The Role of Inflammation in Lymphedema: A Narrative Review of Pathogenesis and Opportunities for Therapeutic Intervention
Abstract
1. Introduction
2. The Lymphatic System
3. Lymphedema: An Overview
3.1. Definition and Epidemiology
3.2. Clinical Characterization
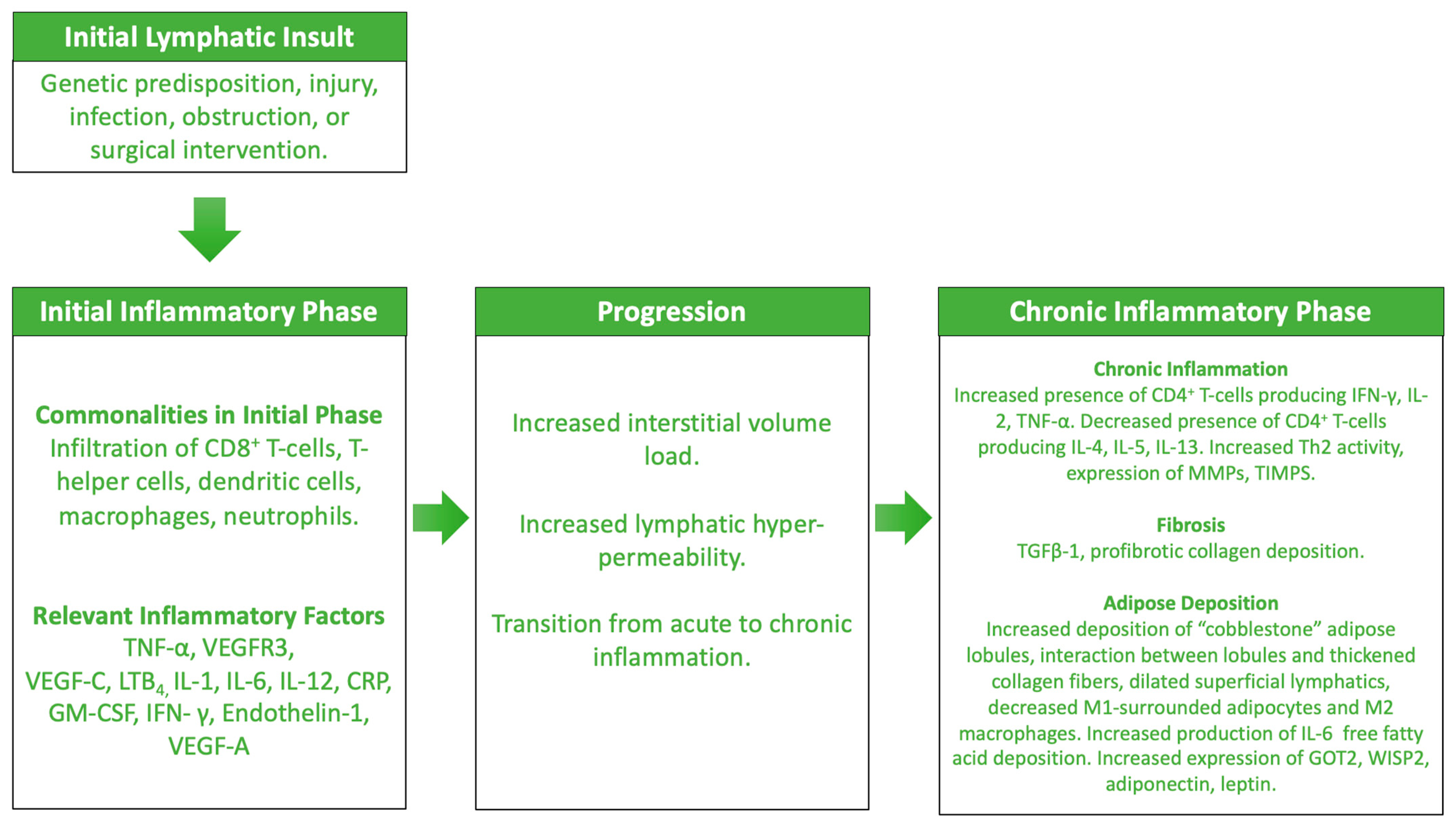
4. Lymphedema: An Inflammatory Pathophysiologic Process
4.1. The Initial Phases of Lymphedema
4.2. The Prolonged Phases of Lymphedema
5. Lymphedema: Current Therapies
5.1. The Relationship between Treatment and Inflammation
5.2. Complete Decongestive Therapy
5.3. Surgical Intervention
6. Lymphedema: Future Therapies and Challenges in Development
6.1. Targetting Inflammation for Future Therapeutics
6.2. 5-Lipoxygenase Targeting Medications
6.3. Antifibrotic Medications
6.4. Tacrolimus
6.5. Challenges for Therapy Development and Translation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, J.E.; Bertram, C.D. Lymphatic System Flows. Annu. Rev. Fluid. Mech. 2018, 50, 459–482. [Google Scholar] [CrossRef]
- Liao, S.; Padera, T.P. Lymphatic function and immune regulation in health and disease. Lymphat. Res. Biol. 2013, 11, 136–143. [Google Scholar] [CrossRef]
- Dixon, J.B. Lymphatic lipid transport: Sewer or subway? Trends Endocrinol. Metab. 2010, 21, 480–487. [Google Scholar] [CrossRef]
- Yuan, Y.; Arcucci, V.; Levy, S.M.; Achen, M.G. Modulation of immunity by lymphatic dysfunction in lymphedema. Front. Immunol. 2019, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.A.; Phillips, T.J. Lymphedema: Pathophysiology and clinical manifestations. J. Am. Acad. Dermatol. 2017, 77, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Rockson, S.G. Advances in Lymphedema. Circ. Res. 2021, 128, 2003–2016. [Google Scholar] [CrossRef]
- Fayyaz Ahmed, R.G. New and Emerging Treatments for Migraine. J. Pain. Reli. 2015, 4, 48–52. [Google Scholar] [CrossRef]
- Dayan, J.H.; Ly, C.L.; Kataru, R.P.; Mehrara, B.J. Lymphedema: Pathogenesis and Novel Therapies. Annu. Rev. Med. 2018, 69, 263–276. [Google Scholar] [CrossRef]
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef]
- Breslin, J.; Yang, Y.; Scallan, J.; Sweat, R.; Adderley, S.; Murfee, W.L. Lymphatic Vessel Network Structure and Physiology. Compr. Physiol. 2019, 9, 207–299. [Google Scholar] [CrossRef]
- Ozdowski, L.; Gupta, V. Physiology, Lymphatic System. In Stat Pearls; StatPearls Publishing: St. Petersburg, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557833/ (accessed on 7 January 2024).
- Levick, J.R.; Michel, C.C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 2010, 87, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Sawdon, M.; Kirkman, E. Capillary dynamics and the interstitial fluid–lymphatic system. Anaesth. Intensive Care Med. 2017, 18, 309–315. [Google Scholar] [CrossRef]
- Von Der Weid, P.Y.; Zawieja, D.C. Lymphatic smooth muscle: The motor unit of lymph drainage. Int. J. Biochem. Cell Biol. 2004, 36, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Trzewik, J.; Mallipattu, S.K.; Artmann, G.M.; Delano, F.A.; Schmid-Schonbein, G.W. Evidence for a second valve system in lymphatics: Endothelial microvalves. Am. Soc. Mech. Eng. Bioeng. Div. BED. 2001, 50, 455. [Google Scholar] [CrossRef] [PubMed]
- Nipper, M.E.; Dixon, J.B. Engineering the Lymphatic System. Cardiovasc. Eng. Technol. 2011, 2, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Ballard, M.; Wolf, K.T.; Nepiyushchikh, Z.; Dixon, J.B.; Alexeev, A. Probing the effect of morphology on lymphatic valve dynamic function. Biomech. Model. Mechanobiol. 2018, 17, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Ulvmar, M.H.; Werth, K.; Braun, A.; Kelay, P.; Hub, E.; Eller, K.; Chan, L.; Lucas, B.; Novitzky-Basso, I.; Nakamura, K.; et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat. Immunol. 2014, 15, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Sibler, E.; He, Y.; Ducoli, L.; Rihs, V.; Sidler, P.; Puig-Moreno, C.; Frey, J.; Fujimoto, N.; Detmar, M.; Dieterich, L.C. Immunomodulatory Responses of Subcapsular Sinus Floor Lymphatic Endothelial Cells in Tumor-Draining Lymph Nodes. Cancers 2022, 14, 3602. [Google Scholar] [CrossRef] [PubMed]
- Calabro, S.; Liu, D.; Gallman, A.; Nascimento, M.S.L.; Yu, Z.; Zhang, T.-T.; Chen, P.; Zhang, B.; Xu, L.; Gowthaman, U.; et al. Differential Intrasplenic Migration of Dendritic Cell Subsets Tailors Adaptive Immunity. Cell Rep. 2016, 16, 2472–2485. [Google Scholar] [CrossRef]
- Randolph, G.J.; Ivanov, S.; Zinselmeyer, B.; Scallan, J. The Lymphatic System: Integral Roles in Immunity. Annu. Rev. Immunol. 2017, 35, 31–52. [Google Scholar] [CrossRef]
- Sinha, R.K.; Park, C.; Hwang, I.Y.; Davis, M.D.; Kehrl, J.H. B Lymphocytes Exit Lymph Nodes through Cortical Lymphatic Sinusoids by a Mechanism Independent of Sphingosine-1-Phosphate-Mediated Chemotaxis. Immunity 2009, 30, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Cheng, G.; Conner, D.A.; Huang, Y.; Kucherlapati, R.S.; Munn, L.L.; Ruddle, N.H.; Jain, R.K.; Fukumura, D.; Padera, T.P. Impaired lymphatic contraction associated with immunosuppression. Proc. Natl. Acad. Sci. USA 2011, 108, 18784–18789, Correction in Proc. Natl. Acad. Sci. USA 2011, 113, E5992. [Google Scholar] [CrossRef] [PubMed]
- Lahdenranta, J.; Hagendoorn, J.; Padera, T.P.; Hoshida, T.; Nelson, G.; Kashiwagi, S.; Jain, R.K.; Fukumura, D. Endothelial nitric oxide synthase mediates lymphangiogenesis and lymphatic metastasis. Cancer Res. 2009, 69, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rehal, S.; Roizes, S.; Zhu, H.L.; Cole, W.C.; von der Weid, P.Y. The pro-inflammatory cytokine TNF-α inhibits lymphatic pumping via activation of the NF-κB-iNOS signaling pathway. Microcirculation 2017, 24, e12364. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, K.; Tirronen, A.; Ylä-Herttuala, S. Intestinal lymphatic vessels and their role in chylomicron absorption and lipid homeostasis. Curr. Opin. Lipidol. 2019, 30, 370–376. [Google Scholar] [CrossRef]
- Dixon, J.B. Mechanisms of chylomicron uptake into lacteals. Ann. N. Y. Acad. Sci. 2010, 1207 (Suppl. S1), 52–57. [Google Scholar] [CrossRef] [PubMed]
- Paupert, J.; Sounni, N.E.; Noël, A. Lymphangiogenesis in post-natal tissue remodeling: Lymphatic endothelial cell connection with its environment. Mol. Aspects Med. 2011, 32, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Gracia, G.; Cao, E.; Feeney, O.M.; Johnston, A.P.R.; Porter, C.J.H.; Trevaskis, N.L. High-Density Lipoprotein Composition Influences Lymphatic Transport after Subcutaneous Administration. Mol. Pharm. 2020, 17, 2938–2951. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Elvington, A.; Randolph, G.J. The role of the lymphatic system in cholesterol transport. Front. Pharmacol. 2015, 6, 182. [Google Scholar] [CrossRef]
- Martel, C.; Li, W.; Fulp, B.; Platt, A.M.; Gautier, E.L.; Westerterp, M.; Bittman, R.; Tall, A.R.; Chen, S.-H.; Thomas, M.J.; et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J. Clin. Investig. 2013, 123, 1571–1579. [Google Scholar] [CrossRef]
- Lim, H.Y.; Thiam, C.H.; Yeo, K.P.; Bisoendial, R.; Hii, C.S.; McGrath, K.C.; Tan, K.W.; Heather, A.; Alexander, J.S.J.; Angeli, V. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-Mediated transport of HDL. Cell Metab. 2013, 17, 671–684. [Google Scholar] [CrossRef]
- Greene, A.K.; Borud, L.J.; Slavin, S.A. Lymphedema. In Plastic Surgery Secrets Plus, 2nd ed.; Weinzweg, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 630–635. [Google Scholar] [CrossRef]
- Warren, A.G.; Brorson, H.; Borud, L.J.; Slavin, S.A. Lymphedema: A comprehensive review. Ann. Plast. Surg. 2007, 59, 464–472. [Google Scholar] [CrossRef]
- Rockson, S.G.; Rivera, K.K. Estimating the population burden of lymphedema. Ann. N. Y. Acad. Sci. 2008, 1131, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Bona, A.; Ferreira, K.; Carvalho, R.; Thuler, L.; Bergmann, A. Incidence, prevalence, and factors associated with lymphedema after treatment for cervical cancer: A systematic review. Int. J. Gynecol. Cancer 2020, 11, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Torgbenu, E.; Luckett, T.; Buhagiar, M.A.; Chang, S.; Phillips, J.L. Prevalence and incidence of cancer related lymphedema in low and middle-income countries: A systematic review and meta-analysis. BMC Cancer 2020, 20, 604. [Google Scholar] [CrossRef]
- Keast, D.H.; Moffatt, C.; Janmohammad, A. Lymphedema impact and prevalence international study: The canadian data. Lymphat. Res. Biol. 2019, 17, 178–186. [Google Scholar] [CrossRef]
- Shallwani, S.M.; Hodgson, P.; Towers, A. Examining obesity in lymphedema: A retrospective study of 178 new patients with suspected lymphedema at a Canadian hospital-based clinic. Physiother. Canada 2020, 72, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Sudduth, C.L.; Greene, A.K. Current Overview of Obesity-Induced Lymphedema. Adv. Wound Care 2022, 11, 392–398. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Anuradha, R.; Manokaran, G.; Bethunaickan, R. An overview of lymphatic filariasis lymphedema. Lymphology 2017, 50, 164–182. [Google Scholar]
- Taghian, N.R.; Miller, C.L.; Jammallo, L.S.; O’Toole, J.; Skolny, M.N. Lymphedema following breast cancer treatment and impact on quality of life: A review. Crit. Rev. Oncol. Hematol. 2014, 92, 227–234. [Google Scholar] [CrossRef]
- Stolldorf, D.P.; Dietrich, M.S.; Ridner, S.H. A Comparison of the Quality of Life in Patients with Primary and Secondary Lower Limb Lymphedema: A Mixed-Methods Study. West. J. Nurs. Res. 2016, 38, 1313–1334. [Google Scholar] [CrossRef] [PubMed]
- Anbari, A.B.; Wanchai, A.; Armer, J.M. Breast cancer-related lymphedema and quality of life: A qualitative analysis over years of survivorship. Chronic Illn. 2021, 17, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Nakagami, G.; Sugama, J.; Kobayashi, N.; Kimura, E.; Arai, Y.; Sato, A.; Mercier, G.; Moffatt, C.; Murray, S.; et al. The prevalence and functional impact of chronic edema and lymphedema in Japan: Limprint study. Lymphat. Res. Biol. 2019, 17, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.; Oberoi, D.; Radke, L.; Francis, G.J.; Carlson, L.E. Living with leg lymphedema: Developing a novel model of quality lymphedema care for cancer survivors. J. Cancer Surviv. 2021, 15, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.; Piedalue, K.A.; Baydoun, M.; Carlson, L.E. The quality of life and psychosocial implications of cancer-related lower-extremity lymphedema: A systematic review of the literature. J. Clin. Med. 2020, 9, 3200. [Google Scholar] [CrossRef] [PubMed]
- Teerachaisakul, M.; Ekataksin, W.; Durongwatana, S.; Taneepanichskul, S. Risk factors for cellulitis in patients with lymphedema: A case-controlled study. Lymphology 2013, 46, 150–156. [Google Scholar] [PubMed]
- Vignes, S.; Poizeau, F.; Dupuy, A. Cellulitis risk factors for patients with primary or secondary lymphedema. J. Vasc. Surg. Venous Lymphat. Disord. 2022, 10, 179–185.e1. [Google Scholar] [CrossRef] [PubMed]
- Chlebicki, M.P.; Oh, C.C. Recurrent cellulitis: Risk factors, etiology, pathogenesis and treatment. Curr. Infect. Dis. Rep. 2014, 16, 422. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Yang, E.J.; Kim, D.K.; Jeong, H.J.; Kim, G.C.; Sim, Y.J. Prevalence and epidemiological factors involved in cellulitis in korean patients with lymphedema. Ann. Rehabil. Med. 2016, 40, 326–333. [Google Scholar] [CrossRef]
- Kataru, R.P.; Baik, J.E.; Park, H.J.; Wiser, I.; Rehal, S.; Shin, J.Y.; Mehrara, B.J. Regulation of immune function by the lymphatic system in lymphedema. Front. Immunol. 2019, 10, 470. [Google Scholar] [CrossRef]
- Gnanasekaran, V.; Perumal, V.; Periyasamy, M.; Ramanathan, M.; De Britto, L. Stigma and stigma-induced stress in filarial lymphoedema patients in Puducherry, India. J. Health Sci. 2023, 13, 5–11. [Google Scholar] [CrossRef]
- Eneanya, O.A.; Garske, T.; Donnelly, C.A. The social, physical and economic impact of lymphedema and hydrocele: A matched cross-sectional study in rural Nigeria. BMC Infect. Dis. 2019, 19, 332. [Google Scholar] [CrossRef] [PubMed]
- Maxeiner, A.M.; Saga, E.; Downer, C.; Arthur, L. Comparing the psychosocial issues experienced by individuals with primary vs. secondary lymphedema. Rehabil. Oncol. 2009, 27, 9–15. [Google Scholar] [CrossRef]
- Eaton, L.H.; Narkthong, N.; Hulett, J.M. Psychosocial Issues Associated with Breast Cancer-Related Lymphedema: A Literature Review. Curr. Breast Cancer Rep. 2020, 12, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Mercier, G.; Pastor, J.; Clément, V.; Rodts, U.; Moffat, C.; Quéré, I. Out-of-pocket payments, vertical equity and unmet medical needs in France: A national multicenter prospective study on lymphedema. PLoS ONE 2019, 14, e0216386. [Google Scholar] [CrossRef] [PubMed]
- Sawers, L.; Stillwaggon, E. Economic costs and benefits of community-based lymphedema-management programs for lymphatic filariasis in India. Am. J. Trop. Med. Hyg. 2020, 103, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Executive Committee. The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the international society of lymphology. Lymphology 2016, 49, 170–184. [Google Scholar]
- Li, C.Y.; Kataru, R.P.; Mehrara, B.J. Histopathologic features of lymphedema: A molecular review. Int. J. Mol. Sci. 2020, 21, 2546. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Dayan, J.H.; Kataru, R.P.; Mehrara, B.J. The Vicious Circle of Stasis, Inflammation, and Fibrosis in Lymphedema. Plast. Reconstr. Surg. 2023, 151, 330E–341E. [Google Scholar] [CrossRef]
- Mendez, U.; Brown, E.M.; Ongstad, E.L.; Slis, J.R.; Goldman, J. Functional recovery of fluid drainage precedes lymphangiogenesis in acute murine foreleg lymphedema. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, 2250–2256. [Google Scholar] [CrossRef]
- Babu, S.; Anuradha, R.; Kumar, N.P.; George, P.J.; Kumaraswami, V.; Nutman, T.B. Filarial lymphatic pathology reflects augmented Toll-Like receptor-mediated, mitogen-activated protein kinase-mediated proinflammatory cytokine production. Infect. Immun. 2011, 79, 4600–4608. [Google Scholar] [CrossRef] [PubMed]
- Bennuru, S.; Nutman, T.B. Lymphangiogenesis and lymphatic remodeling induced by filarial parasites: Implications for pathogenesis. PLoS Pathog. 2009, 5, e1000688. [Google Scholar] [CrossRef] [PubMed]
- Rockson, S.G.; Keeley, V.; Kilbreath, S.; Szuba, A.; Towers, A. Cancer-associated secondary lymphoedema. Nat. Rev. Dis. Prim. 2019, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Nicolls, M.R.; Tian, W.; Rockson, S.G. Lymphatic Dysfunction, Leukotrienes, and Lymphedema. Annu. Rev. Physiol. 2018, 80, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Rockson, S.G.; Jiang, X.; Kim, J.; Begaye, A.; Shuffle, E.M.; Tu, A.B.; Cribb, M.; Nepiyushchikh, Z.; Feroze, A.H.; et al. Leukotriene B4 antagonism ameliorates experimental lymphedema. Sci. Transl. Med. 2017, 9, eaal3920. [Google Scholar] [CrossRef] [PubMed]
- Ridner, S.H.; Dietrich, M.S.; Sonis, S.T.; Murphy, B. Biomarkers Associated with Lymphedema and Fibrosis in Patients with Cancer of the Head and Neck. Lymphat. Res. Biol. 2018, 16, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Leung, G.; Baggott, C.; West, C.; Elboim, C.; Paul, S.M.; Cooper, B.A.; Abrams, G.; Dhruva, A.; Schmidt, B.L.; Kober, K.; et al. Cytokine candidate genes predict the development of secondary lymphedema following breast cancer surgery. Lymphat. Res. Biol. 2014, 12, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Das, B.K.; Sahoo, P.K.; Ravindran, B. A role for tumour necrosis factor-α in acute lymphatic filariasis. Parasite Immunol. 1996, 18, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, V.M.; Brouillard, P.; Korhonen, E.A.; Sipilä, T.; Jha, S.K.; Revencu, N.; Labarque, V.; Fastré, E.; Schlögel, M.; Ravoet, M.; et al. Characterization of ANGPT2 mutations associated with primary lymphedema. Sci. Transl. Med. 2020, 12, eaax8013. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.T.; Khankin, E.V.; Karumanchi, S.A.; Parikh, S.M. Angiopoietin 2 Is a Partial Agonist/Antagonist of Tie2 Signaling in the Endothelium. Mol. Cell Biol. 2009, 29, 2011–2022. [Google Scholar] [CrossRef]
- Choi, D.; Park, E.; Jung, E.; Cha, B.; Lee, S.; Yu, J.; Kim, P.M.; Lee, S.; Hong, Y.J.; Koh, C.J.; et al. Piezo1 incorporates mechanical force signals into the genetic program that governs lymphatic valve development and maintenance. JCI Insight 2019, 4, e125068. [Google Scholar] [CrossRef] [PubMed]
- Burrows, P.E.; Gonzalez-Garay, M.L.; Rasmussen, J.C.; Aldrich, M.B.; Guilliod, R.; Maus, E.A.; Fife, C.E.; Kwon, S.; Lapinski, P.E.; King, P.D.; et al. Lymphatic abnormalities are associated with RASA1 gene mutations in mouse and man. Proc. Natl. Acad. Sci. USA 2013, 110, 8621–8626. [Google Scholar] [CrossRef] [PubMed]
- Finegold, D.N.; Schacht, V.; Kimak, M.A.; Lawrence, E.C.; Foeldi, E.; Karlsson, J.M.; Baty, C.J.; Ferrell, R.E. HGF and MET mutations in primary and secondary lymphedema. Lymphat. Res. Biol. 2008, 6, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Au, A.C.; Hernandez, P.A.; Lieber, E.; Nadroo, A.M.; Shen, Y.-M.; Kelley, K.A.; Gelb, B.D.; Diaz, G.A. Protein tyrosine phosphatase PTPN14 Is a regulator of lymphatic function and choanal development in humans. Am. J. Hum. Genet. 2010, 87, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Finegold, D.N.; Baty, C.J.; Knickelbein, K.Z.; Perschke, S.; Noon, S.E.; Campbell, D.; Karlsson, J.M.; Huang, D.; Kimak, M.A.; Lawrence, E.C.; et al. Connexin 47 mutations increase risk for secondary lymphedema following breast cancer treatment. Clin. Cancer Res. 2013, 18, 2382–2390. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, M.; Lu, T.; Yu, D.; Liu, C.; Wang, Z.; Hu, G. RAF1 promotes lymphatic metastasis of hypopharyngeal carcinoma via regulating LAGE1: An experimental research. J. Transl. Med. 2022, 20, 255. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.B.; Brouillard, P.; Sutton, D.L.; Kazenwadel, J.; Montazaribarforoushi, S.; Secker, G.A.; Oszmiana, A.; Babic, M.; Betterman, K.L.; Brautigan, P.J.; et al. Pathogenic variants in MDFIC cause recessive central conducting lymphatic anomaly with lymphedema. Sci. Transl. Med. 2022, 14, eabm4869. [Google Scholar] [CrossRef]
- Dellinger, M.; Hunter, R.; Bernas, M.; Gale, N.; Yancopoulos, G.; Erickson, R.; Witte, M. Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice. Dev. Biol. 2008, 319, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Martin-Almedina, S.; Ogmen, K.; Sackey, E.; Grigoriadis, D.; Karapouliou, C.; Nadarajah, N.; Ebbing, C.; Lord, J.; Mellis, R.; Kortuem, F.; et al. Janus-faced EPHB4-associated disorders: Novel pathogenic variants and unreported intrafamilial overlapping phenotypes. Genet. Med. 2021, 23, 1315–1324, Correction in Genet. Med. 2021, 23, 1376–1377. [Google Scholar] [CrossRef]
- Shen, B.; Shang, Z.; Wang, B.; Zhang, L.; Zhou, F.; Li, T.; Chu, M.; Jiang, H.; Wang, Y.; Qiao, T.; et al. Genetic dissection of tie pathway in mouse lymphatic maturation and valve development. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1221–1230. [Google Scholar] [CrossRef]
- Zhou, F.; Chang, Z.; Zhang, L.; Hong, Y.-K.; Shen, B.; Wang, B.; Zhang, F.; Lu, G.; Tvorogov, D.; Alitalo, K.; et al. Akt/protein kinase B is required for lymphatic network formation, remodeling, and valve development. Am. J. Pathol. 2010, 177, 2124–2133. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Anuradha, R.; Pavan Kumar, N.P.; George, P.J.; Kumaraswami, V.; Nutman, T.B. Toll-like receptor- and filarial antigen-mediated, mitogen-activated protein kinase- and NF-κB-dependent regulation of angiogenic growth factors in filarial lymphatic pathology. Infect. Immun. 2012, 80, 2509–2518. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bennuru, S.; Nutman, T.B. Lymphatics in human lymphatic filariasis: In vitro models of parasite-induced lymphatic remodeling. Lymphat. Res. Biol. 2009, 7, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Bennuru, S.; Maldarelli, G.; Kumaraswami, V.; Klion, A.D.; Nutman, T.B. Elevated levels of plasma angiogenic factors are associated with human lymphatic filarial infections. Am. J. Trop. Med. Hyg. 2010, 83, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Vickery, A.C.; Albertine, K.H.; Nayar, J.K.; Kwa, B.H. Histopathology of Brugia malayi-infected nude mice after immune-reconstitution. Acta Trop. 1991, 49, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Kenneth Nelson, F.; Greiner, D.L.; Shultz, L.D.; Rajan, T.V. The immunodeficient scid mouse as a model for human lymphatic filariasis. J. Exp. Med. 1991, 173, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Rao, U.R.; Vickery, A.C.; Kwa, B.H.; Nayar, J.K. Regulatory cytokines in the lymphatic pathology of athymic mice infected with Brugia malayi. Int. J. Parasitol. 1996, 26, 561–565. [Google Scholar] [CrossRef]
- Verma, S.K.; Kushwaha, V.; Dubey, V.; Saxena, K.; Sharma, A.; Murthy, P.K. Inflammatory mediator release by Brugia malayi from macrophages of susceptible host Mastomys coucha and THP-1 and RAW 264.7 cell lines. Asian Pac. J. Trop. Med. 2011, 4, 92–96. [Google Scholar] [CrossRef]
- Satapathy, A.K.; Sartono, E.; Sahoo, P.K.; Dentener, M.A.; Michael, E.; Yazdanbakhsh, M.; Ravindran, B. Human bancroftian filariasis: Immunological markers of morbidity and infection. Microbes Infect. 2006, 8, 2414–2423. [Google Scholar] [CrossRef]
- Babu, S.; Nutman, T.B. Immunopathogenesis of lymphatic filarial disease. Semin. Immunopathol. 2012, 34, 847–861. [Google Scholar] [CrossRef]
- Esterre, P.; Plichart, C.; Huin-Blondey, M.O.; Nguyen, L.N. Soluble cellular adhesion molecules, selectins, VEGF and endothelin-1 in patients with Wuchereria bancrofti infection and association with clinical status. Parasite Immunol. 2005, 27, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, R.; George, P.J.; Hanna, L.E.; Chandrasekaran, V.; Kumaran, P.; Nutman, T.B.; Babu, S. IL-4-, TGF-β-, and IL-1-Dependent Expansion of Parasite Antigen-Specific Th9 Cells Is Associated with Clinical Pathology in Human Lymphatic Filariasis. J. Immunol. 2013, 191, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Semnani, R.T.; Liu, A.Y.; Sabzevari, H.; Kubofcik, J.; Zhou, J.; Gilden, J.K.; Nutman, T.B. Brugia malayi Microfilariae Induce Cell Death in Human Dendritic Cells, Inhibit Their Ability to Make IL-12 and IL-10, and Reduce Their Capacity to Activate CD4+ T Cells. J. Immunol. 2003, 171, 1950–1960. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, R.; George, P.J.; Kumaran, P.; Nutman, T.B.; Babu, S. Interleukin-10-and transforming growth factor β-independent regulation of CD8+ T cells expressing type 1 and type 2 cytokines in human lymphatic Filariasis. Clin. Vaccine Immunol. 2014, 21, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Borrero-Wolff, D.; Ritter, M.; Arndts, K.; Wiszniewsky, A.; Debrah, L.B.; Debrah, A.Y.; Osei-Mensah, J.; Chachage, M.; Hoerauf, A.; et al. Distinct Immune Profiles of Exhausted Effector and Memory CD8+ T Cells in Individuals with Filarial Lymphedema. Front. Cell. Infect. Microbiol. 2021, 11, 680832. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, W.; Jamal, S.; Manokaran, G.; Lukomska, B.; Kubicka, U. Skin changes in filarial and non-filarial lymphoedema of the lower extremities. Trop. Med. Parasitol. 1993, 44, 40–44. [Google Scholar] [PubMed]
- Horn, S.; Ritter, M.; Arndts, K.; Borrero-Wolff, D.; Wiszniewsky, A.; Debrah, L.B.; Debrah, A.Y.; Osei-Mensah, J.; Chachage, M.; Hoerauf, A.; et al. Filarial Lymphedema Patients Are Characterized by Exhausted CD4+ T Cells. Front. Cell Infect. Microbiol. 2022, 11, 767306. [Google Scholar] [CrossRef] [PubMed]
- Semnani, R.T.; Mahapatra, L.; Dembele, B.; Konate, S.; Metenou, S.; Dolo, H.; Coulibaly, M.E.; Soumaoro, L.; Coulibaly, S.Y.; Sanogo, D.; et al. Expanded Numbers of Circulating Myeloid Dendritic Cells in Patent Human Filarial Infection Reflect Lower CCR1 Expression. J. Immunol. 2010, 185, 6364–6372. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Karnam, A.; Das, M.; Babu, S.P.S.; Bayry, J. Wuchereria bancrofti filaria activates human dendritic cells and polarizes T helper 1 and regulatory T cells via toll-like receptor 4. Commun. Biol. 2019, 2, 169. [Google Scholar] [CrossRef]
- Sharma, R.; Hoti, S.L.; Meena, R.L.; Vasuki, V.; Sankari, T.; Kaliraj, P. Molecular and functional characterization of macrophage migration inhibitory factor (MIF) homolog of human from lymphatic filarial parasite Wuchereria bancrofti. Parasitol. Res. 2012, 111, 2035–2047. [Google Scholar] [CrossRef]
- Ghanta, S.; Cuzzone, D.A.; Torrisi, J.S.; Albano, N.J.; Joseph, W.J.; Savetsky, I.L.; Gardenier, J.C.; Chang, D.; Zampell, J.C.; Mehrara, B.J. Regulation of inflammation and fibrosis by macrophages in lymphedema. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1065–H1077. [Google Scholar] [CrossRef] [PubMed]
- Gousopoulos, E.; Proulx, S.T.; Bachmann, S.B.; Dieterich, L.C.; Scholl, J.; Karaman, S.; Bianchi, R.; Detmar, M. An Important Role of VEGF-C in Promoting Lymphedema Development. J. Investig. Dermatol. 2017, 137, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qin, J.; Wei, P.; Zhang, J.; Li, Q.; Fu, L.; Li, S.; Ma, C.; Cong, B. Effects of leukotriene B4 and prostaglandin E2 on the differentiation of murine Foxp3+ T regulatory cells and Th17 cells. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Dev, K.; Agarwal, B.; Das, P.; Syed, M.A. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 2020, 223, 383–396. [Google Scholar] [CrossRef]
- Baik, J.E.; Park, H.J.; Kataru, R.P.; Savetsky, I.L.; Ly, C.L.; Shin, J.; Encarnacion, E.M.; Cavali, M.R.; Klang, M.G.; Riedel, E.; et al. TGF-β1 mediates pathologic changes of secondary lymphedema by promoting fibrosis and inflammation. Clin. Transl. Med. 2022, 12, e758. [Google Scholar] [CrossRef] [PubMed]
- Avraham, T.; Zampell, J.C.; Yan, A.; Elhadad, S.; Weitman, E.S.; Rockson, S.G.; Bromberg, J.; Mehrara, B.J. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. J. Fed. Am. Soc. Exp. Biol. 2013, 27, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Yuk, C.M.; Shin, K.; Lee, S.H. Interleukin-17A negatively regulates lymphangiogenesis in T helper 17 cell-mediated inflammation. Mucosal Immunol. 2018, 11, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Hirakawa, S.; Sasaki, T.; Inuzuka, K.; Katahashi, K.; Kayama, T.; Yamanaka, Y.; Tsuyuki, H.; Endo, Y.; Naruse, E.; et al. Role of Subcutaneous Adipose Tissues in the Pathophysiology of Secondary Lymphedema. Lymphat. Res. Biol. 2022, 20, 593–599. [Google Scholar] [CrossRef]
- Koc, M.; Wald, M.; Varaliová, Z.; Ondrůjová, B.; Čížková, T.; Brychta, M.; Kračmerová, J.; Beranová, L.; Pala, J.; Šrámková, V.; et al. Lymphedema alters lipolytic, lipogenic, immune and angiogenic properties of adipose tissue: A hypothesis-generating study in breast cancer survivors. Sci. Rep. 2021, 11, 8171. [Google Scholar] [CrossRef]
- Tashiro, K.; Feng, J.; Wu, S.H.; Mashiko, T.; Kanayama, K.; Narushima, M.; Uda, H.; Miyamoto, S.; Koshima, I.; Yoshimura, K. Pathological changes of adipose tissue in secondary lymphoedema. Br. J. Dermatol. 2017, 177, 158–167. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, M.; Xiang, Q.; Li, Z.; Xu, F.; Chen, W.; Chen, J.; Huang, J.; Yu, N.; Zhou, Z.; et al. Single-cell RNA sequencing of subcutaneous adipose tissues identifies therapeutic targets for cancer-associated lymphedema. Cell Discov. 2022, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Yusof, K.M.; Groen, K.; Rosli, R.; Abdullah, M.; Mahmud, R.; Avery-Kiejda, K.A. Evaluation of Circulating MicroRNAs and Adipokines in Breast Cancer Survivors with Arm Lymphedema. Int. J. Mol. Sci. 2022, 23, 11359. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Pedrosa, B.C.; de Castro, P.M.M.; e Santos, L.V.S.; de Andrade, D.L.; Vilaça, A.F.; Pinheiro Júnior, J.E.G.; Ferreira, A.P.d.L.; Lins, E.M.; Maia, J.N.; Andrade, M.D.A.; et al. Effects of complex decongestive therapy and aquatic physiotherapy on markers of the inflammatory process in individuals with lymphedema. Physiother Theory Pract. 2022. [Google Scholar] [CrossRef] [PubMed]
- Brix, B.; Apich, G.; Rössler, A.; Walbrodt, S.; Goswami, N. Effects of physical therapy on hyaluronan clearance and volume regulating hormones in lower limb lymphedema patients: A pilot study. Sci. Prog. 2021, 104, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ridner, S.H.; Dietrich, M.S.; Deng, J.; Ettema, S.L.; Murphy, B. Advanced pneumatic compression for treatment of lymphedema of the head and neck: A randomized wait-list controlled trial. Support. Care Cancer 2021, 29, 795–803. [Google Scholar] [CrossRef]
- Rannikko, E.H.; Leppäpuska, I.M.; Laukka, M.; Saarikko, A.; Hartiala, P. Short Duration of Upper Extremity Lymphedema Correlates With a Favorable Cytokine Response After Lymph Node Transfer Surgery. Lymphology 2022, 55, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Viitanen, T.P.; Visuri, M.T.; Sulo, E.; Saarikko, A.M.; Hartiala, P. Anti-inflammatory effects of flap and lymph node transfer. J. Surg. Res. 2015, 199, 718–725. [Google Scholar] [CrossRef]
- Torrisi, J.S.; Joseph, W.J.; Ghanta, S.; Cuzzone, D.A.; Albano, N.J.; Savetsky, I.L.; Gardenier, J.C.; Skoracki, R.; Chang, D.; Mehrara, B.J. Lymphaticovenous bypass decreases pathologic skin changes in upper extremity breast cancer-related lymphedema. Lymphat. Res. Biol. 2015, 13, 46–53. [Google Scholar] [CrossRef]
- Imai, H.; Kawase, T.; Yoshida, S.; Mese, T.; Roh, S.; Fujita, A.; Uchiki, T.; Sasaki, A.; Nagamatsu, S.; Takazawa, A.; et al. Peripheral T cell profiling reveals downregulated exhaustion marker and increased diversity in lymphedema post-lymphatic venous anastomosis. iScience. 2023, 26, 106822. [Google Scholar] [CrossRef] [PubMed]
- Rockson, S.G.; Tian, W.; Jiang, X.; Kuznetsova, T.; Haddad, F.; Zampell, J.; Mehrara, B.; Sampson, J.P.; Roche, L.; Kim, J.; et al. Pilot studies demonstrate the potential benefits of antiinflammatory therapy in human lymphedema. JCI Insight 2018, 3, e123775. [Google Scholar] [CrossRef]
- Nakamura, K.; Radhakrishnan, K.; Wong, Y.M.; Rockson, S.G. Anti-inflammatory pharmacotherapy with ketoprofen ameliorates experimental lymphatic vascular insufficiency in mice. PLoS ONE 2009, 4, e8380. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, K.Y.; Wang, Z.; Park, J.-H.; Bae, S.M.; Kim, S.-Y.; Song, H.-Y.; Jeon, J.Y. EW-7197, a Transforming Growth Factor-Beta Type i Receptor Kinase Inhibitor, Ameliorates Acquired Lymphedema in a Mouse Tail Model. Lymphat. Res. Biol. 2020, 18, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Gardenier, J.C.; Kataru, R.P.; Hespe, G.E.; Savetsky, I.L.; Torrisi, J.S.; Nores, G.D.G.; Jowhar, D.K.; Nitti, M.D.; Schofield, R.C.; Carlow, D.C.; et al. Topical tacrolimus for the treatment of secondary lymphedema. Nat. Commun. 2017, 8, 14345. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Campbell, A.C.; Kuonqui, K.; Sarker, A.; Park, H.J.; Shin, J.; Kataru, R.P.; Coriddi, M.; Dayan, J.H.; Mehrara, B.J. The Future of Lymphedema: Potential Therapeutic Targets for Treatment. Curr. Breast Cancer Rep. 2023, 15, 233–241. [Google Scholar] [CrossRef]
- Gulmark Hansen, F.C.; Jørgensen, M.G.; Sørensen, J.A. Treatment of Breast Cancer-Related Lymphedema With Topical Tacrolimus: A Prospective, Open-Label, Single-Arm, Phase II Pilot Trial. J. Breast Cancer 2023, 26, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Borman, P.; Yaman, A.; Yasrebi, S.; Pınar İnanlı, A.; Arıkan Dönmez, A. Combined Complete Decongestive Therapy Reduces Volume and Improves Quality of Life and Functional Status in Patients With Breast Cancer-Related Lymphedema. Clin. Breast Cancer. 2022, 22, e270–e277. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.C.; Maus, E.A.; Rasmussen, J.C.; Marshall, M.V.; Adams, K.E.; Fife, C.E.; Smith, L.A.; Chan, W.; Sevick-Muraca, E.M. Assessment of lymphatic contractile function after manual lymphatic drainage using near-infrared fluorescence imaging. Arch. Phys. Med. Rehabil. 2011, 92, 756–764.e1. [Google Scholar] [CrossRef] [PubMed]
- Badger, C.M.A.; Peacock, J.L.; Mortimer, P.S. A randomized, controlled, parallel-group clinical trial comparing multilayer bandaging followed by hosiery versus hosiery alone in the treatment of patients with lymphedema of the limb. Cancer 2000, 88, 2832–2837. [Google Scholar] [CrossRef]
- Cho, S.; Roh, K.; Park, J.; Park, Y.S.; Lee, M.; Cho, S.; Kil, E.-J.; Cho, M.-J.; Oh, J.S.; Byun, H.-S.; et al. Hydrolysis of hyaluronic acid in lymphedematous tissue alleviates fibrogenesis via TH1 cell-mediated cytokine expression. Sci. Rep. 2017, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Granzow, J.W.; Soderberg, J.M.; Kaji, A.H.; Dauphine, C. Review of current surgical treatments for lymphedema. Ann. Surg. Oncol. 2014, 21, 1195–1201. [Google Scholar] [CrossRef]
- Carl, H.M.; Walia, G.; Bello, R.; Clarke-Pearson, E.; Hassanein, A.H.; Cho, B.; Pedreira, R.; Sacks, J.M. Systematic Review of the Surgical Treatment of Extremity Lymphedema. J. Reconstr. Microsurg. 2017, 33, 412–425. [Google Scholar] [CrossRef]
- Gallagher, K.K.; Lopez, M.; Iles, K.; Kugar, M. Surgical Approach to Lymphedema Reduction. Curr. Oncol. Rep. 2020, 22, 97. [Google Scholar] [CrossRef]
- Wiegand, S.; Wichmann, G.; DIetz, A. Treatment of Lymphatic Malformations with the mTOR Inhibitor Sirolimus: A Systematic Review. Lymphat. Res. Biol. 2018, 16, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.; Gökakın, A.K.; Üreyen, O.; Altınel, Ö.; Tezcan, E.; Gür, A.; Muslu, Ü.; Atabey, M. The efficacy of micronized flavonoid fraction (450 mg diosmin plus 50 mg hesperidin) in the treatment of lymphedema after axillary dissection. Cumhur. Tip. Derg. 2011, 33, 312–317. [Google Scholar]
- Bello, A.E.; Holt, R.J. Cardiovascular Risk with Non-steroidal Anti-inflammatory Drugs: Clinical Implications. Drug Saf. 2014, 37, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Rockson, S.G. Trial of Acebilustat for the Treatment of Upper Arm Lymphedema (HEAL). ClinicalTrials.Gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05203835 (accessed on 7 January 2024).
- Jørgensen, M.G.; Toyserkani, N.M.; Hansen, C.R.; Hvidsten, S.M.; Baun, C.M.; Hejbøl, E.K.M.; Schrøder, H.D.M.; Sørensen, J.A. Quantification of Chronic Lymphedema in a Revised Mouse Model. Ann. Plast. Surg. 2018, 81, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.F.; Yu, R.P.; Stanton, E.W.; Wang, J.; Wong, A.K. Current Advancements in Animal Models of Postsurgical Lymphedema: A Systematic Review. Adv. Wound Care 2022, 11, 399–418. [Google Scholar] [CrossRef]
- Ito, R.; Suami, H. Lymphatic territories (Lymphosomes) in Swine: An animal model for future lymphatic research. Plast. Reconstr. Surg. 2015, 136, 297–304. [Google Scholar] [CrossRef]
| Current Therapies | ||
| Treatment | Description | Reported Impact on Inflammatory Mechanisms |
| Complete Decongestive Therapy | Manual physical therapies (e.g., compression, massage) that aim to mobilize accumulated interstitial fluid from affected regions back into blood vascular circulation. | Complete Decongestive Therapy (CDT) Decreased circulating levels of TNF-α, IL-10, monocytes [115]. Increased aldosterone, no significant change in hyaluronic acid levels after 3-weeks of CDT [116]. Pneumatic Compression No significant difference (head and neck) in blood levels of IFN-γ, TNF-α, TGFβ-1, IL-1β , IL-6 after 8-weeks of pneumatic compression [117]. |
| Surgical Interventions | Physiologic and reductive techniques, including lymphaticovenous anastomosis, vascularized lymph node transfer, breast reconstruction, combined approaches. | Lymph Node Transfer and Combined Techniques Increased production of IL-10 (after combined lymph node transfer and anastomosis) [118]. Modulation of VEGF-C production, correlation between IL-10, TNF-α, TGFβ-1 and lymphedema-related factors following lymph node transfer [119]. Lymphaticovenous anastomosis Decreased CD4+ cell inflammation, hyperkeratosis, epidermal proliferation, collagen type I deposition and TGFβ-1 expression (biopsy) [120]. One-year post-operative decrease in IFN-γ and IL-17A expression, increased T-cell receptor diversity. Downregulation of PD-1, Tim-3, PD-1+Tim-3+ on CD4+ and CD8+ T cells [121]. |
| Novel Therapies | ||
| Treatment | Description | Reported Impact on Inflammatory Mechanisms |
| 5-Lipoxgenase Targeting Medications | Ketoprofen, bestatin (Ubenimex), and Acebilustat are known modifiers of the 5-lipoxygenase pathway that leads to lymphedema progression and worsening. | Ketoprofen Decreased dermal thickness, improved histopathological scores (dermal thickness, collagen thickness, intercellular mucin deposits, perivascular inflammation), decreased plasma G-CSF (human) [122]. Upregulation of VEGF-C, VEGFR-3, PROX-1 expression and paradoxical increase in TNF-α. Normalized histopathological findings of hyperkeratosis, epidermal spongiosis, edema, irregularity of epidermal/dermal junction, elongation of dermal papillae of tail (murine) [123]. Bestatin Improved lymphatic flow, decreased lymphatic permeability, diminished macrophage and neutrophil infiltration in skin sample, decreased IL-6, IL-4, IL-13, and IL-17A, elevated IL-10 (murine) [67]. |
| Antifibrotic Medications | Anti-fibrotic medications target tissue transformation that has been found in later stages of lymphedema. | Neutralizing anti-TGF Antibodies Decreased ECM deposition, increased collateral lymphatic formation, inhibition of T-cell infiltration. Decreased tail edema, fibroadipose tissue deposition, and expression of TGFβ-1 and pSmad3 in skin, decreased expression of all TGF-β isoforms and downstream signaling molecules (Sp1, RhoA, Cfl1, Map3k7, Mapk14, RelA, Nfκb2 and Akt1) and inflammatory mediators (IL-1β, TNF-α, IL-6, -4, -13, -10, -17α) in tail tissue. Decreased skin leukocyte, CD4+, Th1, and Th2 cells, and neutrophils (murine) [107]. EW-7197 Improvements in fibrosis, interstitial flow, lymphangiogenesis, decreased tail diameter (murine) [124]. |
| Tacrolimus | Tacrolimus is an anti-T-cell agent approved for topic treatment of skin inflammation and fibrosis. | Improved lymphatic contractility, swelling, T-cell infiltration, tissue fibrosis. Increased formation of lymphatic collateral vessels, decreased backflow (murine) [125,126,127]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowman, C.; Rockson, S.G. The Role of Inflammation in Lymphedema: A Narrative Review of Pathogenesis and Opportunities for Therapeutic Intervention. Int. J. Mol. Sci. 2024, 25, 3907. https://doi.org/10.3390/ijms25073907
Bowman C, Rockson SG. The Role of Inflammation in Lymphedema: A Narrative Review of Pathogenesis and Opportunities for Therapeutic Intervention. International Journal of Molecular Sciences. 2024; 25(7):3907. https://doi.org/10.3390/ijms25073907
Chicago/Turabian StyleBowman, Catharine, and Stanley G. Rockson. 2024. "The Role of Inflammation in Lymphedema: A Narrative Review of Pathogenesis and Opportunities for Therapeutic Intervention" International Journal of Molecular Sciences 25, no. 7: 3907. https://doi.org/10.3390/ijms25073907
APA StyleBowman, C., & Rockson, S. G. (2024). The Role of Inflammation in Lymphedema: A Narrative Review of Pathogenesis and Opportunities for Therapeutic Intervention. International Journal of Molecular Sciences, 25(7), 3907. https://doi.org/10.3390/ijms25073907






