Difference between Keratinized- and Non-Keratinized-Originating Epithelium in the Process of Immune Escape of Oral Squamous Cell Carcinoma
Abstract
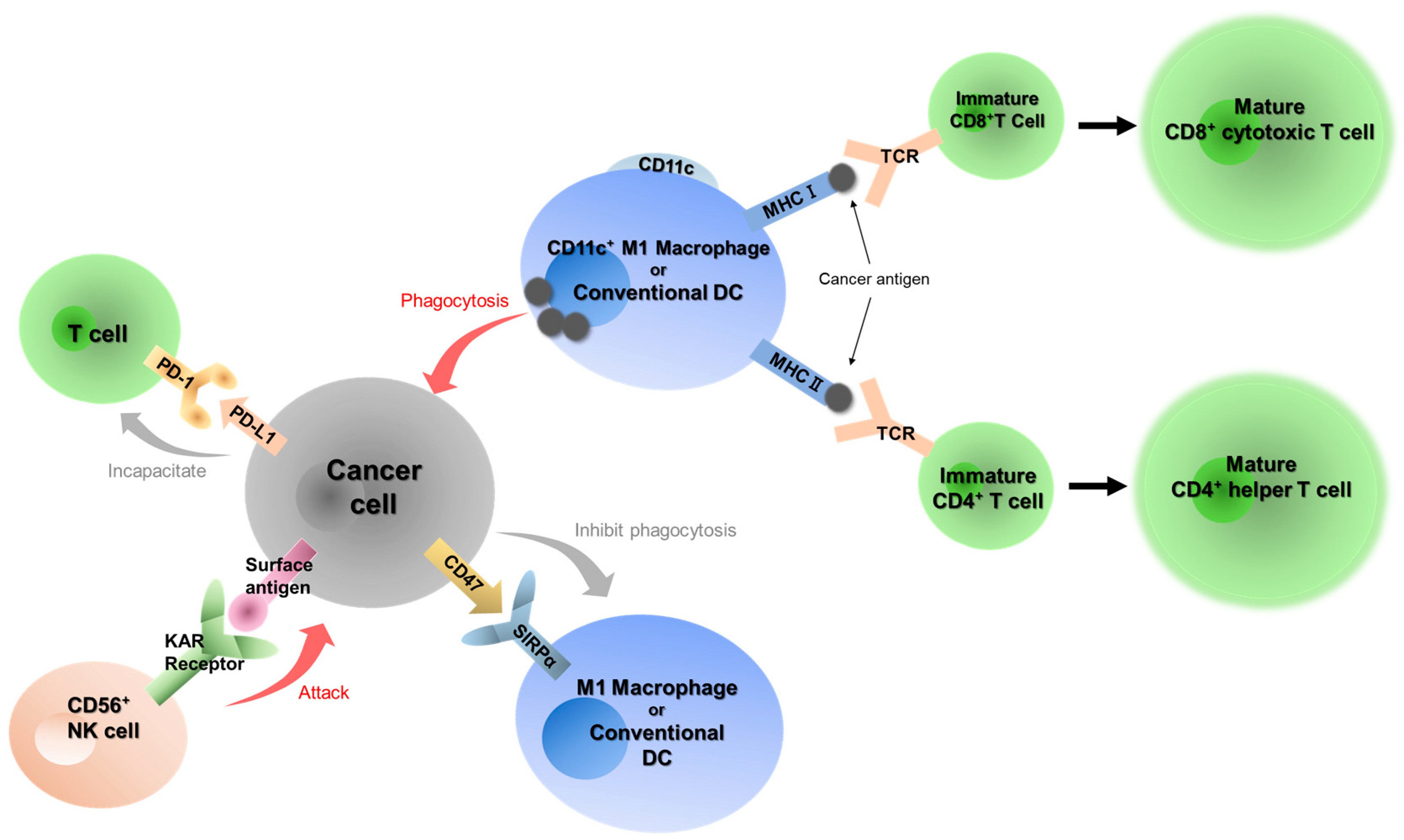
1. Introduction
2. Results
2.1. Characteristics of Patients with OSCC
2.2. Immunohistochemical Staining of Seven Immune-Related Factors
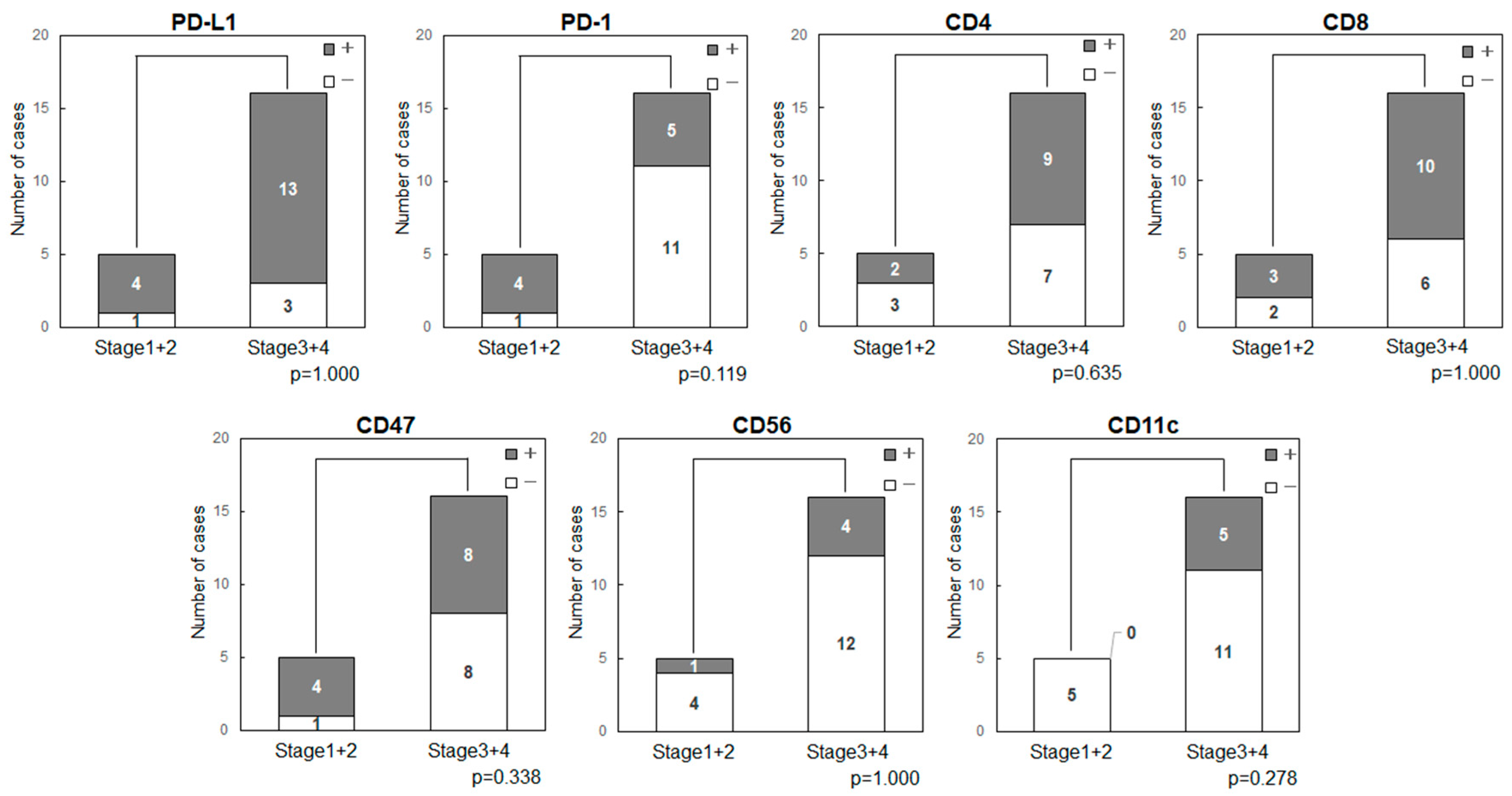
2.3. Cancer Stage
Cancer Stage and Expression of Immune-Related Factors in OSCC
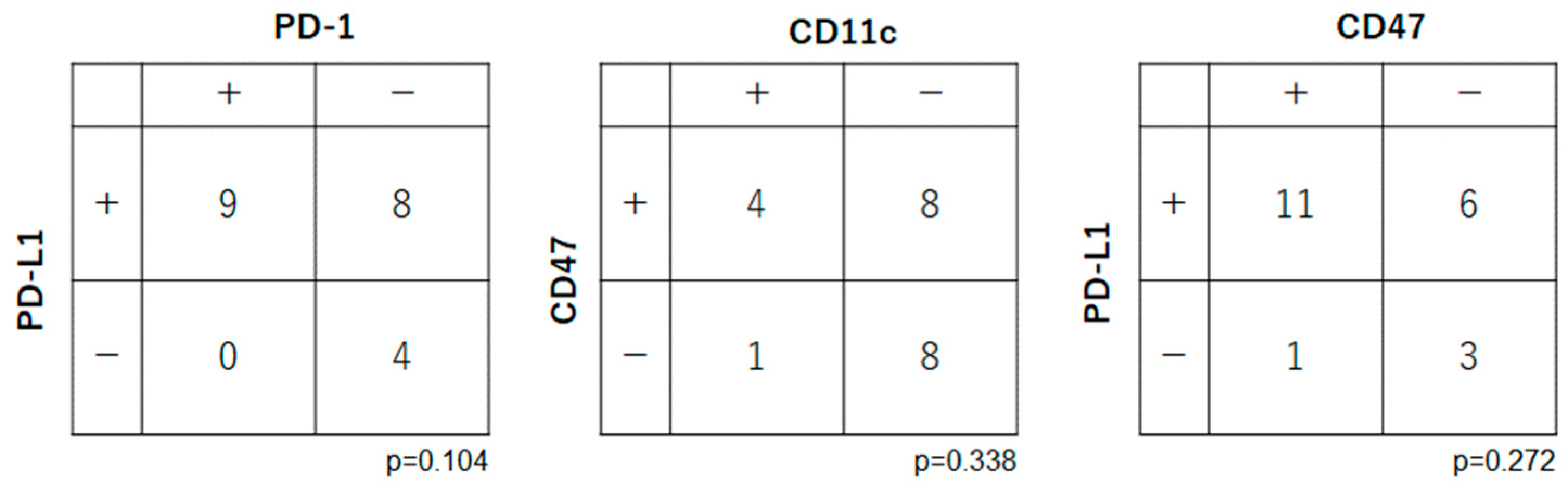
2.4. Relationships among Expression Levels of Immune Checkpoint-Related Factors in OSCC
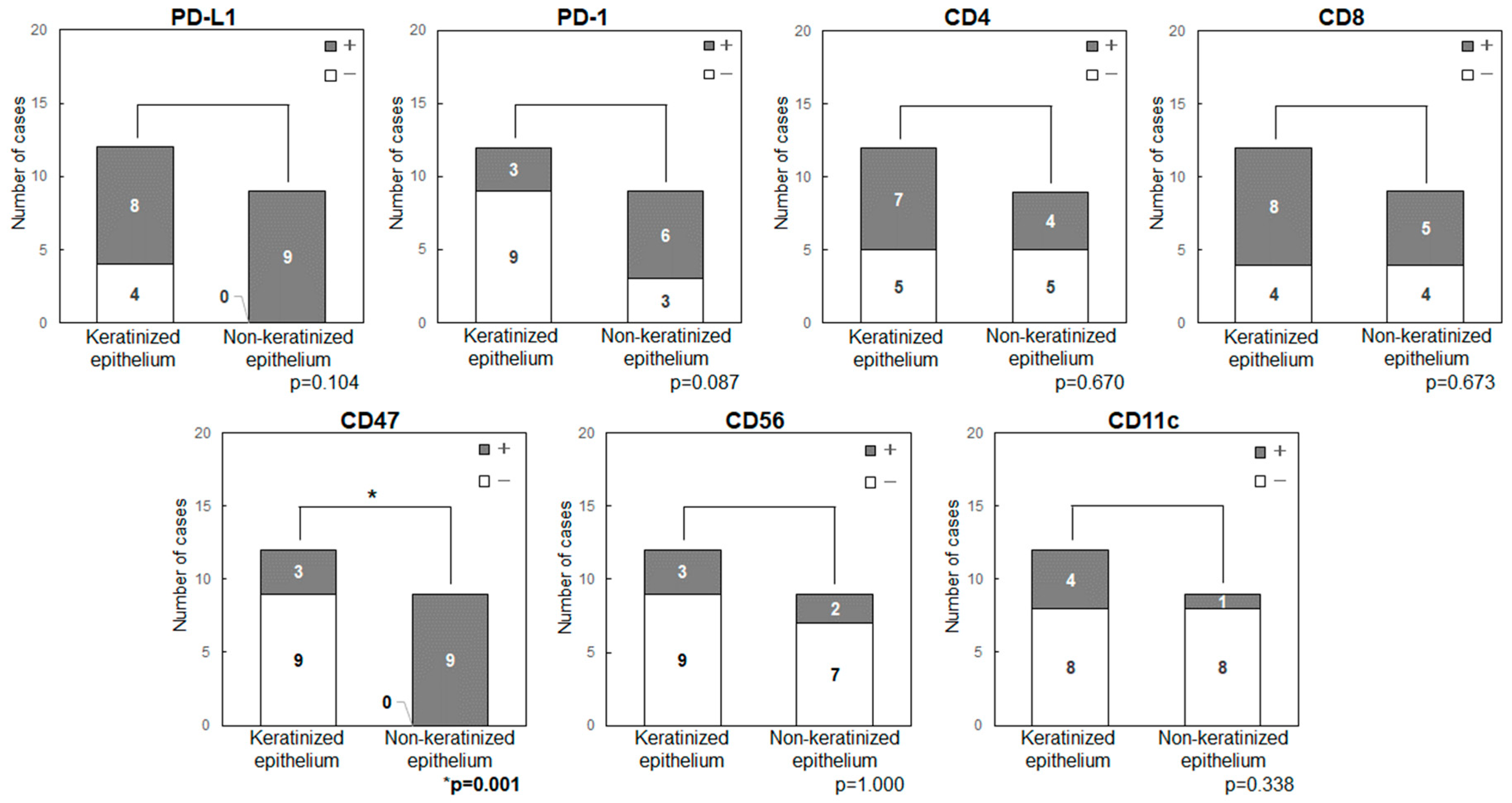
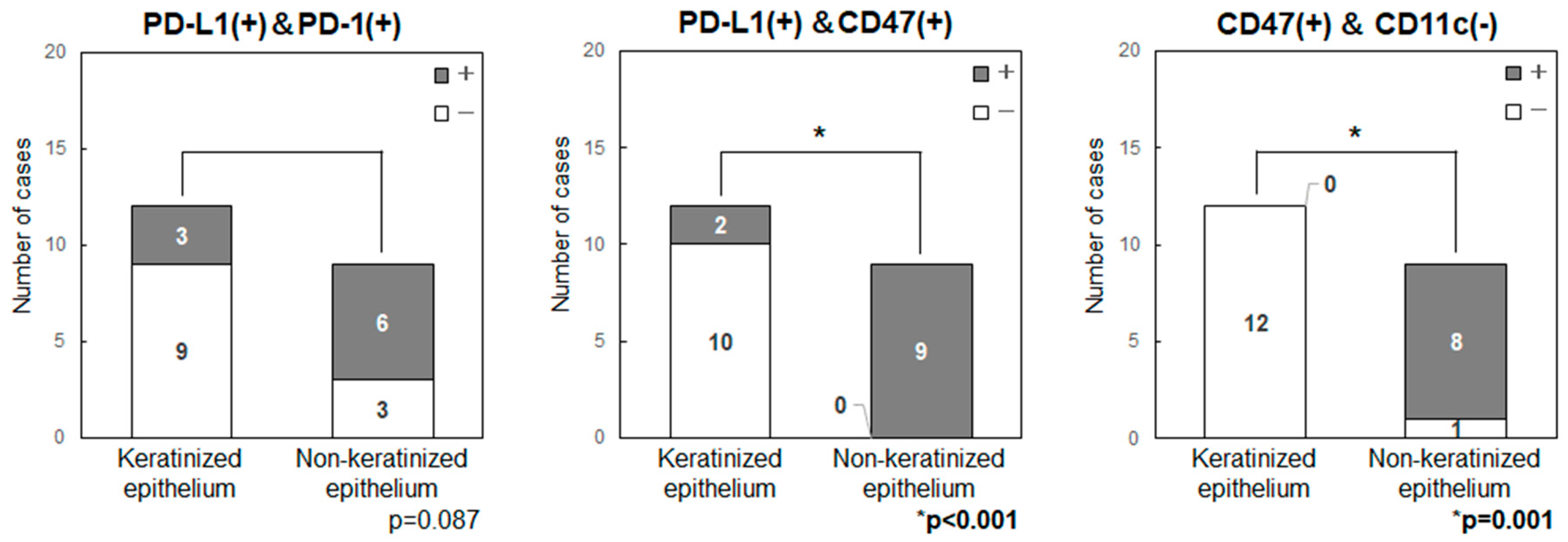
2.5. Comparison of the Expression Levels of Immune Checkpoint-Related Factors in Primary Tumor Sites (Keratinized or Non-Keratinized Epithelium)
3. Discussion
4. Materials and Methods
4.1. Patients and Clinical Data
4.2. Immunohistochemical Staining
4.3. Statistical Analysis
4.4. Ethical Standards
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 4 December 2023).
- Bishop, J.A.; Sciubba, J.J.; Westra, W.H. Squamous cell carcinoma of the oral cavity and oropharynx. Surg. Pathol. Clin. 2011, 4, 1127–1151. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLO-BOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittenkind, C. TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons, Inc.: HobLoken, NJ, USA, 2017. [Google Scholar]
- Komori, T.; Ono, M.; Hara, E.S.; Ueda, J.; Nguyen, H.T.T.; Nguyen, H.T.; Yonezawa, T.; Maeba, T.; Kimura-Ono, A.; Takarada, T.; et al. Type IV collagen α6 chain is a regulator of keratin 10 in keratinization of oral mucosal epithelium. Sci. Rep. 2018, 8, 2612. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- NCCN Guidelines for Head and Neck Cancers (Version 1.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 20 June 2022).
- Fukumoto, C.; Ogisawa, S.; Tani, M.; Hyodo, T.; Kamimura, R.; Sawatani, Y.; Hasegawa, T.; Komiyama, Y.; Fujita, A.; Wakui, T.; et al. Clinical characteristics, treatment methods and prognoses of patients with oral squamous cell carcinoma in Japanese population: A single institution retrospective cohort study. BMC Geriatr. 2020, 20, 487. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, C.; Sawatani, Y.; Shiraishi, R.; Zama, M.; Shimura, M.; Hasegawa, T.; Komiyama, Y.; Fujita, A.; Wakui, T.; Kawamata, H. Effectiveness of cetuximab as preemptive postsurgical therapy for oral squamous cell carcinoma patients with major risk: A single-center retrospective cohort study. Investig. New Drugs 2021, 39, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, C.; Oshima, R.; Sawatani, Y.; Shiraishi, R.; Hyodo, T.; Kamimura, R.; Hasegawa, T.; Komiyama, Y.; Izumi, S.; Fujita, A.; et al. Surveillance for patients with oral squamous cell carcinoma after complete surgical resection as primary treatment: A single-center retrospective cohort study. Cancers 2021, 13, 5843. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Baste, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Chamoto, K.; Al-Habsi, M.; Honjo, T. Role of PD-1 in immunity and diseases. Emerging concepts targeting immune checkpoints in cancer and autoimmunity. Curr. Top. Microbiol. Immunol. 2017, 410, 75–97. [Google Scholar]
- Topalian, S.L.; Taube, J.M.; Pardoll, D.M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020, 367, 6477. [Google Scholar] [CrossRef] [PubMed]
- Abril-Rodriguez, G.; Ribas, A. SnapShot: Immune checkpoint inhibitors. Cancer Cell 2017, 31, 848–848.e1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, Q.; Pan, Y.; Fang, X.; Xu, H.; Zhao, T.; Zhu, G.; Jiang, T.; Li, S.; Cao, H. Efficacy and safety of PD-1/PD-L1 checkpoint inhibitors versus anti-PD-1/PD-L1 combined with other therapies for tumors: A systematic review. Cancers 2023, 15, 682. [Google Scholar] [CrossRef] [PubMed]
- Korman, A.J.; Garrett-Thomson, S.C.; Lonberg, N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat. Rev. Drug Discov. 2021, 21, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Moy, J.; Ferris, R.L. Immunotherapy for head and neck squamous cell carcinoma. Curr. Oncol. Rep. 2018, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Kujan, O.; van Schaijik, B.; Farah, C.S. Immune Checkpoint Inhibitors in Oral Cavity Squamous Cell Carcinoma and Oral Po-tentially Malignant Disorders: A Systematic Review. Cancers 2020, 12, 1937. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.T.; Xia, R.H.; Zhu, D.W.; Dou, S.J.; Zhu, G.P.; Dong, M.J.; Wang, L.Z.; Sun, Q.; Zhao, T.C.; Zhou, Z.H.; et al. A pilot study of neoadjuvant combination of anti-PD-1 camrelizumab and VEGFR2 inhibitor apatinib for locally advanced resectable oral squamous cell carcinoma. Nat. Commun. 2022, 13, 5378. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, J.D.; Hanna, G.J.; Jo, V.Y.; Rawal, B.; Chen, Y.H.; Catalano, P.S.; Lako, A.; Ciantra, Z.; Weirather, J.L.; Criscitiello, S.; et al. Neoadjuvant Nivolumab or Nivolumab Plus Ipilimumab in Untreated Oral Cavity Squamous Cell Carcinoma: A Phase 2 Open-Label Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Wise-Draper, T.M.; Gulati, S.; Palackdharry, S.; Hinrichs, B.H.; Worden, F.P.; Old, M.O.; Dunlap, N.E.; Kaczmar, J.M.; Patil, Y.; Riaz, M.K.; et al. Phase II Clinical Trial of Neoadjuvant and Adjuvant Pembrolizumab in Resectable Local-Regionally Advanced Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2022, 28, 1345–1352. [Google Scholar] [CrossRef]
- Haddad, R.I.; Harrington, K.; Tahara, M.; Ferris, R.L.; Gillison, M.; Fayette, J.; Daste, A.; Koralewski, P.; Zurawski, B.; Taberna, M.; et al. Nivolumab Plus Ipilimumab Versus EXTREME Regimen as First-Line Treatment for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: The Final Results of CheckMate 651. J. Clin. Oncol. 2023, 41, 2166–2180. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, C.; Cao, Y.; Wang, J.; Jiao, S.; Zhang, J.; Wang, M.; Tang, P.; Ouyang, Z.; Liang, W.; et al. Blockade of dual immune checkpoint inhibitory signals with a CD47/PD-L1 bispecific antibody for cancer treatment. Theranostics 2023, 13, 148–160. [Google Scholar] [CrossRef]
- Chen, S.H.; Dominik, P.K.; Stanfield, J.; Ding, S.; Yang, W.; Kurd, N.; Llewellyn, R.; Heyen, J.; Wang, C.; Melton, Z.; et al. Dual checkpoint blockade of CD47 and PD-L1 using an affinity-tuned bispecific antibody maximizes antitumor immunity. J. Immunother. Cancer 2021, 9, e003464. [Google Scholar] [CrossRef]
- Son, J.; Hsieh, R.C.; Lin, H.Y.; Krause, K.J.; Yuan, Y.; Biter, A.B.; Welsh, J.; Curran, M.A.; Hong, D.S. Inhibition of the CD47-SIRPα axis for cancer therapy: A systematic review and meta-analysis of emerging clinical data. Front. Immunol. 2022, 13, 1027235. [Google Scholar] [CrossRef] [PubMed]
- Veillette, A.; Chen, J. SIRPα-CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol. 2018, 39, 173–184. [Google Scholar] [CrossRef]
- Weiskopf, K. Cancer immunotherapy targeting the CD47/SIRPα axis. Eur. J. Cancer 2017, 76, 100–109. [Google Scholar] [CrossRef]
- Yang, H.; Xun, Y.; You, H. The landscape overview of CD47-based immunotherapy for hematological malignancies. Biomark. Res. 2023, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Ochiai, T.; Shen, F.C.; Hasegawa, H. Phenotypic alteration of basal cells in oral lichen planus; switching keratin 19 and desmoglein 1 expression. J. Oral Sci. 2018, 60, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, I.; Neves, J.F.; Carrero, D.; Peng, Q.; Palasz, N.; Liakath-Ali, K.; Lord, G.M.; Morgan, P.R.; Lombardi, G.; Watt, F.M. Immunomodulatory role of keratin 76 in oral and gastric cancer. Nat. Commun. 2018, 9, 3437. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, I.; Watt, F.M. The role of keratins in modulating carcinogenesis via communication with cells of the immune system. Cell Stress 2019, 3, 136–138. [Google Scholar] [CrossRef]
- Lin, D.; Hu, Q.; Yang, L.; Zeng, X.; Xiao, Y.; Wang, D.; Dai, W.; Lu, H.; Fang, J.; Tang, Z.; et al. The niche-specialist and age-related oral microbial ecosystem: Crosstalk with host immune cells in homeostasis. Microb. Genom. 2022, 8, mgen000811. [Google Scholar] [CrossRef]
- Qu, T.; Zhong, T.; Pang, X.; Huang, Z.; Jin, C.; Wang, Z.M.; Li, B.; Xia, Y. Ligufalimab, a novel anti-CD47 antibody with no hemagglutination demonstrates both monotherapy and combo antitumor activity. J. Immunother. Cancer 2022, 10, e005517. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Chen, W.; Wang, W.; Yang, F.; Liu, X.; Sheng, Y.; Du, K.; He, M.; Lyu, X.; et al. A pH-dependent anti-CD47 antibody that selectively targets solid tumors and improves therapeutic efficacy and safety. J. Hematol. Oncol. 2023, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Matlung, H.L.; Szilagyi, K.; Barclay, N.A.; van den Berg, T.K. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 2017, 276, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, N.J.; Patnaik, A.; Liao, J.B.; Moroney, J.W.; Miller, D.S.; Fleming, G.F.; Axt, M.; Wang, Y.V.; Agoram, B.; Volkmer, J.-P.; et al. A phase Ib study of the anti-CD47 antibody magrolimab with the PD-L1 inhibitor avelumab (a) in solid tumor (ST) and ovarian cancer (OC) patients. J. Clin. Oncol. 2020, 38, 18. [Google Scholar] [CrossRef]
- Matoba, T.; Imai, M.; Ohkura, N.; Kawakita, D.; Ijichi, K.; Toyama, T.; Morita, A.; Murakami, S.; Sakaguchi, S.; Yamazaki, S. Regulatory T cells expressing abundant CTLA-4 on the cell surface with a proliferative gene profile are key features of human head and neck cancer. Int. J. Cancer 2019, 144, 2811–2822. [Google Scholar] [CrossRef]
- Minohara, K.; Imai, M.; Matoba, T.; Wing, J.B.; Shime, H.; Odanaka, M.; Uraki, R.; Kawakita, D.; Toyama, T.; Takahashi, S.; et al. Mature dendritic cells enriched in regulatory molecules may control regulatory T cells and the prognosis of head and neck cancer. Cancer Sci. 2023, 114, 1256–1269. [Google Scholar] [CrossRef]

| Variable | n | % |
|---|---|---|
| Gender, male, n (%) | 13 | (61.9) |
| Gender, female, n (%) | 8 | (38.1) |
| Age, mean (SD) y | 64.9 | (15.4) |
| Age, median y | 66.5 | |
| Primary site, n (%) | ||
| Tongue | 6 | (28.6) |
| Buccal mucosa | 3 | (14.3) |
| Mandibular gingiva | 8 | (38.1) |
| Maxillary gingiva | 4 | (19.0) |
| Clinical T stage, n (%) | ||
| T1 | 2 | (9.5) |
| T2 | 4 | (19.0) |
| T4a | 14 | (66.7) |
| T4b | 1 | (4.8) |
| Clinical N stage, n (%) | ||
| N0 | 9 | (42.9) |
| N1 | 5 | (23.8) |
| N2a | 1 | (4.8) |
| N2b | 4 | (19.0) |
| N2c | 2 | (9.5) |
| Clinical Stage, n (%) | ||
| Stage 1 | 2 | (9.5) |
| Stage 2 | 3 | (14.3) |
| Stage 3 | 1 | (4.8) |
| Stage 4a | 15 | (71.4) |
| Death in 5-year period, n (%) | 5 | (23.8) |
| Recurrence or metastasis in 5-year period, n (%) | 3 | (14.3) |
| No. | Gender | Age | Primary Site | T | N | Stage | PD-L1 | PD-1 | CD4 | CD8 | CD47 | CD56 | CD11c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 75 | Maxillary gingiva | 4a | 1 | 4a | ++ | ++ | +++ | +++ | +++ | + | + |
| 2 | M | 65 | Tongue | 1 | 0 | 1 | + | ++ | + | + | +++ | ND | ND |
| 3 | M | 61 | Mandibular gingiva | 4a | 0 | 4a | + | +++ | +++ | +++ | +++ | ND | + |
| 4 | M | 72 | Mandibular gingiva | 4a | 1 | 4a | ++ | ++ | +++ | +++ | + | + | ND |
| 5 | F | 75 | Buccal mucosa | 2 | 0 | 2 | + | +++ | +++ | ++ | +++ | + | ND |
| 6 | M | 40 | Tongue | 2 | 0 | 2 | ++ | ++ | + | ++ | +++ | ND | ND |
| 7 | M | 40 | Maxillary gingiva | 4b | 2c | 4a | + | ND | ND | ND | ++ | ND | ND |
| 8 | M | 57 | Tongue | 4a | 2a | 4a | + | + | ND | + | +++ | ND | ND |
| 9 | F | 67 | Mandibular gingiva | 4a | 0 | 4a | ND | + | + | ++ | +++ | ND | + |
| 10 | F | 72 | Maxillary gingiva | 4a | 1 | 4a | ND | + | + | +++ | + | ND | ND |
| 11 | M | 71 | Tongue | 4a | 2b | 4a | + | ND | ++ | ND | +++ | ND | + |
| 12 | M | 72 | Buccal mucosa | 4a | 2b | 4a | + | ND | ++ | ++ | +++ | ND | ND |
| 13 | M | 41 | Tongue | 2 | 0 | 2 | + | ++ | ND | ++ | +++ | ND | ND |
| 14 | F | 46 | Maxillary gingiva | 4a | 2b | 4a | ++ | ND | +++ | ++ | + | + | + |
| 15 | M | 73 | Mandibular gingiva | 4a | 1 | 4a | ++ | ND | ++ | ++ | ++ | ND | ND |
| 16 | F | 56 | Tongue | 2 | 1 | 3 | + | ++ | ++ | +++ | +++ | + | ND |
| 17 | M | 72 | Buccal mucosa | 4a | 2b | 4a | + | ++ | ND | + | +++ | ND | ND |
| 18 | M | 66 | Mandibular gingiva | 4a | 2c | 4a | + | ND | + | + | ++ | ND | ND |
| 19 | F | 78 | Mandibular gingiva | 4a | 0 | 4a | ND | ND | +++ | +++ | ++ | ND | ND |
| 20 | F | 90 | Mandibular gingiva | 4a | 0 | 4a | + | ND | + | + | ++ | ND | ND |
| 21 | M | 75 | Mandibular gingiva | 1 | 0 | 1 | ND | + | +++ | ND | ++ | ND | ND |
| Immune-Related Factors | Summary of Factors | Criteria for Immunohistochemical Staining | Criteria for Immunohistochemical Staining (Two Classifications: Negative/Positive) |
|---|---|---|---|
| PD-L1 | Immune escape from T cells in tumors. | ND: CPS < 1, +: 1 ≤ CPS < 20, ++: CPS ≥20 | negative: CPS < 1, positive: 1 ≤ CPS |
| PD-1 | Receptor on the surface of active T cells. Increased by T cell exhaustion. | Percentage of positive cells (×200) ND: <5%, +: 5–25%, ++: 26–50%, +++: ≥51% | negative: ND, +, positive: ++, +++ |
| CD4 | Marker of helper T cells. | Percentage of positive cells (×200) ND: ≤1 cell, +: 2–4 cells, ++: ≥5 cells, +++: cellular aggregate | negative: ND, +, positive: ++, +++ |
| CD8 | Marker of cytotoxic T cells. | Percentage of positive cells (×200) ND: <5%, +: 5–25%, ++: 26–50%, +++: ≥51% | negative: ND, +, positive: ++, +++ |
| CD47 | Immune escape from cDCs and M1 macrophages in tumors. | Percentage of positive cells (×200) ND: <5%, +: 5–25%, ++: 26–50%, +++: ≥51% | negative: ND, +, ++ positive: +++ |
| CD56 | Marker of NK cells. | Percentage of positive cells (×400) ND: negative, +: positive | same as on the left |
| CD11c | Markers of conventional dendritic cells (cDCs) and M1 macrophages. | Percentage of positive cells (×400) ND: <10 cells, +: 10–50 cells, ++: ≥51 cells | negative: ND, positive: +, ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitsukawa, Y.; Fukumoto, C.; Hyodo, T.; Komiyama, Y.; Shiraishi, R.; Koike, A.; Yagisawa, S.; Kunitomi, Y.; Hasegawa, T.; Kotani, W.; et al. Difference between Keratinized- and Non-Keratinized-Originating Epithelium in the Process of Immune Escape of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2024, 25, 3821. https://doi.org/10.3390/ijms25073821
Kitsukawa Y, Fukumoto C, Hyodo T, Komiyama Y, Shiraishi R, Koike A, Yagisawa S, Kunitomi Y, Hasegawa T, Kotani W, et al. Difference between Keratinized- and Non-Keratinized-Originating Epithelium in the Process of Immune Escape of Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2024; 25(7):3821. https://doi.org/10.3390/ijms25073821
Chicago/Turabian StyleKitsukawa, Yoshiaki, Chonji Fukumoto, Toshiki Hyodo, Yuske Komiyama, Ryo Shiraishi, Aya Koike, Shuma Yagisawa, Yosuke Kunitomi, Tomonori Hasegawa, Wataru Kotani, and et al. 2024. "Difference between Keratinized- and Non-Keratinized-Originating Epithelium in the Process of Immune Escape of Oral Squamous Cell Carcinoma" International Journal of Molecular Sciences 25, no. 7: 3821. https://doi.org/10.3390/ijms25073821
APA StyleKitsukawa, Y., Fukumoto, C., Hyodo, T., Komiyama, Y., Shiraishi, R., Koike, A., Yagisawa, S., Kunitomi, Y., Hasegawa, T., Kotani, W., Ishida, K., Wakui, T., & Kawamata, H. (2024). Difference between Keratinized- and Non-Keratinized-Originating Epithelium in the Process of Immune Escape of Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences, 25(7), 3821. https://doi.org/10.3390/ijms25073821






