Design, Synthesis and Biological Evaluation of Novel Phenyl-Substituted Naphthoic Acid Ethyl Ester Derivatives as Strigolactone Receptor Inhibitor
Abstract
1. Introduction
2. Results and Discussion
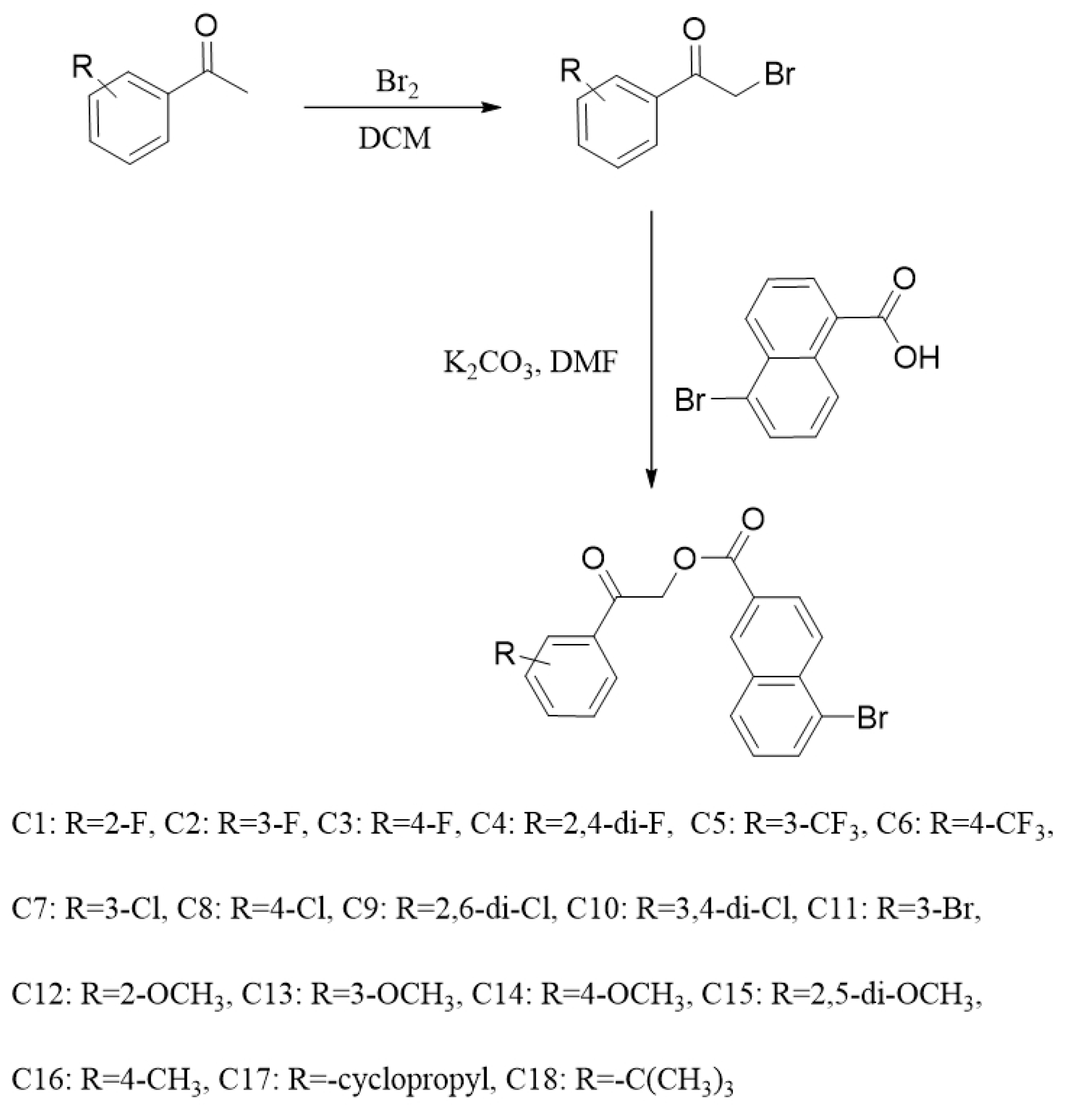
2.1. Chemistry
2.2. Biological Analysis
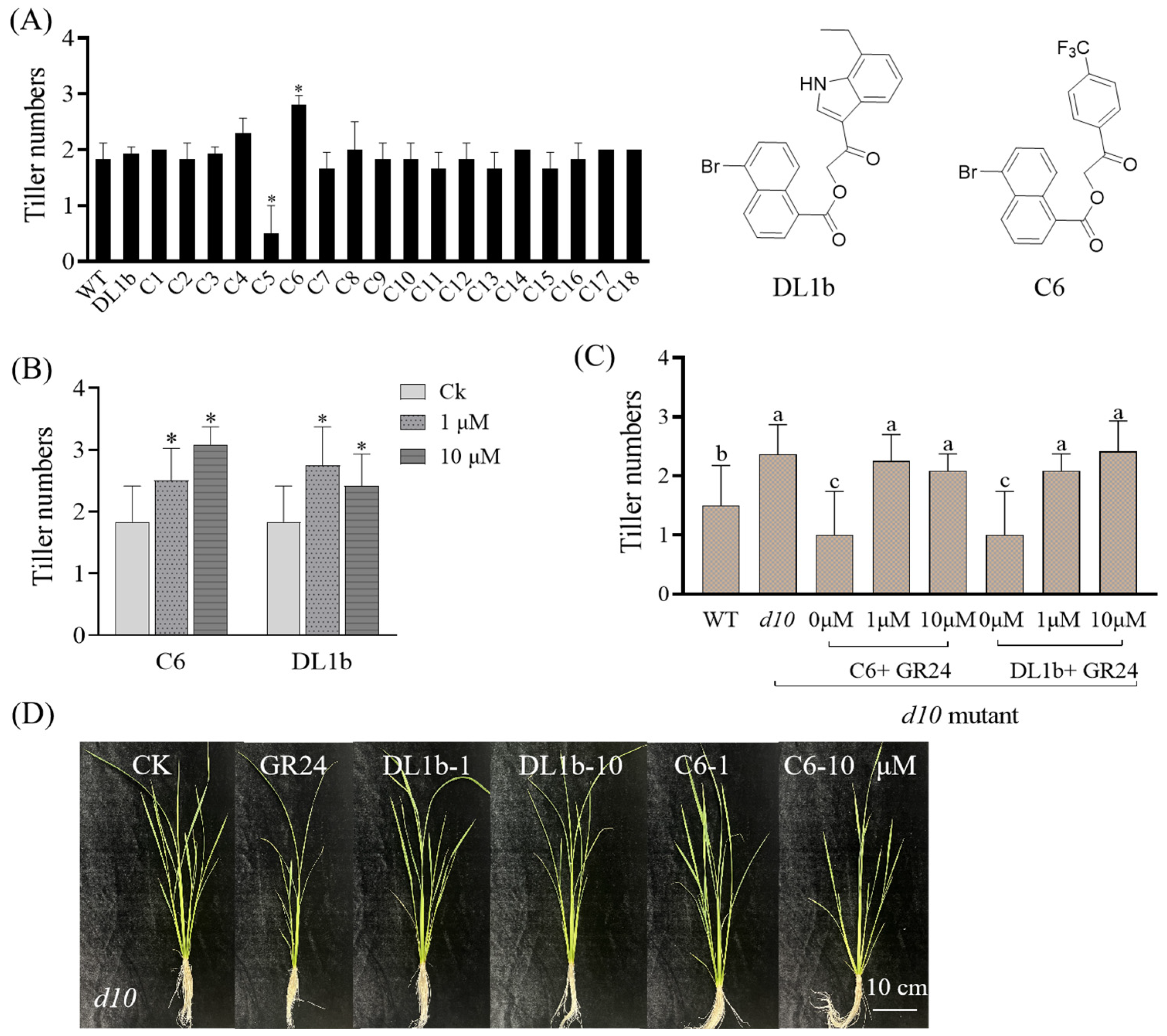
2.2.1. Effect of the Target Compounds on Rice Tillering of Wild Type and d10 Mutant
2.2.2. Parasitic Seed Germination in Response to Cs Application
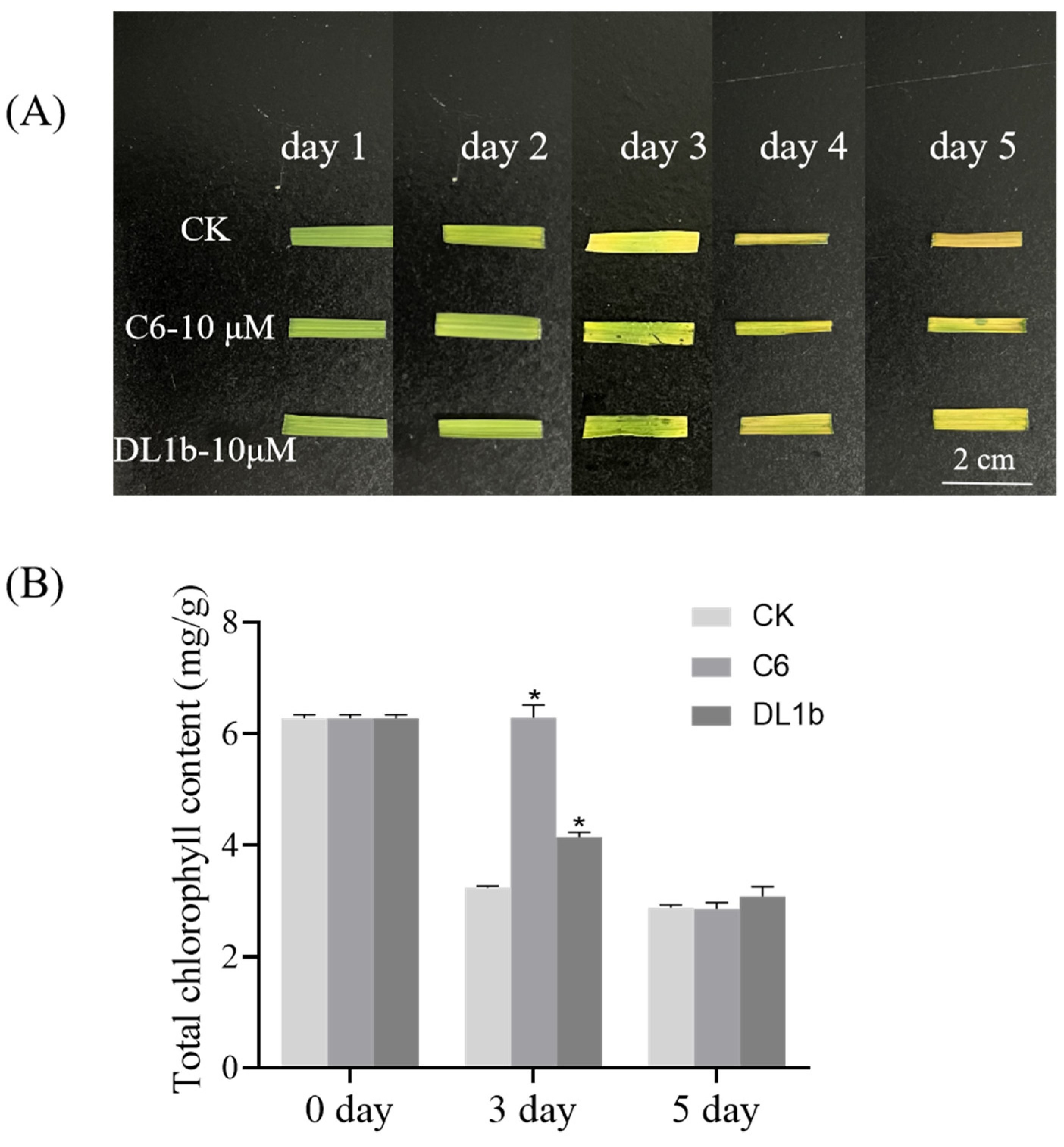
2.2.3. Bioactivity of C6 in Dark-Induced Leaf Senescence
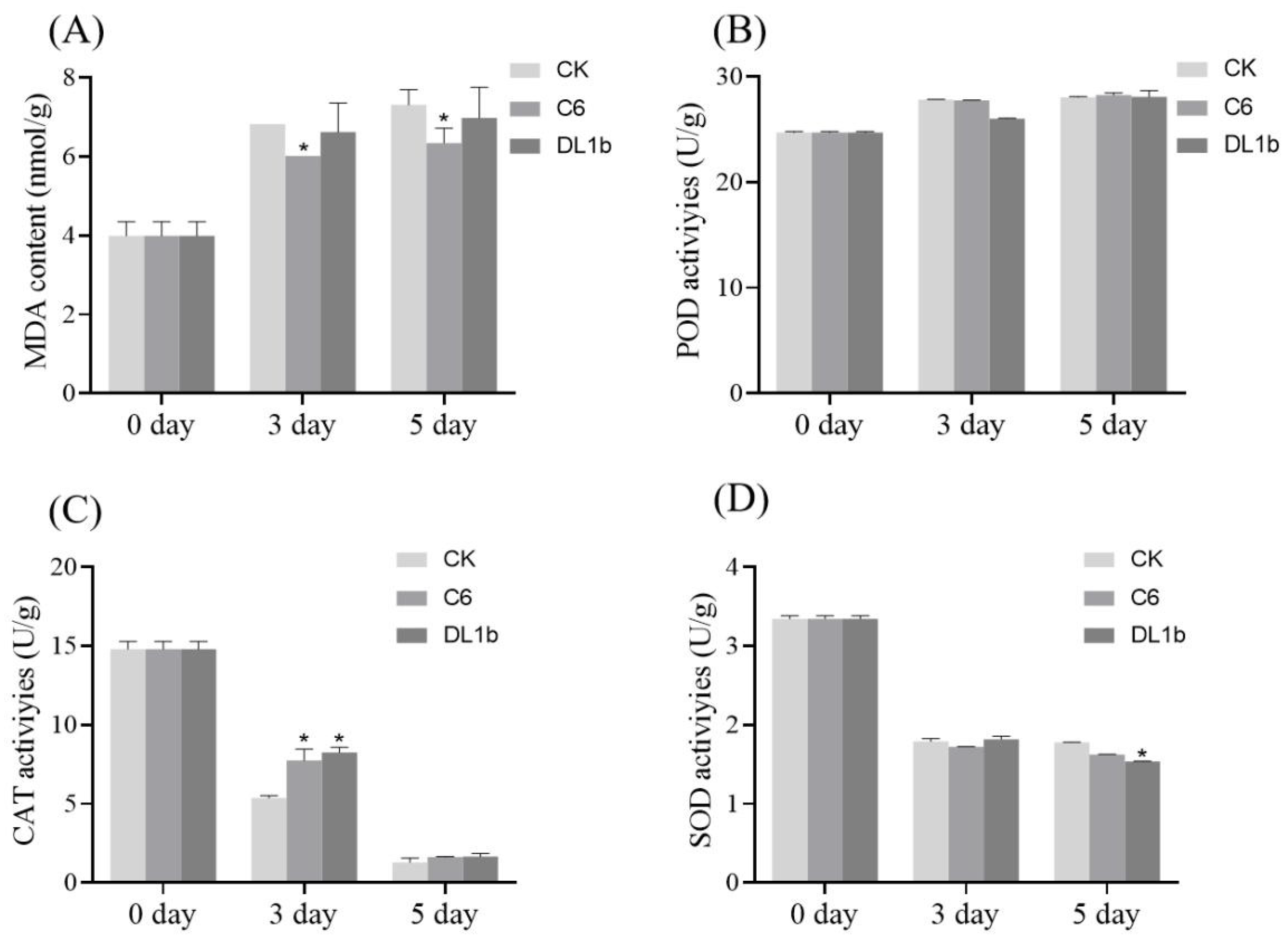
2.2.4. Effect on MDA (Malondialdehyde) Levels and SOD (Superoxide Dismutase), POD (Peroxidase), CAT (Catalase) Activities in Detached Rice Leaves in Response to C6 Application
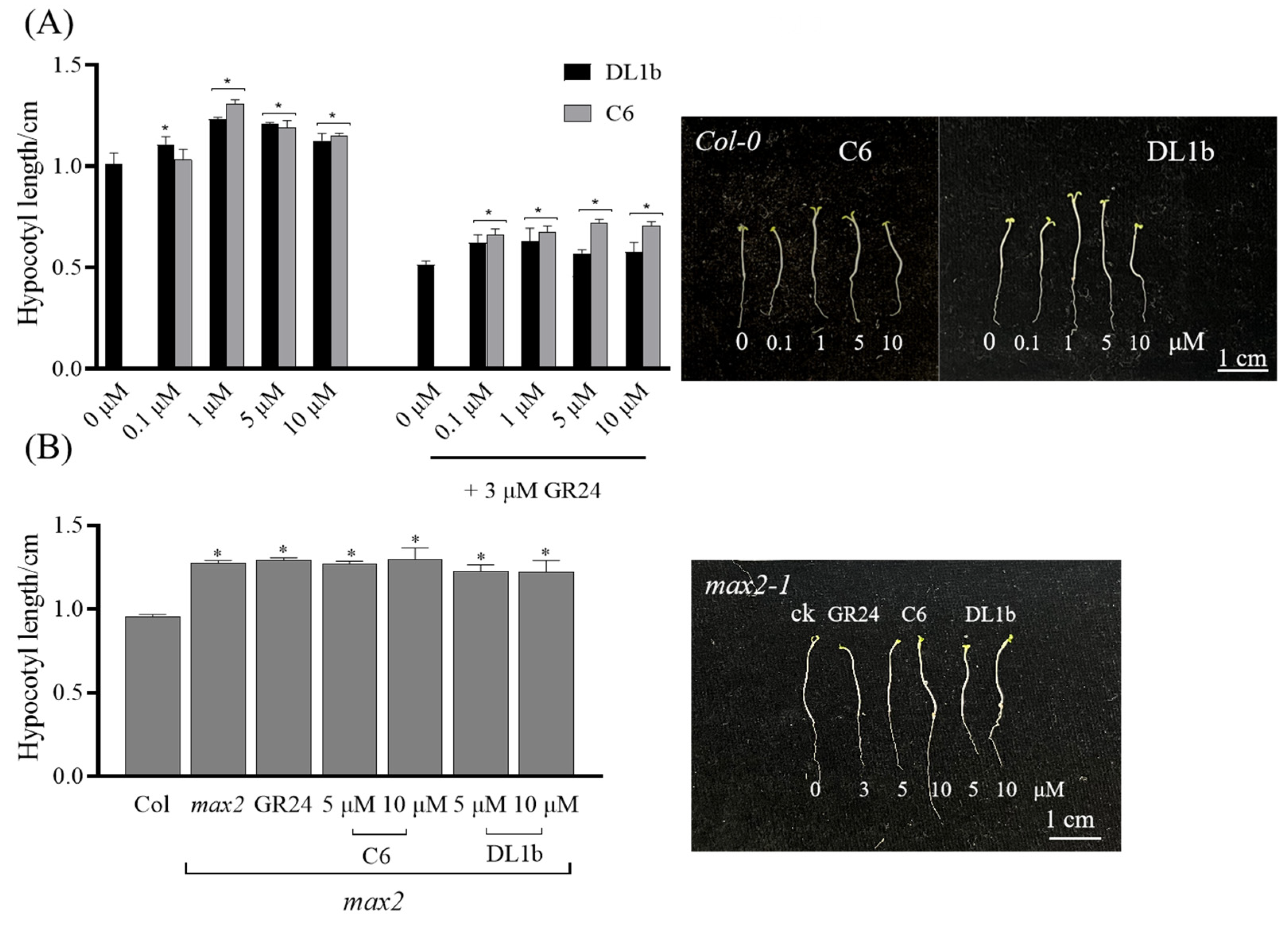
2.2.5. Activity of C6 on Arabidopsis Hypocotyl Elongation of Wild Type and max2 Mutant
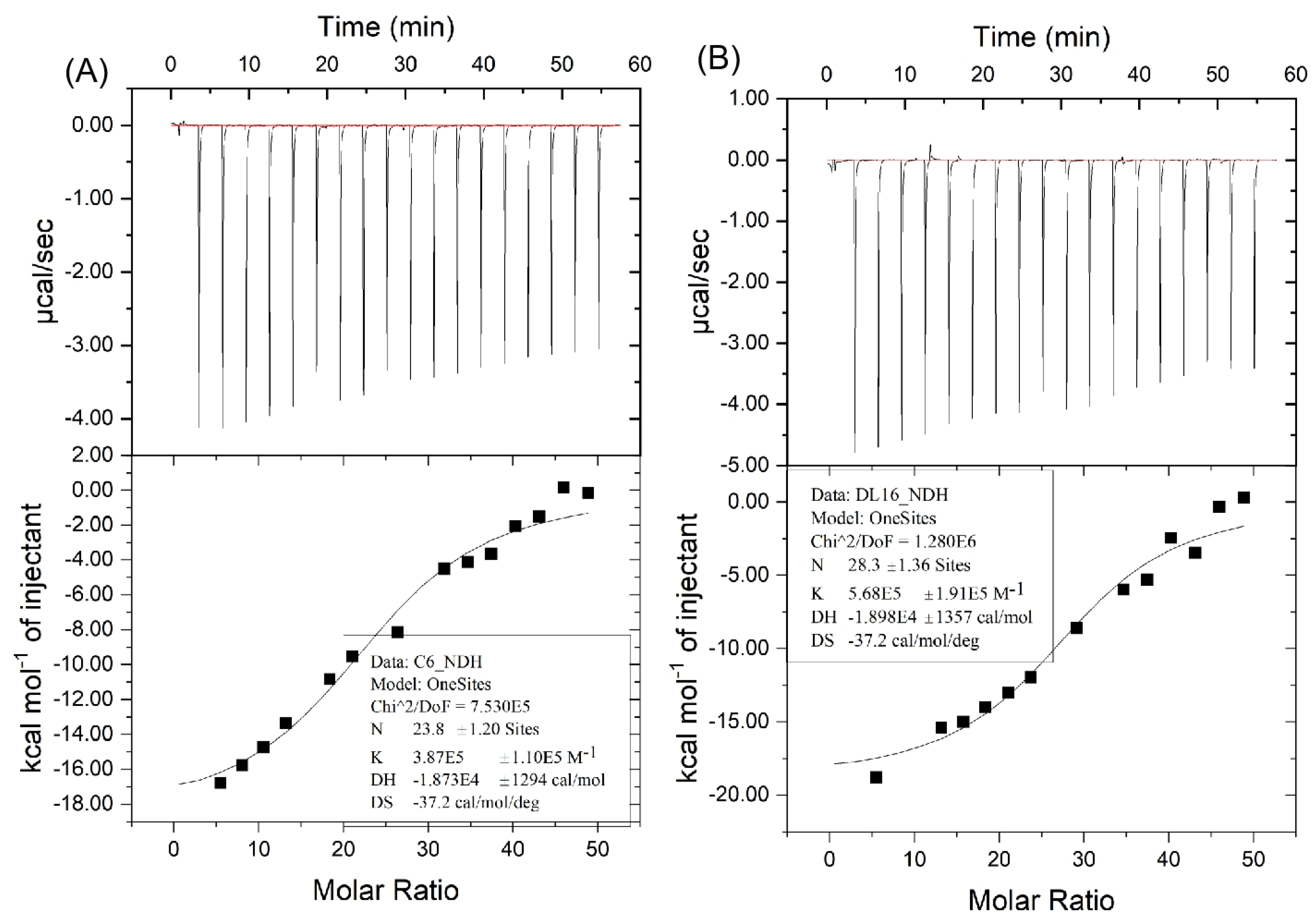
2.3. Analysis of ITC Assay
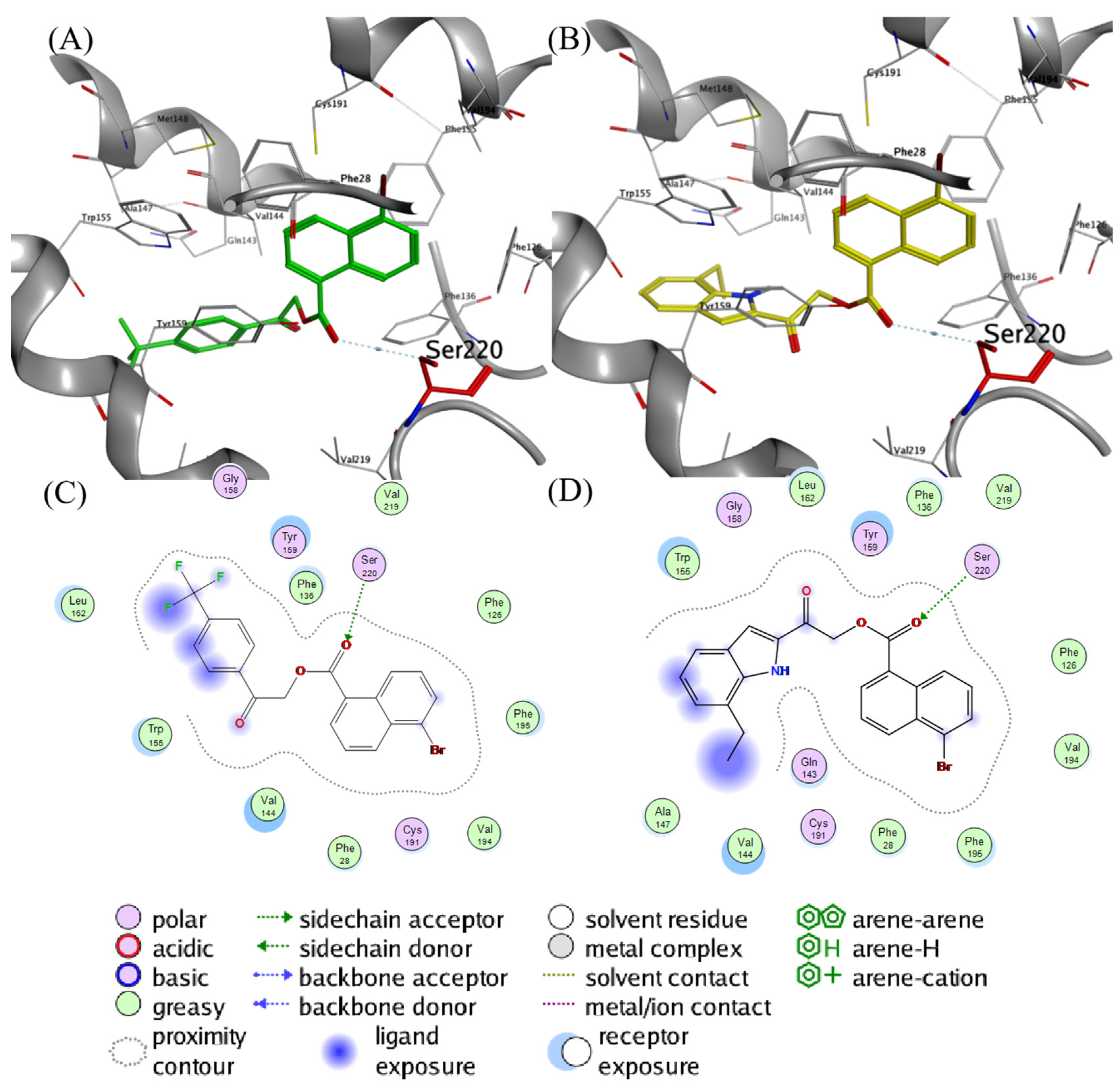
2.4. Molecular Docking Analysis
3. Materials and Methods
3.1. Chemicals, Analytical Instruments and Plant Materials
3.2. General Synthesis
3.2.1. The Preparation of 2-Bromo-1-Phenylethanones
3.2.2. The Preparation of Target Compounds C1–C18
3.3. Biological Assay
3.3.1. O. sativa Branching Assays
3.3.2. Seed Germination Bioassays against P. aegyptiaca
3.3.3. Analysis of A. thaliana Hypocotyl Elongation
3.3.4. Dark-Induced Rice Leaf Senescence
3.3.5. Determination of SOD, POD, CAT, and MDA Levels in Detached Rice Leaves
3.4. Isothermal Titration Calorimetry (ITC) Assay
3.5. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef]
- Ruyter-Spira, C.; Kohlen, W.; Charnikhova, T.; van Zeijl, A.; van Bezouwen, L.; de Ruijter, N.; Cardoso, C.; Lopez-Raez, J.A.; Matusova, R.; Bours, R.; et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: Another belowground role for strigolactones? Plant Physiol. 2011, 155, 721–734. [Google Scholar] [CrossRef]
- Ha, C.V.; Leyva-González, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Dong, N.V.; et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar] [CrossRef]
- Gutjahr, C.; Parniske, M. Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annu. Rev. Cell Dev. Biol. 2013, 29, 593–617. [Google Scholar] [CrossRef]
- Valentina Fiorilli, J.Y.W.P. Apocarotenoids: Old and new mediators of the arbuscular mycorrhizal symbiosis. Front. Plant Sci. 2019, 10, 1186. [Google Scholar] [CrossRef]
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of witchweed (Striga lutea Lour.): Isolation and properties of a potent stimulant. Science 1966, 154, 1189. [Google Scholar] [CrossRef]
- Gressel, J.; Hanafi, A.; Head, G.; Marasas, W.; Obilana, A.B.; Ochanda, J.; Souissi, T.; Tzotzos, G. Major heretofore intractable biotic constraints to African food security that may be amenable to novel biotechnological solutions. Crop Prot. 2004, 23, 661–689. [Google Scholar] [CrossRef]
- Ejeta, G. Breeding for Striga resistance in sorghum: Exploitation of an intricate host–parasite biology. Crop Sci. 2007, 47, 216. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Fonne-Pfister, R.; Screpanti, C.; De Mesmaeker, A. Strigolactones: New plant hormone with promising features. Angew. Chem.-Int. Edit. 2019, 58, 12778–12786. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yan, J.; Yu, C.; Wang, C.; Tan, W.; Duan, L. Design, synthesis and biological evaluation of novel 1H-1,2,4-Triazole derivatives as strigolactone biosynthesis inhibitors. J. Plant Growth Regul. 2023, 43, 741–754. [Google Scholar] [CrossRef]
- Nakamura, H.; Asami, T. Target sites for chemical regulation of strigolactone signaling. Front. Plant Sci. 2014, 5, 623. [Google Scholar] [CrossRef]
- Nakamura, H.; Hirabayashi, K.; Miyakawa, T.; Kikuzato, K.; Hu, W.; Xu, Y.; Jiang, K.; Takahashi, I.; Niiyama, R.; Dohmae, N.; et al. Triazole ureas covalently bind to strigolactone receptor and antagonize strigolactone responses. Mol. Plant. 2019, 12, 44–58. [Google Scholar] [CrossRef]
- Alan, W.; Johnson, G.G.A.H. The preparation of synthetic analogues of Strigol. J. Chem. Soc. Perkin Trans. I 1981, 1734–1743. [Google Scholar] [CrossRef]
- Uraguchi, D.; Kuwata, K.; Hijikata, Y.; Yamaguchi, R.; Imaizumi, H.; Am, S.; Rakers, C.; Mori, N.; Akiyama, K.; Irle, S.; et al. A femtomolar-range suicide germination stimulant for the parasitic plant Striga hermonthica. Science 2018, 362, 1301–1305. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Chen, L.; Zhang, C.; Wang, F.; Li, H.; Wang, M.; Wang, Y.; Nan, F.; Xie, D.; et al. Strigolactone mimic 2-nitrodebranone is highly active in Arabidopsis growth and development. Plant J. 2021, 107, 67–76. [Google Scholar] [CrossRef]
- Jamil, M.; Kountche, B.A.; Wang, J.Y.; Haider, I.; Jia, K.; Takahashi, I.; Ota, T.; Asami, T.; Al-Babili, S. A new series of carlactonoic acid based strigolactone analogs for fundamental and applied research. Front. Plant Sci. 2020, 11, 434. [Google Scholar] [CrossRef]
- Holbrook-Smith, D.; Toh, S.; Tsuchiya, Y.; McCourt, P. Small-molecule antagonists of germination of the parasitic plant Striga hermonthica. Nat. Chem. Biol. 2016, 12, 724–729. [Google Scholar] [CrossRef]
- Yoshimura, M.; Kim, S.F.; Takise, R.; Kusano, S.; Nakamura, S.; Izumi, M.; Yagi, A.; Itami, K.; Hagihara, S. Development of potent inhibitors for strigolactone receptor DWARF 14. Chem. Commun. 2020, 56, 14917–14919. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, A.; Waters, M.T.; Sun, Y.K.; Skelton, B.W.; Dixon, K.W.; Ghisalberti, E.L.; Flematti, G.R.; Smith, S.M. Strigolactone hormones and their stereoisomers signal through two related receptor proteins to induce different physiological responses in Arabidopsis. Plant Physiol. 2014, 165, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Kountche, B.A.; Haider, I.; Wang, J.Y.; Aldossary, F.; Zarban, R.A.; Jia, K.; Yonli, D.; Shahul Hameed, U.F.; Takahashi, I.; et al. Methylation at the C-3′ in D-Ring of strigolactone analogs reduces biological activity in root parasitic plants and rice. Front. Plant Sci. 2019, 10, 353. [Google Scholar] [CrossRef]
- Hamiaux, C.; Drummond, R.S.M.; Janssen, B.J.; Ledger, S.E.; Cooney, J.M.; Newcomb, R.D.; Snowden, K.C. DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr. Biol. 2012, 22, 2032–2036. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Yoshimura, M.; Sato, Y.; Kuwata, K.; Toh, S.; Holbrook-Smith, D.; Zhang, H.; McCourt, P.; Itami, K.; Kinoshita, T.; et al. Probing strigolactone receptors in Striga hermonthica with fluorescence. Science 2015, 349, 864–868. [Google Scholar] [CrossRef]
- Shahul, H.U.; Haider, I.; Jamil, M.; Kountche, B.A.; Guo, X.; Zarban, R.A.; Kim, D.; Al-Babili, S.; Arold, S.T. Structural basis for specific inhibition of the highly sensitive ShHTL7 receptor. Embo Rep. 2018, 19, e45619. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Sato, A.; Kuwata, K.; Inukai, Y.; Kinoshita, T.; Itami, K.; Tsuchiya, Y.; Hagihara, S. Discovery of shoot branching regulator targeting strigolactone receptor DWARF14. Acs Cent. Sci. 2018, 4, 230–234. [Google Scholar] [CrossRef]
- Wu, Y.; Guan, Q.; Zheng, D.; Yan, P.; Feng, D.; Du, J.; Zhang, J.; Zuo, D.; Bao, K.; Zhang, W. Conformation impacts on the bioactivities of SMART analogues. Eur. J. Med. Chem. 2018, 158, 733–742. [Google Scholar] [CrossRef]
- Bi, X.; Li, J.; Li, J.; Shi, W.; Dai, Y.; Li, Q.; Zhang, W.; Huang, W.; Qian, H.; Jiang, C. Design, synthesis and biological evaluation of novel 4,5-dihydro-[1,2,4]triazolo[4,3-f]pteridine derivatives as potential BRD4 inhibitors. Bioorg. Med. Chem. 2019, 17, 2813–2821. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.; Taghizadeh-Ghehi, M. Comment on “Effect of imatinib treatment on renal anemia in chronic myeloid leukemia patients”. J. Oncol. Pharm. Pract. 2023, 29, 1. [Google Scholar] [CrossRef] [PubMed]
- Beirne, D.F.; Farkas, B.; Donati, C.; Gandin, V.; Rozas, I.; Velasco-Torrijos, T.; Montagner, D. Novel design of dual-action Pt(IV) anticancer pro-drugs based on cisplatin and derivatives of the tyrosine kinase inhibitors imatinib and nilotinib. Dalton Trans. 2023, 52, 14110–14122. [Google Scholar] [CrossRef]
- Manjunath, M.S.; Koshariya, A.K.; Sharma, N.; Rajput, A.; Pandey, S.K.; Singh, S.; Kumar, R.; Singh, B.V. Exploring the use of aromatic compounds in crop growth and protection. Int. J. Plant Soil. Sci. 2023, 35, 78–89. [Google Scholar] [CrossRef]
- Tibbetts, J.D.; Hutchby, M.; Cunningham, W.B.; Chapman, R.S.L.; Kociok-Kohn, G.; Davidson, M.G.; Bull, S.D. Sustainable syntheses of Paracetamol and Ibuprofen from biorenewable beta-pinene. Chemsuschem 2023, 16, e202300670. [Google Scholar] [CrossRef]
- Neuburger, J.E.; Gazizova, A.; Tiedemann, S.; von Langermann, J. Chemoenzymatic synthesis of enantiopure amino alcohols from simple methyl ketones. Eur. J. Org. Chem. 2023, 26, e202201471. [Google Scholar] [CrossRef]
- Arite, T.; Iwata, H.; Ohshima, K.; Maekawa, M.; Nakajima, M.; Kojima, M.; Sakakibara, H.; Kyozuka, J. DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. 2007, 51, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Arite, T.; Umehara, M.; Ishikawa, S.; Hanada, A.; Maekawa, M.; Yamaguchi, S.; Kyozuka, J. D14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol. 2009, 50, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Yao, R.; Quan, T.; Wang, F.; Chen, L.; Du, X.; Zhang, W.; Deng, H.; Xie, D.; Luo, T. Simple β-lactones are potent irreversible antagonists for strigolactone receptors. Cell Res. 2017, 27, 1525–1528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- HhhAh, A.; Tp, A.; Ls, A.; Jjm, B.; Ba, B.; Bza, B. New hybrid type strigolactone mimics derived from plant growth regulator auxin. New Biotechnol. 2019, 48, 76–82. [Google Scholar]
- Yao, R.; Ming, Z.; Yan, L.; Li, S.; Wang, F.; Ma, S.; Yu, K.; Yang, M.; Chen, L.; Chen, L.; et al. DWARF14 is a non-canonical hormone receptor for strigolactone. Nature 2016, 536, 469–473. [Google Scholar] [CrossRef]
- Xu, P.; Jinbo, H.; Cai, W. Karrikin signaling regulates hypocotyl shade avoidance response by modulating auxin homeostasis in Arabidopsis. New Phytol. 2022, 236, 1748–1761. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Shinozawa, A.; Sasaki, Y.; Yajima, S.; Braguy, J.; Wang, J.Y.; Jamil, M.; Felemban, A.; Chen, G.E.; Balakrishna, A.; et al. Canonical strigolactones are not the major determinant of tillering but important rhizospheric signals in rice. Sci. Adv. 2022, 8, 1278. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Awad, A.A.; Xie, X.; Yoneyama, K.; Takeuchi, Y. Strigolactones as germination stimulants for root parasitic plants. Plant Cell Physiol. 2010, 51, 1095–1103. [Google Scholar] [CrossRef]



| Compd. | P. aegyptiaca Germination Rate (%) | IC50 (μmol/L) | |||
|---|---|---|---|---|---|
| 40 μM | 80 μM | 160 μM | 320 μM | ||
| C6 | 88.6 ± 1.2 b | 36.4 ± 3.2 a | 9.3 ± 3.1 b | 8.3 ± 1.9 b | 82.8 |
| DL1b | 82.0 ± 4.2 a | 53.1 ± 3.4 b | 4.2 ± 1.2 a | 3.2 ± 1.8 a | 83.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, L.; Li, X.; Ding, Y.; Ma, D.; Yu, C.; Zhao, H.; Wang, Y.; Liu, Z.; Duan, L. Design, Synthesis and Biological Evaluation of Novel Phenyl-Substituted Naphthoic Acid Ethyl Ester Derivatives as Strigolactone Receptor Inhibitor. Int. J. Mol. Sci. 2024, 25, 3902. https://doi.org/10.3390/ijms25073902
Du L, Li X, Ding Y, Ma D, Yu C, Zhao H, Wang Y, Liu Z, Duan L. Design, Synthesis and Biological Evaluation of Novel Phenyl-Substituted Naphthoic Acid Ethyl Ester Derivatives as Strigolactone Receptor Inhibitor. International Journal of Molecular Sciences. 2024; 25(7):3902. https://doi.org/10.3390/ijms25073902
Chicago/Turabian StyleDu, Lin, Xingjia Li, Yimin Ding, Dengke Ma, Chunxin Yu, Hanqing Zhao, Ye Wang, Ziyan Liu, and Liusheng Duan. 2024. "Design, Synthesis and Biological Evaluation of Novel Phenyl-Substituted Naphthoic Acid Ethyl Ester Derivatives as Strigolactone Receptor Inhibitor" International Journal of Molecular Sciences 25, no. 7: 3902. https://doi.org/10.3390/ijms25073902
APA StyleDu, L., Li, X., Ding, Y., Ma, D., Yu, C., Zhao, H., Wang, Y., Liu, Z., & Duan, L. (2024). Design, Synthesis and Biological Evaluation of Novel Phenyl-Substituted Naphthoic Acid Ethyl Ester Derivatives as Strigolactone Receptor Inhibitor. International Journal of Molecular Sciences, 25(7), 3902. https://doi.org/10.3390/ijms25073902






