak, S.S.; Cheong, S.A.; Yoon, J.D.; Jeon, Y.; Hyun, S.H. Expression patterns of sirtuin genes in porcine preimplantation embryos and effects of sirtuin inhibitors on in vitro embryonic development after parthenogenetic activation and in vitro fertilization. Theriogenology 2012, 78, 1597–1610. [Google Scholar] [CrossRef]
- Pollard, C.-L.; Gibb, Z.; Swegen, A.; Grupen, C.G. NAD+, Sirtuins and PARPs: Enhancing oocyte developmental competence. J. Reprod. Dev. 2022, 68, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Iljas, J.D.; Wei, Z.; Homer, H.A. Sirt1 sustains female fertility by slowing age-related decline in oocyte quality required for post-fertilization embryo development. Aging Cell 2020, 19, e13204. [Google Scholar] [CrossRef] [PubMed]
- Seli, E.; Babayev, E.; Collins, S.C.; Nemeth, G.; Horvath, T.L. Minireview: Metabolism of female reproduction: Regulatory mechanisms and clinical implications. Mol. Endocrinol. 2014, 28, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Rigor, P.; Cervantes, M.; Ceglia, N.; Sebastian, C.; Xiao, C.; Roqueta-Rivera, M.; Deng, C.; Osborne, T.F.; Mostoslavsky, R.; et al. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell 2014, 158, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, H.; Dessain, S.K.; Eaton, E.N.; Imai, S.-I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Cantó, C.; Mottis, A.; Jo, Y.-S.; Viswanathan, M.; Schoonjans, K. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- McBurney, M.W.; Yang, X.; Jardine, K.; Hixon, M.; Boekelheide, K.; Webb, J.R.; Lansdorp, P.M.; Lemieux, M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol. Cell. Biol. 2003, 23, 38–54. [Google Scholar] [CrossRef]
- Bordone, L.; Cohen, D.; Robinson, A.; Motta, M.C.; Van Veen, E.; Czopik, A.; Steele, A.D.; Crowe, H.; Marmor, S.; Luo, J. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 2007, 6, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Cinco, R.; Digman, M.A.; Gratton, E.; Luderer, U. Spatial characterization of bioenergetics and metabolism of primordial to preovulatory follicles in whole ex vivo murine ovary. Biol. Reprod. 2016, 95, 129. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Dekanová, P.; Harrath, A.H.; Alwasel, S.H.; Vašíček, D. Interrelationships between sirtuin 1 and transcription factors p53 and NF-κB (p50/p65) in the control of ovarian cell apoptosis and proliferation. Cell Tissue Res. 2014, 358, 627–632. [Google Scholar] [CrossRef]
- de Oliveira, R.M.; Sarkander, J.; Kazantsev, A.G.; Outeiro, T.F. SIRT2 as a therapeutic target for age-related disorders. Front. Pharmacol. 2012, 3, 82. [Google Scholar] [CrossRef]
- Dryden, S.C.; Nahhas, F.A.; Nowak, J.E.; Goustin, A.-S.; Tainsky, M.A. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol. Cell. Biol. 2003, 23, 3173–3185. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, S.; Xiao, M.; Lin, Y.; Zhou, L.; Lei, Q.; Xiong, Y.; Guan, K.-L.; Zhao, S. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Mol. Cell 2011, 43, 33–44. [Google Scholar] [CrossRef]
- North, B.J.; Rosenberg, M.A.; Jeganathan, K.B.; Hafner, A.V.; Michan, S.; Dai, J.; Baker, D.J.; Cen, Y.; Wu, L.E.; Sauve, A.A. SIRT 2 induces the checkpoint kinase BubR1 to increase lifespan. EMBO J. 2014, 33, 1438–1453. [Google Scholar] [CrossRef]
- Riepsamen, A.; Wu, L.; Lau, L.; Listijono, D.; Ledger, W.; Sinclair, D.; Homer, H. Nicotinamide impairs entry into and exit from meiosis I in mouse oocytes. PLoS ONE 2015, 10, e0126194. [Google Scholar]
- Zhang, L.; Hou, X.; Ma, R.; Moley, K.; Schedl, T.; Wang, Q. Sirt2 functions in spindle organization and chromosome alignment in mouse oocyte meiosis. FASEB J. 2014, 28, 1435. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Hou, X.; Han, L.; Li, X.; Ge, J.; Wang, Q. Sirt2-BubR1 acetylation pathway mediates the effects of advanced maternal age on oocyte quality. Aging Cell 2018, 17, e12698. [Google Scholar] [CrossRef]
- Lombard, D.B.; Zwaans, B.M. SIRT3: As simple as it seems? Gerontology 2013, 60, 56–64. [Google Scholar] [CrossRef]
- Di Emidio, G.; Falone, S.; Artini, P.G.; Amicarelli, F.; D’Alessandro, A.M.; Tatone, C. Mitochondrial sirtuins in reproduction. Antioxidants 2021, 10, 1047. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Bindu, S.; Pillai, V.B.; Samant, S.; Pan, Y.; Huang, J.-Y.; Gupta, M.; Nagalingam, R.S.; Wolfgeher, D.; Verdin, E. SIRT3 blocks aging-associated tissue fibrosis in mice by deacetylating and activating glycogen synthase kinase 3β. Mol. Cell. Biol. 2016, 36, 678–692. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Gupta, M.; Kim, G.; Rajamohan, S.B.; Isbatan, A.; Gupta, M.P. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Investig. 2009, 119, 2758–2771. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Patel, K.; Muldoon-Jacobs, K.; Bisht, K.S.; Aykin-Burns, N.; Pennington, J.D.; van der Meer, R.; Nguyen, P.; Savage, J.; Owens, K.M. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell 2010, 17, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, L.; Ma, R.; Hou, X.; Yu, Y.; Sun, S.; Xu, Y.; Schedl, T.; Moley, K.H.; Wang, Q. Sirt3 prevents maternal obesity-associated oxidative stress and meiotic defects in mouse oocytes. Cell Cycle 2015, 14, 2959–2968. [Google Scholar] [CrossRef]
- Zhao, H.-C.; Ding, T.; Ren, Y.; Li, T.-J.; Li, R.; Fan, Y.; Yan, J.; Zhao, Y.; Li, M.; Yu, Y. Role of Sirt3 in mitochondrial biogenesis and developmental competence of human in vitro matured oocytes. Hum. Reprod. 2016, 31, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Kratz, E.M.; Kokot, I.; Dymicka-Piekarska, V.; Piwowar, A. Sirtuins—The new important players in women’s gynecological health. Antioxidants 2021, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Pacella-Ince, L.; Zander-Fox, D.; Lane, M. Mitochondrial SIRT3 and its target glutamate dehydrogenase are altered in follicular cells of women with reduced ovarian reserve or advanced maternal age. Hum. Reprod. 2014, 29, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Itami, N.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Palmitic acid induces ceramide accumulation, mitochondrial protein hyperacetylation, and mitochondrial dysfunction in porcine oocytes. Biol. Reprod. 2018, 98, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Mostoslavsky, R.; Haigis, K.M.; Fahie, K.; Christodoulou, D.C.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Karow, M.; Blander, G. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic β cells. Cell 2006, 126, 941–954. [Google Scholar] [CrossRef]
- Jeong, S.M.; Xiao, C.; Finley, L.W.; Lahusen, T.; Souza, A.L.; Pierce, K.; Li, Y.-H.; Wang, X.; Laurent, G.; German, N.J. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell 2013, 23, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Mathias, R.A.; Greco, T.M.; Oberstein, A.; Budayeva, H.G.; Chakrabarti, R.; Rowland, E.A.; Kang, Y.; Shenk, T.; Cristea, I.M. Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell 2014, 159, 1615–1625. [Google Scholar] [CrossRef]
- Zeng, J.; Jiang, M.; Wu, X.; Diao, F.; Qiu, D.; Hou, X.; Wang, H.; Li, L.; Li, C.; Ge, J. SIRT 4 is essential for metabolic control and meiotic structure during mouse oocyte maturation. Aging Cell 2018, 17, e12789. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.A.; Huynh, F.K.; Fisher-Wellman, K.; Stuart, J.D.; Peterson, B.S.; Douros, J.D.; Wagner, G.R.; Thompson, J.W.; Madsen, A.S.; Green, M.F. SIRT4 is a lysine deacylase that controls leucine metabolism and insulin secretion. Cell Metab. 2017, 25, 838–855.e15. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Zhang, J.; Zhang, J.; Wang, Y.; Wang, J.; Kang, J.; Quan, F.; Su, J.; Zhang, Y. Coenzyme Q10 supplement rescues postovulatory oocyte aging by regulating SIRT4 expression. Curr. Mol. Pharmacol. 2022, 15, 190–203. [Google Scholar] [PubMed]
- Tan, M.; Peng, C.; Anderson, K.A.; Chhoy, P.; Xie, Z.; Dai, L.; Park, J.; Chen, Y.; Huang, H.; Zhang, Y. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014, 19, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhou, Y.; Su, X.; Yu, J.J.; Khan, S.; Jiang, H.; Kim, J.; Woo, J.; Kim, J.H.; Choi, B.H. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 2011, 334, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, T.; Ciarlo, E.; Le Roy, D.; Roger, T. Impact of the dual deletion of the mitochondrial sirtuins SIRT3 and SIRT5 on anti-microbial host defenses. Front. Immunol. 2019, 10, 2341. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, R.; Martín-Ramírez, R.; Rotoli, D.; Hernández, J.; Naftolin, F.; Martín-Vasallo, P.; Palumbo, A.; Ávila, J. Granulosa-lutein cell sirtuin gene expression profiles differ between normal donors and infertile women. Int. J. Mol. Sci. 2019, 21, 295. [Google Scholar] [CrossRef]
- Han, L.; Ge, J.; Zhang, L.; Ma, R.; Hou, X.; Li, B.; Moley, K.; Wang, Q. Sirt6 depletion causes spindle defects and chromosome misalignment during meiosis of mouse oocyte. Sci. Rep. 2015, 5, 15366. [Google Scholar] [CrossRef]
- Ge, J.; Li, C.; Li, C.; Huang, Z.; Zeng, J.; Han, L.; Wang, Q. SIRT6 participates in the quality control of aged oocytes via modulating telomere function. Aging 2019, 11, 1965. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, J. SIRT7 an emerging sirtuin: Deciphering newer roles. J. Physiol. Pharmacol. 2013, 64, 531–534. [Google Scholar]
- Tsai, Y.-C.; Greco, T.M.; Cristea, I.M. Sirtuin 7 plays a role in ribosome biogenesis and protein synthesis. Mol. Cell. Proteom. 2014, 13, 73–83. [Google Scholar] [CrossRef]
- Grob, A.; Roussel, P.; Wright, J.E.; McStay, B.; Hernandez-Verdun, D.; Sirri, V. Involvement of SIRT7 in resumption of rDNA transcription at the exit from mitosis. J. Cell Sci. 2009, 122, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, B.N.; Thackray, J.K.; Simonet, N.G.; Chahar, S.; Kane-Goldsmith, N.; Newkirk, S.J.; Lee, S.; Xing, J.; Verzi, M.P.; An, W. SIRT7 mediates L1 elements transcriptional repression and their association with the nuclear lamina. Nucleic Acids Res. 2019, 47, 7870–7885. [Google Scholar] [CrossRef]
- Tharp, M.E.; Malki, S.; Bortvin, A. Maximizing the ovarian reserve in mice by evading LINE-1 genotoxicity. Nat. Commun. 2020, 11, 330. [Google Scholar] [CrossRef]
- Barber, M.F.; Michishita-Kioi, E.; Xi, Y.; Tasselli, L.; Kioi, M.; Moqtaderi, Z.; Tennen, R.I.; Paredes, S.; Young, N.L.; Chen, K. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature 2012, 487, 114–118. [Google Scholar] [CrossRef]
- Ray Chaudhuri, A.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Cantó, C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. 2012, 16, 290–295. [Google Scholar] [CrossRef]
- Boehler, C.; Gauthier, L.R.; Mortusewicz, O.; Biard, D.S.; Saliou, J.-M.; Bresson, A.; Sanglier-Cianferani, S.; Smith, S.; Schreiber, V.; Boussin, F. Poly (ADP-ribose) polymerase 3 (PARP3), a newcomer in cellular response to DNA damage and mitotic progression. Proc. Natl. Acad. Sci. USA 2011, 108, 2783–2788. [Google Scholar] [CrossRef]
- Beck, C.; Robert, I.; Reina-San-Martin, B.; Schreiber, V.; Dantzer, F. Poly(ADP-ribose) polymerases in double-strand break repair: Focus on PARP1, PARP2 and PARP3. Exp. Cell Res. 2014, 329, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.; Bai, P.; De Murcia, J.M.; De Murcia, G. PARP-1, PARP-2 and ATM in the DNA damage response: Functional synergy in mouse development. DNA Repair 2004, 3, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Su, X.; Quinn, W.J.; Hui, S.; Krukenberg, K.; Frederick, D.W.; Redpath, P.; Zhan, L.; Chellappa, K.; White, E. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab. 2018, 27, 1067–1080.e5. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.L. PARPs and ADP-ribosylation: 60 years on. Genes Dev. 2020, 34, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Bai, P. Biology of poly (ADP-ribose) polymerases: The factotums of cell maintenance. Mol. Cell 2015, 58, 947–958. [Google Scholar] [CrossRef]
- Yang, F.; Baumann, C.; De La Fuente, R. Persistence of histone H2AX phosphorylation after meiotic chromosome synapsis and abnormal centromere cohesion in poly (ADP-ribose) polymerase (Parp-1) null oocytes. Dev. Biol. 2009, 331, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Veuger, S.J.; Hunter, J.E.; Durkacz, B.W. Ionizing radiation-induced NF-κB activation requires PARP-1 function to confer radioresistance. Oncogene 2009, 28, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, H.R.; Kim, M.G.; Lee, J.S.; Jin, S.J.; Lee, H.T. The effect of poly (ADP-ribosyl) ation inhibition on the porcine cumulus-oocyte complex during in vitro maturation. Biochem. Biophys. Res. Commun. 2017, 483, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Osada, T.; Ogino, H.; Hino, T.; Ichinose, S.; Nakamura, K.; Omori, A.; Noce, T.; Masutani, M. PolyADP-ribosylation is required for pronuclear fusion during postfertilization in mice. PLoS ONE 2010, 5, e12526. [Google Scholar] [CrossRef]
- Kondratska, O.; Grushka, N.; Pavlovych, S.; Krasutska, N.; Tsyhankov, S.; Yanchii, R. Effects of poly (ADP-ribose) polymerase inhibition on DNA integrity and gene expression in ovarian follicular cells in mice with endotoxemia. Iran. Biomed. J. 2022, 26, 44. [Google Scholar]
- Kelleher, A.M.; Setlem, R.; Dantzer, F.; DeMayo, F.J.; Lydon, J.P.; Kraus, W.L. Deficiency of PARP-1 and PARP-2 in the mouse uterus results in decidualization failure and pregnancy loss. Proc. Natl. Acad. Sci. USA 2021, 118, e2109252118. [Google Scholar] [CrossRef]
- Winship, A.L.; Griffiths, M.; Lliberos Requesens, C.; Sarma, U.; Phillips, K.-A.; Hutt, K.J. The PARP inhibitor, olaparib, depletes the ovarian reserve in mice: Implications for fertility preservation. Hum. Reprod. 2020, 35, 1864–1874. [Google Scholar] [CrossRef]
- Nakamura, K.; Takae, S.; Shiraishi, E.; Shinya, K.; Igualada, A.J.; Suzuki, N. Poly (ADP-ribose) polymerase inhibitor exposure reduces ovarian reserve followed by dysfunction in granulosa cells. Sci. Rep. 2020, 10, 17058. [Google Scholar] [CrossRef]
- Chini, C.C.; Peclat, T.R.; Warner, G.M.; Kashyap, S.; Espindola-Netto, J.M.; de Oliveira, G.C.; Gomez, L.S.; Hogan, K.A.; Tarragó, M.G.; Puranik, A.S. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels. Nat. Metab. 2020, 2, 1284–1304. [Google Scholar] [CrossRef]
- Aksoy, P.; White, T.A.; Thompson, M.; Chini, E.N. Regulation of intracellular levels of NAD: A novel role for CD38. Biochem. Biophys. Res. Commun. 2006, 345, 1386–1392. [Google Scholar] [CrossRef]
- Chini, E.N.; Chini, C.C.; Netto, J.M.E.; de Oliveira, G.C.; van Schooten, W. The pharmacology of CD38/NADase: An emerging target in cancer and diseases of aging. Trends Pharmacol. Sci. 2018, 39, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Pereira, J.; Tarragó, M.G.; Chini, C.C.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Adebanjo, O.A.; Anandatheerthavarada, H.K.; Koval, A.P.; Moonga, B.S.; Biswas, G.; Sun, L.; Sodam, B.R.; Bevis, P.J.; Huang, C.L.-H.; Epstein, S. A new function for CD38/ADP-ribosyl cyclase in nuclear Ca2+ homeostasis. Nat. Cell Biol. 1999, 1, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Scheibye-Knudsen, M.; Chua, K.F.; Mattson, M.P.; Croteau, D.L.; Bohr, V.A. Nuclear DNA damage signalling to mitochondria in ageing. Nat. Rev. Mol. Cell Biol. 2016, 17, 308–321. [Google Scholar] [CrossRef]
- Lee, H.C.; Deng, Q.W.; Zhao, Y.J. The calcium signaling enzyme CD38-a paradigm for membrane topology defining distinct protein functions. Cell Calcium 2022, 101, 102514. [Google Scholar] [CrossRef]
- Partida-Sánchez, S.; Cockayne, D.A.; Monard, S.; Jacobson, E.L.; Oppenheimer, N.; Garvy, B.; Kusser, K.; Goodrich, S.; Howard, M.; Harmsen, A. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat. Med. 2001, 7, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Jiang, L.; Ram Sairam, M. Follicle-stimulating hormone mediated calcium signaling by the alternatively spliced growth factor type I receptor. Biol. Reprod. 2000, 62, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Carvacho, I.; Piesche, M.; Maier, T.J.; Machaca, K. Ion channel function during oocyte maturation and fertilization. Front. Cell Dev. Biol. 2018, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Verduci, E.; Cena, H.; Magenes, V.C.; Todisco, C.F.; Tenuta, E.; Gregorio, C.; De Giuseppe, R.; Bosetti, A.; Di Profio, E. Polycystic ovary syndrome in insulin-resistant adolescents with obesity: The role of nutrition therapy and food supplements as a strategy to protect fertility. Nutrients 2021, 13, 1848. [Google Scholar] [CrossRef] [PubMed]
- van Helden, J.; Evliyaoglu, O.; Küberl, A.; Weiskirchen, R. Disorders of the glucose metabolism correlate with the phenotype and the severity in women with polycystic ovary syndrome. Clin. Endocrinol. 2020, 93, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Deshpande, D.A.; White, T.A.; Walseth, T.F.; Kannan, M.S. Regulation of CD38 expression and function by steroid hormones in myometrium. Mol. Cell. Endocrinol. 2006, 246, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, S.; Chiao, B.; Lei, Z.; Chew, S.H.; Ebstein, R.P. A novel role of CD38 and oxytocin as tandem molecular moderators of human social behavior. Neurosci. Biobehav. Rev. 2020, 115, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Araki, S.-i.; Koya, D. The Role of CD38 in the Pathogenesis of Cardiorenal Metabolic Disease and Aging, an Approach from Basic Research. Cells 2023, 12, 595. [Google Scholar] [CrossRef] [PubMed]
- Agorrody, G.; Peclat, T.R.; Peluso, G.; Gonano, L.A.; Santos, L.; van Schooten, W.; Chini, C.C.; Escande, C.; Chini, E.N.; Contreras, P. Benefits in cardiac function by CD38 suppression: Improvement in NAD+ levels, exercise capacity, heart rate variability and protection against catecholamine-induced ventricular arrhythmias. J. Mol. Cell. Cardiol. 2022, 166, 11–22. [Google Scholar] [CrossRef]
- Guerreiro, S.; Privat, A.-L.; Bressac, L.; Toulorge, D. CD38 in neurodegeneration and neuroinflammation. Cells 2020, 9, 471. [Google Scholar] [CrossRef]
- Zeidler, J.D.; Hogan, K.A.; Agorrody, G.; Peclat, T.R.; Kashyap, S.; Kanamori, K.S.; Gomez, L.S.; Mazdeh, D.Z.; Warner, G.M.; Thompson, K.L. The CD38 glycohydrolase and the NAD sink: Implications for pathological conditions. Am. J. Physiol.-Cell Physiol. 2022, 322, C521–C545. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, F.; Deaglio, S.; Funaro, A.; Ferrero, E.; Horenstein, A.L.; Ortolan, E.; Vaisitti, T.; Aydin, S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol. Rev. 2008, 88, 841–886. [Google Scholar] [CrossRef] [PubMed]
- Matalonga, J.; Glaria, E.; Bresque, M.; Escande, C.; Carbó, J.M.; Kiefer, K.; Vicente, R.; León, T.E.; Beceiro, S.; Pascual-García, M. The nuclear receptor LXR limits bacterial infection of host macrophages through a mechanism that impacts cellular NAD metabolism. Cell Rep. 2017, 18, 1241–1255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Q.; Wang, H.; Zhu, J.; Cong, L.; Li, H.; Sun, Y. NAD+ deficiency and mitochondrial dysfunction in granulosa cells of women with polycystic ovary syndrome. Biol. Reprod. 2021, 105, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Zhuan, Q.; Li, J.; Du, X.; Zhang, L.; Meng, L.; Cheng, K.; Zhu, S.; Hou, Y.; Fu, X. Nampt affects mitochondrial function in aged oocytes by mediating the downstream effector FoxO3a. J. Cell. Physiol. 2022, 237, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Pollard, C.-L.; Younan, A.; Swegen, A.; Gibb, Z.; Grupen, C.G. Insights into the NAD+ biosynthesis pathways involved during meiotic maturation and spindle formation in porcine oocytes. J. Reprod. Dev. 2022, 68, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Ruszkiewicz, J.A.; Bürkle, A.; Mangerich, A. Fueling genome maintenance: On the versatile roles of NAD+ in preserving DNA integrity. J. Biol. Chem. 2022, 298, 102037. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Q.; Zhang, L.; Zhang, S.; Dai, Y. Poly-ADP-ribose polymerase (PARP) inhibitors and ovarian function. Biomed. Pharmacother. 2023, 157, 114028. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, X.; Wang, X.; Zheng, Y.; Qiu, J.; Peng, Y.; Xu, J.; Chai, Z.; Liu, C.; Khawar, M.B. Multiple roles of SIRT2 in regulating physiological and pathological signal transduction. Genet. Res. 2022, 2022, e38. [Google Scholar] [CrossRef]
- Radak, Z.; Koltai, E.; Taylor, A.W.; Higuchi, M.; Kumagai, S.; Ohno, H.; Goto, S.; Boldogh, I. Redox-regulating sirtuins in aging, caloric restriction, and exercise. Free Radic. Biol. Med. 2013, 58, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Syed, H.; Amjad, S.; Baig, M.; Khan, T.A.; Rehman, R. Interplay between oxidative stress, SIRT1, reproductive and metabolic functions. Curr. Res. Physiol. 2021, 4, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhou, S.; Coetzee, S.; Chen, A. SIRT4 and its roles in energy and redox metabolism in health, disease and during exercise. Front. Physiol. 2019, 10, 1006. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Wu, S.; Zhang, P.; Gan, M.; Chen, L.; Zhao, Y.; Niu, L.; Zhang, S.; Jiang, Y. Regulation of SIRT1 in Ovarian Function: PCOS Treatment. Curr. Issues Mol. Biol. 2023, 45, 2073–2089. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-A.; Joo, N.-R.; Park, J.-H.; Oh, S.-M. Role of the SIRT1/p53 regulatory axis in oxidative stress-mediated granulosa cell apoptosis. Mol. Med. Rep. 2021, 23, 20. [Google Scholar] [CrossRef]
- Szymanska, M.; Manthe, S.; Shrestha, K.; Girsh, E.; Harlev, A.; Meidan, R. The cAMP pathway promotes sirtuin-1 expression in human granulosa-lutein cells. Reprod. Biol. 2020, 20, 273–281. [Google Scholar] [CrossRef]
- Schmid, N.; Dietrich, K.-G.; Forne, I.; Burges, A.; Szymanska, M.; Meidan, R.; Mayr, D.; Mayerhofer, A. Sirtuin 1 and Sirtuin 3 in granulosa cell tumors. Int. J. Mol. Sci. 2021, 22, 2047. [Google Scholar] [CrossRef]
- Luo, L.-L.; Chen, X.-C.; Fu, Y.-C.; Xu, J.-J.; Li, L.; Lin, X.-H.; Xiang, Y.-F.; Zhang, X.-M. The effects of caloric restriction and a high-fat diet on ovarian lifespan and the expression of SIRT1 and SIRT6 proteins in rats. Aging Clin. Exp. Res. 2012, 24, 125–133. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takahashi, Y. The essential role of SIRT1 in hypothalamic-pituitary axis. Front. Endocrinol. 2018, 9, 605. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Alexa, R.; Dekanova, P.; Kadasi, A.; Stochmalova, A.; Grossmann, R.; Alwasel, S.H.; Harrath, A.H. The mTOR system can affect basic ovarian cell functions and mediate the effect of ovarian hormonal regulators. Int. J. Pharmacol. 2015, 11, 570–578. [Google Scholar] [CrossRef]
- Karolczak, K.; Watala, C. Estradiol as the Trigger of Sirtuin-1-Dependent Cell Signaling with a Potential Utility in Anti-Aging Therapies. Int. J. Mol. Sci. 2023, 24, 13753. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; He, H.; Jiang, X.; Hua, R.; Chen, H.; Yang, L.; Cheng, J.; Duan, J.; Li, Q. SIRT2 plays a novel role on progesterone, estradiol and testosterone synthesis via PPARs/LXRα pathways in bovine ovarian granular cells. J. Steroid Biochem. Mol. Biol. 2019, 185, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Mahfooz, S.; Maurya, V.K.; Kumar, V.; Basanna, C.S.; Kaur, G.; Hanif, K.; Jha, R.K. PARP1 during embryo implantation and its upregulation by oestradiol in mice. Reproduction 2014, 147, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Soni, U.K.; Chadchan, S.B.; Joshi, A.; Kumar, V.; Maurya, V.K.; Verma, R.K.; Jha, R.K. Poly (ADP-ribose) polymerase-2 is essential for endometrial receptivity and blastocyst implantation, and regulated by caspase-8. Mol. Cell. Endocrinol. 2020, 518, 110946. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Lin, H. Sirtuins in epigenetic regulation. Chem. Rev. 2015, 115, 2350–2375. [Google Scholar] [CrossRef] [PubMed]
- Rauh, D.; Fischer, F.; Gertz, M.; Lakshminarasimhan, M.; Bergbrede, T.; Aladini, F.; Kambach, C.; Becker, C.F.; Zerweck, J.; Schutkowski, M. An acetylome peptide microarray reveals specificities and deacetylation substrates for all human sirtuin isoforms. Nat. Commun. 2013, 4, 2327. [Google Scholar] [CrossRef]
- Pan, L.-Z.; Ahn, D.-G.; Sharif, T.; Clements, D.; Gujar, S.A.; Lee, P.W. The NAD+ synthesizing enzyme nicotinamide mononucleotide adenylyltransferase 2 (NMNAT-2) is a p53 downstream target. Cell Cycle 2014, 13, 1041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Glaría, E.; Valledor, A.F. Roles of CD38 in the Immune Response to Infection. Cells 2020, 9, 228. [Google Scholar] [CrossRef]
- Wang, R.-H.; Kim, H.-S.; Xiao, C.; Xu, X.; Gavrilova, O.; Deng, C.-X. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J. Clin. Investig. 2011, 121, 4477–4490. [Google Scholar] [CrossRef]
- Ahuja, N.; Schwer, B.; Carobbio, S.; Waltregny, D.; North, B.J.; Castronovo, V.; Maechler, P.; Verdin, E. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J. Biol. Chem. 2007, 282, 33583–33592. [Google Scholar] [CrossRef]
- Gulshan, M.; Yaku, K.; Okabe, K.; Mahmood, A.; Sasaki, T.; Yamamoto, M.; Hikosaka, K.; Usui, I.; Kitamura, T.; Tobe, K. Overexpression of Nmnat3 efficiently increases NAD and NGD levels and ameliorates age-associated insulin resistance. Aging Cell 2018, 17, e12798. [Google Scholar] [CrossRef]
- Stromsdorfer, K.L.; Yamaguchi, S.; Yoon, M.J.; Moseley, A.C.; Franczyk, M.P.; Kelly, S.C.; Qi, N.; Imai, S.-i.; Yoshino, J. NAMPT-mediated NAD+ biosynthesis in adipocytes regulates adipose tissue function and multi-organ insulin sensitivity in mice. Cell Rep. 2016, 16, 1851–1860. [Google Scholar] [CrossRef]
- Escande, C.; Nin, V.; Price, N.L.; Capellini, V.; Gomes, A.P.; Barbosa, M.T.; O’Neil, L.; White, T.A.; Sinclair, D.A.; Chini, E.N. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: Implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes 2013, 62, 1084–1093. [Google Scholar] [CrossRef]
- Hogan, K.A.; Chini, C.C.; Chini, E.N. The multi-faceted ecto-enzyme CD38: Roles in immunomodulation, cancer, aging, and metabolic diseases. Front. Immunol. 2019, 10, 1187. [Google Scholar] [CrossRef]
- Schilling, E.; Wehrhahn, J.; Klein, C.; Raulien, N.; Ceglarek, U.; Hauschildt, S. Inhibition of nicotinamide phosphoribosyltransferase modifies LPS-induced inflammatory responses of human monocytes. Innate Immun. 2012, 18, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, H.; Wang, H.; Chen, W.; Zeng, X.; Luo, X.; Xu, J.; Sun, Y. Deletion of enzymes for de novo NAD+ biosynthesis accelerated ovarian aging. Aging Cell 2023, 22, e13904. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, M.; Yu, L.; Wang, Y.; Yao, Y.; Wang, D. Niacin inhibits apoptosis and rescues premature ovarian failure. Cell. Physiol. Biochem. 2018, 50, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Almubarak, A.M.; Kim, E.; Yu, I.-J.; Jeon, Y. Supplementation with Niacin during in vitro maturation improves the quality of porcine embryos. Theriogenology 2021, 169, 36–46. [Google Scholar] [CrossRef]
- Kafi, M.; Ashrafi, M.; Azari, M.; Jandarroodi, B.; Abouhamzeh, B.; Asl, A.R. Niacin improves maturation and cryo-tolerance of bovine in vitro matured oocytes: An experimental study. Int. J. Reprod. BioMed. 2019, 17, 621. [Google Scholar] [CrossRef]
- Huang, P.; Zhou, Y.; Tang, W.; Ren, C.; Jiang, A.; Wang, X.; Qian, X.; Zhou, Z.; Gong, A. Long-term treatment of Nicotinamide mononucleotide improved age-related diminished ovary reserve through enhancing the mitophagy level of granulosa cells in mice. J. Nutr. Biochem. 2022, 101, 108911. [Google Scholar] [CrossRef]
- Bertoldo, M.; Uddin, G.; Youngson, N.; Agapiou, D.; Walters, K.; Sinclair, D.; Morris, M.; Gilchrist, R. Multigenerational obesity-induced perturbations in oocyte-secreted factor signalling can be ameliorated by exercise and nicotinamide mononucleotide. Hum. Reprod. Open 2018, 2018, hoy010. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Cui, Z.; Gao, Q.; Rui, R.; Xiong, B. Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes. Cell Rep. 2020, 32, 107987. [Google Scholar] [CrossRef] [PubMed]
- Pollard, C.-L.; Gibb, Z.; Hawdon, A.; Swegen, A.; Grupen, C.G. Supplementing media with NAD+ precursors enhances the in vitro maturation of porcine oocytes. J. Reprod. Dev. 2021, 67, 319–326. [Google Scholar] [CrossRef]
- Krisher, R.; Brad, A.; Herrick, J.; Sparman, M.; Swain, J. A comparative analysis of metabolism and viability in porcine oocytes during in vitro maturation. Anim. Reprod. Sci. 2007, 98, 72–96. [Google Scholar] [PubMed]
- Wen, J.; Wang, G.-L.; Yuan, H.-J.; Zhang, J.; Xie, H.-L.; Gong, S.; Han, X.; Tan, J.-H. Effects of glucose metabolism pathways on nuclear and cytoplasmic maturation of pig oocytes. Sci. Rep. 2020, 10, 2782. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chen, W.; Cong, L.; Wang, M.; Li, H.; Wang, H.; Luo, X.; Zhu, J.; Zeng, X.; Zhu, Z. NADase CD38 is a key determinant of ovarian aging. Nat. Aging 2024, 4, 110–128. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intake and its Panel on Folate, Other B Vitamins and Choline; The National Academies Collection: Reports funded by National Institutes of Health. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B(6), Folate, Vitamin B(12), Pantothenic Acid, Biotin, and Choline; National Academy of Sciences: Washington, DC, USA, 1998. [Google Scholar]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.; Riaz, U.; Lv, H.; Yang, L. A Molecular Perspective and Role of NAD+ in Ovarian Aging. Int. J. Mol. Sci. 2024, 25, 4680. https://doi.org/10.3390/ijms25094680
Ahmed M, Riaz U, Lv H, Yang L. A Molecular Perspective and Role of NAD+ in Ovarian Aging. International Journal of Molecular Sciences. 2024; 25(9):4680. https://doi.org/10.3390/ijms25094680
Chicago/Turabian StyleAhmed, Mehboob, Umair Riaz, Haimiao Lv, and Liguo Yang. 2024. "A Molecular Perspective and Role of NAD+ in Ovarian Aging" International Journal of Molecular Sciences 25, no. 9: 4680. https://doi.org/10.3390/ijms25094680
APA StyleAhmed, M., Riaz, U., Lv, H., & Yang, L. (2024). A Molecular Perspective and Role of NAD+ in Ovarian Aging. International Journal of Molecular Sciences, 25(9), 4680. https://doi.org/10.3390/ijms25094680





