Antifungal Properties of Bio-AgNPs against D. pinodes and F. avenaceum Infection of Pea (Pisum sativum L.) Seedlings
Abstract
1. Introduction
2. Results
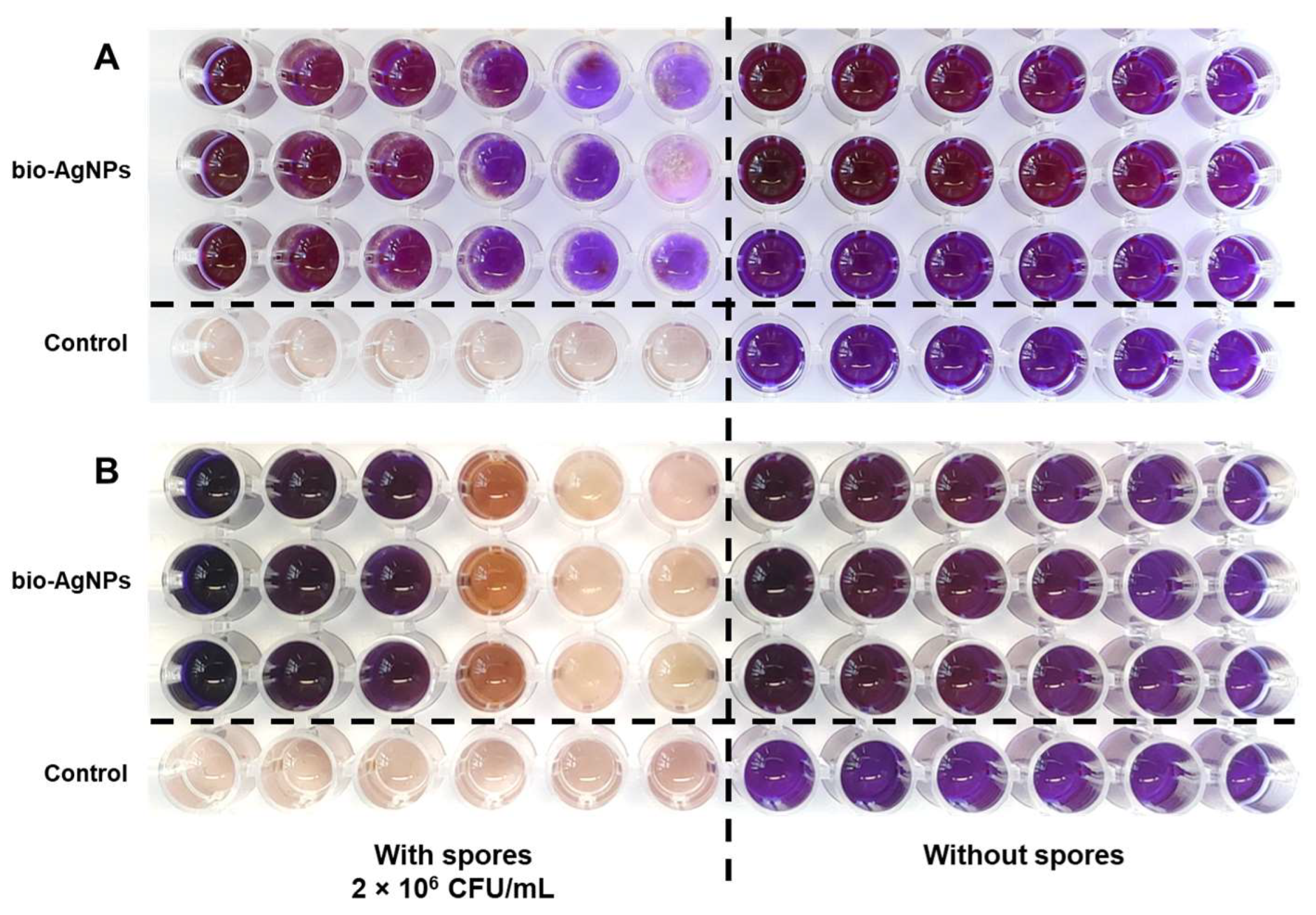
2.1. Antifungal Properties of Bio-AgNPs In Vitro
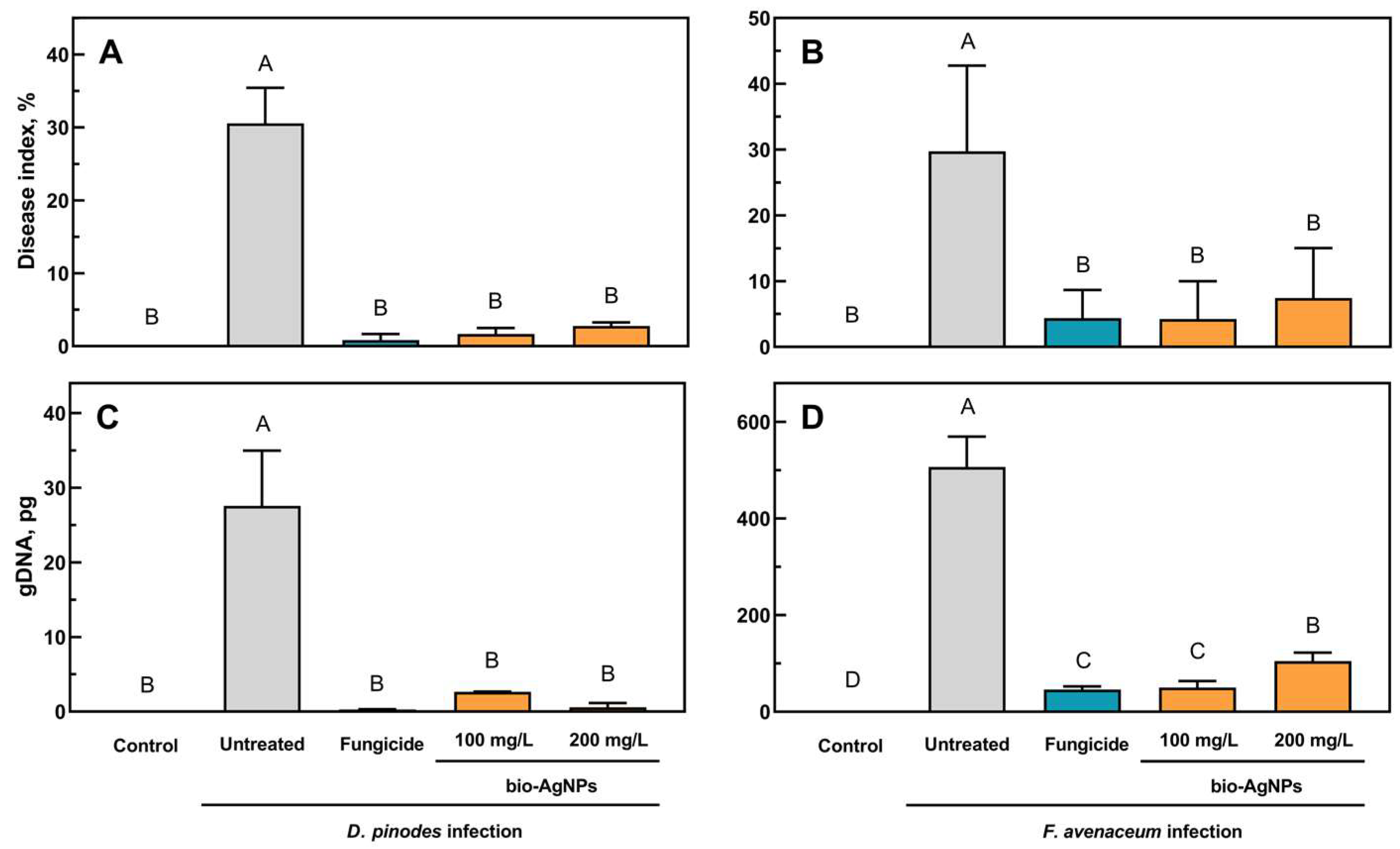
2.2. Pea Seedlings Infection and Antifungal Properties of Bio-AgNPs In Vivo
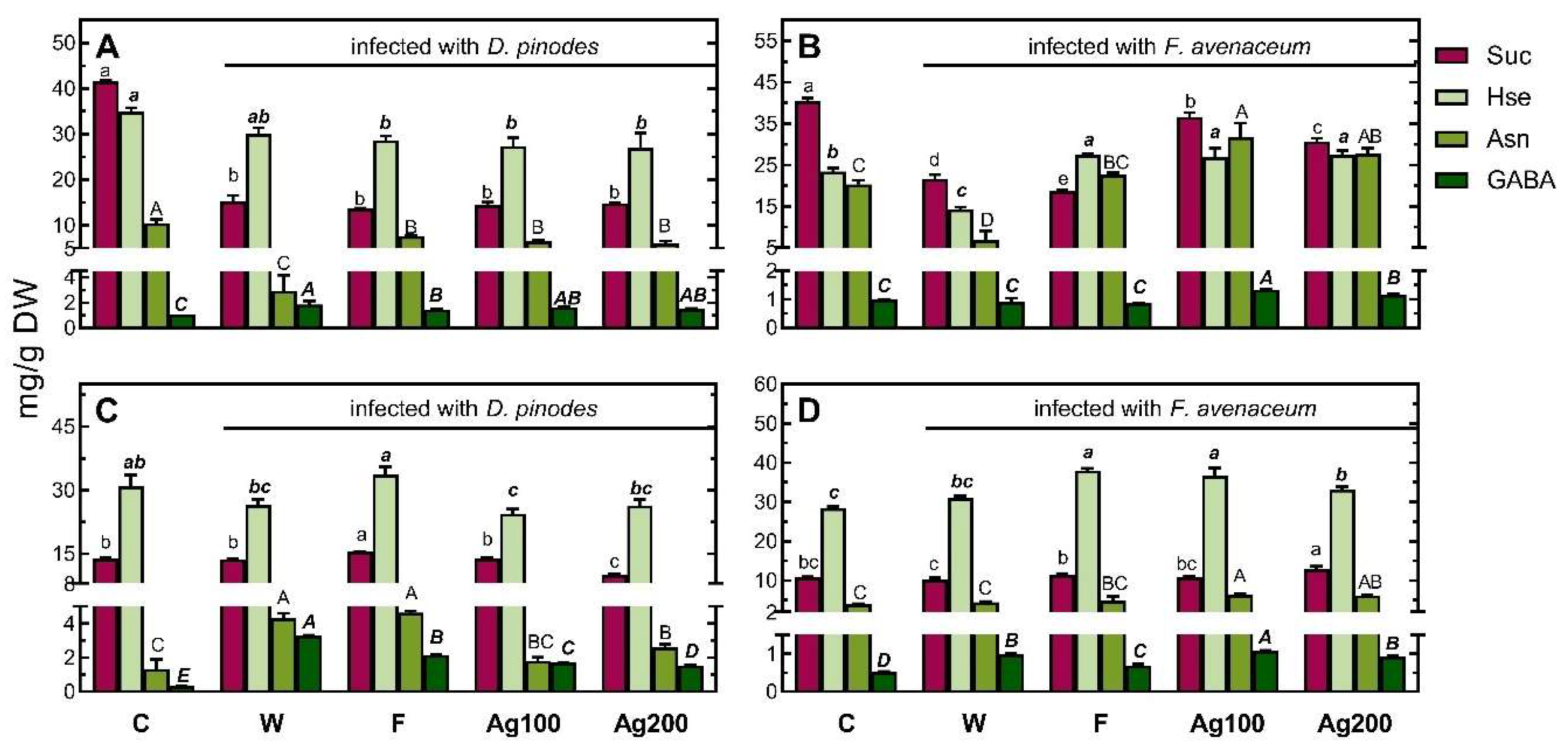
2.3. Polar Metabolic Profiles
2.3.1. Metabolic Profiles of Control Seedlings
2.3.2. Metabolic Profiles of Infected Seedlings
PCA
Changes in the Concentrations of Metabolites
3. Discussion
3.1. Antifungal Activity of Bio-AgNPs against D. pinodes and F. avenaceum
3.2. Changes in Polar Metabolic Profiles of Pea Seedlings after Infections
3.3. Effect of Bio-AgNP Pre-Treatment on Changes in Polar Metabolic Profiles of Pea Seedlings after Infections
4. Materials and Methods
4.1. Bio-Synthesized Silver Nanoparticles and Their Characterization
4.2. Fungal Cultures and Spore Preparation
4.3. MIC Value Determination—Resazurin Assay
4.4. Inhibition of Mycelial Fungal Growth—Poisoned Food Technique
4.5. Plant Material
4.6. Plant Infection
4.7. Disease Index
4.8. qPCR Analysis
4.9. Polar Metabolite Analyses
4.9.1. Extraction of Polar Metabolites
4.9.2. GC-FID and GC-MS Analyses
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jha, A.B.; Gali, K.K.; Alam, Z.; Lachagari, V.B.R.; Warkentin, T.D. Potential application of genomic technologies in breeding for fungal and oomycete disease resistance in pea. Agronomy 2021, 11, 1260. [Google Scholar] [CrossRef]
- Joshi, S.; Pandey, B.R.; Rosewarne, G. Characterization of field pea (Pisum sativum) resistance against Peyronellaea pinodes and Didymella pinodella that cause ascochyta blight. Front. Plant Sci. 2022, 13, 976375. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The current situation of pea protein and its application in the food industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization Corporate Statistical Database. Available online: http://faostat.fao.org/ (accessed on 15 January 2024).
- Rubiales, D.; Barilli, E.; Rispail, N. Breeding for biotic stress resistance in pea. Agriculture 2023, 13, 1825. [Google Scholar] [CrossRef]
- Wille, L.; Messmer, M.M.; Bodenhausen, N.; Studer, B.; Hohmann, P. Heritable variation in pea for resistance against a root rot complex and its characterization by amplicon sequencing. Front. Plant Sci. 2020, 11, 542153. [Google Scholar] [CrossRef] [PubMed]
- Tivoli, B.; Banniza, S. Comparison of the epidemiology of ascochyta blights on grain legumes. Eur. J. Plant Pathol. 2007, 119, 59–76. [Google Scholar] [CrossRef]
- Barbetti, M.J.; Khan, T.N.; Pritchard, I.; Lamichhane, J.R.; Aubertot, J.N.; Corrales, D.C.; You, M.P. Challenges with managing disease complexes during application of different measures against foliar diseases of field pea. Plant Dis. 2021, 105, 616–627. [Google Scholar] [CrossRef]
- Chilvers, M.I.; Rogers, J.D.; Dugan, F.M.; Stewart, J.E.; Chen, W.; Peever, T.L. Didymella pisi sp. nov., the teleomorph of Ascochyta pisi. Mycol. Res. 2009, 113, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Index Fungorum. Available online: https://www.indexfungorum.org/ (accessed on 15 January 2024).
- Davidson, J.A.; Hartley, D.; Priest, M.; Krysinska-Kaczmarek, M.; Herdina; McKay, A.; Scott, E.S. A new species of Phoma causes ascochyta blight symptoms on field peas (Pisum sativum) in South Australia. Mycologia 2009, 101, 120–128. [Google Scholar] [CrossRef]
- Davidson, J.A.; Krysinska-Kaczmarek, M.; Wilmshurst, C.J.; McKay, A.; Herdina; Scott, E.S. Distribution and survival of ascochyta blight pathogens in field-pea-cropping soils of Australia. Plant Dis. 2011, 95, 1217–1223. [Google Scholar] [CrossRef]
- Keirnan, E.C.; Tan, Y.P.; Laurence, M.H.; Mertin, A.A.; Liew, E.C.Y.; Summerell, B.A.; Shivas, R.G. Cryptic diversity found in Didymellaceae from Australian native legumes. MycoKeys 2021, 78, 1–20. [Google Scholar] [CrossRef]
- Tran, H.S.; You, M.P.; Khan, T.N.; Barbetti, M.J. Relative host resistance to black spot disease in field pea (Pisum sativum) is determined by individual pathogens. Plant Dis. 2015, 99, 580–587. [Google Scholar] [CrossRef]
- Coyne, C.J.; Porter, L.D.; Boutet, G.; Ma, Y.; McGee, R.J.; Lesné, A.; Baranger, A.; Pilet-Nayel, M.L. Confirmation of Fusarium root rot resistance QTL Fsp-Ps 2.1 of pea under controlled conditions. BMC Plant Biol. 2019, 19, 4–11. [Google Scholar] [CrossRef]
- Basu, P.K.; Brown, N.J.; Crête, R.; Gourley, C.O.; Johnston, H.W.; Pepin, H.S.; Seaman, W.L. Yield loss conversion factors for Fusarium root rot of pea. Can. Plant Dis. Surv. 1976, 56, 25–32. [Google Scholar]
- Wu, L.; Fredua-Agyeman, R.; Strelkov, S.E.; Chang, K.F.; Hwang, S.F. Identification of quantitative trait loci associated with partial resistance to fusarium root rot and wilt caused by Fusarium graminearum in field pea. Front. Plant Sci. 2022, 12, 784593. [Google Scholar] [CrossRef] [PubMed]
- Dohroo, N.P.; Verma, S.; Bharat, N.K. Fusarium wilt and root rot of pea. Int. J. Trop. Plant Dis. 1998, 16, 1–20. [Google Scholar]
- Feng, J.; Hwang, R.; Chang, K.F.; Hwang, S.F.; Strelkov, S.E.; Gossen, B.D.; Conner, R.L.; Turnbull, G.D. Genetic variation in Fusarium avenaceum causing root rot on field pea. Plant Pathol. 2010, 59, 845–852. [Google Scholar] [CrossRef]
- Chittem, K.; Mathew, F.M.; Gregoire, M.; Lamppa, R.S.; Chang, Y.W.; Markell, S.G.; Bradley, C.A.; Barasubiye, T.; Goswami, R.S. Identification and characterization of Fusarium spp. associated with root rots of field pea in North Dakota. Eur. J. Plant Pathol. 2015, 143, 641–649. [Google Scholar] [CrossRef]
- Pflughöft, O.; Merker, C.; von Tiedemann, A.; Schäfer, B.C. Zur Verbreitung und Bedeutung von Pilzkrankheiten in Körnerfuttererbsen (Pisum sativum L.) in Deutschland. Gesunde Pflanz. 2012, 64, 39–48. [Google Scholar] [CrossRef]
- Šišić, A.; Baćanović, J.; Finckh, M.R. Endophytic Fusarium equiseti stimulates plant growth and reduces root rot disease of pea (Pisum sativum L.) caused by Fusarium avenaceum and Peyronellaea pinodella. Eur. J. Plant Pathol. 2017, 148, 271–282. [Google Scholar] [CrossRef]
- Fernandez, M.R. Fusarium populations in roots of oilseed and pulse crops grown in eastern Saskatchewan. Can. J. Plant Sci. 2007, 87, 945–952. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 0813819199. [Google Scholar]
- Barilli, E.; Cobos, M.J.; Rubiales, D. Clarification on host range of Didymella pinodes the causal agent of pea ascochyta blight. Front. Plant Sci. 2016, 7, 579. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, J.G.; Jacobsen, H.J.; Chatterton, S.; Hassan, F.; Bowness, R.; Hall, L.M. Lack of efficacy of transgenic pea (Pisum sativum L.) stably expressing antifungal genes against Fusarium spp. in three years of confined field trials. GM Crop. Food 2018, 9, 90–108. [Google Scholar] [CrossRef] [PubMed]
- Fondevilla, S.; Krezdorn, N.; Rubiales, D.; Rotter, B.; Winter, P. Bulked segregant transcriptome analysis in pea identifies key expression markers for resistance to Peyronellaea pinodes. Sci. Rep. 2022, 12, 18159. [Google Scholar] [CrossRef] [PubMed]
- Bodah, E.T.; Porter, L.D.; Chaves, B.; Dhingra, A. Evaluation of pea accessions and commercial cultivars for fusarium root rot resistance. Euphytica 2016, 208, 63–72. [Google Scholar] [CrossRef]
- Liu, N.; Xu, S.; Yao, X.; Zhang, G.; Mao, W.; Hu, Q.; Feng, Z.; Gong, Y. Studies on the control of ascochyta blight in field peas (Pisum sativum L.) caused by Ascochyta pinodes in Zhejiang Province, China. Front. Microbiol. 2016, 7, 18159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Modderman, C.T.; Markell, S.; Wunsch, M.; Pasche, J.S. Efficacy of in-furrow fungicides for management of field pea root rot caused by Fusarium avenaceum and F. solani under greenhouse and field conditions. Plant Health Prog. 2018, 19, 212–219. [Google Scholar] [CrossRef]
- Davidson, J.A.; Walela, C.; Day, S.; Roberts, P.; McMurray, L. Evaluation of economic fungicide strategies for control of ascochyta blight in field pea in southern Australia. Australas. Plant Pathol. 2022, 51, 495–505. [Google Scholar] [CrossRef]
- Nazir, N.; Badri, Z.A.; Bhat, N.A.; Bhat, F.A.; Sultan, P.; Bhat, T.A.; Rather, M.A.; Sakina, A. Effect of the combination of biological, chemical control and agronomic technique in integrated management pea root rot and its productivity. Sci. Rep. 2022, 12, 11348. [Google Scholar] [CrossRef]
- Fungicide Resistance Action Committee, FRAC Code List© 2023: Fungal Control Agents Sorted by Cross-Resistance Pattern and Mode of Action. Available online: https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2023---final.pdf (accessed on 15 January 2024).
- Hu, M.; Chen, S. Non-target site mechanisms of fungicide resistance in crop pathogens: A review. Microorganisms 2021, 9, 502. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Metal nanoparticles against fungicide resistance: Alternatives or partners? Pest Manag. Sci. 2022, 78, 3953–3956. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Luna, A.R.; Cruz-Martínez, H.; Vásquez-López, A.; Medina, D.I. Metal nanoparticles as novel antifungal agents for sustainable agriculture: Current advances and future directions. J. Fungi 2021, 7, 1033. [Google Scholar] [CrossRef] [PubMed]
- Elmer, W.; White, J.C. The future of nanotechnology in plant pathology. Annu. Rev. Phytopathol. 2018, 56, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Bach, H. Mechanisms of antifungal properties of metal nanoparticles. Nanomaterials 2022, 12, 4470. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.A.; Alrajhi, A.M.; Fouda, M.M.; Abdelkareem, E.M.; Thabit, T.M.; Bouqellah, N.A. Effect of silver nanoparticles on toxigenic Fusarium spp. and deoxynivalenol secretion in some grains. J. AOAC Int. 2018, 101, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, A.; Gómez, J.V.; Mateo, E.M.; Jiménez, M.; Mateo, F. Antifungal effect of engineered silver nanoparticles on phytopathogenic and toxigenic Fusarium spp. and their impact on mycotoxin accumulation. Int. J. Food Microbiol. 2019, 306, 108259. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Chen, X.; Ahmed, T.; Shang, Q.; Zhang, S.; Ma, Z.; Yin, Y. Toxicity and action mechanisms of silver nanoparticles against the mycotoxin-producing fungus Fusarium graminearum. J. Adv. Res. 2022, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Salaria, N.; Thakur, K.; Kukreja, S.; Yadav, N.; Yadav, R.; Goutam, U. Green silver nanoparticles for phytopathogen control. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 439–446. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Use of copper, silver and zinc nanoparticles against foliar and soil-borne plant pathogens. Sci. Total Environ. 2019, 670, 292–299. [Google Scholar] [CrossRef]
- Macías Sánchez, K.L.; González Martínez, H.D.R.; Carrera Cerritos, R.; Martínez Espinosa, J.C. In vitro evaluation of the antifungal effect of AgNPs on Fusarium oxysporum f. sp. lycopersici. Nanomaterials 2023, 13, 1274. [Google Scholar] [CrossRef] [PubMed]
- Matras, E.; Gorczyca, A.; Przemieniecki, S.W.; Oćwieja, M. Surface properties-dependent antifungal activity of silver nanoparticles. Sci. Rep. 2022, 12, 18046. [Google Scholar] [CrossRef] [PubMed]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green synthesis of metallic nanoparticles: Applications and limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Saeri, M.R.; Azizi, M. Synthesis, characterization and biocompatibility of silver nanoparticles synthesized from Nigella sativa leaf extract in comparison with chemical silver nanoparticles. Ecotoxicol. Environ. Saf. 2015, 120, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Saif, S.; Tahir, A.; Asim, T.; Chen, Y. Plant mediated green synthesis of CuO nanoparticles: Comparison of toxicity of engineered and plant mediated CuO nanoparticles towards Daphnia magna. Nanomaterials 2016, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, E.; Kahrizi, D.; Arkan, E. Comparison of different properties of zinc oxide nanoparticles synthesized by the green (using Juglans regia L. leaf extract) and chemical methods. J. Mol. Liq. 2019, 286, 110831. [Google Scholar] [CrossRef]
- Rashidian, G.; Lazado, C.C.; Mahboub, H.H.; Mohammadi-Aloucheh, R.; Prokić, M.D.; Nada, H.S.; Faggio, C. Chemically and green synthesized ZnO nanoparticles alter key immunological molecules in common carp (Cyprinus carpio) skin mucus. Int. J. Mol. Sci. 2021, 22, 3270. [Google Scholar] [CrossRef] [PubMed]
- Gunalan, S.; Sivaraj, R.; Rajendran, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. Mater. Int. 2012, 22, 693–700. [Google Scholar] [CrossRef]
- Dowlath, M.J.H.; Musthafa, S.A.; Mohamed Khalith, S.B.; Varjani, S.; Karuppannan, S.K.; Ramanujam, G.M.; Arunachalam, A.M.; Arunachalam, K.D.; Chandrasekaran, M.; Chang, S.W.; et al. Comparison of characteristics and biocompatibility of green synthesized iron oxide nanoparticles with chemical synthesized nanoparticles. Environ. Res. 2021, 201, 111585. [Google Scholar] [CrossRef]
- Railean-Plugaru, V.; Pomastowski, P.; Buszewski, B. Use of Lactobacillus paracasei isolated from whey for silver nanocomposite synthesis: Antiradical and antimicrobial properties against selected pathogens. J. Dairy Sci. 2021, 104, 2480–2498. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, H.; Nam, H.C.; Park, S.R.; Jung, J.Y.; Park, W.H. Injectable methylcellulose hydrogel containing silver oxide nanoparticles for burn wound healing. Carbohydr. Polym. 2018, 181, 579–586. [Google Scholar] [CrossRef]
- Railean, V.; Pomastowski, P.; Płociński, T.; Gloc, M.; Dobrucka, R.; Kurzydłowski, K.J.; Buszewski, B. Consideration of a new approach to clarify the mechanism formation of AgNPs, AgNCl and AgNPs@AgNCl synthesized by biological method. Discov. Nano 2023, 18, 2. [Google Scholar] [CrossRef]
- Mansoor, S.; Zahoor, I.; Baba, T.R.; Padder, S.A.; Bhat, Z.A.; Koul, A.M.; Jiang, L. Fabrication of silver nanoparticles against fungal pathogens. Front. Nanotechnol. 2021, 3, 1–12. [Google Scholar] [CrossRef]
- Luan, L.Q.; Xo, D.H. In vitro and in vivo fungicidal effects of γ-irradiation synthesized silver nanoparticles against Phytophthora capsici causing the foot rot disease of pepper plant. J. Plant Pathol. 2018, 100, 241–248. [Google Scholar] [CrossRef]
- Alvarez-Carvajal, F.; Gonzalez-Soto, T.E.; Armenta-Calderón, A.; Mendez Ibarra, R.; Esquer-Miranda, E.; Juarez, J.; Encinas-Basurto, D. Silver nanoparticles coated with chitosan against Fusarium oxysporum causing the tomato wilt. Biotecnia 2020, 22, 73–80. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Pluskota, W.E.; Stelmaszewska, J.; Szablińska, J. Dehydration induces expression of GALACTINOL SYNTHASE and RAFFINOSE SYNTHASE in seedlings of pea (Pisum sativum L.). J. Plant Physiol. 2014, 171, 1306–1314. [Google Scholar] [CrossRef]
- Pluskota, W.E.; Szablińska, J.; Obendorf, R.L.; Górecki, R.J.; Lahuta, L.B. Osmotic stress induces genes, enzymes and accumulation of galactinol, raffinose and stachyose in seedlings of pea (Pisum sativum L.). Acta Physiol. Plant. 2015, 37, 156. [Google Scholar] [CrossRef]
- Szablińska-Piernik, J.; Lahuta, L.B. Changes in polar metabolites during seed germination and early seedling development of pea, cucumber, and wheat. Agriculture 2023, 13, 2278. [Google Scholar] [CrossRef]
- Szablińska-Piernik, J.; Lahuta, L.B. Metabolite profiling of semi-leafless pea (Pisum sativum L.) under progressive soil drought and subsequent re-watering. J. Plant Physiol. 2021, 256, 156. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Szablińska-Piernik, J.; Horbowicz, M. Changes in metabolic profiles of pea (Pisum sativum L.) as a result of repeated short-term soil drought and subsequent re-watering. Int. J. Mol. Sci. 2022, 23, 1704. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.C.; Joy, K.W.; Ireland, R.J. Amino acid metabolism of pea leaves. Plant Physiol. 1984, 74, 822–826. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, L.; Garneau, M.G.; Majumdar, R.; Grant, J.; Tegeder, M. Improvement of pea biomass and seed productivity by simultaneous increase of phloem and embryo loading with amino acids. Plant J. 2015, 81, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Aragón, R.; Palacios, J.M.; Ramírez-Parra, E. Rhizobial symbiosis promotes drought tolerance in Vicia sativa and Pisum sativum. Environ. Exp. Bot. 2023, 208, 105268. [Google Scholar] [CrossRef]
- Ballesteros-Gutiérrez, M.; Albareda, M.; Barbas, C.; López-Gonzálvez, Á.; Dunn, M.F.; Palacios, J.M. A host-specific diaminobutyrate aminotransferase contributes to symbiotic performance, homoserine metabolism, and competitiveness in the Rhizobium leguminosarum/Pisum sativum system. Front. Microbiol. 2023, 14, 1–16. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Rojas, C.M.; Senthil-Kumar, M.; Tzin, V.; Mysore, K.S. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front. Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Faubert, D.; Jabaji, S. A Metabolic profiling strategy for the dissection of plant defense against fungal pathogens. PLoS ONE 2014, 9, e111930. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Roychoudhury, A. Go green to protect plants: Repurposing the antimicrobial activity of biosynthesized silver nanoparticles to combat phytopathogens. Nanotechnol. Environ. Eng. 2021, 6, 10. [Google Scholar] [CrossRef]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef]
- Castillejo, M.Á.; Curto, M.; Fondevilla, S.; Rubiales, D.; Jorrín, J.V. Two-dimensional electrophoresis based proteomic analysis of the pea (Pisum sativum) in response to Mycosphaerella pinodes. J. Agric. Food Chem. 2010, 58, 12822–12832. [Google Scholar] [CrossRef] [PubMed]
- Castillejo, M.Á.; Bani, M.; Rubiales, D. Understanding pea resistance mechanisms in response to Fusarium oxysporum through proteomic analysis. Phytochemistry 2015, 115, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Castillejo, M.Á.; Fondevilla-Aparicio, S.; Fuentes-Almagro, C.; Rubiales, D. Quantitative analysis of target peptides related to resistance against ascochyta blight (Peyronellaea pinodes) in pea. J. Proteome Res. 2020, 19, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Fondevilla, S.; Küster, H.; Krajinski, F.; Cubero, J.I.; Rubiales, D. Identification of genes differentially expressed in a resistant reaction to Mycosphaerella pinodes in pea using microarray technology. BMC Genom. 2011, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Maeda, A.; Hirose, M.; Ichinose, Y.; Shiraishi, T.; Toyoda, K. Ultrastructural and cytological studies on Mycosphaerella pinodes infection of the model legume Medicago truncatula. Front. Plant Sci. 2017, 8, 1132. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P.; Proctor, R.H.; Moretti, A. Mycotoxin production in Fusarium according to contemporary species concepts. Annu. Rev. Phytopathol. 2021, 59, 373–402. [Google Scholar] [CrossRef] [PubMed]
- Desalegn, G.; Turetschek, R.; Kaul, H.P.; Wienkoop, S. Microbial symbionts affect Pisum sativum proteome and metabolome under Didymella pinodes infection. J. Proteom. 2016, 143, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, B.; Ding, H.; Zhang, J.; Li, S. Review: The role of NADP-malic enzyme in plants under stress. Plant Sci. 2019, 281, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Han, G.; Meng, Z.; Lin, L.; Sui, N. Roles of malic enzymes in plant development and stress responses. Plant Signal. Behav. 2019, 14, e1644596. [Google Scholar] [CrossRef] [PubMed]
- Garry, G.; Tivoli, B.; Jeuffroy, M.H.; Citharel, J. Effects of Ascochyta blight caused by Mycosphaerella pinodes on the translocation of carbohydrates and nitrogenous compounds from the leaf and hull to the seed of dried-pea. Plant Pathol. 1996, 45, 769–777. [Google Scholar] [CrossRef]
- Béasse, C.; Ney, B.; Tivoli, B. A simple model of pea (Pisum sativum) growth affected by Mycosphaerella pinodes. Plant Pathol. 2000, 49, 187–200. [Google Scholar] [CrossRef]
- Leclerc, M.; Jumel, S.; Hamelin, F.M.; Treilhaud, R.; Parisey, N.; Mammeri, Y. Imaging with spatio-temporal modelling to characterize the dynamics of plant-pathogen lesions. PLoS Comput. Biol. 2023, 19, e1011627. [Google Scholar] [CrossRef] [PubMed]
- Turetschek, R.; Desalegn, G.; Epple, T.; Kaul, H.P.; Wienkoop, S. Key metabolic traits of Pisum sativum maintain cell vitality during Didymella pinodes infection: Cultivar resistance and the microsymbionts’ influence. J. Proteom. 2017, 169, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Rosati, R.G.; Lario, L.D.; Hourcade, M.E.; Cervigni, G.D.L.; Luque, A.G.; Scandiani, M.M.; Spampinato, C.P. Primary metabolism changes triggered in soybean leaves by Fusarium tucumaniae infection. Plant Sci. 2018, 274, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Scandiani, M.M.; Luque, A.G.; Razori, M.V.; Casalini, L.C.; Aoki, T.; O’Donnell, K.; Cervigni, G.D.L.; Spampinato, C.P. Metabolic profiles of soybean roots during early stages of Fusarium tucumaniae infection. J. Exp. Bot. 2015, 66, 391–402. [Google Scholar] [CrossRef]
- Fagard, M.; Launay, A.; Clément, G.; Courtial, J.; Dellagi, A.; Farjad, M.; Krapp, A.; Soulié, M.C.; Masclaux-Daubresse, C. Nitrogen metabolism meets phytopathology. J. Exp. Bot. 2014, 65, 5643–5656. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Rogers, L.M.; Song, Y.; Guo, W.; Kolattukudy, P.E. Homoserine and asparagine are host signals that trigger in planta expression of a pathogenesis gene in Nectria haematococca. Proc. Natl. Acad. Sci. USA 2005, 102, 4197–4202. [Google Scholar] [CrossRef] [PubMed]
- Melcher, I.M. Homoserine synthesis in dark-grown and light-grown seedlings of Pisum sativum. New Phytol. 1985, 100, 157–162. [Google Scholar] [CrossRef]
- Mitchell, D.J.; Bidwell, R.G.S. Synthesis and metabolism of homoserine in developing pea seedlings. Can. J. Bot. 1970, 48, 2037–2042. [Google Scholar] [CrossRef]
- Lea, P.J.; Sodek, L.; Parry, M.A.J.; Shewry, P.R.; Halford, N.G. Asparagine in plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- Seifi, H.S.; De Vleesschauwer, D.; Aziz, A.; Höfte, M. Modulating plant primary amino acid metabolism as a necrotrophic virulence strategy: The immune-regulatory role of asparagine synthetase in botrytis cinerea-tomato interaction. Plant Signal. Behav. 2014, 9, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Al-Quraan, N.A.; Al-Ajlouni, Z.I.; Qawasma, N.F. Physiological and biochemical characterization of the GABA shunt pathway in pea (Pisum sativum L.) seedlings under drought stress. Horticulturae 2021, 7, 125. [Google Scholar] [CrossRef]
- Shelp, B.J.; Aghdam, M.S.; Flaherty, E.J. γ-aminobutyrate (GABA) regulated plant defense: Mechanisms and opportunities. Plants 2021, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Seifi, H.S.; Curvers, K.; De Vleesschauwer, D.; Delaere, I.; Aziz, A.; Höfte, M. Concurrent overactivation of the cytosolic glutamine synthetase and the GABA shunt in the ABA-deficient sitiens mutant of tomato leads to resistance against Botrytis cinerea. New Phytol. 2013, 199, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline metabolism as regulatory hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Ratajczak, L. The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiol. Plant. 2014, 36, 1607–1619. [Google Scholar] [CrossRef]
- Liu, Y.H.; Song, Y.H.; Ruan, Y.L. Sugar conundrum in plant-pathogen interactions: Roles of invertase and sugar transporters depend on pathosystems. J. Exp. Bot. 2022, 73, 1910–1925. [Google Scholar] [CrossRef] [PubMed]
- Kopczewski, T.; Kuźniak, E.; Ciereszko, I.; Kornaś, A. Alterations in primary carbon metabolism in cucumber infected with Pseudomonas syringae pv lachrymans: Local and systemic responses. Int. J. Mol. Sci. 2022, 23, 12418. [Google Scholar] [CrossRef] [PubMed]
- Agudo-Jurado, F.J.; Reveglia, P.; Rubiales, D.; Evidente, A.; Barilli, E. Status of phytotoxins isolated from necrotrophic fungi causing diseases on grain legumes. Int. J. Mol. Sci. 2023, 24, 5116. [Google Scholar] [CrossRef]
- Perincherry, L.; Witaszak, N.; Urbaniak, M.; Waśkiewicz, A.; Stępień, Ł. Effects of secondary metabolites from pea on Fusarium growth and mycotoxin biosynthesis. J. Fungi 2021, 7, 1004. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, transport and physiological activity in leaf and root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Vishwakarma, K.; Kumar, V.; Arif, N.; Das, S.; Johnson, R.; Janeeshma, E.; Puthur, J.T.; Aliniaeifard, S.; Chauhan, D.K.; et al. Metal/Metalloid-based nanomaterials for plant abiotic stress tolerance: An overview of the mechanisms. Plants 2022, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Dilnawaz, F.; Misra, A.N.; Apostolova, E. Involvement of nanoparticles in mitigating plant’s abiotic stress. Plant Stress 2023, 10, 100280. [Google Scholar] [CrossRef]
- Rajwade, J.M.; Chikte, R.G.; Paknikar, K.M. Nanomaterials: New weapons in a crusade against phytopathogens. Appl. Microbiol. Biotechnol. 2020, 104, 1437–1461. [Google Scholar] [CrossRef]
- El-Shetehy, M.; Moradi, A.; Maceroni, M.; Reinhardt, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Mauch, F.; Schwab, F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Kim, H.J.; Su Kim, J.; Kim, M.S.; Yoon, B.D.; Park, H.J.; Kim, C.Y. A nanosized Ag–silica hybrid complex prepared by γ-irradiation activates the defense response in Arabidopsis. Radiat. Phys. Chem. 2012, 81, 180–184. [Google Scholar] [CrossRef]
- Jiang, L.; Xiang, S.; Lv, X.; Wang, X.; Li, F.; Liu, W.; Liu, C.; Ran, M.; Huang, J.; Xu, X.; et al. Biosynthesized silver nanoparticles inhibit Pseudomonas syringae pv. tabaci by directly destroying bacteria and inducing plant resistance in Nicotiana benthamiana. Phytopathol. Res. 2022, 4, 43. [Google Scholar] [CrossRef]
- Mercado-Meza, D.Y.; Guevara-González, R.G.; Esquivel, K.; Carbajal-Valenzuela, I.; Avila-Quezada, G.D. Green silver nanoparticles display protection against Clavibacter michiganensis subsp. michiganensis in tomato plants (Solanum lycopersicum L.). Plant Stress 2023, 10, 100256. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Szablińska-Piernik, J.; Głowacka, K.; Stałanowska, K.; Railean-Plugaru, V.; Hrobowicz, M.; Pomastowski, P.; Buszewski, B. The effect of bio-synthesized silver nanoparticles on germination, early seedling development, and metabolome of wheat (Triticum aestivum L.). Molecules 2022, 27, 2303. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hillstrand, D.S.; Auld, D.L. Comparative evaluation of four techniques for screening winter peas for resistance to Phoma medicaginis var. pinodella. Crop. Sci. 1982, 22, 282–287. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.; Bufo, S.A.; Camele, I. An attempt of biocontrol the tomato-wilt disease caused by Verticillium dahliae using Burkholderia gladioli pv. agaricicola and its bioactive secondary metabolites. Int. J. Plant Biol. 2017, 8, 57–60. [Google Scholar] [CrossRef][Green Version]
- Kulik, T.; Jestoi, M.; Okorski, A. Development of TaqMan assays for the quantitative detection of Fusarium avenaceum/Fusarium tricinctum and Fusarium poae esyn1 genotypes from cereal grain. FEMS Microbiol. Lett. 2011, 314, 49–56. [Google Scholar] [CrossRef]
- Okorski, A.; Milewska, A.; Pszczółkowska, A.; Karpiesiuk, K.; Kozera, W.; Dąbrowska, J.A.; Radwińska, J. Prevalence of Fusarium fungi and deoxynivalenol levels in winter wheat grain in different climatic regions of Poland. Toxins 2022, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolite profiling in Arabidopsis. In Arabidopsis Protocol. Methods in Molecular Biology; Salinas, J., Sanchez-Serrano, J.J., Eds.; Humana Press: Totowa, NJ, USA, 2006; Volume 323, pp. 439–447. [Google Scholar]
- Sun, X.; Weckwerth, W. COVAIN: A toolbox for uni- and multivariate statistics, time-series and correlation network analysis and inverse estimation of the differential Jacobian from metabolomics covariance data. Metabolomics 2012, 8, 81–93. [Google Scholar] [CrossRef]





| After 7 Days | After 14 Days | |||||
|---|---|---|---|---|---|---|
| Mycelium Diameter (mm) | MGI (%) | Mycelium Diameter (mm) | MGI (%) | |||
| D. pinodes | Control | 41.5 A | - | 73.2 A | - | |
| Fungicide | 6.0 D | 100.00 A | 12.0 E | 91.06 A | ||
| bio-AgNPs | 10 mg/L | 41.2 AB | 0.10 B | 64.7 B | 12.82 C | |
| 100 mg/L | 33.6 AB | 21.81 B | 49.4 C | 22.73 BC | ||
| 150 mg/L | 29.2 B | 33.68 B | 49.4 C | 21.95 BC | ||
| 200 mg/L | 23.8 C | 49.23 AB | 38.5 D | 45.06 B | ||
| F. avenaceum | Control | 62.6 A | - | 78.9 A | - | |
| Fungicide | 6.0 C | 100.00 A | 6.0 C | 100.00 A | ||
| bio-AgNPs | 10 mg/L | 63.7 A | −2.24 C | 85.0 A | −8.96 C | |
| 100 mg/L | 37.4 B | 44.89 B | 69.3 B | 13.30 B | ||
| 150 mg/L | 31.3 B | 55.31 B | 64.1 B | 19.99 B | ||
| 200 mg/L | 31.3 B | 55.43 B | 59.8 B | 25.92 B | ||
| Control | Seedlings Infected after Short-Term Immersion in | ||||||
|---|---|---|---|---|---|---|---|
| Water | Fungicide * | Bio-AgNPs | |||||
| 100 mg/L | 200 mg/L | ||||||
| D. pinodes infection | FW (mg) | shoots | 211.1 a | 136.5 b | 160.1 ab | 171.3 ab | 198.0 ab |
| roots | 195.0 ab | 159.3 b | 205.5 ab | 210.2 ab | 218.6 a | ||
| Length (mm) | shoots | 33.4 a | 31.7 a | 30.3 a | 36.3 a | 38.5 a | |
| roots | 54.7 bc | 47.7 c | 55.0 b | 59.9 ab | 62.2 a | ||
| F. avenaceum infection | FW (mg) | shoots | 307.2 ab | 212.7 b | 286.0 ab | 331.4 a | 307.4 a |
| roots | 363.6 a | 364.9 a | 333.0 a | 354.2 a | 290.3 a | ||
| Length (mm) | shoots | 56.7 ab | 44.0 b | 57.4 ab | 60.6 a | 64.4 a | |
| roots | 97.6 a | 95.3 a | 97.4 a | 99.0 a | 96.7 a | ||
| Control | Seedlings Infected after Short-Term Immersion in | ||||||
|---|---|---|---|---|---|---|---|
| Water | Fungicide * | Bio-AgNPs | |||||
| 100 mg/L | 200 mg/L | ||||||
| Metabolites | mg/g DW | ||||||
| D. pinodes infection | Roots | TIPMs, including the following: | 64.90 b | 65.53 b | 74.17 a | 58.12 c | 56.21 c |
| TSCs | 17.16 ab | 15.74 c | 17.70 a | 16.83 b | 12.29 d | ||
| TAAs | 36.19 bc | 39.06 b | 45.25 a | 31.33 c | 34.11 bc | ||
| TOAs | 3.57 bc | 3.74 a | 3.68 ab | 3.44 c | 3.24 d | ||
| TRCs | 7.98 a | 6.99 c | 7.55 b | 6.53 d | 6.57 d | ||
| Shoots | TIPMs, including the following: | 121.26 a | 82.87 b | 80.22 b | 80.25 b | 80.84 b | |
| TSCs | 47.41 a | 20.46 b | 18.00 c | 18.77 c | 19.01 bc | ||
| TAAs | 59.63 a | 44.82 b | 46.27 b | 45.21 b | 45.48 b | ||
| TOAs | 3.54 d | 4.32 b | 3.91 c | 3.94 c | 5.06 a | ||
| TRCs | 10.68 d | 13.25 a | 12.03 b | 12.34 b | 11.29 c | ||
| F. avenaceum infection | Roots | TIPMs, including the following: | 59.52 c | 64.05 b | 72.73 a | 73.31 a | 71.45 a |
| TSCs | 14.65 b | 12.84 c | 13.63 bc | 14.80 b | 17.11 a | ||
| TAAs | 36.28 c | 41.36 b | 48.75 a | 48.21 a | 43.96 b | ||
| TOAs | 2.86 c | 4.08 a | 4.20 a | 3.40 b | 3.03 c | ||
| TRCs | 5.72 c | 5.78 c | 6.15 c | 6.90 b | 7.34 a | ||
| Shoots | TIPMs, including the following: | 117.80 b | 72.23 d | 103.45 c | 132.60 a | 119.60 b | |
| TSCs | 47.72 a | 29.64 c | 27.86 c | 46.12 a | 38.52 b | ||
| TAAs | 58.82 c | 30.31 d | 61.82 bc | 73.94 a | 68.74 ab | ||
| TOAs | 3.12 b | 3.08 b | 3.68 a | 3.12 b | 3.25 b | ||
| TRCs | 8.13 c | 9.20 b | 10.10 a | 9.42 b | 9.10 b | ||
| Genotype/Gene | Primer/Probe | Sequence (5′-3′) | Regression Equation, Efficiency of qPCR (E) | References |
|---|---|---|---|---|
| D. pinodes ITS | Forward | 5′-AGAGACCGATAGCGCACAAG-3′ | y = −3.77x + 23.9 R2 = 0.96; E = 91.9 | [13] |
| Reverse | 5′-AGTCCAGGCTGGTTGCAGGA-3′ | |||
| Probe | FAM—CATGTACCTCTCTTCGGG—MGB | |||
| F. avenaceum Esyn1 | Forward | 5′-AGCAGTCGAGTTCGTCAACAGA-3′ | y = −3.44x + 19.7 R2 = 0.99; E = 95.3 | [119] |
| Reverse | 5′-GGCYTTTCCTGCGAACTTG-3′ | |||
| Probe | FAM—CCGTCGAGTCCTCT—MGB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stałanowska, K.; Szablińska-Piernik, J.; Pszczółkowska, A.; Railean, V.; Wasicki, M.; Pomastowski, P.; Lahuta, L.B.; Okorski, A. Antifungal Properties of Bio-AgNPs against D. pinodes and F. avenaceum Infection of Pea (Pisum sativum L.) Seedlings. Int. J. Mol. Sci. 2024, 25, 4525. https://doi.org/10.3390/ijms25084525
Stałanowska K, Szablińska-Piernik J, Pszczółkowska A, Railean V, Wasicki M, Pomastowski P, Lahuta LB, Okorski A. Antifungal Properties of Bio-AgNPs against D. pinodes and F. avenaceum Infection of Pea (Pisum sativum L.) Seedlings. International Journal of Molecular Sciences. 2024; 25(8):4525. https://doi.org/10.3390/ijms25084525
Chicago/Turabian StyleStałanowska, Karolina, Joanna Szablińska-Piernik, Agnieszka Pszczółkowska, Viorica Railean, Miłosz Wasicki, Paweł Pomastowski, Lesław Bernard Lahuta, and Adam Okorski. 2024. "Antifungal Properties of Bio-AgNPs against D. pinodes and F. avenaceum Infection of Pea (Pisum sativum L.) Seedlings" International Journal of Molecular Sciences 25, no. 8: 4525. https://doi.org/10.3390/ijms25084525
APA StyleStałanowska, K., Szablińska-Piernik, J., Pszczółkowska, A., Railean, V., Wasicki, M., Pomastowski, P., Lahuta, L. B., & Okorski, A. (2024). Antifungal Properties of Bio-AgNPs against D. pinodes and F. avenaceum Infection of Pea (Pisum sativum L.) Seedlings. International Journal of Molecular Sciences, 25(8), 4525. https://doi.org/10.3390/ijms25084525








