Zinc, Copper, and Iron in Selected Skin Diseases
Abstract
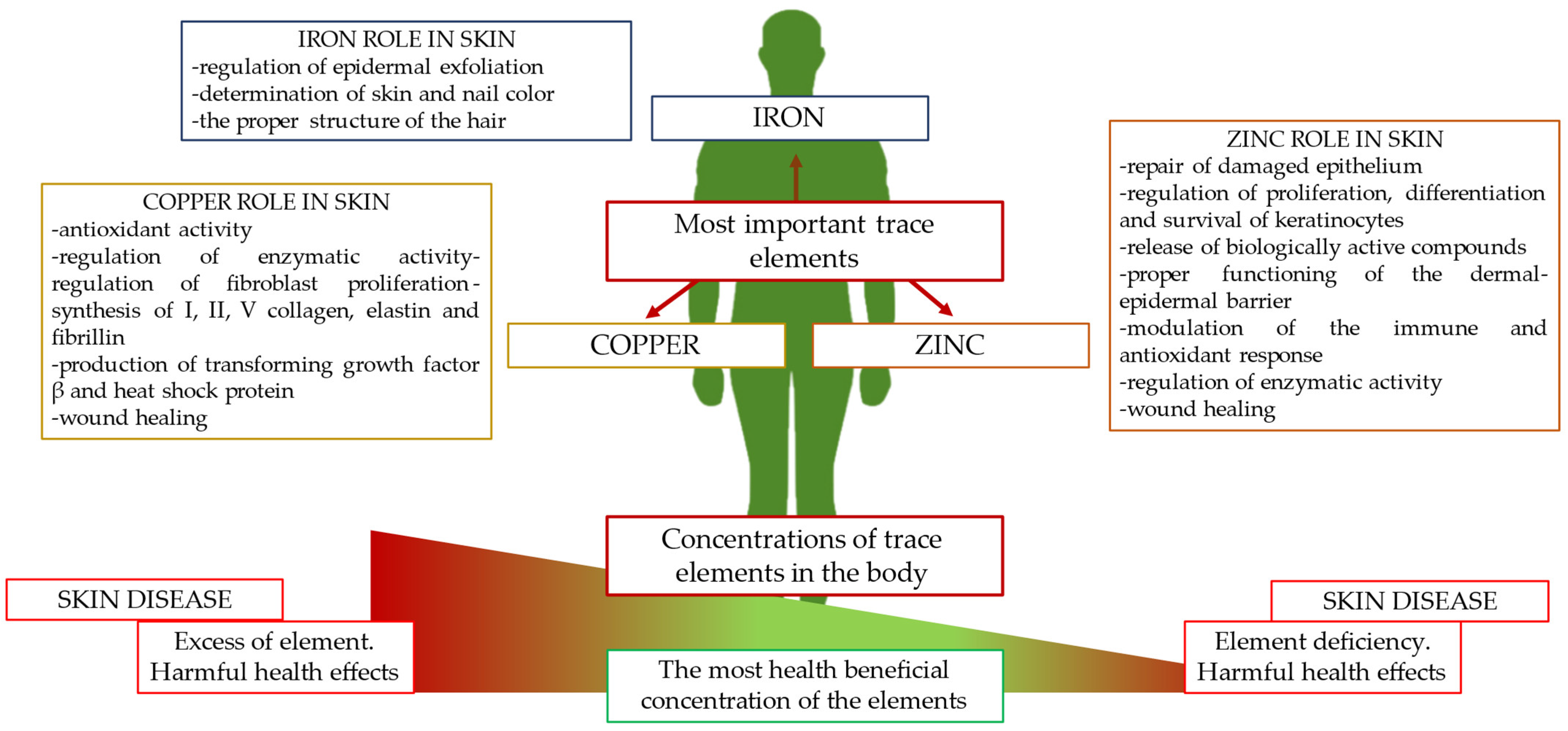
1. Introduction
2. Zinc
2.1. Biological Roles of Zinc in the Human Body
2.2. Zinc in Skin Physiology
2.3. Zinc in Psoriasis and Pemphigus Vulgaris
2.4. Zinc in Atopic Dermatitis
2.5. Zinc in Acne Vulgaris
2.6. Zinc in Seborrheic Dermatitis
3. Copper
3.1. Biological Roles of Copper in the Human Body
3.2. Copper in Skin Physiology
3.3. Copper in Psoriasis and Pemphigus Vulgaris
3.4. Copper in Atopic Dermatitis
3.5. Copper in Acne Vulgaris
3.6. Copper in Seborrheic Dermatitis
4. Iron
4.1. Biological Roles of Iron in the Human Body
4.2. Iron in Skin Physiology
4.3. Iron in Psoriasis and Pemphigus Vulgaris
4.4. Iron in Atopic Dermatitis
4.5. Iron in Acne Vulgaris
4.6. Iron in Seborrheic Dermatitis
5. Further Research Directions: Medical Ultrasonography of Skin
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jin, J.; Mulesa, L.; Carrilero Rouillet, M. Trace Elements in Parenteral Nutrition: Considerations for the Prescribing Clinician. Nutrients 2017, 9, 440. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Wu, X.; Cui, L.; Mao, S. Editorial: Trace Element Chemistry and Health. Front. Nutr. 2022, 9, 1034577. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The Essential Metals for Humans: A Brief Overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, E.; Marley, A.; Samaan, M.A.; Brookes, M.J. Iron Deficiency Anaemia: Pathophysiology, Assessment, Practical Management. BMJ Open Gastroenterol. 2022, 9, e000759. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Min, J.; Wang, F. Copper Homeostasis and Cuproptosis in Health and Disease. Sig. Transduct. Target Ther. 2022, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. Zinc in Dermatology. J. Dermatol. Treat. 2022, 33, 2455–2458. [Google Scholar] [CrossRef]
- Zemrani, B.; Bines, J.E. Recent Insights into Trace Element Deficiencies: Causes, Recognition and Correction. Curr. Opin. Gastroenterol. 2020, 36, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [PubMed]
- Antoniadis, V.; Shaheen, S.M.; Levizou, E.; Shahid, M.; Niazi, N.K.; Vithanage, M.; Ok, Y.S.; Bolan, N.; Rinklebe, J. A Critical Prospective Analysis of the Potential Toxicity of Trace Element Regulation Limits in Soils Worldwide: Are They Protective Concerning Health Risk Assessment?—A Review. Environ. Int. 2019, 127, 819–847. [Google Scholar] [CrossRef]
- Kumar, P.; Lal, N.R.; Mondal, A.K.; Mondal, A.; Gharami, R.C.; Maiti, A. Zinc and skin: A brief summary. Dermatol. Online J. 2012, 18, 1. [Google Scholar] [CrossRef]
- Rodríguez-Tomàs, E.; Baiges-Gaya, G.; Castañé, H.; Arenas, M.; Camps, J.; Joven, J. Trace Elements under the Spotlight: A Powerful Nutritional Tool in Cancer. J. Trace Elem. Med. Biol. 2021, 68, 126858. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G. Using Copper to Improve the Well-Being of the Skin. Curr. Chem. Biol. 2014, 8, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Surbek, M.; Sukseree, S.; Eckhart, L. Iron Metabolism of the Skin: Recycling versus Release. Metabolites 2023, 13, 1005. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.; Lio, P. The role of trace elements in dermatology: A systematic review. J. Integr. Dermatol. 2023, 5, 1. [Google Scholar]
- Nazik, H.; Bengü, A.Å.; Gül, F.Ç.; Demir, B.; Öztürk, P.; Mülayim, M.K. Evaluation of the levels of trace elements in the blood and hair of patients with seborrheic dermatitis. JTEMIN 2019, 36, 120–125. [Google Scholar] [CrossRef]
- Lei, L.; Su, J.; Chen, J.; Chen, W.; Chen, X.; Peng, C. Abnormal Serum Copper and Zinc Levels in Patients with Psoriasis: A Meta-Analysis. Indian J. Dermatol. 2019, 64, 224. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Nastaran, N.; Sara, K.; Shima, Y. Trace elements status in psoriasis and their relationship with the severity of the disease. Iran J. Dermatol. 2012, 15, 38–41. [Google Scholar]
- Kogan, S.; Sood, A.; Garnick, M.S. Zinc and Wound Healing: A Review of Zinc Physiology and Clinical Applications. Wounds 2017, 29, 102–106. [Google Scholar] [PubMed]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Skalny, A.V.; Aschner, M.; Tinkov, A.A. Zinc. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 96, pp. 251–310. ISBN 9780128206485. [Google Scholar]
- Maywald, M.; Rink, L. Zinc in Human Health and Infectious Diseases. Biomolecules 2022, 12, 1748. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Costa, M.I.; Sarmento-Ribeiro, A.B.; Gonçalves, A.C. Zinc: From Biological Functions to Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 4822. [Google Scholar] [CrossRef]
- Stiles, L.I.; Ferrao, K.; Mehta, K.J. Role of Zinc in Health and Disease. Clin. Exp. Med. 2024, 24, 38. [Google Scholar] [CrossRef]
- Deep, V.; Sondhi, S.; Gupta, S. Assessment of Serum Zinc Levels in Patients With Decompensated Cirrhosis of the Liver and Its Association With Disease Severity and Hepatic Encephalopathy: A Prospective Observational Study From North India. Cureus 2023, 15, e41207. [Google Scholar] [CrossRef]
- Nascimento Marreiro, D.D.; Martins, M.D.P.S.C.; Sousa, S.S.R.D.; Ibiapina, V.; Torres, S.; Pires, L.V.; Nascimento Nogueira, N.D.; Lima, J.M.C.; Monte, S.J.H.D. Urinary Excretion of Zinc and Metabolic Control of Patients with Diabetes Type 2. Biol. Trace Elem. Res. 2007, 120, 42–50. [Google Scholar] [CrossRef]
- Al-Khafaji, Z.; Brito, S.; Bin, B.-H. Zinc and Zinc Transporters in Dermatology. IJMS 2022, 23, 16165. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kinoshita, M.; Shimada, S.; Kawamura, T. Zinc in Keratinocytes and Langerhans Cells: Relevance to the Epidermal Homeostasis. J. Immunol. Res. 2018, 2018, 5404093. [Google Scholar] [CrossRef]
- Glutsch, V.; Hamm, H.; Goebeler, M. Zinc and Skin: An Update. J. Dtsch. Derma Gesell. 2019, 17, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S. Zinc therapy in dermatology: A review. Dermatol. Res. Pract. 2014, 2014, 709152. [Google Scholar] [CrossRef]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in wound healing modulation. Nutrients 2018, 10, 16. [Google Scholar] [CrossRef]
- Nitzan, Y.B.; Cohen, A.D. Zinc in skin pathology and care. J. Dermatolog. Treat. 2006, 17, 205–210. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945. [Google Scholar] [CrossRef]
- Raharja, A.; Mahil, S.K.; Barker, J.N. Psoriasis: A Brief Overview. Clin. Med. 2021, 21, 170–173. [Google Scholar] [CrossRef]
- Zwain, A.; Aldiwani, M.; Taqi, H. The Association Between Psoriasis and Cardiovascular Diseases. Eur. Cardiol. 2021, 16, e19. [Google Scholar] [CrossRef]
- Garshick, M.S.; Ward, N.L.; Krueger, J.G.; Berger, J.S. Cardiovascular Risk in Patients With Psoriasis. J. Am. Coll. Cardiol. 2021, 77, 1670–1680. [Google Scholar] [CrossRef]
- Hao, Y.; Zhu, Y.; Zou, S.; Zhou, P.; Hu, Y.; Zhao, Q.; Gu, L.; Zhang, H.; Wang, Z.; Li, J. Metabolic Syndrome and Psoriasis: Mechanisms and Future Directions. Front. Immunol. 2021, 12, 711060. [Google Scholar] [CrossRef]
- Kirmit, A.; Kader, S.; Aksoy, M.; Bal, C.; Nural, C.; Aslan, O. Trace Elements and Oxidative Stress Status in Patients with Psoriasis. Postepy. Dermatol. Alergol. 2020, 37, 333–339. [Google Scholar] [CrossRef]
- Sheikh, G.; Masood, Q.; Majeed, S.; Hassan, I. Comparison of Levels of Serum Copper, Zinc, Albumin, Globulin and Alkaline Phosphatase in Psoriatic Patients and Controls: A Hospital Based Casecontrol Study. Indian Dermatol. Online J. 2015, 6, 81. [Google Scholar] [CrossRef]
- Payasvi, B.; Agarwal, B.K.; Sharma, V.K.; Anna, A. Analysis of Serum Copper, Zinc and Iron Levels in Psoriasis and Psoriasis with Hyper Tension Patients. Int. J. Innov. Res. Dev. 1990, 2, 544–553. [Google Scholar]
- Bor, N.M.; Karabiyikoglu, A.; Dereagzi, H. Zinc in treatment of psoriasis. J. Islam. World Acad. Sci. 1991, 4, 78–82. [Google Scholar]
- Own, A. Zinc serum in psoriatic patients. Mustansiriya Med. J. 2012, 11, 20–23. [Google Scholar]
- Saxena, N.; Sharma, R.; Singh, V.S.; Sharma, N. Serum zinc and copper levels in psoriasis. IJDVL 1990, 56, 3. [Google Scholar]
- Arora, P.; Dhillon, K.; Rajan, S.; Sayal, S.; Das, A. Serum Zinc Levels in Cutaneous Disorders. Med. J. Armed Forces India 2002, 58, 304–306. [Google Scholar] [CrossRef]
- Khan, F.; Naeem, S.; Ahmad, Z.; Zuberi, N.A. Association of serum levels of zinc and copper with degree of severity in patients with psoriasis. J. Saidu Med. Coll. Swat. 2018, 7, 2. [Google Scholar]
- Morgan, M.E.I.; Hughes, M.A.; Mcmillan, E.M.; King, I.; Mackie, R.M. Plasma Zinc in Psoriatic In-Patients Treated with Local Zinc Applications. Br. J. Dermatol. 1980, 102, 579–583. [Google Scholar] [CrossRef]
- Hasan, N.S.; Hadeel, S.B.; Rawaa, F.J. Evaluation of trace elements zinc & copper in Iraqi patients with psoriasis & extent of the disease. Int. J. Res. Pharm. Chem. 2016, 6, 9–14. [Google Scholar]
- Nigam, P. Serum Zinc and Copper Levels and Cu: Zn Ratio in Psoriasis. Indian J. Dermatol. Venereol. Leprol. 2005, 71, 205. [Google Scholar] [CrossRef]
- Kılıç, S.; Şehitoğlu, H. Correlation between psoriasis and ZIP2 and ZIP3 Zinc transporters. Turk. J. Med. Sci. 2020, 14, 61. [Google Scholar]
- Aggarwal, J.; Singh, A.; Gupta, S.; Prasad, R. Copper and Zinc Status in Psoriasis: Correlation with Severity. Ind. J. Clin. Biochem. 2021, 36, 120–123. [Google Scholar] [CrossRef]
- Ala, S.; Shokrzadeh, M.; Golpour, M.; Salehifar, E.; Alami, M.; Ahmadi, A. Zinc and Copper Levels in Iranian Patients with Psoriasis: A Case Control Study. Biol. Trace Elem. Res. 2013, 153, 22–27. [Google Scholar] [CrossRef]
- Wacewicz, M.; Socha, K.; Soroczyńska, J.; Niczyporuk, M.; Aleksiejczuk, P.; Ostrowska, J.; Borawska, M.H. Concentration of Selenium, Zinc, Copper, Cu/Zn Ratio, Total Antioxidant Status and c-Reactive Protein in the Serum of Patients with Psoriasis Treated by Narrow-Band Ultraviolet B Phototherapy: A Case-Control Study. J. Trace Elem. Med. Biol. 2017, 44, 109–114. [Google Scholar] [CrossRef]
- Di Lernia, V.; Casanova, D.M.; Goldust, M.; Ricci, C. Pemphigus Vulgaris and Bullous Pemphigoid: Update on Diagnosis and Treatment. Dermatol. Pract. Concept. 2020, 10, e2020050. [Google Scholar] [CrossRef]
- Didona, D.; Paolino, G.; Di Zenzo, G.; Didona, B.; Pampena, R.; Di Nicola, M.R.; Mercuri, S.R. Pemphigus Vulgaris: Present and Future Therapeutic Strategies. Dermatol. Pract. Concept 2022, 12, e2022037. [Google Scholar] [CrossRef]
- Rosi-Schumacher, M.; Baker, J.; Waris, J.; Seiffert-Sinha, K.; Sinha, A.A. Worldwide Epidemiologic Factors in Pemphigus Vulgaris and Bullous Pemphigoid. Front. Immunol. 2023, 14, 1159351. [Google Scholar] [CrossRef]
- Javanbakht, M.; Daneshpazhooh, M.; Chams-Davatchi, C.; Eshraghian, M.; Zarei, M.; Chamari, M.D. Serum selenium, zinc, and copper in early diagnosed patients with pemphigus vulgaris. Iran J. Public Health 2012, 41, 105–109. [Google Scholar]
- Yazdanpanah, M.J.; Ghayour-Mobarhan, M.; Taji, A.; Javidi, Z.; Pezeshkpoor, F.; Tavallaie, S.; Momenzadeh, A.; Esmaili, H.; Shojaie-Noori, S.; Khoddami, M.; et al. Serum Zinc and Copper Status in Iranian Patients with Pemphigus Vulgaris. Int. J. Dermatol. 2011, 50, 1343–1346. [Google Scholar] [CrossRef]
- Esmaeili, N.; Soori, T.; Nooraei, Z.; Karimi, A. Serum levels of copper and zinc in patients with pemphigus vulgaris admitted to Razi Hospital, Tehran, Iran in 2012 and 2013. J. Cosmet. Dermatol. 2015, 6, 93–99. [Google Scholar]
- La Serra, L.; Salathiel, A.M.; Trevilato, T.M.B.; Alves, R.I.S.; Segura-Muñoz, S.I.; De Oliveira Souza, V.C.; Barbosa, F., Jr.; Roselino, A.M. Trace Element Profile in Pemphigus Foliaceus and in Pemphigus Vulgaris Patients from Southeastern Brazil. J. Trace Elem. Med. Biol. 2019, 51, 31–35. [Google Scholar] [CrossRef]
- Wollenberg, A.; Werfel, T.; Ring, J.; Ott, H.; Gieler, U.; Weidinger, S. Atopic Dermatitis in Children and Adults. Dtsch. Ärzteblatt. Int. 2023, 120, 224. [Google Scholar] [CrossRef]
- Clebak, K.T.; Helm, L.; Uppal, P.; Davis, C.R.; Helm, M.F. Atopic Dermatitis. Prim. Care Clin. Off. Pract. 2023, 50, 191–203. [Google Scholar] [CrossRef]
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. IJMS 2021, 22, 4130. [Google Scholar] [CrossRef]
- David, T.J.; Wells, F.E.; Sharpe, T.C.; Gibbs, A.C.; Devlin, J. Serum levels of trace metals in children with atopic eczema. Br. J. Dermatol. 1990, 122, 485–495. [Google Scholar] [CrossRef]
- Toro, R.D.; Capotorti, M.G.; Gialanella, G.; Del Giudice, M.M.; Moro, R.; Perrone, L. Zinc and Copper Status of Allergic Children. Acta Paediatr. 1987, 76, 612–617. [Google Scholar] [CrossRef]
- Gray, N.A.; Esterhuizen, T.M.; Khumalo, N.P.; Stein, D.J. Investigating Hair Zinc Concentrations in Children with and without Atopic Dermatitis. S. Afr. Med. J. 2020, 110, 409. [Google Scholar] [CrossRef]
- Farhood, I.G.; Ahmed, M.H.; Al-Bandar, R.T.; Farhood, R.G. Assessment of Serum Zinc Level in Patients with Atopic Dermatitis. Iraqi J. Med. Sci. 2019, 17, 103–107. [Google Scholar] [CrossRef]
- Esenboga, S.; Gur Cetinkaya, P.; Sahiner, N.; Birben, E.; Soyer, O.; Enis Sekerel, B.; Murat Sahiner, U. Infantile Atopic Dermatitis: Serum Vitamin D, Zinc and TARC Levels and Their Relationship with Disease Phenotype and Severity. Allergol. Immunopathol. 2021, 49, 162–168. [Google Scholar] [CrossRef]
- Karabacak, E.; Aydin, E.; Kutlu, A.; Ozcan, O.; Muftuoglu, T.; Gunes, A.; Dogan, B.; Ozturk, S. Erythrocyte Zinc Level in Patients with Atopic Dermatitis and Its Relation to SCORAD Index. Postepy Dermatol Alergol. 2016, 5, 349–352. [Google Scholar] [CrossRef]
- el-Kholy, M.S.; Gas Allah, M.A.; el-Shimi, S.; el-Baz, F.; el-Tayeb, H.; Abdel-Hamid, M.S. Zinc and copper status in children with bronchial asthma and atopic dermatitis. J. Egypt Public Health Assoc. 1990, 65, 657–668. [Google Scholar]
- Toyran, M.; Kaymak, M.; Vezir, E.; Harmanci, K.; Kaya, A.; Giniş, T.; Köse, G.; Kocabaş, C.N. Trace element levels in children with atopic dermatitis. J. Investig. Allergol. Clin. Immunol. 2012, 22, 341–344. [Google Scholar]
- Hon, K.-L.E.; Wang, S.S.; Hung, E.C.W.; Lam, H.S.; Lui, H.H.K.; Chow, C.-M.; Ching, G.K.W.; Fok, T.; Ng, P.-C.; Leung, T.-F. Serum Levels of Heavy Metals in Childhood Eczema and Skin Diseases: Friends or Foes: Heavy Metals in Childhood Eczema. Pediatr. Allergy Immunol. 2010, 21, 831–836. [Google Scholar] [CrossRef]
- Gray, N.A.; Dhana, A.; Stein, D.J.; Khumalo, N.P. Zinc and Atopic Dermatitis: A Systematic Review and Meta-analysis. Acad. Dermatol. Venereol. 2019, 33, 1042–1050. [Google Scholar] [CrossRef]
- David, T.J.; Wells, F.E.; Sharpe, T.C.; Gibbs, A.C.C. Low Serum Zinc in Children with Atopic Eczema. Br. J. Dermatol. 1984, 111, 597–601. [Google Scholar] [CrossRef]
- Landiasari, D.A.; Diah Lintang Kawuryan, D.L.; Hidayah, D. Correlation between Serum Zinc Levels and Severity of Atopic Dermatitis. APJPCH 2020, 3, 114–118. [Google Scholar]
- Atay, O.; Asılsoy, S.; Sırın, S.; Atakul, G.; Al, S.; Boyacıoglu, O.K.; Uzuner, N.; Karaman, O. The Effect of Nutrition and Micronutrients on Children with Atopic Dermatitis. Asthma Allergy Immunol. 2023, 21, 1089–1094. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, S.; Jeong, M.; Ko, J.; Ro, Y. Hair Zinc Levels and the Efficacy of Oral Zinc Supplementation in Patients with Atopic Dermatitis. Acta Derm. Venerol. 2014, 94, 558–562. [Google Scholar] [CrossRef]
- Vasam, M.; Korutla, S.; Bohara, R.A. Acne Vulgaris: A Review of the Pathophysiology, Treatment, and Recent Nanotechnology Based Advances. Biochem. Biophys. Rep. 2023, 36, 101578. [Google Scholar] [CrossRef]
- Layton, A.M.; Ravenscroft, J. Adolescent Acne Vulgaris: Current and Emerging Treatments. Lancet Child Adolesc. Health 2023, 7, 136–144. [Google Scholar] [CrossRef]
- Gaber, H.A.A.; Abozied, A.A.-H.; Abd-Elkareem, I.M.; El-Shazly, Y.N.Y. Serum Zinc Levels in Patients with Acne Vulgaris and Its Relation to The Severity of Disease. Egypt. J. Hosp. Med. 2019, 75, 2845–2848. [Google Scholar] [CrossRef]
- Butool, F.; Amanullah, M.; Syed, P.A.; Mohammed, R.A. Role of serum Zinc and Copper levels in patients with acne vulgaris. J. Orofac. Res 2019, 8, 71–75. [Google Scholar]
- Saleh, B.; Zainab, A.; Ali, M. Serum Trace Elements (Zinc, Copper and Magnesium) Status in Iraqi Patients with Acne Vulgaris: (Case-Controlled Study). Iraqi J. Pharm. Sci. 2011, 20, 2. [Google Scholar] [CrossRef]
- Manor, B.; Bhawna, B.; Huma, N.; Anand, D.; Ashish, M.; Bubul, K.; Vikas, K. Copper and zinc microminerals levels in patients of acne vulgaris: A case control study. Int. J. Sci. Res. 2023, 12, 55–56. [Google Scholar]
- Salma, A.; Rubaiya, A.; Reazul, I.; Hoque, M.; Abul, H.; Zabun, N. Effect of Serum Trace Elements, Macro-minerals and Antioxidants in Acne Vulgaris Patients: A Case-Control Study. Dhaka Univ. J. Pharm. Sci. 2016, 15, 215–220. [Google Scholar]
- Kaymak, Y.; Adişen, E.; Erhan, M.; Çelik, B.; Gürer, M.A. Inc Levels in Patients with Acne Vulgaris. J. Turk. Acad. Dermatol. 2007, 1, 71302a. [Google Scholar]
- Goodarzi, A.; Roohaninasab, M.; Atefi, N.S.; Sadeghzadeh Bazargan, A.; Ghassemi, M.; Ghahremani, A.P.; Teymoori, N.; Biglari Abhari, M. Determination of Serum Levels of Zinc in Acne Vulgaris Patients: A Case Control Study. Iran. J. Dermatol. 2020, 23, 28–31. [Google Scholar] [CrossRef]
- Yee, B.E.; Richards, P.; Sui, J.Y.; Marsch, A.F. Serum Zinc Levels and Efficacy of Zinc Treatment in Acne Vulgaris: A Systematic Review and Meta-analysis. Dermatol. Ther. 2020, 33, e14252. [Google Scholar] [CrossRef]
- Rostami Mogaddam, M.; Safavi Ardabili, N.; Maleki, N.; Soflaee, M. Correlation between the Severity and Type of Acne Lesions with Serum Zinc Levels in Patients with Acne Vulgaris. BioMed. Res. Int. 2014, 2014, 474108. [Google Scholar] [CrossRef]
- Ahmed, L.; Al-Timimi, D.; Sharaf, B. Association between acne vulgaris and circulatory zinc status among adolescents and young adults. Duhok Med. J. 2022, 16, 23–37. [Google Scholar] [CrossRef]
- Amer, M.; Bahgat, M.R.; Tosson, Z.; Mowla, M.Y.M.A.; Amer, K. Serum Zinc in Acne Vulgaris. Int. J. Dermatol. 1982, 21, 481–484. [Google Scholar] [CrossRef]
- Fitzherbert, J.C. Zinc deficiency in acne vulgaris. Med. J. Aust. 1977, 2, 685–686. [Google Scholar] [CrossRef]
- Ozuguz, P.; Dogruk Kacar, S.; Ekiz, O.; Takci, Z.; Balta, I.; Kalkan, G. Evaluation of Serum Vitamins A and E and Zinc Levels According to the Severity of Acne Vulgaris. Cutan. Ocul. Toxicol. 2014, 33, 99–102. [Google Scholar] [CrossRef]
- Cervantes, J.; Eber, A.E.; Perper, M.; Nascimento, V.M.; Nouri, K.; Keri, J.E. The Role of Zinc in the Treatment of Acne: A Review of the Literature. Dermatol. Ther. 2018, 31, e12576. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J. Role of zinc in acne: A study of 77 patients. Int. J. Res. Dermatol. 2018, 4, 1–5. [Google Scholar] [CrossRef]
- Dall’Oglio, F.; Nasca, M.R.; Gerbino, C.; Micali, G. An Overview of the Diagnosis and Management of Seborrheic Dermatitis. CCID 2022, 15, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Aktaş Karabay, E.; Aksu Çerman, A. Serum Zinc Levels in Seborrheic Dermatitis: A Case-Control Study. Turk. J. Med. Sci. 2019, 49, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Zohreh, H.; Majid, S.; Mohammad, H. The Relationship of Serum Selenium, Zinc, and Copper Levels with Seborrheic Dermatitis: A Case-Control Study. Iran. J. Dermatol. 2019, 22, 7–12. [Google Scholar] [CrossRef]
- Burkhead, J.L.; Collins, J.F. Nutrition Information Brief—Copper. Adv. Nutr. 2022, 13, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Y.; Liu, X. Copper Homeostasis and Copper-Induced Cell Death: Novel Targeting for Intervention in the Pathogenesis of Vascular Aging. Biomed. Pharmacother. 2023, 169, 115839. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Copper Homeostasis: Emerging Target for Cancer Treatment. IUBMB Life 2020, 72, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, C.; Arnesen, E.K. Copper—A Scoping Review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67. [Google Scholar] [CrossRef]
- Ciosek, Ż.; Kot, K.; Rotter, I. Iron, Zinc, Copper, Cadmium, Mercury, and Bone Tissue. IJERPH 2023, 20, 2197. [Google Scholar] [CrossRef]
- Osredkar, J. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clin. Toxicol. 2011, s3. [Google Scholar] [CrossRef]
- Myint, Z.W.; Oo, T.H.; Thein, K.Z.; Tun, A.M.; Saeed, H. Copper Deficiency Anemia: Review Article. Ann. Hematol. 2018, 97, 1527–1534. [Google Scholar] [CrossRef]
- Focarelli, F.; Giachino, A.; Waldron, K.J. Copper Microenvironments in the Human Body Define Patterns of Copper Adaptation in Pathogenic Bacteria. PLoS Pathog. 2022, 18, e1010617. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.C. Copper Homeostasis in Mammals, with Emphasis on Secretion and Excretion. A Review. IJMS 2020, 21, 4932. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, Y.; Shi, H.; Peng, Y.; Fan, X.; Li, C. The Molecular Mechanisms of Copper Metabolism and Its Roles in Human Diseases. Pflug. Arch.-Eur. J. Physiol. 2020, 472, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. GHK-Cu may Prevent Oxidative Stress in Skin by Regulating Copper and Modifying Expression of Numerous Antioxidant Genes. Cosmetics 2015, 2, 236–247. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin Whitening Agents: Medicinal Chemistry Perspective of Tyrosinase Inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Alizadeh, S.; Seyedalipour, B.; Shafieyan, S.; Kheime, A.; Mohammadi, P.; Aghdami, N. Copper Nanoparticles Promote Rapid Wound Healing in Acute Full Thickness Defect via Acceleration of Skin Cell Migration, Proliferation, and Neovascularization. Biochem. Biophys. Res. Commun. 2019, 517, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Hwang, H.; Chauhan, S.; Leonardi, D.; Gonzalez, S. Stimulation of Cell Proliferation and Expression of Matrixmetalloproteinase-1 and Interluekin-8 Genes in Dermal Fibroblasts by Copper. Connect. Tissue Res. 2010, 51, 224–229. [Google Scholar] [CrossRef]
- Philips, N.; Samuel, P.; Parakandi, H.; Gopal, S.; Siomyk, H.; Ministro, A.; Thompson, T.; Borkow, G. Beneficial Regulation of Fibrillar Collagens, Heat Shock Protein-47, Elastin Fiber Components, Transforming Growth Factor-Β1, Vascular Endothelial Growth Factor and Oxidative Stress Effects by Copper in Dermal Fibroblasts. Connect. Tissue Res. 2012, 53, 373–378. [Google Scholar] [CrossRef]
- Ramirez, H.; Patel, S.B.; Pastar, I. The Role of TGFβ Signaling in Wound Epithelialization. Adv. Wound Care 2014, 3, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Wu, T.; Wu, W.; Tu, W.; Jiang, S.; Chen, S.; Ma, Y.; Liu, Q.; Zhou, X.; Jin, L.; et al. Involvement of Collagen-Binding Heat Shock Protein 47 in Scleroderma-Associated Fibrosis. Protein Cell 2015, 6, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-L.; Zhou, Z.-F.; Liu, J.-F.; Hou, X.-D.; Zhou, Z.; Dai, Y.-L.; Hou, Z.-Y.; Chen, F.; Zheng, L.-P. Donut-like MOFs of Copper/Nicotinic Acid and Composite Hydrogels with Superior Bioactivity for Rh-bFGF Delivering and Skin Wound Healing. J. Nanobiotechnol. 2021, 19, 275. [Google Scholar] [CrossRef] [PubMed]
- Salvo, J.; Sandoval, C. Role of Copper Nanoparticles in Wound Healing for Chronic Wounds: Literature Review. Burn. Trauma 2022, 10, tkab047. [Google Scholar] [CrossRef] [PubMed]
- Zackheim, H.S.; Wolf, P. Serum Copper in Psoriasis and Other Dermatoses. J. Investig. Dermatol. 1972, 58, 28–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shahidi-Dadras, M.; Namazi, N.; Younespour, S. Comparative Analysis of Serum Copper, Iron, Ceruloplasmin, and Transferrin Levels in Mild and Severe Psoriasis Vulgaris in Iranian Patients. Indian Dermatol. Online J. 2017, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, R.; Yuti, A.M.; Basavaraj, K.H. Relevance of Copper and Ceruloplasmin in Psoriasis. Clin. Chim. Acta 2010, 411, 1390–1392. [Google Scholar] [CrossRef] [PubMed]
- Doǧan, P.; Soyuer, Ü.; Tanrikulu, G. Superoxide Dismutase and Myeloperoxidase Activity in Polymorphonuclear Leukocytes, and Serum Ceruloplasmin and Copper Levels, in Psoriasis. Br. J. Dermatol. 2006, 120, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Butnaru, C.; Pascu, M.; Mircea, C.; Agoroaei, L.; Solovăstru, L.; Vâţă, D.; Butnaru, E.; Petrescu, Z. Serum zinc and copper levels in some dermatological diseases. Rev. Med. Chir. Soc. Med. Nat. Iasi 2008, 112, 253–537. [Google Scholar]
- Martínez-Morillo, E.; Bauça, J.M. Biochemical Diagnosis of Wilson’s Disease: An Update. Adv. Lab. Med./Av. Med. Lab. 2022, 3, 103–113. [Google Scholar] [CrossRef]
- Torkian, S.; Khanjani, N.; Mahmoodi, M.R.; Khosravi, V. A Review of Copper Concentrations in Iranian Populations. Env. Monit. Assess 2019, 191, 537. [Google Scholar] [CrossRef] [PubMed]
- Mashaly, H.M.; Sayed, K.S.; Mohammad, F.N.; Shaker, O.G.; Saeed, M.G. Estimation of serum zinc and copper in Egyptian patients with pemphigus vulgaris. J. Egypt Women’s Dermatol. Soc. 2014, 11, 32–35. [Google Scholar] [CrossRef]
- Al-Ghurabi, B.; Al-Hassan, A.; Al-Waiz, M. Serum levels of copper and zinc in patients with atopic dermatitis in Iraq. Iraqi J. Sci. 2007, 20, 69–72. [Google Scholar]
- Butool, F.; Amanullah, M. Significance of Serum Copper Levels in Patients with Acne Vulgaris. Cosmetol. Oro. Facial. Surg. 2018, 4, 130. [Google Scholar]
- El-Saaiee, L.; Abdel-Aal, H.; El-Mahdy, H.; Abdel-Aal, A.M. Serum copper, iron and zinc in cases of acne vulgaris. J. Med. 1983, 14, 125–136. [Google Scholar] [PubMed]
- Khayyat, M.I.; Khaddam, J.; Alshaer, R.H. Evaluation of Serum Copper Level in Acne Vulgaris Patients. TUJ-ESS 2023, 45, 569–577. [Google Scholar]
- Amanullah, M. Significance of Serum Copper Levels in Patients with Acne Vulgaris. J. Clin. Exp. Transpl. 2020, 5, 2. [Google Scholar]
- Ikaraoha, C.I.; Mbadiwe, N.C.; Anyanwu, C.J.; Odekhian, J.; Nwadike, C.N.; Amah, H.C. The Role of Blood Lead, Cadmium, Zinc and Copper in Development and Severity of Acne Vulgaris in a Nigerian Population. Biol. Trace Elem. Res. 2017, 176, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Islam, M.R.; Islam, M.R.; Ali, R.; Rahman, S.M.M.; Nahar, Z.; Hasnat, A.; Islam, M.S. Altered Serum Elements, Antioxidants, MDA, and Immunoglobulins Are Associated with an Increased Risk of Seborrheic Dermatitis. Heliyon 2021, 7, e06621. [Google Scholar] [CrossRef]
- Stewart, J.C.M.; Horrobin, D.F.; Morse, P.; Ward, N.; Abouk Shakra, F. Serum copper levels are elevated in seborrhoeic dermatitis. Br. J. Dermatol. 1989, 121, 40–41. [Google Scholar] [CrossRef]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The Roles of Iron in Health and Disease. Mol. Asp. Med. 2001, 22, 1–87. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Gutiérrez, A.G.; González-Rosendo, G.; Sánchez-Muñoz, J.; Polo-Pozo, J.; Rodríguez-Jerez, J.J. Bioavailability of Heme Iron in Biscuit Filling Using Piglets as an Animal Model for Humans. Int. J. Biol. Sci. 2008, 4, 58–62. [Google Scholar] [CrossRef]
- Hunt, J.R.; Roughead, Z.K. Adaptation of Iron Absorption in Men Consuming Diets with High or Low Iron Bioavailability. Am. J. Clin. Nutr. 2000, 71, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Han, O. Molecular Mechanism of Intestinal Iron Absorption. Metallomics 2011, 3, 103. [Google Scholar] [CrossRef] [PubMed]
- Dasa, F.; Abera, T. Factors Affecting Iron Absorption and Mitigation Mechanisms: A Review. Int. J. Agric. Sc Food Technol. 2018, 4, 024–030. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Wang, J.; Huang, R.; Wan, D. Regulation of Iron Homeostasis and Related Diseases. Mediat. Inflamm. 2020, 2020, 6062094. [Google Scholar] [CrossRef] [PubMed]
- Ems, T.; St Lucia, K.; Huecker, M.R. Biochemistry, Iron Absorption. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Andrews, N.C. Disorders of Iron Metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Friel, J.; Qasem, W.; Cai, C. Iron and the Breastfed Infant. Antioxidants 2018, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.A.; Richards, T.; Srai, S.K.S. The Role of Iron in the Skin and Cutaneous Wound Healing. Front. Pharmacol. 2014, 5, 156. [Google Scholar] [CrossRef]
- Youssry, I.; Mohsen, N.A.; Shaker, O.G.; El-Hennawy, A.; Fawzy, R.; Abu-Zeid, N.M.; El-Beshlawy, A. Skin Iron Concentration: A Simple, Highly Sensitive Method for Iron Stores Evaluation in Thalassemia Patients. Hemoglobin 2007, 31, 357–365. [Google Scholar] [CrossRef]
- Coger, V.; Million, N.; Rehbock, C.; Sures, B.; Nachev, M.; Barcikowski, S.; Wistuba, N.; Strauß, S.; Vogt, P.M. Tissue Concentrations of Zinc, Iron, Copper, and Magnesium During the Phases of Full Thickness Wound Healing in a Rodent Model. Biol. Trace Elem. Res. 2019, 191, 167–176. [Google Scholar] [CrossRef]
- Leveque, N.; Robin, S.; Makki, S.; Muret, P.; Rougier, A.; Humbert, P. Iron and Ascorbic Acid Concentrations in Human Dermis with Regard to Age and Body Sites. Gerontology 2003, 49, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Yokouchi, M.; Kubo, A. Maintenance of Tight Junction Barrier Integrity in Cell Turnover and Skin Diseases. Exp. Dermatol. 2018, 27, 876–883. [Google Scholar] [CrossRef]
- Pelle, E.; Jian, J.; Declercq, L.; Dong, K.; Yang, Q.; Pourzand, C.; Maes, D.; Pernodet, N.; Yarosh, D.B.; Huang, X. Protection against Ultraviolet A-induced Oxidative Damage in Normal Human Epidermal Keratinocytes under Post-menopausal Conditions by an Ultraviolet A-activated Caged-iron Chelator: A Pilot Study. Photoderm. Photoimm. Photomed. 2011, 27, 231–235. [Google Scholar] [CrossRef]
- Aroun, A.; Zhong, J.L.; Tyrrell, R.M.; Pourzand, C. Iron, Oxidative Stress and the Example of Solar Ultraviolet A Radiation. Photochem. Photobiol. Sci. 2012, 11, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Dao, E.; Zeller, M.P.; Wainman, B.C.; Farquharson, M.J. Feasibility of the Use of a Handheld XRF Analyzer to Measure Skin Iron to Monitor Iron Levels in Critical Organs. J. Trace Elem. Med. Biol. 2018, 50, 305–311. [Google Scholar] [CrossRef]
- Daszkiewicz, M. The Role of Nutrition in the Prevention and Therapy of Selected Skin Diseases. Aesth. Cosmetol. Med. 2021, 10, 175–179. [Google Scholar] [CrossRef]
- Behrangi, E.; Baniasadi, F.; Esmaeeli, S.; Hedayat, K.; Goodarzi, A.; Azizian, Z. Serum Iron Level, Ferritin and Total Iron Binding Capacity Level among Nonpregnant Women with and without Melasma. J. Res. Med. Sci. 2015, 20, 281–283. [Google Scholar] [PubMed]
- Kalantar-Zadeh, K.; Rodriguez, R.A.; Humphreys, M.H. Association between Serum Ferritin and Measures of Inflammation, Nutrition and Iron in Haemodialysis Patients. Nephrol. Dial. Transplant. 2004, 19, 141–149. [Google Scholar] [CrossRef]
- Rashmi, R.; Yuti, A.M.; Basavaraj, K.H. Enhanced Ferritin/Iron Ratio in Psoriasis. Indian. J. Med. Res. 2012, 135, 662–665. [Google Scholar]
- Elhaddad, H.; Morsy, R.; Mourad, B.; Elnimr, T. A comprehensive study o the content of serum trace elements in psoriasis. J. Elem. 2017, 22, 31–42. [Google Scholar] [CrossRef]
- Ponikowska, M.; Tupikowska, M.; Kasztura, M.; Jankowska, E.A.; Szepietowski, J.C. Deranged Iron Status in Psoriasis: The Impact of Low Body Mass: Deranged Iron Status in Psoriasis. J. Cachexia Sarcopenia Muscle 2015, 6, 358–364. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Hayes, H.; Gambling, L.; Craig, L.C.A.; Allan, K.; Prabhu, N.; Turner, S.W.; McNeill, G.; Erkkola, M.; Seaton, A.; et al. An Exploratory Study of the Associations between Maternal Iron Status in Pregnancy and Childhood Wheeze and Atopy. Br. J. Nutr. 2014, 112, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Bédard, A.; Lewis, S.J.; Burgess, S.; Henderson, A.J.; Shaheen, S.O. Maternal Iron Status during Pregnancy and Respiratory and Atopic Outcomes in the Offspring: A Mendelian Randomisation Study. BMJ Open Resp. Res. 2018, 5, e000275. [Google Scholar] [CrossRef]
- Fortes, C.; Mastroeni, S.; Mannooranparampil, T.J.; Di Lallo, D. Pre-Natal Folic Acid and Iron Supplementation and Atopic Dermatitis in the First 6 Years of Life. Arch. Dermatol. Res. 2019, 311, 361–367. [Google Scholar] [CrossRef]
- Roth-Walter, F. Iron-Deficiency in Atopic Diseases: Innate Immune Priming by Allergens and Siderophores. Front. Allergy 2022, 3, 859922. [Google Scholar] [CrossRef]
- Peroni, D.G.; Hufnagl, K.; Comberiati, P.; Roth-Walter, F. Lack of Iron, Zinc, and Vitamins as a Contributor to the Etiology of Atopic Diseases. Front. Nutr. 2023, 9, 1032481. [Google Scholar] [CrossRef]
- Hamad, N.S.; Tofiq, D.I. Effect of Serum Trace Elements in Hand Eczema Patients in Sulaimani Province: A Case-Control Study. Technium. BioChemMed. 2021, 2, 8–27. [Google Scholar]
- Onur, A.; Levent Cinar, S.; Abanci Ayhan, N. Is There Any Relationship Between Acne Vulgaris and Diet Inflammatory Index in Women? Nutr. Clín. Diet Hosp. 2023, 43, 120–128. [Google Scholar]
- Kwiatek, W.M.; Drewniak, T.; Gajda, M.; Gałka, M.; Hanson, A.L.; Cichocki, T. Preliminary Study on the Distribution of Selected Elements in Cancerous and Non-Cancerous Kidney Tissues. J. Trace Elem. Med. Biol. 2002, 16, 155–160. [Google Scholar] [CrossRef]
- Csány, G.; Gergely, L.H.; Kiss, N.; Szalai, K.; Lőrincz, K.; Strobel, L.; Csabai, D.; Hegedüs, I.; Marosán-Vilimszky, P.; Füzesi, K.; et al. Preliminary Clinical Experience with a Novel Optical–Ultrasound Imaging Device on Various Skin Lesions. Diagnostics 2022, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Barcaui, E.D.O.; Carvalho, A.C.P.; Lopes, F.P.P.L.; Piñeiro-Maceira, J.; Barcaui, C.B. High Frequency Ultrasound with Color Doppler in Dermatology. Bras Dermatol 2016, 91, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, E.; Mikailova, D.; Krakhaleva, J.; Krinitsyna, J.; Yakubovich, A.; Sergeeva, I. Ultrasonography Patterns of Atopic Dermatitis in Children. Ski. Res. Technol. 2020, 26, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Șomlea, M.; Boca, A.; Pop, A.; Ilieș, R.; Vesa, S.; Buzoianu, A.; Tătaru, A. High-Frequency Ultrasonography of Psoriatic Skin: A Non-Invasive Technique in the Evaluation of the Entire Skin of Patients with Psoriasis: A Pilot Study. Exp. Ther. Med. 2019, 18, 4981–4986. [Google Scholar] [CrossRef] [PubMed]
| Psoriasis | |
|---|---|
| ZINC | |
| Material | Observations |
| Serum | Conflicting reports - Lower zinc levels in patients with psoriasis [16,38,39,40,41,42,43,44,45,46,47] or - No differences in zinc levels between psoriasis patients and healthy patients [17,48,49,50,51,52] - Lower zinc concentrations in patients with more than 20% skin involvement and a positive family history [51] - Zinc concentrations correlated with disease severity and psoriasis type [17,42] or - No correlation between zinc concentrations and the course of psoriasis [48] |
| COPPER | |
| Serum | Conflicting reports - Higher copper levels in patients with psoriasis [16,17,38,39,43,48,50,51,52,116,117,118,119,120,121] or - No differences in copper levels between psoriasis patients and healthy patients [117] - Higher copper levels in patients with more advanced forms of the disease [50,117,118] or - No correlation between zinc concentrations and the course of psoriasis [17,48] |
| Urine or hair | No studies available, but this type of material can be used in the determination of copper concentrations in patients with psoriasis |
| IRON | |
| Skin | - Lower iron concentrations in the epidermis and higher in the dermis of psoriasis patients [151,152] |
| Serum | Reports sparse but consistent - Lower iron levels in patients with psoriasis [117,153] |
| Pemphigus vulgaris | |
| ZINC | |
| Serum | Reports sparse but consistent - Lower zinc levels in patients with pemphigus [56,57,58,59] - No correlation between zinc concentrations and course of pemphigus [59] |
| COPPER | |
| Serum | Conflicting reports - Lower zinc levels in patients with pemphigus [57,123] or - No differences in zinc levels between pemphigus patients and healthy patients [56,58,59,122] - Negative correlation between copper concentrations and pemphigus duration in men [56] |
| IRON | |
| No literature reports | |
| Atopic Dermatitis | |
| ZINC | |
| Serum | Conflicting reports - Lower zinc levels in patients with AD [69,70,71,72,73,74] or - No differences in zinc levels between AD patients and healthy patients [63,64,65,66,67,68] - Lower zinc levels in patients with more advanced AD [74,75] or - No correlation between zinc concentrations and course of AD [67] |
| Erythrocytes | Reports sparse but consistent - Lower zinc concentrations in AD patients [68,70] - Negative correlation between zinc concentrations and SCORAD index [68,70] |
| Hair | Reports sparse but consistent - Lower zinc levels in AD patients [64,69,76] |
| COPPER | |
| Serum | Conflicting reports - Higher copper levels in patients with AD [63,69,124] or - Lower copper levels in patients with AD [71] or - No differences in copper levels between AD patients and healthy patients [64,70,116] |
| Hair | Single study - Higher copper levels in patients with AD [69] |
| IRON | |
| Serum | Reports sparse but consistent - No differences in copper levels between AD patients and healthy patients [63,70,160] - No correlation between zinc concentrations and the course of AD [75] |
| Acne Vulgaris | |
| ZINC | |
| Serum | Consistent reports - Lower zinc levels in patients with acne [79,80,81,82,83,84,85,86,87,88,89,90,91] Conflicting reports - Lower zinc levels in patients with more advanced acne [81,87,91] - Correlation of zinc concentration with the occurrence of individual acne lesions [87] or - No correlation between zinc concentrations and the course of acne [84,85] |
| COPPER | |
| Serum | Conflicting report - Lower zinc levels in patients with acne [80,125,126,127,128,129] or - No differences in copper levels between acne patients and healthy patients [81,83] Single study - Correlation between copper concentrations and acne severity [127] |
| IRON | |
| Serum | Reports sparse but consistent - No differences in copper levels between acne patients and healthy patients [126,161] |
| Seborrheic Dermatitis | |
| ZINC | |
| Serum | Conflicting report - Lower zinc levels in patients with SD [95] or - No differences in copper levels between SD patients and healthy patients [15,96] |
| Hair | Single study - Higher zinc levels in patients with SD [15] |
| COPPER | |
| Serum | Reports sparse but consistent - Higher copper levels in patients with SD [15,96,130,131] |
| Hair | Single study -Higher copper levels in patients with SD [15] |
| IRON | |
| Serum | Reports sparse but consistent - Higher iron levels in patients with SD [15,130,162] |
| Hair | Single study - Higher iron levels in patients with SD [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podgórska, A.; Kicman, A.; Naliwajko, S.; Wacewicz-Muczyńska, M.; Niczyporuk, M. Zinc, Copper, and Iron in Selected Skin Diseases. Int. J. Mol. Sci. 2024, 25, 3823. https://doi.org/10.3390/ijms25073823
Podgórska A, Kicman A, Naliwajko S, Wacewicz-Muczyńska M, Niczyporuk M. Zinc, Copper, and Iron in Selected Skin Diseases. International Journal of Molecular Sciences. 2024; 25(7):3823. https://doi.org/10.3390/ijms25073823
Chicago/Turabian StylePodgórska, Aleksandra, Aleksandra Kicman, Sylwia Naliwajko, Marta Wacewicz-Muczyńska, and Marek Niczyporuk. 2024. "Zinc, Copper, and Iron in Selected Skin Diseases" International Journal of Molecular Sciences 25, no. 7: 3823. https://doi.org/10.3390/ijms25073823
APA StylePodgórska, A., Kicman, A., Naliwajko, S., Wacewicz-Muczyńska, M., & Niczyporuk, M. (2024). Zinc, Copper, and Iron in Selected Skin Diseases. International Journal of Molecular Sciences, 25(7), 3823. https://doi.org/10.3390/ijms25073823








