Biominerals and Bioinspired Materials in Biosensing: Recent Advancements and Applications
Abstract
1. Introduction
2. Silica Biominerals and Their Biomimetic Analogues for Biosensing Applications
2.1. Biomineralized Silica Structures as Platforms for Biosensors
| Mineral Name | Sensor Type | Sensor Platform | Receptor | Target | Limit of Detection | Reference |
|---|---|---|---|---|---|---|
| Silica | Immunosensor | Diatom biosilica frustules | sdAb against Bacillus anthracis S Layer protein EA1 | Bacillus anthracis | ND | [40] |
| Photoluminescence immunosensor | Diatom biosilica functionalized with polydopamine and AuNPs | Goat anti-rabbit IgG | Rabbit IgG | 8 × 10−9 mg mL−1 | [41] | |
| Immunoassay (SERS) | Diatom biosilica with self-assembled plasmonic nanoparticles | Goat anti-mouse IgG | Mouse IgG | 10 pg mL−1 | [42] | |
| Immunoassay (fluorescence spectroscopy and imaging) | Diatom biosilica | Antibody against mouse IgG | Mouse IgG | 140 fg mL−1 | [43] | |
| Optical biosensor | Biomimetic silica nanoparticles | Intracellular enzyme system from Raoultella planticola | Nitrite | 0.25 mg L−1 | [44] | |
| Amperometric biosensor | Graphite rod electrode | Biosilica-entrapped GOx | Glucose | 0.67 mM | [45] | |
| Amperometric biosensor | GCE | Biosilica-entrapped mSOx | Sarcosine | 340 μM | [46] | |
| Amperometric biosensor | Gold nanoparticles | Biosilica-entrapped AChE | Acetylcholine | ND | [47] | |
| Calcium carbonate | Electrochemical biosensor | Au-nanoparticle-coated chicken eggshell composite (Au/CaCO3 nanocomposite) | N/A | 4-Nitrophenol | 0.54 nM | [48] |
| Electrochemiluminescence immunosensor | Au/CaCO3 nanocomposite (eggshell template) | Nanobody (conjugated to Au/CaCO3) | Ochratoxin A | 5.7 pg mL−1 | [49] | |
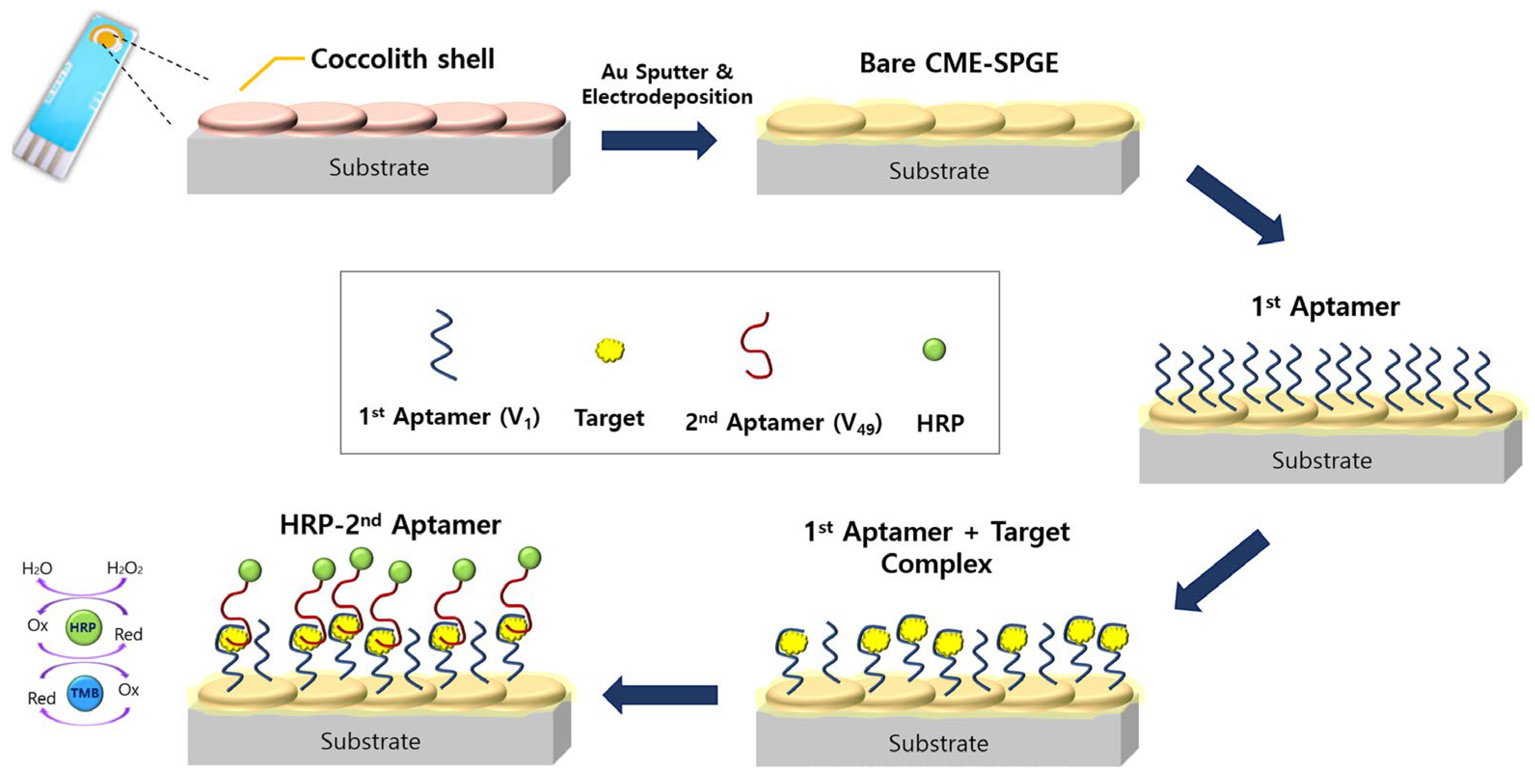
| Electrochemical aptasensor (sandwich-type) | Coccolith-modified screen-printed gold electrode | Cognate pair of aptamers | Vaspin (type 2 diabetes biomarker) | 298 pM | [50] | |
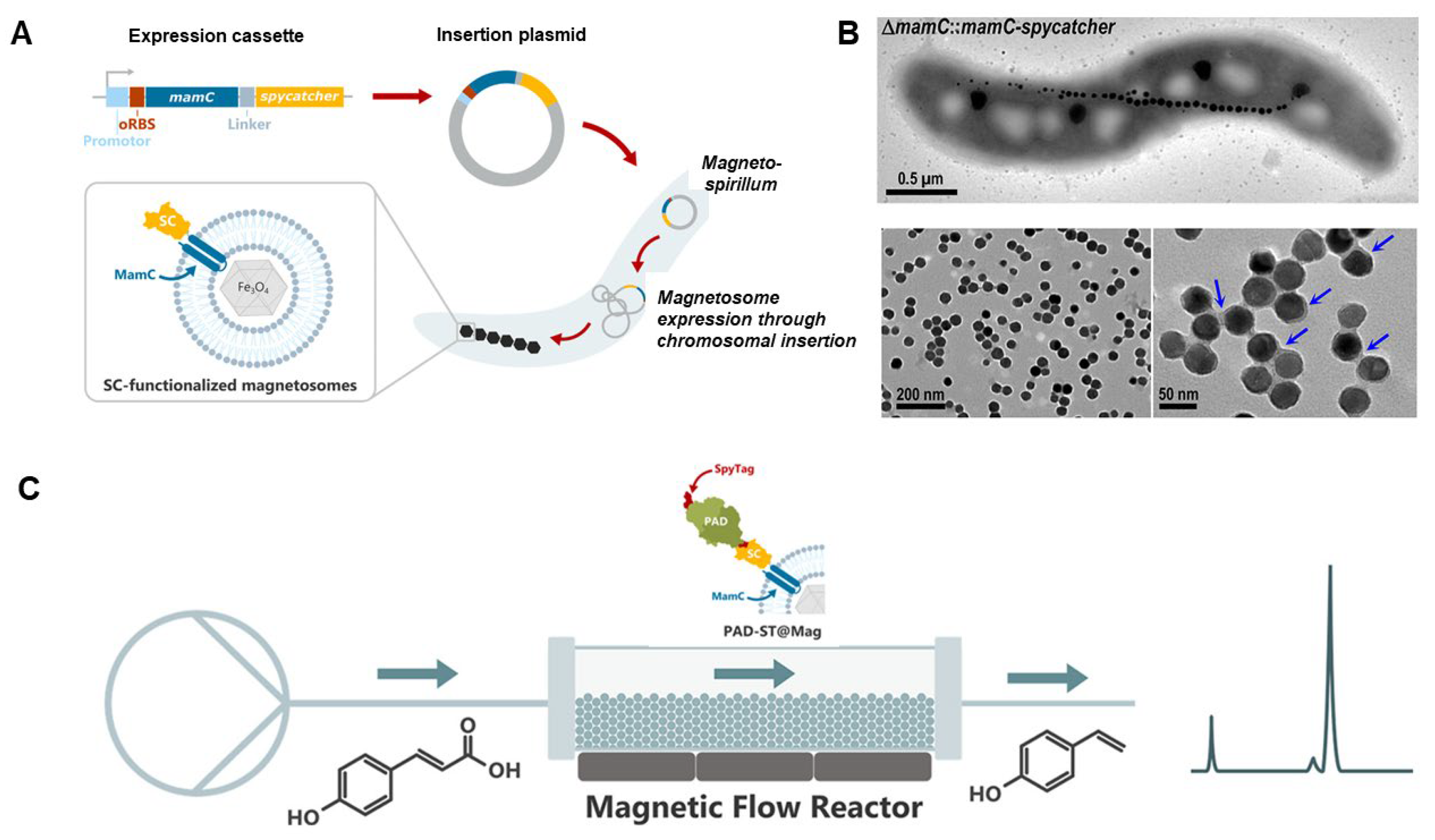
| Magnetosome | Whole-cell magnetic biosensor | Magnetotactic bacteria | Arsenic-inducible promoters | Arsenite | 10 nM | [51] |
| Magnetic ELISA | Magnetosome–nanobody complex | Anti-TBBPA nanobody | TBBPA | 0.025 ng mL−1 | [52] | |
| Magnetic ELISA | Magnetosome–nanobody complex | Anti-fipronil nanobody (requires oxidation for binding) | Fipronil (insecticide) | 2.74 ng mL−1 | [53] | |
| Magnetic immunoassay | BMPs modified with streptavidin | Biotinylated anti-3-PBA Nb2 | 3-PBA | 0.03 ng mL−1 | [54] | |
| ELISA/magneto-impedance spectroscopy | Magnetosome–antibody complex | Anti-Salmonella antibody | Salmonella typhimurium | 101 CFU mL−1 | [55] | |
| ELISA/magneto-impedance spectroscopy | Magnetosome–antibody complex | Anti-listeriolysin antibody | Listeria monocytogenes | 101 CFU mL−1 | [56] | |
| Magnetic concentration/qPCR detection | Biomimetic magnetic nanoparticles (BMNPs) synthesized with MamC protein | N/A (direct electrostatic interaction) | Staphylococcus aureus | 10 CFU mL−1 | [57] | |
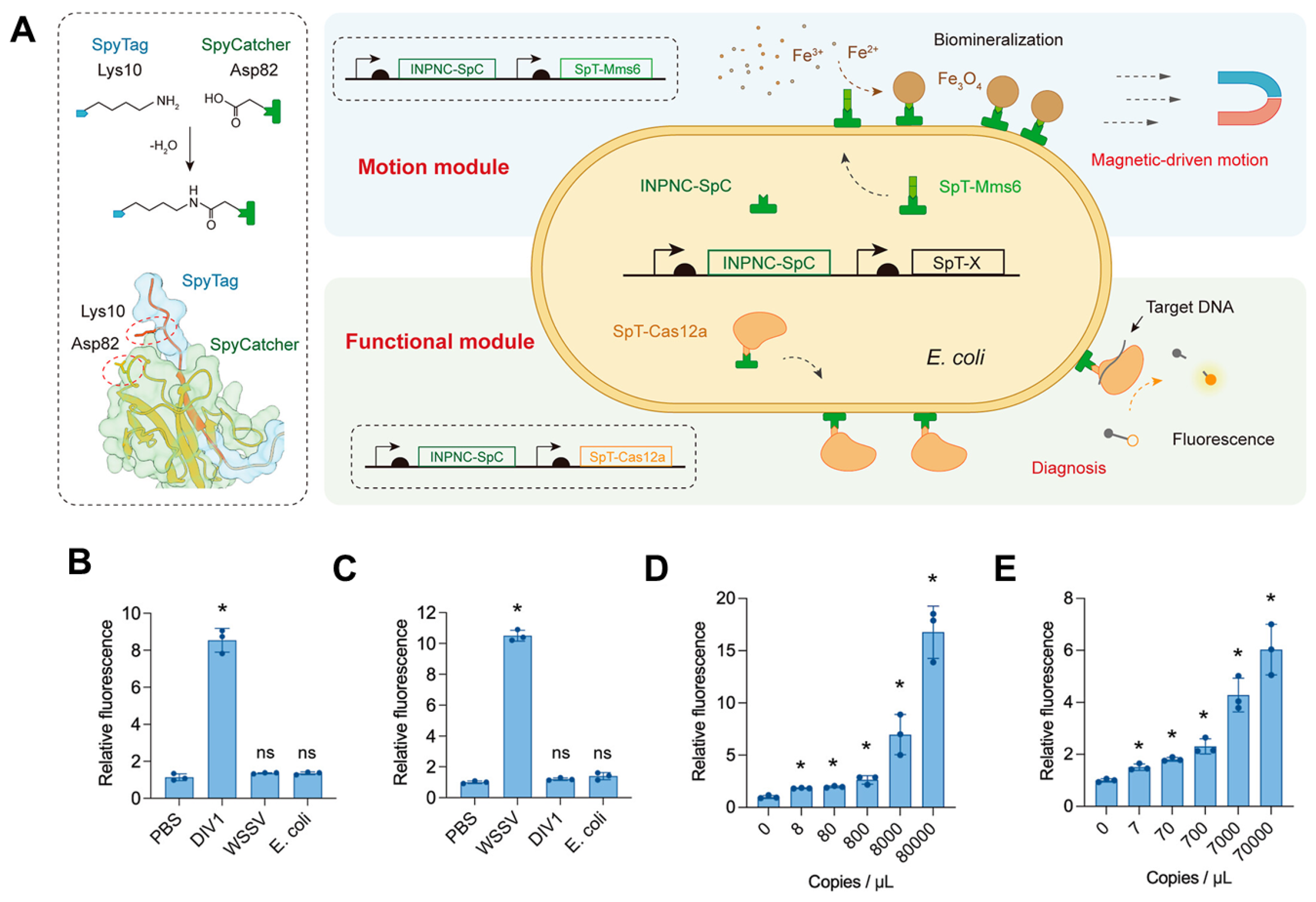
| Nucleic acid detection | Magnetically driven bacterial microrobot | Cas12a protein | Decapod iridescent virus 1 (DIV1) | 8 copies μL−1 | [58] | |
| Nucleic acid detection | Magnetically driven bacterial microrobot | Cas12a protein | White spot syndrome virus (WSSV) | 7 copies μL−1 | [58] | |
| Hydroxyapatite | Electrochemical aptasensor (sandwich-type) | GCE | Anti-PDGF-BB aptamer-modified HAP NPs | PDGF-BB | 50 fg mL−1 | [59] |
| Electrochemical biosensor | GCE/HAp/rGO/AuNPs | Uricase enzyme | Uric acid | 3.9 × 10−6 M | [60] | |
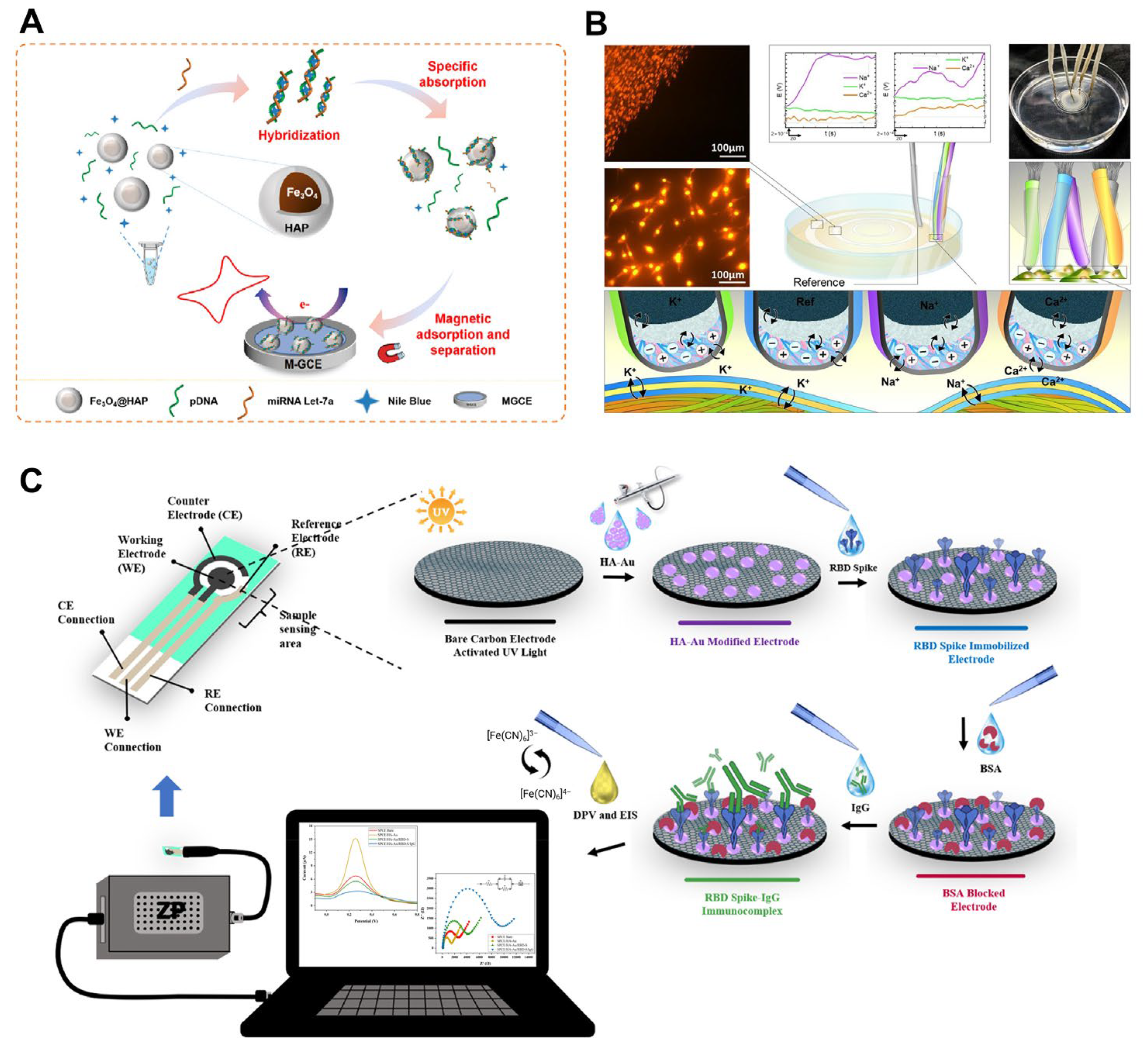
| Electrochemical (label-free) | Magnetic GCE | Fe3O4@HAP | miRNA let-7a | 0.051 fM | [61] | |
| Electrochemical immunosensor | HAP-Au/SPCE | Receptor binding domain of spike protein | IgG antibodies against SARS-CoV-2 | 0.0561 pg mL–1 | [62] | |
| Electrochemical aptasensor (sandwich-type) | GCE/graphene | MNP-TBA1/HAP-TBA2 | Thrombin | 0.03 fM | [63] |
2.2. Biomimetic Silica for Biosensing Platforms
3. Calcium Carbonate Biominerals and Their Biomimetic Analogues for Biosensing Applications
3.1. Biomineralized Calcium Carbonate Structures as Platforms for Biosensors
3.2. Biomimetic Calcium Carbonate for Biosensing Platforms
4. Magnetosome Biominerals and Their Biomimetic Analogues for Biosensing Applications
4.1. Biomineralized Magnetosome Structures as Platforms for Biosensors
4.2. Biomimetic Magnetosomes for Biosensing Platforms
5. Hydroxyapatite Biominerals and Their Biomimetic Analogues for Biosensing Applications
6. Bioinspired Minerals and Materials as Platforms for Biosensors
| Mineral/Material | Sensor Type | Sensor Platform | Receptor | Target | Limit of Detection | Reference |
|---|---|---|---|---|---|---|
| Gold (peptide-templated | Electrochemical | Gr/GO-SH/AuNs | Cholesterol oxidase | Cholesterol | 0.2 nM | [113] |
| Gold (peptide-templated | Fluorescence | Peptide–gold nanocluster/graphene nanocomplex | Peptide (specific to the MMP-9 cleavage site) | MMP-9 | 0.15 nM | [114] |
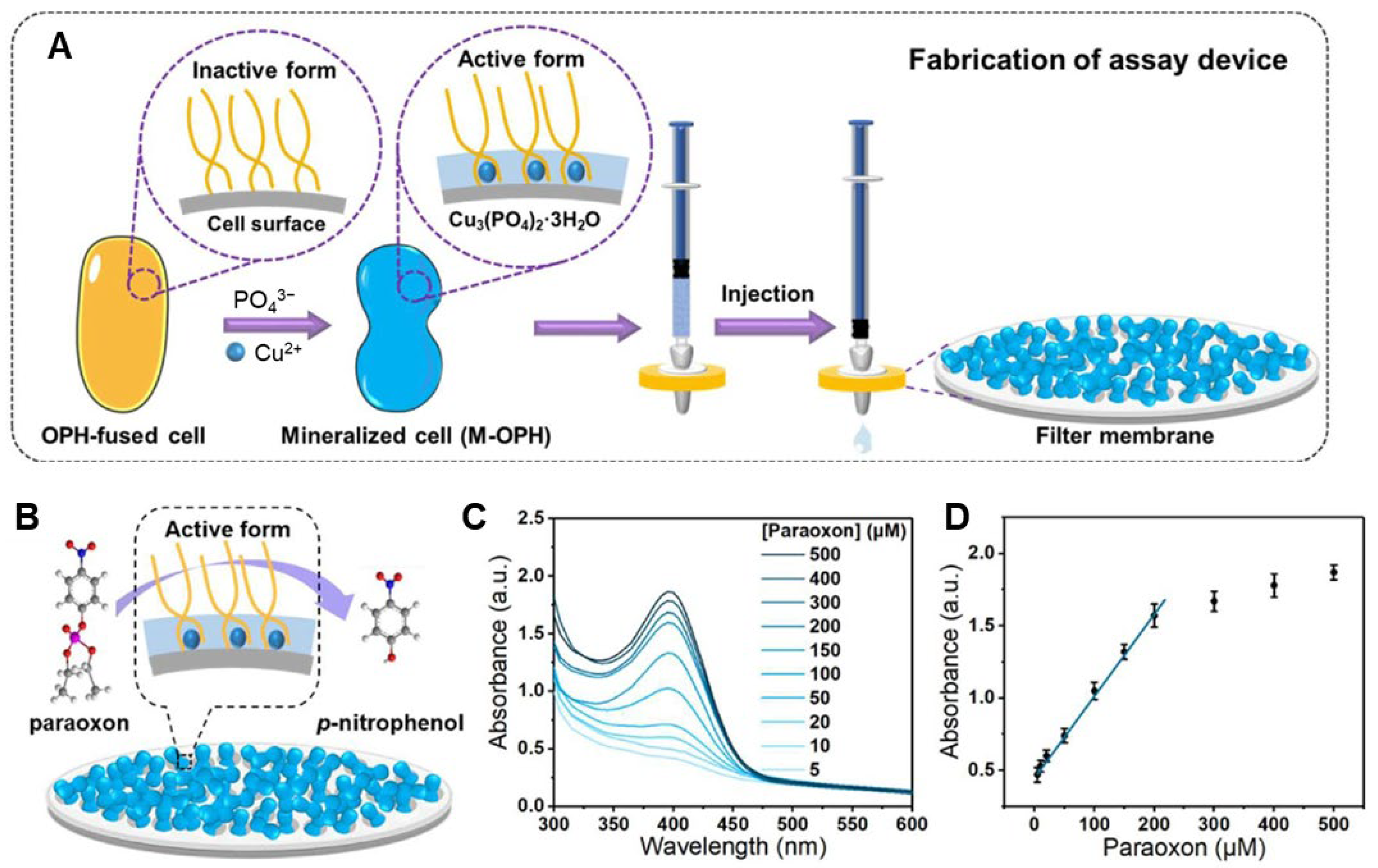
| Cobalt phosphate crystals | Enzymatic | Biomineralized OPH-fused bacteria | OPH enzyme | Paraoxon | 0.08 μM | [117] |
| Fe-MOF | Optical (colorimetric) | Biomimetic mineralized HRP@Fe-MOF | Aptamer (specific to Aβ oligomer) | Aβ oligomer (associated with Alzheimer’s disease) | 0.03 pM | [118] |
| Mineralized MOF | Electrochemical (competitive immunoassay) | Nanobody conjugated with biomimetic mineralized MOF | Nanobody (specific to Aflatoxin B1) | Aflatoxin B1 | 20.0 fg mL−1 | [119] |
| Cu3(PO4)2 | Electrochemical aptasensor | Cu3(PO4)2 nanoflowers/GO | Aptamer (specific to the carcinoembryonic antigen) | Carcinoembryonic antigen | 2.4 fg mL−1 | [120] |
| Ca3(PO4)2 | Colorimetric (potentially) | Paper-based with PAL@Ca3(PO4)2 hybrid nanoflowers | PAL | Phenylalanine | 60 μM | [121] |
| Cu3(PO4)2 | Colorimetric (smartphone-assisted) | Laccase–mineral hybrid microflowers | Laccase enzyme | Epinephrine | 0.6 μM | [122] |
7. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Bhatia, D.; Paul, S.; Acharjee, T.; Ramachairy, S.S. Biosensors and their widespread impact on human health. Sens. Int. 2024, 5, 100257. [Google Scholar] [CrossRef]
- Thakur, A.; Assad, H.; Kaya, S.; Kumar, A. Recent advancements in biosensing and biosensors. In Handbook of Biomolecules; Elsevier: Amsterdam, The Netherlands, 2023; pp. 619–631. [Google Scholar]
- Simkiss, K.; Wilbur, K.M. Biomineralization; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Abdelhamid, M.A.; Pack, S.P. Biomimetic and bioinspired silicifications: Recent advances for biomaterial design and applications. Acta Biomater. 2021, 120, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, C.; Langer, G.; Wheeler, G.L. Coccolithophore calcification: Changing paradigms in changing oceans. Acta Biomater. 2021, 120, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A. Mechanisms of molluscan shell formation. In Calcification in Biological Systems; CRC Press: Boca Raton, FL, USA, 2020; pp. 179–216. [Google Scholar]
- Liu, P.; Zheng, Y.; Zhang, R.; Bai, J.; Zhu, K.; Benzerara, K.; Menguy, N.; Zhao, X.; Roberts, A.P.; Pan, Y. Key gene networks that control magnetosome biomineralization in magnetotactic bacteria. Natl. Sci. Rev. 2023, 10, nwac238. [Google Scholar] [CrossRef] [PubMed]
- Kareem, R.O.; Bulut, N.; Kaygili, O. Hydroxyapatite biomaterials: A comprehensive review of their properties, structures, medical applications, and fabrication methods. J. Chem. Rev. 2024, 6, 1–26. [Google Scholar]
- Yan, T.; Zhang, S.; Yang, Y.; Li, Y.; Xu, L.-P. Biomineralization-inspired magnetic nanoflowers for sensitive miRNA detection based on exonuclease-assisted target recycling amplification. Microchim. Acta 2022, 189, 260. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, Z.; Zhuo, H.; Zhong, C.; Shi, G.; Liu, T.; Huang, X.; Zhong, L.; Peng, X. Ultra-strong and transparent biomimetic nanocomposite through orientation effects and in situ biomineralization. Adv. Funct. Mater. 2024, 34, 2310094. [Google Scholar] [CrossRef]
- Li, H.; Geng, W.; Qi, Z.; Ahmad, W.; Haruna, S.A.; Chen, Q. Stimuli-responsive SERS biosensor for ultrasensitive tetracycline sensing using EDTA-driven PEI@CaCO3 microcapsule and CS@FeMMs. Biosens. Bioelectron. 2023, 226, 115122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, Y.; Wang, P.; Wu, D.; Liu, J.; Huang, R.; Wu, Y.; Li, G. Biomineralization-inspired artificial clickase for portable click SERS immunoassay of Salmonella enterica serovar Paratyphi B in foods. Food Chem. 2023, 413, 135553. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Gao, B.; Zhang, F.; Sun, X.; Zhang, Y.; Li, Z. A novel electrochemical immunosensor based on magnetosomes for detection of staphylococcal enterotoxin B in milk. Talanta 2013, 106, 360–366. [Google Scholar] [CrossRef] [PubMed]
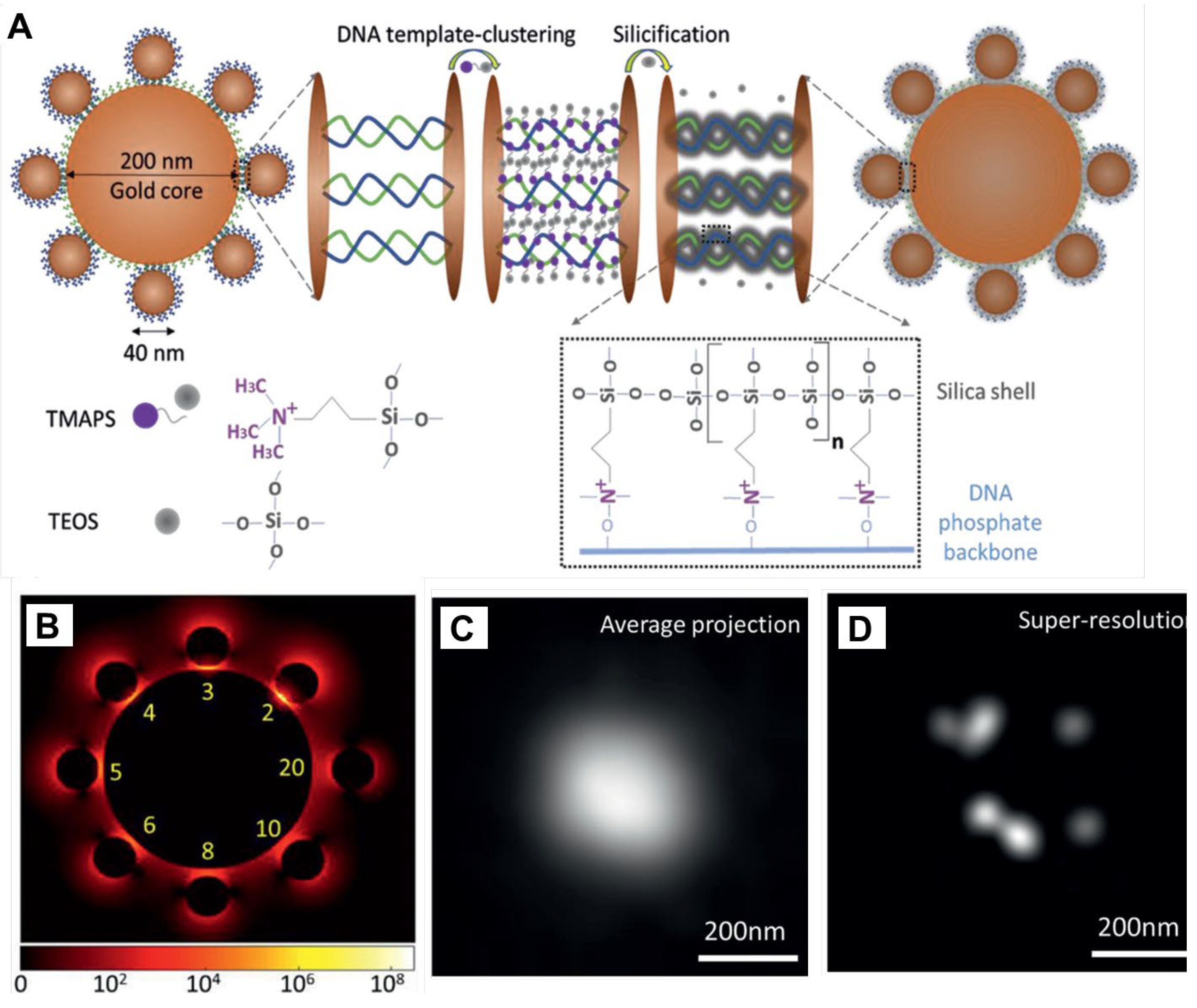
- Liang, L.; Zheng, P.; Zhang, C.; Barman, I. A programmable DNA-silicification-based nanocavity for single-molecule plasmonic sensing. Adv. Mater. 2021, 33, 2005133. [Google Scholar] [CrossRef] [PubMed]
- Selvi, S.V.; Prasannan, A.; Yu, H.; Lincy, V.; Hong, P.-D. Bio-mineralized tin/bismuth oxide nanoparticles with silk fibroins for efficient electrochemical detection of 2-nitroaniline in river water samples. Environ. Res. 2023, 221, 115285. [Google Scholar] [CrossRef] [PubMed]
- Le, L.T.P.; Nguyen, A.H.Q.; Phan, L.M.T.; Ngo, H.T.T.; Wang, X.; Cunningham, B.; Valera, E.; Bashir, R.; Taylor-Robinson, A.W.; Do, C.D. Current smartphone-assisted point-of-care cancer detection: Towards supporting personalized cancer monitoring. TrAC Trends Anal. Chem. 2024, 174, 117681. [Google Scholar] [CrossRef]
- Kou, X.; Tong, L.; Shen, Y.; Zhu, W.; Yin, L.; Huang, S.; Zhu, F.; Chen, G.; Ouyang, G. Smartphone-assisted robust enzymes@MOFs-based paper biosensor for point-of-care detection. Biosens. Bioelectron. 2020, 156, 112095. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.C.; Keeling-Tucker, T. Biosilicification: The role of the organic matrix in structure control. JBIC J. Biol. Inorg. Chem. 2000, 5, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.C.; Morse, D.E. Molecular biology of demosponge axial filaments and their roles in biosilicification. Microsc. Res. Tech. 2003, 62, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.; Davis, A. Heart of glass: Spicule armament and physical defense in temperate reef sponges. Mar. Ecol. Prog. Ser. 2008, 372, 77–86. [Google Scholar] [CrossRef][Green Version]
- Shimizu, K.; Nishi, M.; Sakate, Y.; Kawanami, H.; Bito, T.; Arima, J.; Leria, L.; Maldonado, M. Silica-associated proteins from hexactinellid sponges support an alternative evolutionary scenario for biomineralization in Porifera. Nat. Commun. 2024, 15, 181. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.-R.; Park, K.S.; Abdelhamid, M.A.; Pack, S.P. Novel silicatein-like protein for biosilica production from Amphimedon queenslandica and its use in osteogenic composite fabrication. Korean J. Chem. Eng. 2023, 40, 419–428. [Google Scholar] [CrossRef]
- Shimizu, K.; Cha, J.; Stucky, G.D.; Morse, D.E. Silicatein α: Cathepsin L-like protein in sponge biosilica. Proc. Natl. Acad. Sci. USA 1998, 95, 6234–6238. [Google Scholar] [CrossRef] [PubMed]
- Nishi, M.; Kobayashi, H.; Amano, T.; Sakate, Y.; Bito, T.; Arima, J.; Shimizu, K. Identification of the domains involved in promotion of silica formation in glassin, a protein occluded in hexactinellid sponge biosilica, for development of a tag for purification and immobilization of recombinant proteins. Mar. Biotechnol. 2020, 22, 739–747. [Google Scholar] [CrossRef] [PubMed]
- De Tommasi, E.; Gielis, J.; Rogato, A. Diatom frustule morphogenesis and function: A multidisciplinary survey. Mar. Genom. 2017, 35, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Otzen, D. The role of proteins in biosilicification. Scientifica 2012, 2012, 867562. [Google Scholar] [CrossRef] [PubMed]
- Kroger, N.; Deutzmann, R.; Sumper, M. Silica-precipitating peptides from diatoms: The chemical structure of silaffin-1A from Cylindrotheca fusiformis. J. Biol. Chem. 2001, 276, 26066–26070. [Google Scholar] [CrossRef] [PubMed]
- Richthammer, P.; Börmel, M.; Brunner, E.; van Pée, K.H. Biomineralization in diatoms: The role of silacidins. ChemBioChem 2011, 12, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.A.A.; Meligy, A.M.A.; Yeo, K.B.; Lee, C.-S.; Pack, S.P. Silaffin-3-derived pentalysine cluster as a new fusion tag for one-step immobilization and purification of recombinant Bacillus subtilis catalase on bare silica particles. Int. J. Biol. Macromol. 2020, 159, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.A.A.; Son, R.G.; Park, K.S.; Pack, S.P. Oriented multivalent silaffin-affinity immobilization of recombinant lipase on diatom surface: Reliable loading and high performance of biocatalyst. Colloids Surf. B Biointerfaces 2022, 219, 112830. [Google Scholar] [CrossRef] [PubMed]
- Wenzl, S.; Hett, R.; Richthammer, P.; Sumper, M. Silacidins: Highly acidic phosphopeptides from diatom shells assist in silica precipitation In Vitro. Angew. Chem. Int. Ed. 2008, 47, 1729–1732. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.A.; Yeo, K.B.; Ki, M.-R.; Pack, S.P. Self-encapsulation and controlled release of recombinant proteins using novel silica-forming peptides as fusion linkers. Int. J. Biol. Macromol. 2019, 125, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Ghobara, M.; El-Sheekh, M.; Hamed, A.F.; Abdelhamid, M.A.; Pack, S.P. Diatom nanostructured biosilica. In Value-Added Products from Algae: Phycochemical Production and Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 461–492. [Google Scholar]
- Dhanker, R.; Singh, P.; Sharma, D.; Tyagi, P.; Kumar, M.; Singh, R.; Prakash, S. Diatom silica a potential tool as biosensors and for biomedical field. In Insights into the World of Diatoms: From Essentials to Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 175–193. [Google Scholar]
- Panwar, V.; Dutta, T. Diatom biogenic silica as a felicitous platform for biochemical engineering: Expanding frontiers. ACS Appl. Bio Mater. 2019, 2, 2295–2316. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.; De Stefano, L. Recent advances on diatom-based biosensors. Sensors 2019, 19, 5208. [Google Scholar] [CrossRef] [PubMed]
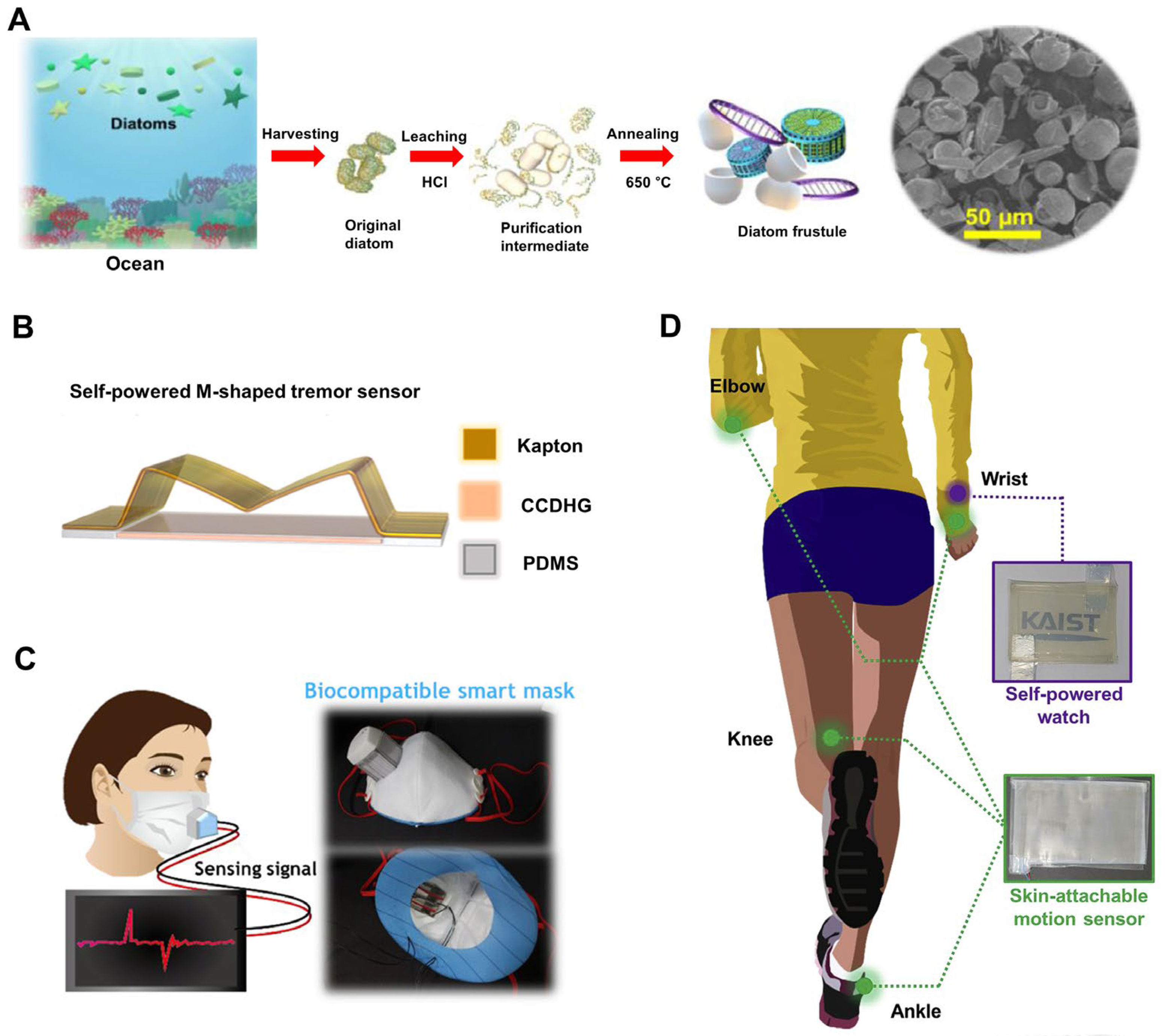
- Kim, J.-N.; Lee, J.; Lee, H.; Oh, I.-K. Stretchable and self-healable catechol-chitosan-diatom hydrogel for triboelectric generator and self-powered tremor sensor targeting at Parkinson disease. Nano Energy 2021, 82, 105705. [Google Scholar] [CrossRef]
- Rajabi-Abhari, A.; Kim, J.-N.; Lee, J.; Tabassian, R.; Mahato, M.; Youn, H.J.; Lee, H.; Oh, I.-K. Diatom bio-silica and cellulose nanofibril for bio-triboelectric nanogenerators and self-powered breath monitoring masks. ACS Appl. Mater. Interfaces 2021, 13, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-N.; Lee, J.; Go, T.W.; Rajabi-Abhari, A.; Mahato, M.; Park, J.Y.; Lee, H.; Oh, I.-K. Skin-attachable and biofriendly chitosan-diatom triboelectric nanogenerator. Nano Energy 2020, 75, 104904. [Google Scholar] [CrossRef]
- Ford, N.R.; Xiong, Y.; Hecht, K.A.; Squier, T.C.; Rorrer, G.L.; Roesijadi, G. Optimizing the design of diatom biosilica-targeted fusion proteins in biosensor construction for Bacillus anthracis detection. Biology 2020, 9, 14. [Google Scholar] [CrossRef]
- Chen, T.; Wu, F.; Li, Y.; Rozan, H.E.; Chen, X.; Feng, C. Gold nanoparticle-functionalized diatom biosilica as label-free biosensor for biomolecule detection. Front. Bioeng. Biotechnol. 2022, 10, 894636. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhen, L.; Ren, F.; Campbell, J.; Rorrer, G.L.; Wang, A.X. Ultra-sensitive immunoassay biosensors using hybrid plasmonic-biosilica nanostructured materials. J. Biophotonics 2015, 8, 659–667. [Google Scholar] [CrossRef]
- Squire, K.; Kong, X.; LeDuff, P.; Rorrer, G.L.; Wang, A.X. Photonic crystal enhanced fluorescence immunoassay on diatom biosilica. J. Biophotonics 2018, 11, e201800009. [Google Scholar] [CrossRef] [PubMed]
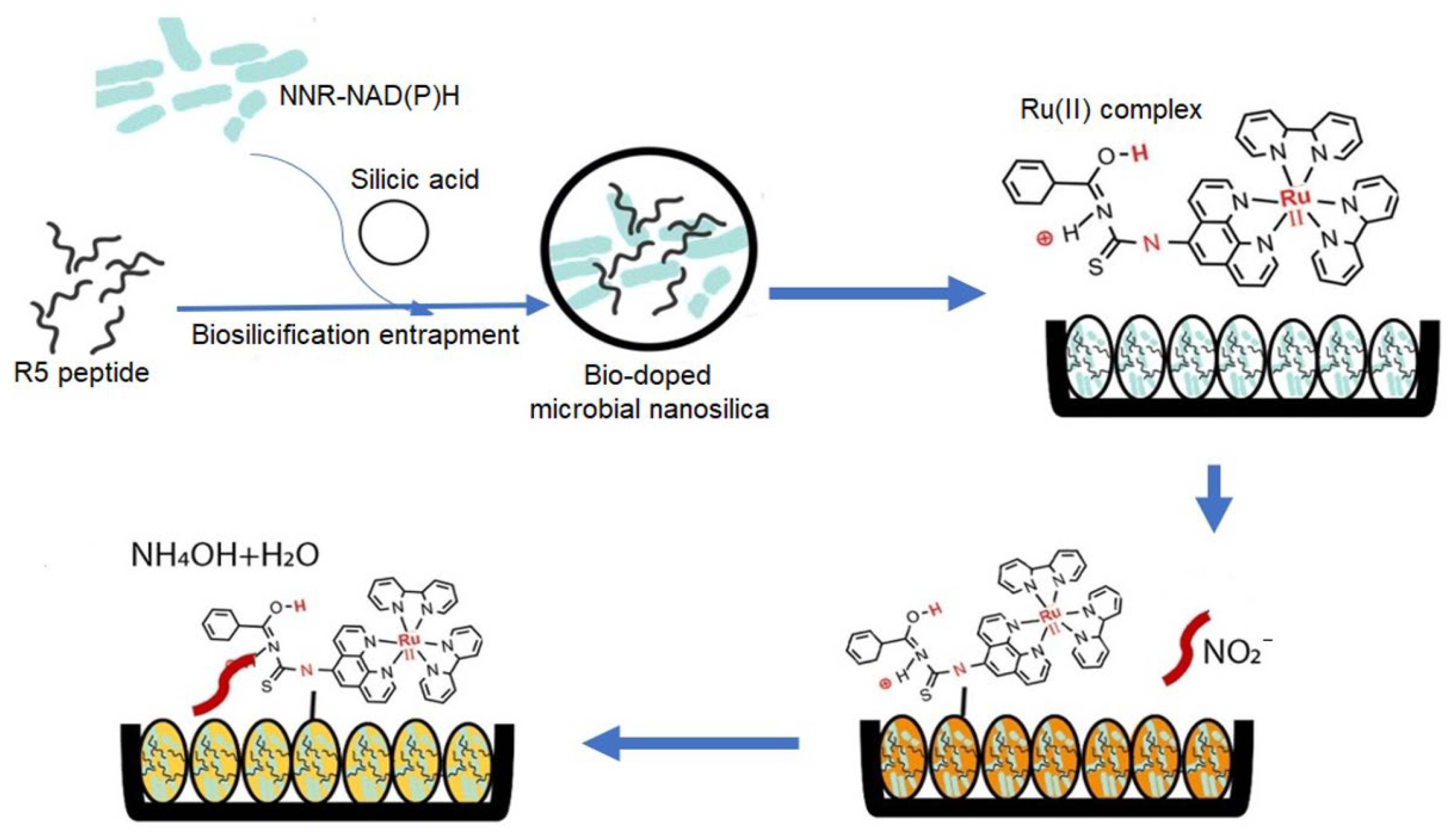
- Mohd Zuki, S.N.S.; Goh, C.T.; Kassim, M.B.; Tan, L.L. Bio-doped microbial nanosilica as optosensing biomaterial for visual quantitation of nitrite in cured meats. Biosensors 2022, 12, 388. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Kim, B.-C.; An, J.-H.; Min, K.; Kim, Y.H.; Um, Y.; Oh, M.-K.; Sang, B.-I. A biosensor based on the self-entrapment of glucose oxidase within biomimetic silica nanoparticles induced by a fusion enzyme. Enzym. Microb. Technol. 2011, 49, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hall, E.A. A biosilification fusion protein for a ‘self-immobilising’sarcosine oxidase amperometric enzyme biosensor. Electroanalysis 2020, 32, 874–884. [Google Scholar] [CrossRef]
- Buiculescu, R.; Chaniotakis, N.A. The stabilization of Au NP–AChE nanocomposites by biosilica encapsulation for the development of a thiocholine biosensor. Bioelectrochemistry 2012, 86, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Kang, Z.; Cao, L.; Lin, M.; Lin, H.; Yang, D.-P. Conversion of waste eggshell into difunctional Au/CaCO3 nanocomposite for 4-Nitrophenol electrochemical detection and catalytic reduction. Appl. Surf. Sci. 2020, 510, 145526. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; He, S.; Cao, H.; Su, B.; Huang, T.; Chen, Q.; Liu, M.; Yang, D.-P. Electrochemiluminescence immunosensor based on nanobody and Au/CaCO3 synthesized using waste eggshells for ultrasensitive detection of ochratoxin A. ACS Omega 2021, 6, 30148–30156. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Nam, O.; Jin, E.; Gu, M.B. A new coccolith modified electrode-based biosensor using a cognate pair of aptamers with sandwich-type binding. Biosens. Bioelectron. 2019, 123, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Dieudonné, A.; Prévéral, S.; Pignol, D. A sensitive magnetic arsenite-specific biosensor hosted in magnetotactic bacteria. Appl. Environ. Microbiol. 2020, 86, e00803–e00820. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tian, J.; Xue, X.; Ma, F.; Li, Q.X.; Morisseau, C.; Hammock, B.D.; Xu, T. Biosynthesis of magnetosome-nanobody complex in Magnetospirillum gryphiswaldense MSR-1 and a magnetosome-nanobody-based enzyme-linked immunosorbent assay for the detection of tetrabromobisphenol A in water. Anal. Bioanal. Chem. 2024, 416, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ma, F.; He, J.; Li, Q.X.; Hammock, B.D.; Tian, J.; Xu, T. Fusion expression of nanobodies specific for the insecticide fipronil on magnetosomes in Magnetospirillum gryphiswaldense MSR-1. J. Nanobiotechnology 2021, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Wang, Y.; Wang, D.; Yang, Y.; Chang, J.; Sun, S.; Gu, S.; He, J. Streptavidin-biotin system-mediated immobilization of bivalent nanobody onto magnetosome for separation and analysis of 3-phenoxybenzoic acid in urine. Anal. Methods 2024, 16, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, S.; Arumugasamy, S.K.; Mathiyarasu, J.; Suthindhiran, K. Magnetosome-anti-Salmonella antibody complex based biosensor for the detection of Salmonella typhimurium. Mater. Sci. Eng. C 2020, 114, 111071. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, S.; Arumugasamy, S.K.; Mathiyarasu, J.; Suthindhiran, K. Development of magnetosomes-based biosensor for the detection of Listeria monocytogenes from food sample. IET Nanobiotechnology 2020, 14, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Carretero, M.; Rodríguez-López, J.; Ropero-Moreno, C.; Granada, J.; Delgado-Martín, J.; Martinez-Bueno, M.; Fernandez-Vivas, A.; Jimenez-Lopez, C. Biomimetic magnetic nanoparticles for bacterial magnetic concentration in liquids and qPCR-detection. Food Control 2023, 147, 109623. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, T.; Li, S.; Feng, J.; Li, W.; Li, L.; Zhou, X.; Wang, M.; Li, F.; Zhao, X.; et al. Living magnetotactic microrobots based on bacteria with a surface-displayed CRISPR/Cas12a system for Penaeus viruses detection. ACS Appl. Mater. Interfaces 2023, 15, 47930–47938. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Tian, D.; Zhang, L.; Guo, Q.; Cui, Y.; Yang, M. Dual signal amplification strategy for amperometric aptasensing using hydroxyapatite nanoparticles: Application to the sensitive detection of the cancer biomarker platelet-derived growth factor BB. Microchim. Acta 2017, 184, 4375–4381. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, W.; Ma, J.; Ruan, F.; Qi, X.; Cai, Y. Potential of a sensitive uric acid biosensor fabricated using hydroxyapatite nanowire/reduced graphene oxide/gold nanoparticle. Microsc. Res. Tech. 2020, 83, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ai, Y.; Yang, W.; Niu, L.; Wang, Q.; Gao, F. Specific adsorption of magnetic Fe3O4@hydroxyapatite nanocomposites to a hybridized duplex for immobilization and label-free electrochemical biosensing of MicroRNA. ACS Appl. Nano Mater. 2023, 6, 8584–8592. [Google Scholar] [CrossRef]
- Syafira, R.S.; Devi, M.J.; Gaffar, S.; Irkham; Kurnia, I.; Arnafia, W.; Einaga, Y.; Syakir, N.; Noviyanti, A.R.; Hartati, Y.W. Hydroxyapatite-gold modified screen-printed carbon electrode for selective SARS-CoV-2 antibody immunosensor. ACS Appl. Bio Mater. 2024, 7, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. Ultrasensitive label-free homogeneous electrochemical aptasensor based on sandwich structure for thrombin detection. Sens. Actuators B Chem. 2018, 267, 412–418. [Google Scholar] [CrossRef]
- Squire, K.J.; Zhao, Y.; Tan, A.; Sivashanmugan, K.; Kraai, J.A.; Rorrer, G.L.; Wang, A.X. Photonic crystal-enhanced fluorescence imaging immunoassay for cardiovascular disease biomarker screening with machine learning analysis. Sens. Actuators B Chem. 2019, 290, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.-R.; Nguyen, T.K.M.; Park, T.-I.; Park, H.-M.; Pack, S.P. Biomimetic Silica Particles with Self-Loading BMP-2 Knuckle Epitope Peptide and Its Delivery for Bone Regeneration. Pharmaceutics 2023, 15, 1061. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Shin, J.W.; Ki, M.-R.; Kim, S.H.; Kim, K.H.; Pack, S.P. Bio-inspired formation of silica particles using the silica-forming peptides found by silica-binding motif sequence, RRSSGGRR. Process Biochem. 2021, 111, 262–269. [Google Scholar] [CrossRef]
- Ki, M.-R.; Kim, S.H.; Rho, S.; Kim, J.K.; Min, K.H.; Yeo, K.B.; Lee, J.; Lee, G.; Jun, S.-H.; Pack, S.P. Use of biosilica to improve loading and delivery of bone morphogenic protein 2. Int. J. Biol. Macromol. 2024, 254, 127876. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.-R.; Kim, S.H.; Park, T.I.; Pack, S.P. Self-entrapment of antimicrobial peptides in silica particles for stable and effective antimicrobial peptide delivery system. Int. J. Mol. Sci. 2023, 24, 16423. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.M.; Ki, M.R.; Lee, C.S.; Pack, S.P. Nanosized and tunable design of biosilica particles using novel silica-forming peptide-modified chimeric ferritin templates. J. Ind. Eng. Chem. 2019, 73, 198–204. [Google Scholar] [CrossRef]
- Nam, D.H.; Lee, J.-O.; Sang, B.-I.; Won, K.; Kim, Y.H. Silaffin peptides as a novel signal enhancer for gravimetric biosensors. Appl. Biochem. Biotechnol. 2013, 170, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Acar, H.; Erkal, T.S.; Bayindir, M.; Guler, M.O. Template-directed synthesis of silica nanotubes for explosive detection. ACS Appl. Mater. Interfaces 2011, 3, 4159–4164. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.S. Molecular mechanisms of biomineralization in marine invertebrates. J. Exp. Biol. 2020, 223, jeb206961. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Morales, J.; Falini, G.; García-Ruiz, J.M. Biological crystallization. In Handbook of Crystal Growth; Elsevier: Amsterdam, The Netherlands, 2015; pp. 873–913. [Google Scholar]
- Murakami, F.S.; Rodrigues, P.O.; Campos, C.M.T.d.; Silva, M.A.S. Physicochemical study of CaCO3 from egg shells. Food Sci. Technol. 2007, 27, 658–662. [Google Scholar] [CrossRef]
- Puspitasari, P.; Utomo, D.M.; Zhorifah, H.F.N.; Permanasari, A.A.; Gayatri, R.W. Physicochemical determination of Calcium Carbonate (CaCO3) from chicken eggshell. Key Eng. Mater. 2020, 840, 478–483. [Google Scholar] [CrossRef]
- Young, J.R.; Henriksen, K. Biomineralization within vesicles: The calcite of coccoliths. Rev. Mineral. Geochem. 2003, 54, 189–215. [Google Scholar] [CrossRef]
- Palmer, A.R. Calcification in marine molluscs: How costly is it? Proc. Natl. Acad. Sci. USA 1992, 89, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Gosling, E. Bivalve Molluscs: Biology, Ecology and Culture; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Ahmed, T.A.; Kulshreshtha, G.; Hincke, M.T. Value-added uses of eggshell and eggshell membranes. In Eggs as Functional Foods and Nutraceuticals for Human Health; The Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Kathiravan, D.; Huang, B.-R.; Saravanan, A. Surface modified highly porous egg-shell membrane derived granular activated carbon coated on paper substrate and its humidity sensing properties. Mater. Chem. Phys. 2022, 277, 125486. [Google Scholar] [CrossRef]
- Gerotto, A.; Zhang, H.; Nagai, R.H.; Stoll, H.M.; Figueira, R.C.L.; Liu, C.; Hernández-Almeida, I. Fossil coccolith morphological attributes as a new proxy for deep ocean carbonate chemistry. Biogeosciences 2023, 20, 1725–1739. [Google Scholar] [CrossRef]
- Sonak, S.M.; Sonak, S.M. Molluscs and their shells. In Marine Shells of Goa: A Guide to Identification; Springer: Cham, Switzerland, 2017; pp. 1–23. [Google Scholar]
- Ahmmed, P.; Reynolds, J.; Levine, J.F.; Bozkurt, A. An accelerometer-based sensing system to study the valve-gaping behavior of bivalves. IEEE Sens. Lett. 2021, 5, 1–4. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, Q.; Sun, S.; Zhu, W.; Wu, P. A bioinspired mineral hydrogel as a self-healable, mechanically adaptable ionic skin for highly sensitive pressure sensing. Adv. Mater. 2017, 29, 1700321. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-h.; Mao, Z.; Chen, Y.; Long, Y.; Wu, H.; Shen, J.; Zhang, R.; Yeung, O.W.H.; Zhou, B.; Zhi, C.; et al. Amorphous biomineral-reinforced hydrogels with dramatically enhanced toughness for strain sensing. Chem. Eng. J. 2023, 468, 143735. [Google Scholar] [CrossRef]
- Li, X.; He, L.; Li, Y.; Chao, M.; Li, M.; Wan, P.; Zhang, L. Healable, degradable, and conductive MXene nanocomposite hydrogel for multifunctional epidermal sensors. ACS Nano 2021, 15, 7765–7773. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lu, Z.; Lu, C.; Sun, X.; Ni, B.; Cölfen, H.; Xiong, R. Bioinspired stabilization of amorphous calcium carbonate by carboxylated nanocellulose enables mechanically robust, healable, and Sensing biocomposites. ACS Nano 2023, 17, 6664–6674. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, C.T.; Bazylinski, D.A. Ecology, diversity, and evolution of magnetotactic bacteria. Microbiol. Mol. Biol. Rev. 2013, 77, 497–526. [Google Scholar] [CrossRef] [PubMed]
- Barr, C.R.; Bedrossian, M.; Lohmann, K.J.; Nealson, K.H. Magnetotactic bacteria: Concepts, conundrums, and insights from a novel In Situ approach using digital holographic microscopy (DHM). J. Comp. Physiol. A 2022, 208, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Hashmi, A.A. Magnetotactic bacteria-synthesized nanoparticles and their applications. In Green Synthesis of Nanomaterials: Biological and Environmental Applications; John Wiley and Sons: Hoboken, NJ, USA, 2024; pp. 187–207. [Google Scholar]
- Masó-Martínez, M.; Topham, P.D.; Fernández-Castané, A. Magnetosomes: Biological synthesis of magnetic nanostructures. In Fundamentals of Low Dimensional Magnets; CRC Press: Boca Raton, FL, USA, 2022; pp. 309–324. [Google Scholar]
- Oestreicher, Z.; Valverde-Tercedor, C.; Mumper, E.; Pérez-Guzmán, L.; Casillas-Ituarte, N.N.; Jimenez-Lopez, C.; Bazylinski, D.A.; Lower, S.K.; Lower, B.H. Localization of native Mms13 to the magnetosome chain of Magnetospirillum magneticum AMB-1 using immunogold electron microscopy, immunofluorescence microscopy and biochemical analysis. Crystals 2021, 11, 874. [Google Scholar] [CrossRef]
- Singappuli-Arachchige, D.; Feng, S.; Wang, L.; Palo, P.E.; Shobade, S.O.; Thomas, M.; Nilsen-Hamilton, M. The magnetosome protein, mms6 from magnetospirillum magneticum strain AMB-1, is a lipid-activated ferric reductase. Int. J. Mol. Sci. 2022, 23, 10305. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Mazuyama, E.; Arakaki, A.; Matsunaga, T. MMS6 protein regulates crystal morphology during nano-sized magnetite biomineralization in vivo. J. Biol. Chem. 2011, 286, 6386–6392. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.E.; Bramble, J.P.; Hounslow, A.M.; Williamson, M.P.; Monnington, A.E.; Cooke, D.J.; Staniland, S.S. Ferrous iron binding key to Mms6 magnetite biomineralisation: A mechanistic study to understand magnetite formation using pH titration and NMR spectroscopy. Chem. Eur. J. 2016, 22, 7885–7894. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, A.; Webb, J.; Matsunaga, T. A Novel Protein Tightly Bound to Bacterial Magnetic Particles in Magnetospirillum magneticum Strain AMB-1. J. Biol. Chem. 2003, 278, 8745–8750. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Tercedor, C.; Abadía-Molina, F.; Martinez-Bueno, M.; Pineda-Molina, E.; Chen, L.; Oestreicher, Z.; Lower, B.H.; Lower, S.K.; Bazylinski, D.A.; Jimenez-Lopez, C. Subcellular localization of the magnetosome protein MamC in the marine magnetotactic bacterium Magnetococcus marinus strain MC-1 using immunoelectron microscopy. Arch. Microbiol. 2014, 196, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.; Cantao, M.E.; Nicolás, M.F.; Barcellos, F.G.; Morillo, V.; Almeida, L.G.; Do Nascimento, F.F.; Lefevre, C.T.; Bazylinski, D.A.; de Vasconcelos, A.T.R. Common ancestry of iron oxide-and iron-sulfide-based biomineralization in magnetotactic bacteria. ISME J. 2011, 5, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Mittmann, E.; Mickoleit, F.; Maier, D.S.; Stäbler, S.Y.; Klein, M.A.; Niemeyer, C.M.; Rabe, K.S.; Schüler, D. A Magnetosome-based platform for flow biocatalysis. ACS Appl. Mater. Interfaces 2022, 14, 22138–22150. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, S.; Kumar, A.S.; Mathiyarasu, J.; Suthindhiran, K. Detection of Escherichia coli in food samples by magnetosome-based biosensor. Biotechnol. Bioprocess Eng. 2023, 28, 152–161. [Google Scholar] [CrossRef]
- Sannigrahi, S.; Arumugasamy, S.K.; Mathiyarasu, J.; Sudhakaran, R.; Suthindhiran, K. Detection of white spot syndrome virus in seafood samples using a magnetosome-based impedimetric biosensor. Arch. Virol. 2021, 166, 2763–2778. [Google Scholar] [CrossRef] [PubMed]
- Ubago-Rodríguez, A.; Casares Atienza, S.; Fernández-Vivas, A.; Peigneux, A.; Jabalera, Y.; de la Cuesta-Rivero, M.; Jimenez-Lopez, C.N.; Azuaga Fortes, A.I. Structure–function of MamC loop and its effect on the in vitro precipitation of biomimetic magnetite nanoparticles. Cryst. Growth Des. 2019, 19, 2927–2935. [Google Scholar] [CrossRef]
- Lopez-Moreno, R.; Fernández-Vivas, A.; Valverde-Tercedor, C.; Azuaga Fortes, A.I.; Casares Atienza, S.; Rodriguez-Navarro, A.B.; Zarivach, R.; Jimenez-Lopez, C. Magnetite nanoparticles biomineralization in the presence of the magnetosome membrane protein MamC: Effect of protein aggregation and protein structure on magnetite formation. Cryst. Growth Des. 2017, 17, 1620–1629. [Google Scholar] [CrossRef]
- Jimenez-Carretero, M.; Jabalera, Y.; Sola-Leyva, A.; Carrasco-Jimenez, M.P.; Jimenez-Lopez, C. Nanoassemblies of acetylcholinesterase and β-lactamase immobilized on magnetic nanoparticles as biosensors to detect pollutants in water. Talanta 2023, 258, 124406. [Google Scholar] [CrossRef] [PubMed]
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, present, and future in bone regeneration. Bone Tissue Regen. Insights 2016, 7, 9. [Google Scholar] [CrossRef]
- Bystrov, V. Piezoelectricity and pyroelectricity in hydroxyapatite. Ferroelectrics 2019, 541, 25–29. [Google Scholar] [CrossRef]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for biomedical applications: A short overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Hartati, Y.W.; Irkham, I.; Zulqaidah, S.; Syafira, R.S.; Kurnia, I.; Noviyanti, A.R.; Topkaya, S.N. Recent advances in hydroxyapatite-based electrochemical biosensors: Applications and future perspectives. Sens. Bio-Sens. Res. 2022, 38, 100542. [Google Scholar] [CrossRef]
- Zyrianova, P.I.; Eltantawy, M.M.; Silin, D.V.; Korolev, I.S.; Nikolaev, K.G.; Kozodaev, D.A.; Slautina, A.S.; Surmenev, R.A.; Kholkin, A.L.; Ulasevich, S.A.; et al. Biomimetic materials based on hydroxyapatite patterns for studying extracellular cell communication. Mater. Des. 2024, 238, 112718. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. A dual-channel homogeneous aptasensor combining colorimetric with electrochemical strategy for thrombin. Biosens. Bioelectron. 2018, 120, 15–21. [Google Scholar] [CrossRef]
- He, Y.; Lv, C.; Hou, X.; Wu, L. Mono-dispersed nano-hydroxyapatite based MRI probe with tetrahedral DNA nanostructures modification for in vitro tumor cell imaging. Anal. Chim. Acta 2020, 1138, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Nandini, S.; Nalini, S.; Reddy, M.B.M.; Suresh, G.S.; Melo, J.S.; Niranjana, P.; Sanetuntikul, J.; Shanmugam, S. Synthesis of one-dimensional gold nanostructures and the electrochemical application of the nanohybrid containing functionalized graphene oxide for cholesterol biosensing. Bioelectrochemistry 2016, 110, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.-D.; Cong, V.T.; Baek, C.; Min, J. Fabrication of peptide stabilized fluorescent gold nanocluster/graphene oxide nanocomplex and its application in turn-on detection of metalloproteinase-9. Biosens. Bioelectron. 2017, 89, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, H.; Wang, A.-J.; Zhong, S.-X.; Fang, K.-M.; Feng, J.-J. Green synthesis of peptide-templated fluorescent copper nanoclusters for temperature sensing and cellular imaging. Analyst 2014, 139, 6536–6541. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lai, G.; Lin, B.; Yu, A.; Yang, N. Enzyme-induced biomineralization of cupric subcarbonate for ultrasensitive colorimetric immunosensing of carcinoembryonic antigen. Sens. Actuators B Chem. 2018, 262, 789–795. [Google Scholar] [CrossRef]
- Han, L.; Liu, A. Novel cell–inorganic hybrid catalytic interfaces with enhanced enzymatic activity and stability for sensitive biosensing of paraoxon. ACS Appl. Mater. Interfaces 2017, 9, 6894–6901. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.-B.; Zhong, Q.; Ren, H.-X. Engineering a thermostable biosensor based on biomimetic mineralization HRP@ Fe-MOF for Alzheimer’s disease. Anal. Bioanal. Chem. 2022, 414, 8331–8339. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhang, X.; Wang, W.; Liu, C.; Yang, W.; Wang, D. Nanobody@Biomimetic mineralized MOF as a sensing immunoprobe in detection of aflatoxin B1. Biosens. Bioelectron. 2023, 220, 114906. [Google Scholar] [CrossRef]
- Xu, L.; Zou, L.; Guo, J.; Cao, Y.; Feng, C.; Ye, B. Simple “signal-off” electrochemical aptasensor based on aptamer-Cu3 (PO4)2 hybrid nanoflowers/graphene oxide for carcinoembryonic antigen detection. ChemElectroChem 2020, 7, 1660–1665. [Google Scholar] [CrossRef]
- Sun, B.; Wang, Z.; Wang, X.; Qiu, M.; Zhang, Z.; Wang, Z.; Cui, J.; Jia, S. Paper-based biosensor based on phenylalnine ammonia lyase hybrid nanoflowers for urinary phenylalanine measurement. Int. J. Biol. Macromol. 2021, 166, 601–610. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Yang, C.; Ma, C.; Tang, J. A smartphone-assisted portable biosensor using laccase-mineral hybrid microflowers for colorimetric determination of epinephrine. Talanta 2021, 224, 121840. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Chen, D.; Han, L. Taking full advantage of the structure and multi-activities of mineralized microbial surface-displayed enzymes: Portable three-in-one organophosphate pesticides assay device. Chem. Eng. J. 2022, 429, 132317. [Google Scholar] [CrossRef]
- Findik, M.; Bingol, H.; Erdem, A. Electrochemical detection of interaction between daunorubicin and DNA by hybrid nanoflowers modified graphite electrodes. Sens. Actuators B Chem. 2021, 329, 129120. [Google Scholar] [CrossRef]
- Zhang, M.; Peltier, R.; Zhang, M.; Lu, H.; Bian, H.; Li, Y.; Xu, Z.; Shen, Y.; Sun, H.; Wang, Z. In situ reduction of silver nanoparticles on hybrid polydopamine–copper phosphate nanoflowers with enhanced antimicrobial activity. J. Mater. Chem. B 2017, 5, 5311–5317. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, D.; Novakovic, K.; Hilkens, C.M.U.; Ferreira, A.M. Interplay between biomaterials and the immune system: Challenges and opportunities in regenerative medicine. Acta Biomater. 2023, 155, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.A.; Halpern, J.M. Guide to selecting a biorecognition element for biosensors. Bioconjugate Chem. 2018, 29, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, H.; Li, Y.; Li, X. Application of artificial intelligence (AI)-enhanced biochemical sensing in molecular diagnosis and imaging analysis: Advancing and challenges. TrAC Trends Anal. Chem. 2024, 174, 117700. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial intelligence biosensors: Challenges and prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhamid, M.A.A.; Ki, M.-R.; Pack, S.P. Biominerals and Bioinspired Materials in Biosensing: Recent Advancements and Applications. Int. J. Mol. Sci. 2024, 25, 4678. https://doi.org/10.3390/ijms25094678
Abdelhamid MAA, Ki M-R, Pack SP. Biominerals and Bioinspired Materials in Biosensing: Recent Advancements and Applications. International Journal of Molecular Sciences. 2024; 25(9):4678. https://doi.org/10.3390/ijms25094678
Chicago/Turabian StyleAbdelhamid, Mohamed A. A., Mi-Ran Ki, and Seung Pil Pack. 2024. "Biominerals and Bioinspired Materials in Biosensing: Recent Advancements and Applications" International Journal of Molecular Sciences 25, no. 9: 4678. https://doi.org/10.3390/ijms25094678
APA StyleAbdelhamid, M. A. A., Ki, M.-R., & Pack, S. P. (2024). Biominerals and Bioinspired Materials in Biosensing: Recent Advancements and Applications. International Journal of Molecular Sciences, 25(9), 4678. https://doi.org/10.3390/ijms25094678






