Hinokitiol Inhibits Breast Cancer Cells In Vitro Stemness-Progression and Self-Renewal with Apoptosis and Autophagy Modulation via the CD44/Nanog/SOX2/Oct4 Pathway
Abstract
1. Introduction
2. Results
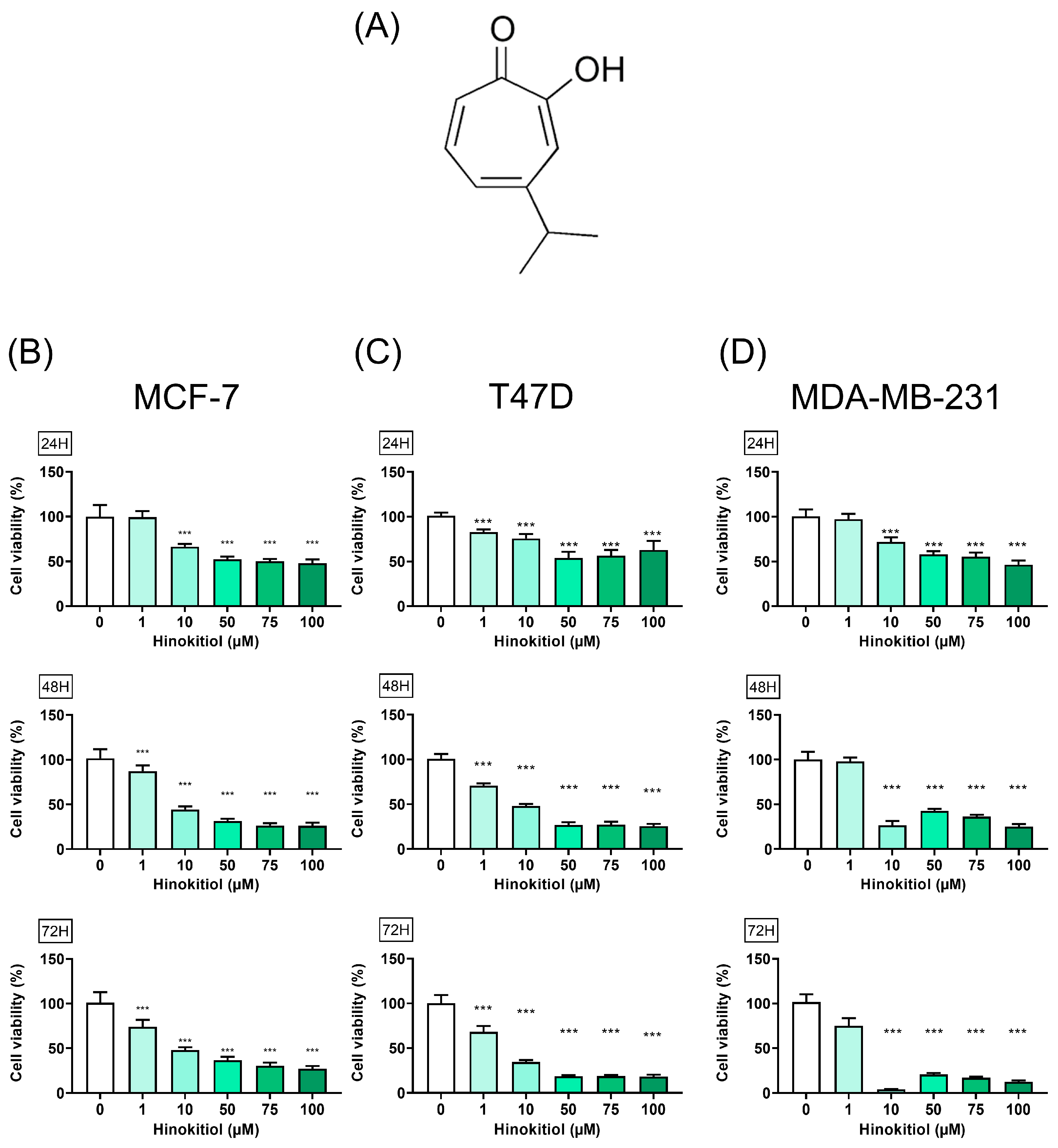
2.1. Hinokitiol on Breast Cancer Cell Viability
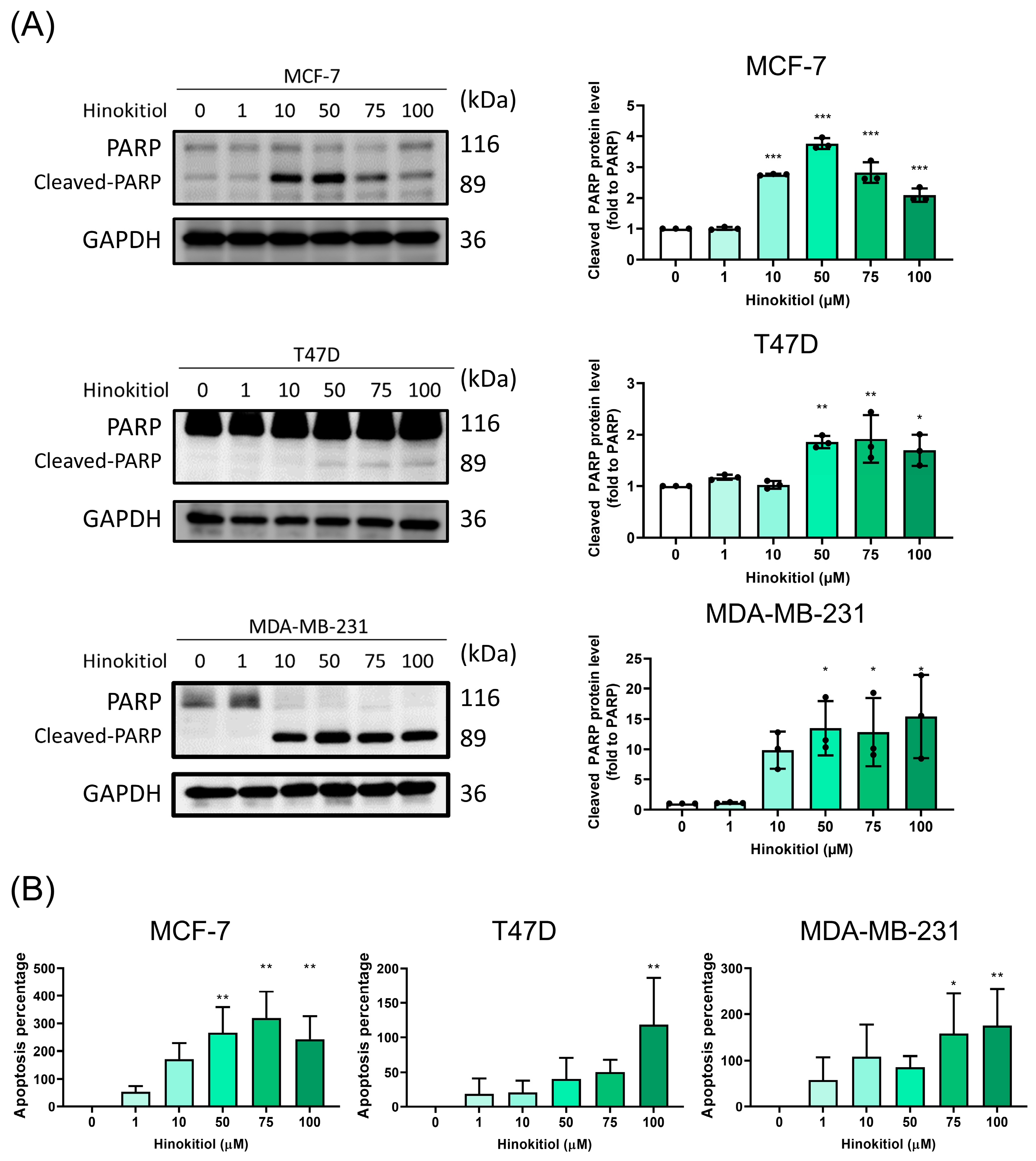
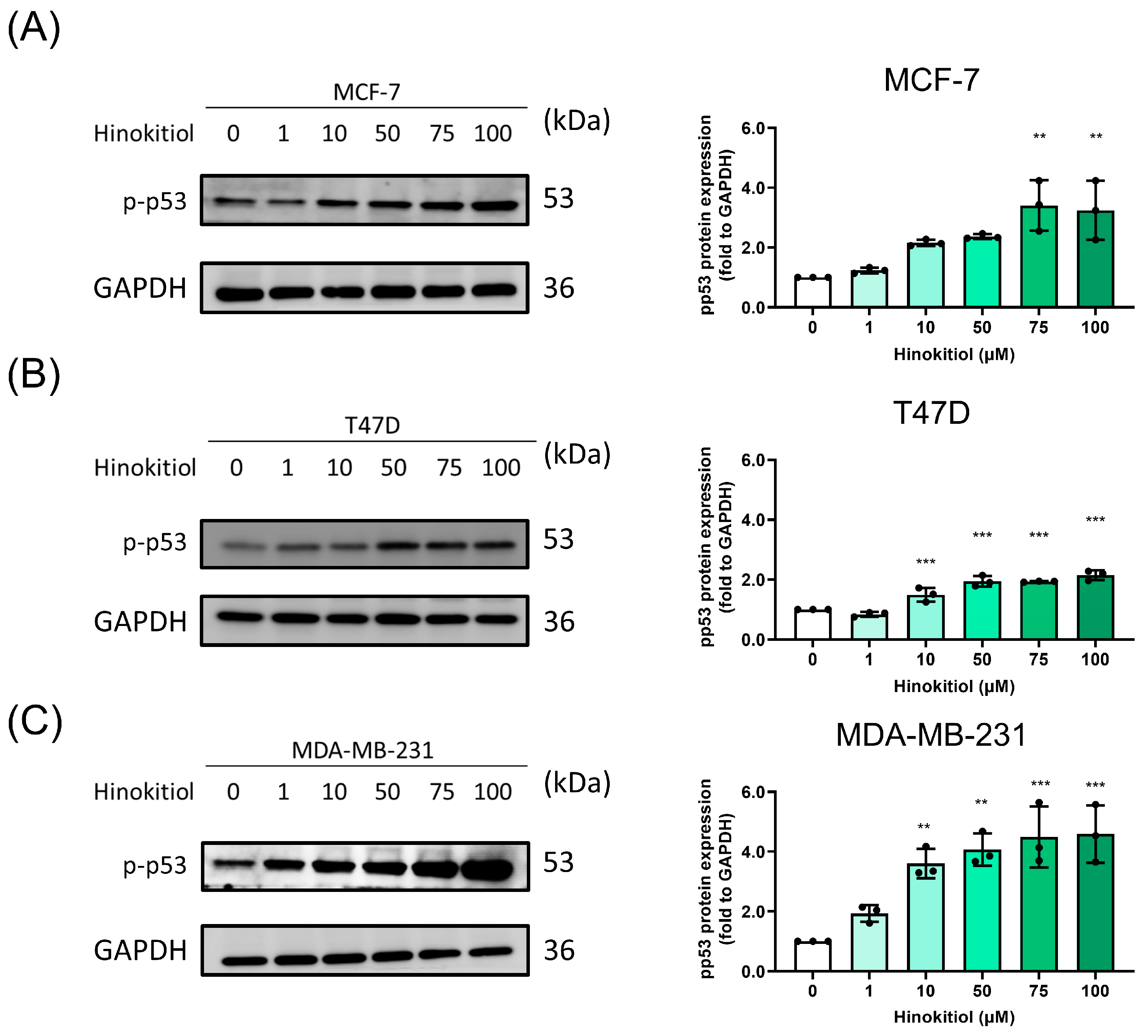
2.2. Hinokitiol Enhanced Breast Cancer Cell Apoptosis via Increasing Cleaved-PARP and p-p53 Protein Expression
2.3. Modulation of Hinokitiol in Autophagy Influx
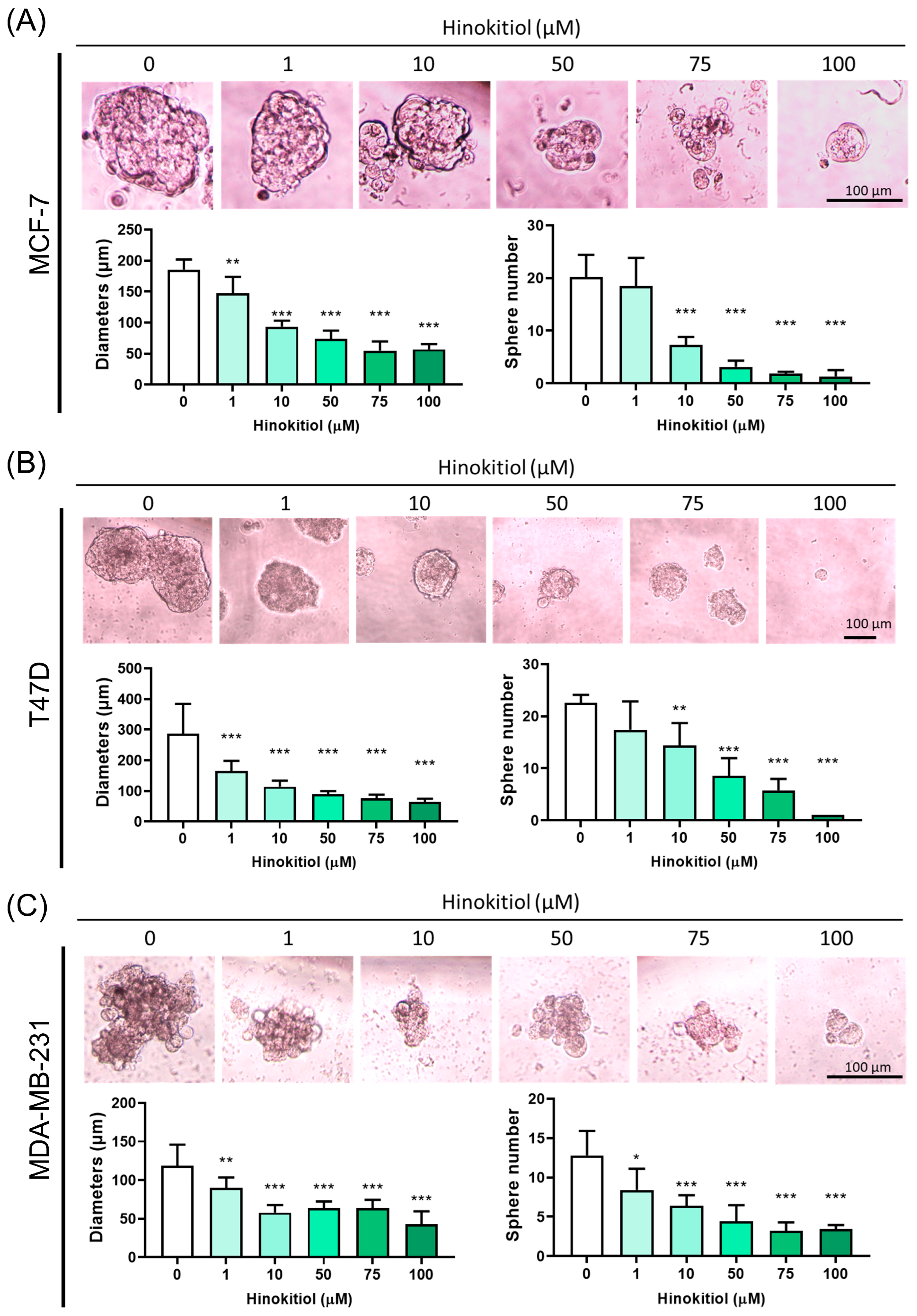
2.4. Effect of Hinokitiol on MCF-7, T47D, MDA-MB-231 Breast Cancer Cell Sphere Number and Diameter Analysis
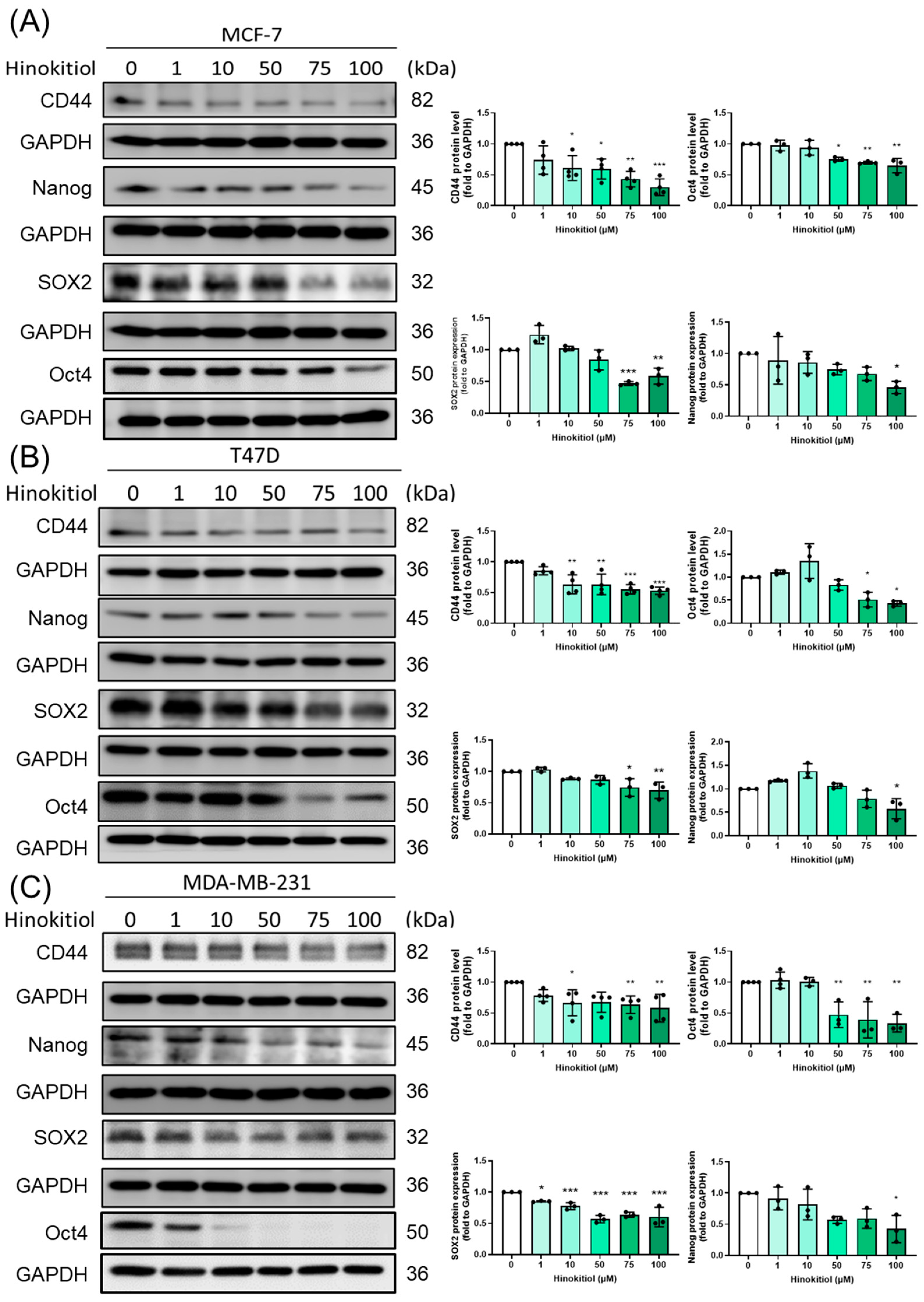
2.5. Effect of Hinokitiol on MCF-7, T47D, MDA-MB-231 Breast Cancer Cell Stemness Related Protein Expression
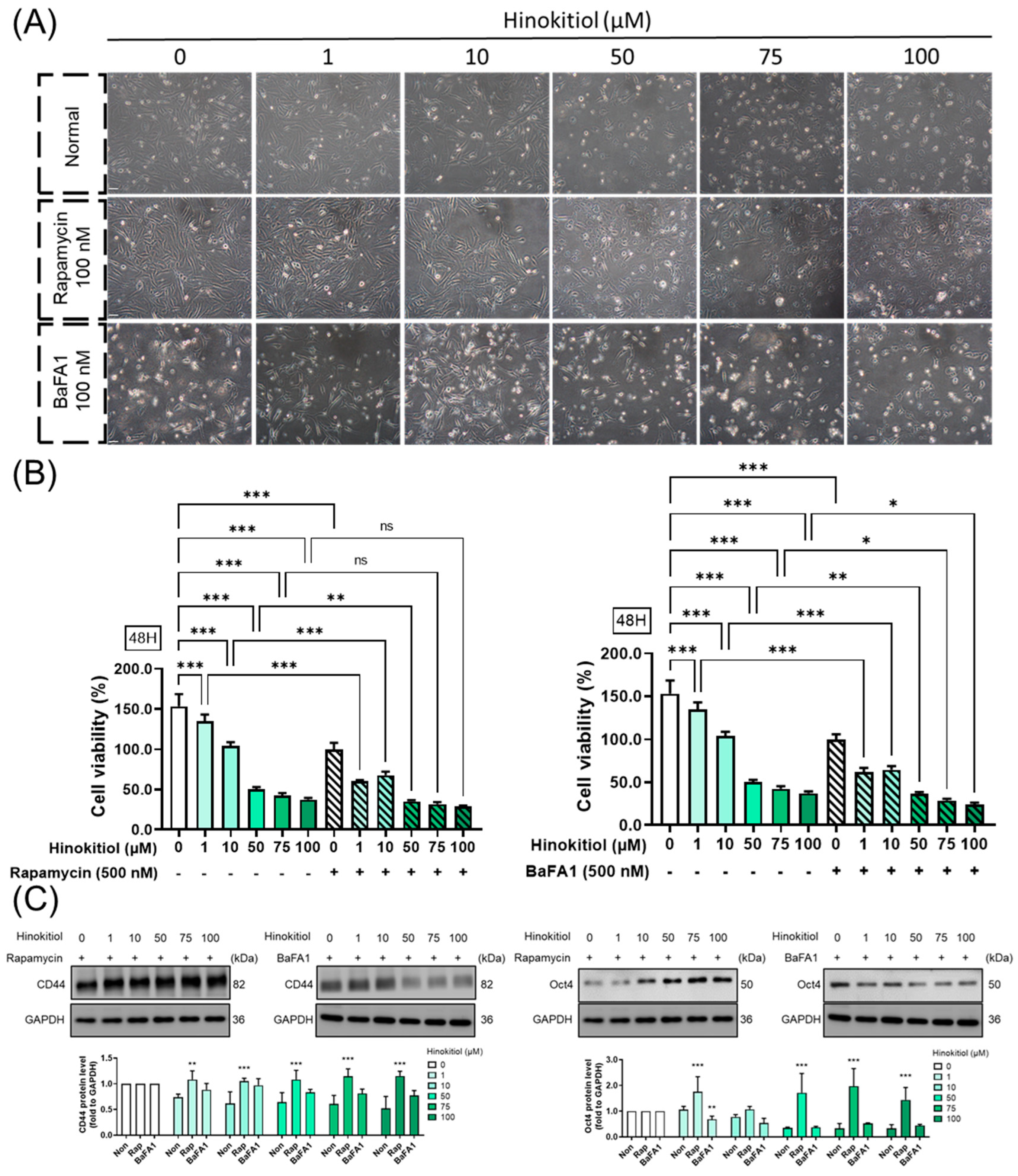
2.6. Effect of Autophagy Modulation in Hinokitiol’s Effect on Proliferation and Stemness Related Protein Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents
4.3. MTT Assay
4.4. Apoptosis Percentage
4.5. Sphere Formation
4.6. Western Blot
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Horlings, H.M.; Kreike, B.; Hayes, M.M.; Hauptmann, M.; Wessels, L.F.; de Jong, D.; Van de Vijver, M.J.; Van’t Veer, L.J.; Peterse, J.L. Refinement of breast cancer classification by molecular characterization of histological special types. J. Pathol. 2008, 216, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.J. Strategies for subtypes—Dealing with the diversity of breast cancer: Highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Nedeljković, M.; Damjanović, A. Mechanisms of chemotherapy resistance in triple-negative breast cancer—How we can rise to the challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [PubMed]
- Zhong, W.; Shen, Z.; Wang, M.; Wang, H.; Sun, Y.; Tao, X.; Hou, D. Tumor microenvironment responsive nanomicelle with folic acid modification co-delivery of doxorubicin/shikonin for triple negative breast cancer treatment. Pharmaceuticals 2023, 16, 374. [Google Scholar] [CrossRef]
- Gao, T.; Yang, X.; Fujisawa, M.; Ohara, T.; Wang, T.; Tomonobu, N.; Sakaguchi, M.; Yoshimura, T.; Matsukawa, A. Spred2: A novel regulator of epithelial-mesenchymal transition and stemness in hepatocellular carcinoma cells. Int. J. Mol. Sci. 2023, 24, 4996. [Google Scholar] [CrossRef]
- Mathews, L.A.; Crea, F.; Farrar, W.L. Epigenetic gene regulation in stem cells and correlation to cancer. Differentiation 2009, 78, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.; Wilson, G.D. The importance of cd44 as a stem cell biomarker and therapeutic target in cancer. Stem Cells Int. 2016, 2016, 2087204. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.H.; Yu, C.C.; Huang, C.Y.; Lin, S.C.; Liu, C.J.; Tsai, T.H.; Chou, S.H.; Chien, C.S.; Ku, H.H.; Lo, J.F. Positive correlations of oct-4 and nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res. 2008, 14, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Wiman, K.G. P53 talks to parp: The increasing complexity of p53-induced cell death. Cell Death Differ. 2013, 20, 1438–1439. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wei, T.; Li, Z.; Zhu, J. P53-dependent apoptosis is essential for the antitumor effect of paclitaxel response to DNA damage in papillary thyroid carcinoma. Int. J. Med. Sci. 2021, 18, 3197–3205. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Wang, C.C.; Wu, C.F.; Lin, Y.H.; Lee, W.C.; Chen, P.J.; Chang, Y.U.; Su, Y.C. Hinokitiol inhibits the viability of oral squamous carcinoma cells by inducing apoptosis and autophagy. Anticancer Res. 2023, 43, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Machino, K.; Sakakibara, Y.; Osada, K.; Ochiai, T.; Uraki, Y.; Shigetomi, K. Pseudomonas bohemica strain ins3 eliminates antibacterial hinokitiol from its culture broth. Biosci. Biotechnol. Biochem. 2023, 87, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Hsu, W.H.; Yen, T.L.; Luo, J.Y.; Kuo, Y.C.; Fong, T.H.; Sheu, J.R. Hinokitiol, a natural tropolone derivative, offers neuroprotection from thromboembolic stroke in vivo. Evid.-Based Complement. Altern. Med. 2013, 2013, 840487. [Google Scholar] [CrossRef]
- Seno, S.; Kimura, M.; Yashiro, Y.; Kimura, R.; Adachi, K.; Terabayashi, A.; Takahashi, M.; Oyama, T.; Abe, H.; Abe, T.; et al. Β-thujaplicin enhances trail-induced apoptosis via the dual effects of xiap inhibition and degradation in nci-h460 human lung cancer cells. Medicines 2021, 8, 26. [Google Scholar] [CrossRef]
- Zhang, G.; He, J.; Ye, X.; Zhu, J.; Hu, X.; Shen, M.; Ma, Y.; Mao, Z.; Song, H.; Chen, F. Β-thujaplicin induces autophagic cell death, apoptosis, and cell cycle arrest through ros-mediated akt and p38/erk mapk signaling in human hepatocellular carcinoma. Cell Death Dis. 2019, 10, 255. [Google Scholar] [CrossRef]
- Chen, J.; Ko, J.; Kim, J.T.; Cho, J.S.; Qiu, S.; Kim, G.-D.; Auh, J.-H.; Lee, H.J. Β-thujaplicin inhibits basal-like mammary tumor growth by regulating glycogen synthase kinase-3β/β-catenin signaling. Food Funct. 2019, 10, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Hsu, W.J.; Wu, L.H.; Liou, H.P.; Pangilinan, C.R.; Tyan, Y.C.; Lee, C.H. Hinokitiol reduces tumor metastasis by inhibiting heparanase via extracellular signal-regulated kinase and protein kinase b pathway. Int. J. Med. Sci. 2020, 17, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yamauchi, H. Hinokitiol, a metal chelator derived from natural plants, suppresses cell growth and disrupts androgen receptor signaling in prostate carcinoma cell lines. Biochem. Biophys. Res. Commun. 2006, 351, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Bao, C.; Park, H.C.; Kim, M.; Choi, H.K.; Kim, Y.S.; Lee, H.J. Β-thujaplicin modulates estrogen receptor signaling and inhibits proliferation of human breast cancer cells. Biosci. Biotechnol. Biochem. 2015, 79, 1011–1017. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, H.; Zhang, M.; Zhang, J.; Sun, Z.; Zhang, W.; Dong, W.; Cheng, C.; Yao, Y.; Li, K. Hinokitiol-iron complex is a ferroptosis inducer to inhibit triple-negative breast tumor growth. Cell Biosci. 2023, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.K.; Lin, S.T.; Chang, W.W.; Liu, L.W.; Li, T.Y.; Kuo, C.Y.; Hsieh, J.L.; Lee, C.H. Hinokitiol induces autophagy in murine breast and colorectal cancer cells. Environ. Toxicol. 2016, 31, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Lu, S.H.; Chang, C.C.; Thomas, P.A.; Jayakumar, T.; Sheu, J.R. Hinokitiol, a tropolone derivative, inhibits mouse melanoma (b16-f10) cell migration and in vivo tumor formation. Eur. J. Pharmacol. 2015, 746, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.H.; Chang, K.W.; Hsia, S.M.; Yu, C.C.; Fuh, L.J.; Chi, T.Y.; Shieh, T.M. In vitro antimicrobial and anticancer potential of hinokitiol against oral pathogens and oral cancer cell lines. Microbiol. Res. 2013, 168, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Pennati, M.; Lopergolo, A.; Profumo, V.; De Cesare, M.; Sbarra, S.; Valdagni, R.; Zaffaroni, N.; Gandellini, P.; Folini, M. Mir-205 impairs the autophagic flux and enhances cisplatin cytotoxicity in castration-resistant prostate cancer cells. Biochem. Pharmacol. 2014, 87, 579–597. [Google Scholar] [CrossRef]
- Lin, P.H.; Chiang, Y.F.; Shieh, T.M.; Chen, H.Y.; Shih, C.K.; Wang, T.H.; Wang, K.L.; Huang, T.C.; Hong, Y.H.; Li, S.C.; et al. Dietary compound isoliquiritigenin, an antioxidant from licorice, suppresses triple-negative breast tumor growth via apoptotic death program activation in cell and xenograft animal models. Antioxidants 2020, 9, 228. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, M.; Zhou, F.; Zhang, L.; Meng, X. The breast cancer stem cells traits and drug resistance. Front. Pharmacol. 2020, 11, 599965. [Google Scholar] [CrossRef]
- Cabarcas, S.M.; Mathews, L.A.; Farrar, W.L. The cancer stem cell niche—There goes the neighborhood? Int. J. Cancer 2011, 129, 2315–2327. [Google Scholar] [CrossRef]
- Kim, M.; Bakyt, L.; Akhmetkaliyev, A.; Toktarkhanova, D.; Bulanin, D. Re-sensitizing cancer stem cells to conventional chemotherapy agents. Int. J. Mol. Sci. 2023, 24, 2122. [Google Scholar] [CrossRef] [PubMed]
- Chanmee, T.; Ontong, P.; Kimata, K.; Itano, N. Key roles of hyaluronan and its cd44 receptor in the stemness and survival of cancer stem cells. Front. Oncol. 2015, 5, 180. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.C.; Liao, Y.W.; Chen, P.N.; Lu, K.H.; Yu, C.C.; Hsieh, P.L. Hinokitiol suppresses cancer stemness and oncogenicity in glioma stem cells by nrf2 regulation. Cancer Chemother. Pharmacol. 2017, 80, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.F.; Huang, K.C.; Chen, H.Y.; Huang, T.C.; Ali, M.; Chang, H.Y.; Shieh, T.M.; Shih, Y.H.; Wang, K.L.; Huang, Y.J.; et al. The adipokine visfatin modulates cancer stem cell properties in triple-negative breast cancer. Biomedicines 2023, 11, 297. [Google Scholar] [CrossRef]
- Salama, S.; Hamdy, N.M.; El-shimy, R.; El-Mesallamy, H. Clinical significance of the transcription factor sox11, cell-cell adhesion protein e-cadherin and zinc finger protein bcl11a in the diagnosis of breast cancer. Arch. Pharm. Sci. Ain Shams Univ. 2021, 5, 97–110. [Google Scholar] [CrossRef]
- Chen, S.M.; Wang, B.Y.; Lee, C.H.; Lee, H.T.; Li, J.J.; Hong, G.C.; Hung, Y.C.; Chien, P.J.; Chang, C.Y.; Hsu, L.S.; et al. Hinokitiol up-regulates mir-494-3p to suppress bmi1 expression and inhibits self-renewal of breast cancer stem/progenitor cells. Oncotarget 2017, 8, 76057–76068. [Google Scholar] [CrossRef]
- Tu, D.G.; Yu, Y.; Lee, C.H.; Kuo, Y.L.; Lu, Y.C.; Tu, C.W.; Chang, W.W. Hinokitiol inhibits vasculogenic mimicry activity of breast cancer stem/progenitor cells through proteasome-mediated degradation of epidermal growth factor receptor. Oncol. Lett. 2016, 11, 2934–2940. [Google Scholar] [CrossRef]
- Gao, W.; Wu, D.; Wang, Y.; Wang, Z.; Zou, C.; Dai, Y.; Ng, C.-F.; Teoh, J.Y.-C.; Chan, F.L. Development of a novel and economical agar-based non-adherent three-dimensional culture method for enrichment of cancer stem-like cells. Stem Cell Res. Ther. 2018, 9, 243. [Google Scholar] [CrossRef]
- Hong, X.; Chedid, K.; Kalkanis, S.N. Glioblastoma cell line-derived spheres in serum-containing medium versus serum-free medium: A comparison of cancer stem cell properties. Int. J. Oncol. 2012, 41, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Cheng, L.; Li, C.; Dai, L.; Zhang, H.; Yan, F.; Shi, H.; Dong, G.; Ning, Z.; et al. Erratum: Enrichment and characterization of cancer stem-like cells in ultra-low concentration of serum and non-adhesive culture system. Am. J. Transl. Res. 2020, 12, 2319–2320. [Google Scholar]
- Wu, D.; Yu, X.; Wang, J.; Hui, X.; Zhang, Y.; Cai, Y.; Ren, M.; Guo, M.; Zhao, F.; Dou, J. Ovarian cancer stem cells with high ror1 expression serve as a new prophylactic vaccine for ovarian cancer. J. Immunol. Res. 2019, 2019, 9394615. [Google Scholar] [CrossRef] [PubMed]
- Niklaus, N.J.; Tokarchuk, I.; Zbinden, M.; Schläfli, A.M.; Maycotte, P.; Tschan, M.P. The multifaceted functions of autophagy in breast cancer development and treatment. Cells 2021, 10, 1447. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Li, X.; Tang, X.; Jiang, J.; Han, Y.; Li, Y.; Ma, C.; Liu, Z.; He, Y. Neferine promotes the apoptosis of hnscc through the accumulation of p62/sqstm1 caused by autophagic flux inhibition. Int. J. Mol. Med. 2021, 48, 124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.A.; Johnson, S.K.; Brown, B.L.; McCarragher, L.M.; Al-Sakkaf, K.; Royds, J.A.; Dobson, P.R. Enhanced anti-cancer effect of a phosphatidylinositol-3 kinase inhibitor and doxorubicin on human breast epithelial cell lines with different p53 and oestrogen receptor status. Int. J. Cancer 2008, 123, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Zheng, Y.; Yan, Y.; Bargonetti, J.; Foster, D.A. Mutant p53 in mda-mb-231 breast cancer cells is stabilized by elevated phospholipase d activity and contributes to survival signals generated by phospholipase d. Oncogene 2006, 25, 7305–7310. [Google Scholar] [CrossRef] [PubMed]
- Sp, N.; Kang, D.Y.; Lee, J.M.; Bae, S.W.; Jang, K.J. Potential antitumor effects of 6-gingerol in p53-dependent mitochondrial apoptosis and inhibition of tumor sphere formation in breast cancer cells. Int. J. Mol. Sci. 2021, 22, 4660. [Google Scholar] [CrossRef]
- Johnson, S.; Chen, H.; Lo, P.-K. In vitro tumorsphere formation assays. Bio-Protocol 2013, 3, e325. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, Y.-F.; Huang, K.-C.; Chen, H.-Y.; Hamdy, N.M.; Huang, T.-C.; Chang, H.-Y.; Shieh, T.-M.; Huang, Y.-J.; Hsia, S.-M. Hinokitiol Inhibits Breast Cancer Cells In Vitro Stemness-Progression and Self-Renewal with Apoptosis and Autophagy Modulation via the CD44/Nanog/SOX2/Oct4 Pathway. Int. J. Mol. Sci. 2024, 25, 3904. https://doi.org/10.3390/ijms25073904
Chiang Y-F, Huang K-C, Chen H-Y, Hamdy NM, Huang T-C, Chang H-Y, Shieh T-M, Huang Y-J, Hsia S-M. Hinokitiol Inhibits Breast Cancer Cells In Vitro Stemness-Progression and Self-Renewal with Apoptosis and Autophagy Modulation via the CD44/Nanog/SOX2/Oct4 Pathway. International Journal of Molecular Sciences. 2024; 25(7):3904. https://doi.org/10.3390/ijms25073904
Chicago/Turabian StyleChiang, Yi-Fen, Ko-Chieh Huang, Hsin-Yuan Chen, Nadia M. Hamdy, Tsui-Chin Huang, Hsin-Yi Chang, Tzong-Ming Shieh, Yun-Ju Huang, and Shih-Min Hsia. 2024. "Hinokitiol Inhibits Breast Cancer Cells In Vitro Stemness-Progression and Self-Renewal with Apoptosis and Autophagy Modulation via the CD44/Nanog/SOX2/Oct4 Pathway" International Journal of Molecular Sciences 25, no. 7: 3904. https://doi.org/10.3390/ijms25073904
APA StyleChiang, Y.-F., Huang, K.-C., Chen, H.-Y., Hamdy, N. M., Huang, T.-C., Chang, H.-Y., Shieh, T.-M., Huang, Y.-J., & Hsia, S.-M. (2024). Hinokitiol Inhibits Breast Cancer Cells In Vitro Stemness-Progression and Self-Renewal with Apoptosis and Autophagy Modulation via the CD44/Nanog/SOX2/Oct4 Pathway. International Journal of Molecular Sciences, 25(7), 3904. https://doi.org/10.3390/ijms25073904








